Amorphous Blue Phase III: Structure, Materials, and Properties
Abstract
:1. Introduction
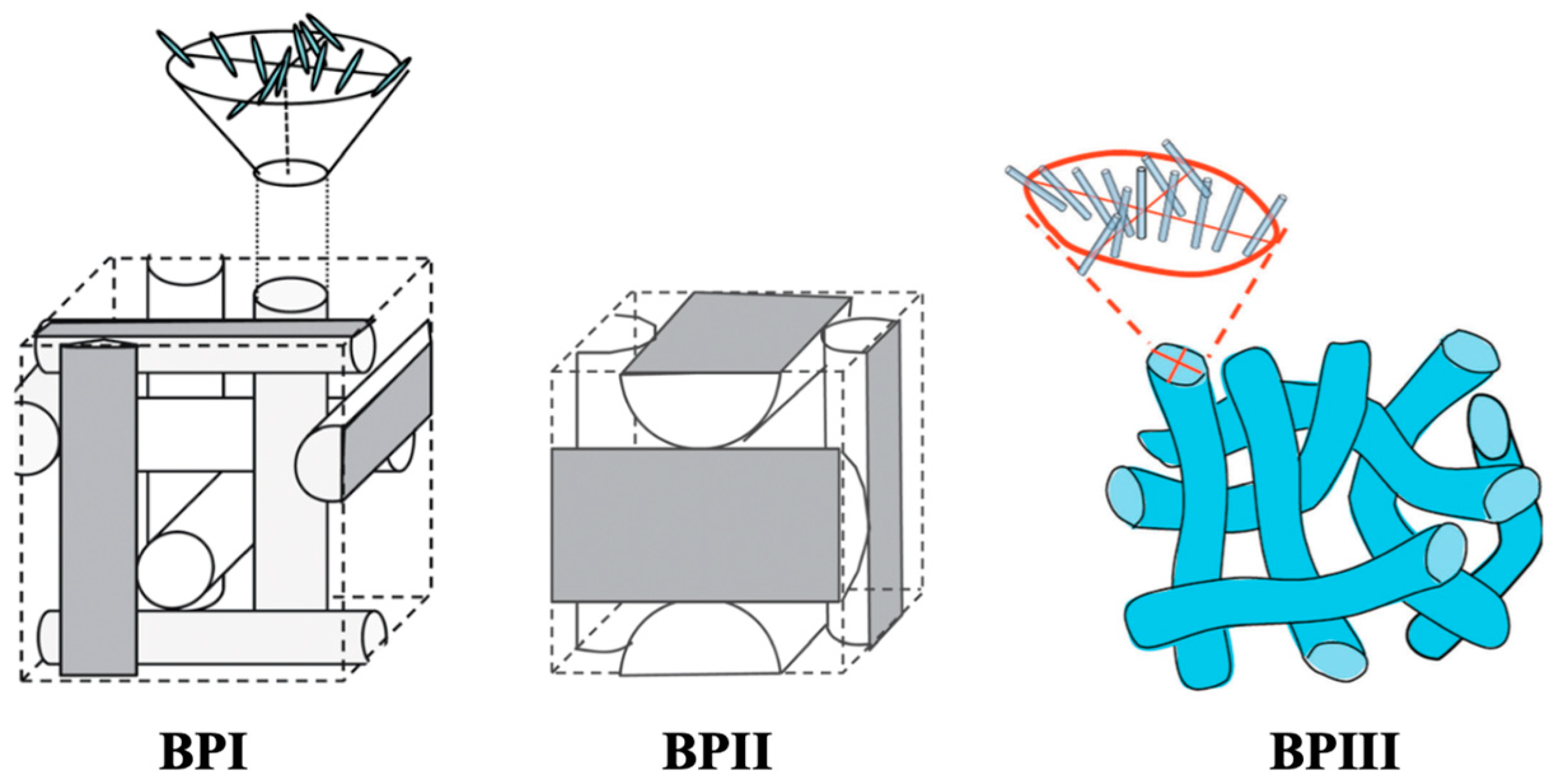
2. Phase Structure of BPIII
- (1)
- BPIII is the highest-temperature blue phase.
- (2)
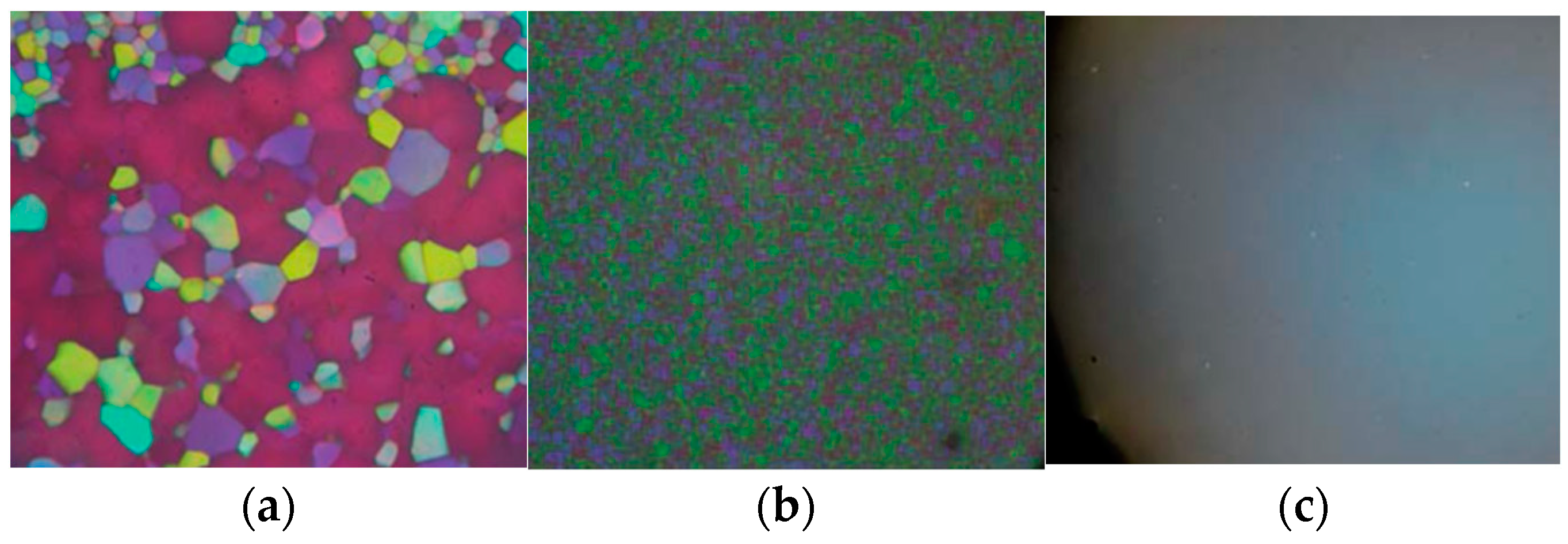
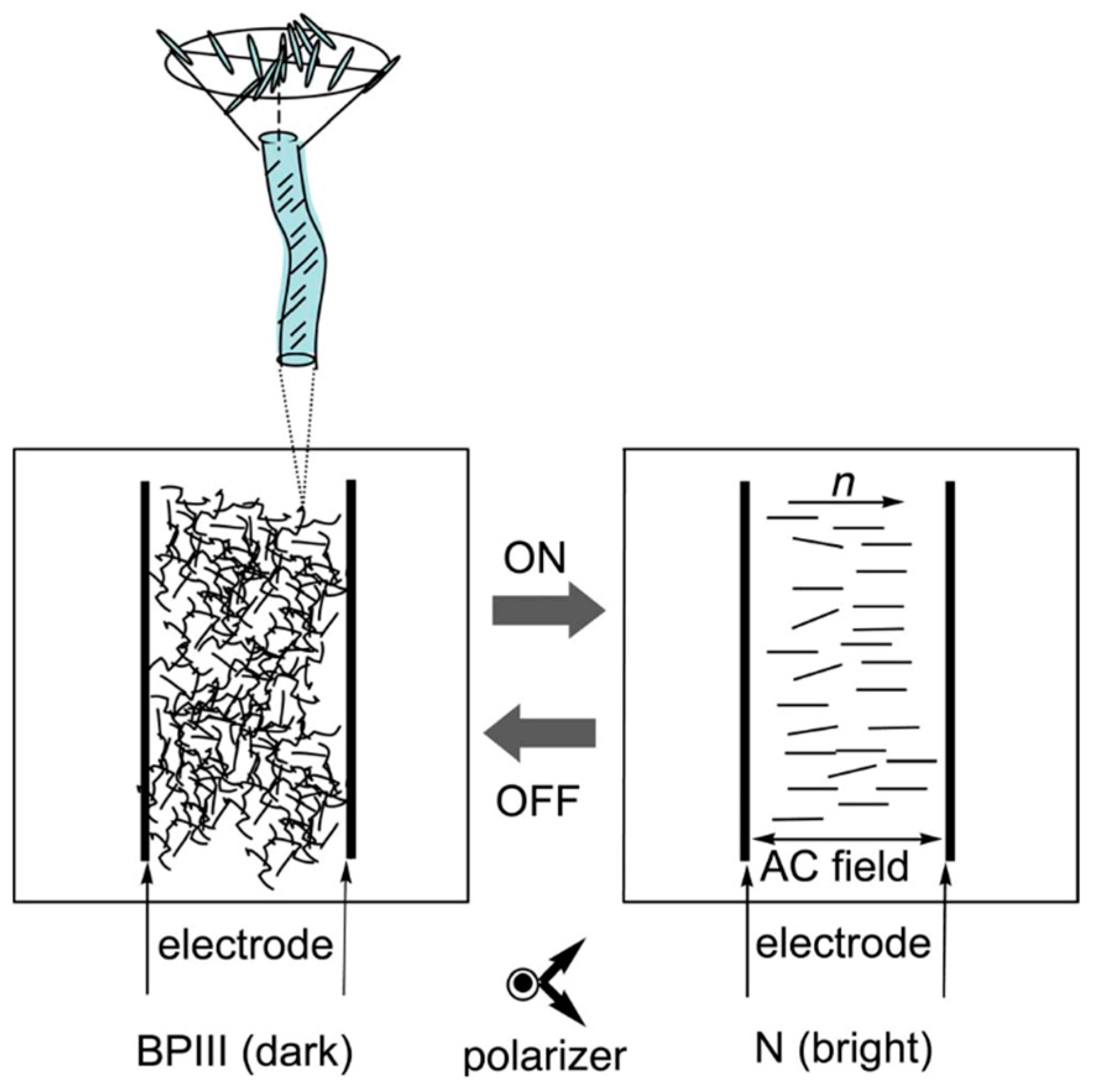
- The visual appearance of BPIII is foggy, and it is often bluish or grayish in color. BPIII appears to be closer in structure to the isotropic liquid.
- (3)
- (4)
- A heat capacity peak between BPIII and the isotropic liquid was much larger than that between BPIII and BPII. Therefore, BPIII appears to be closer in structure to BPII than to the isotropic phase, in contrast to the visual appearance [43].
- (5)
- BPIII shows higher shear elasticity and viscosity than the BPII and isotropic phases [44].
- (6)
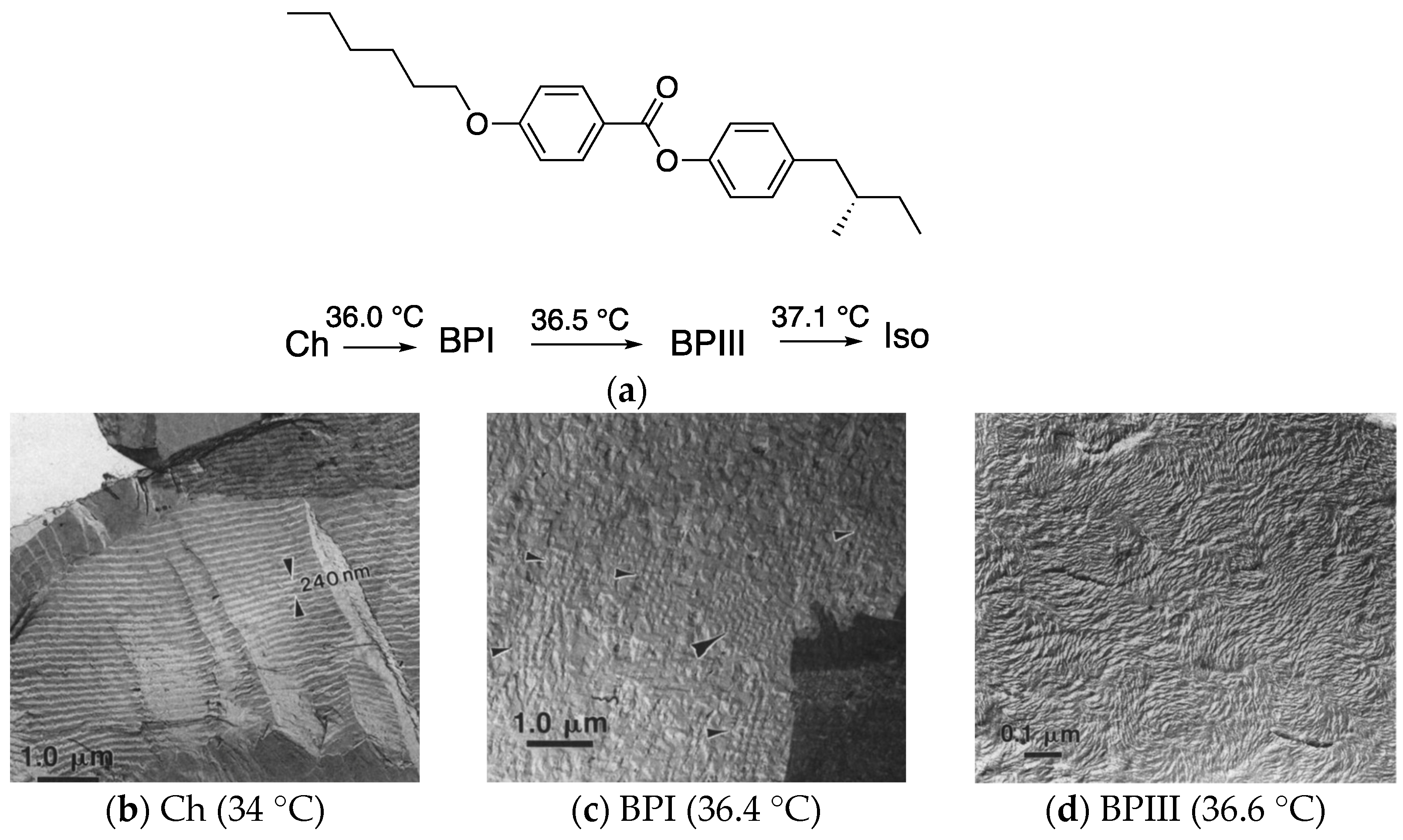
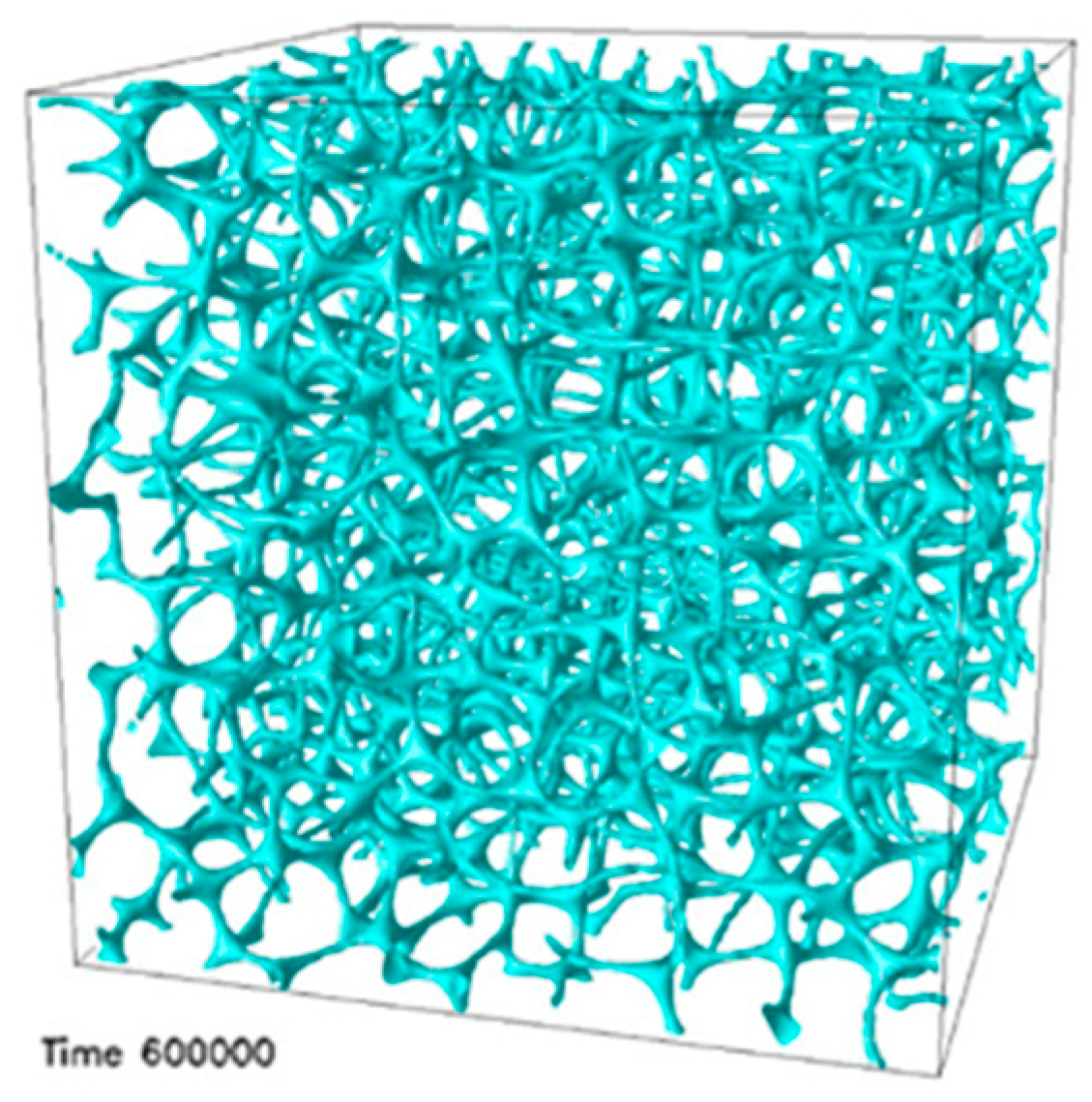
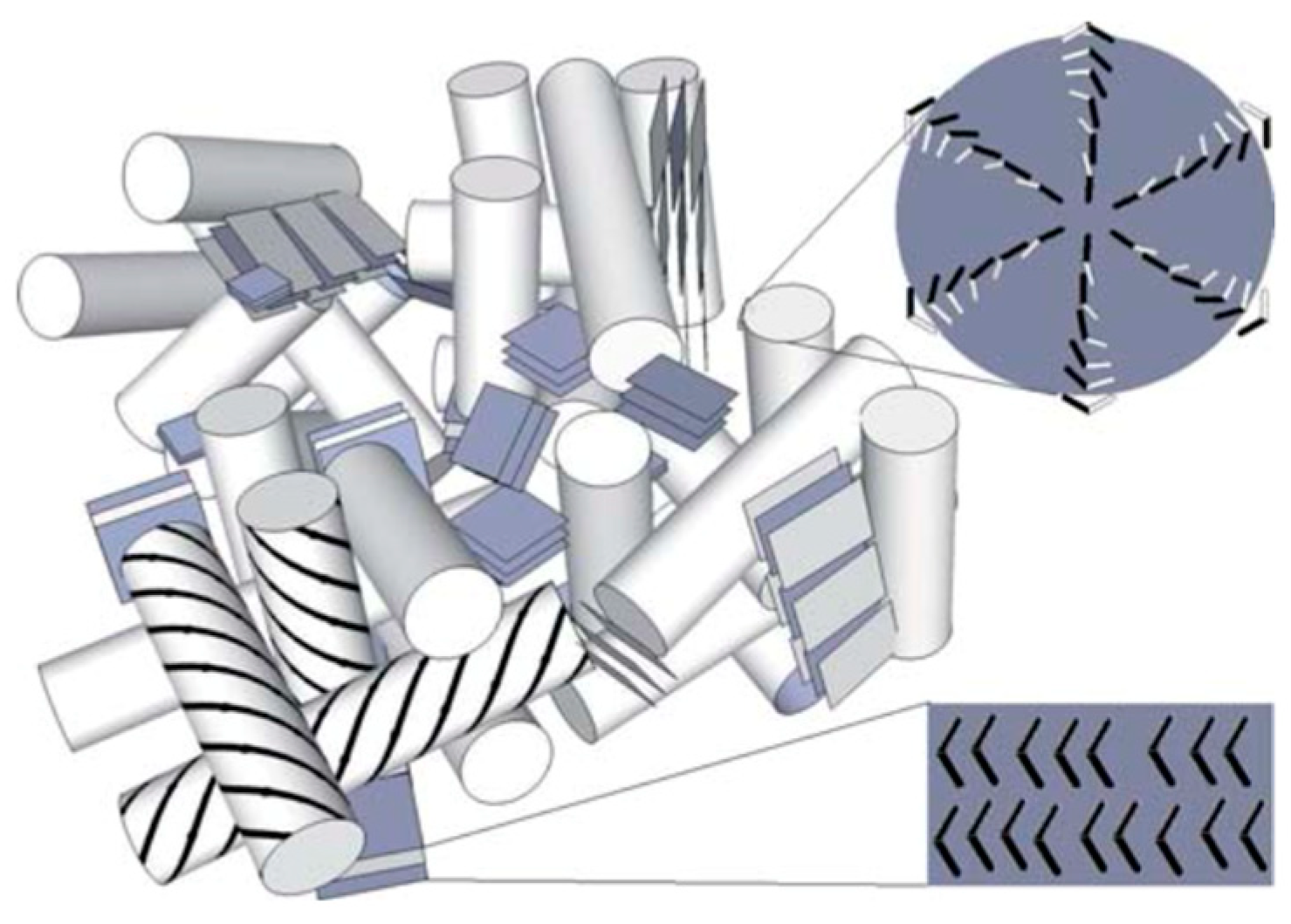
- Transmission electron microscopy (TEM) of a BPIII compound (CE4) shows that the BPIII is a spaghetti-like tangle of DTCs of diameters a fraction of the cholesteric pitch. No long-range structure or periodicity is present, although the DTCs appear aligned over distances of a few microns [45].
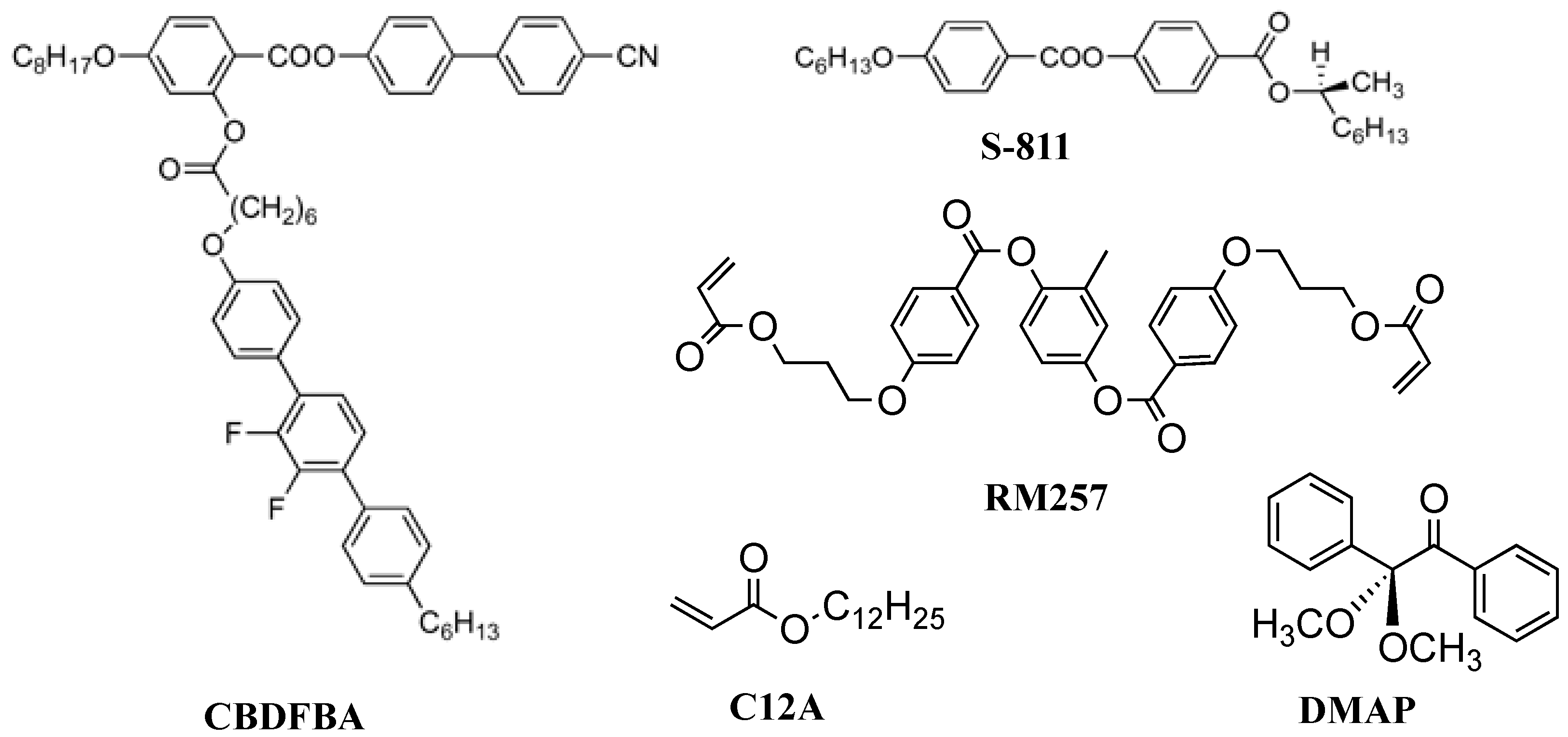
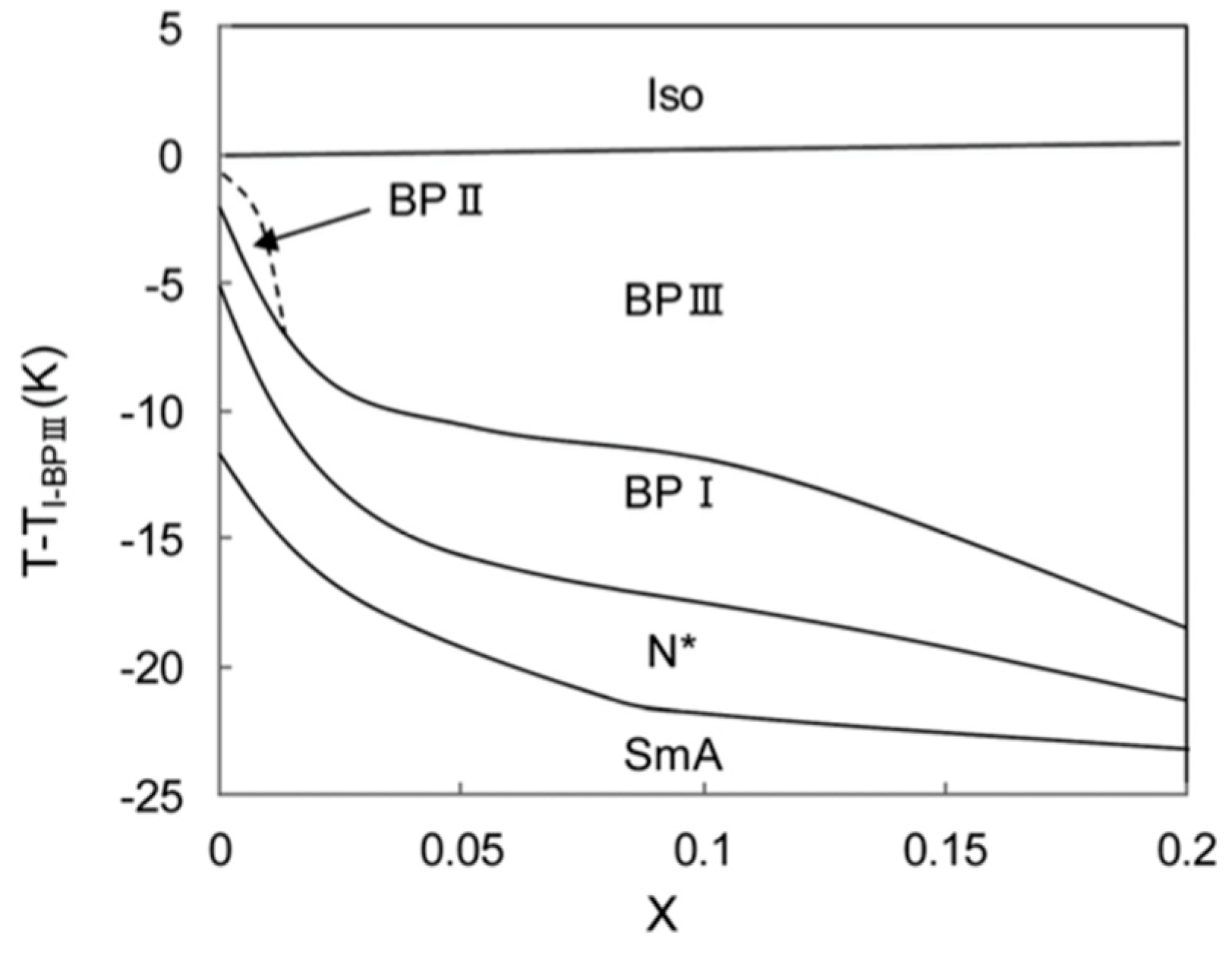
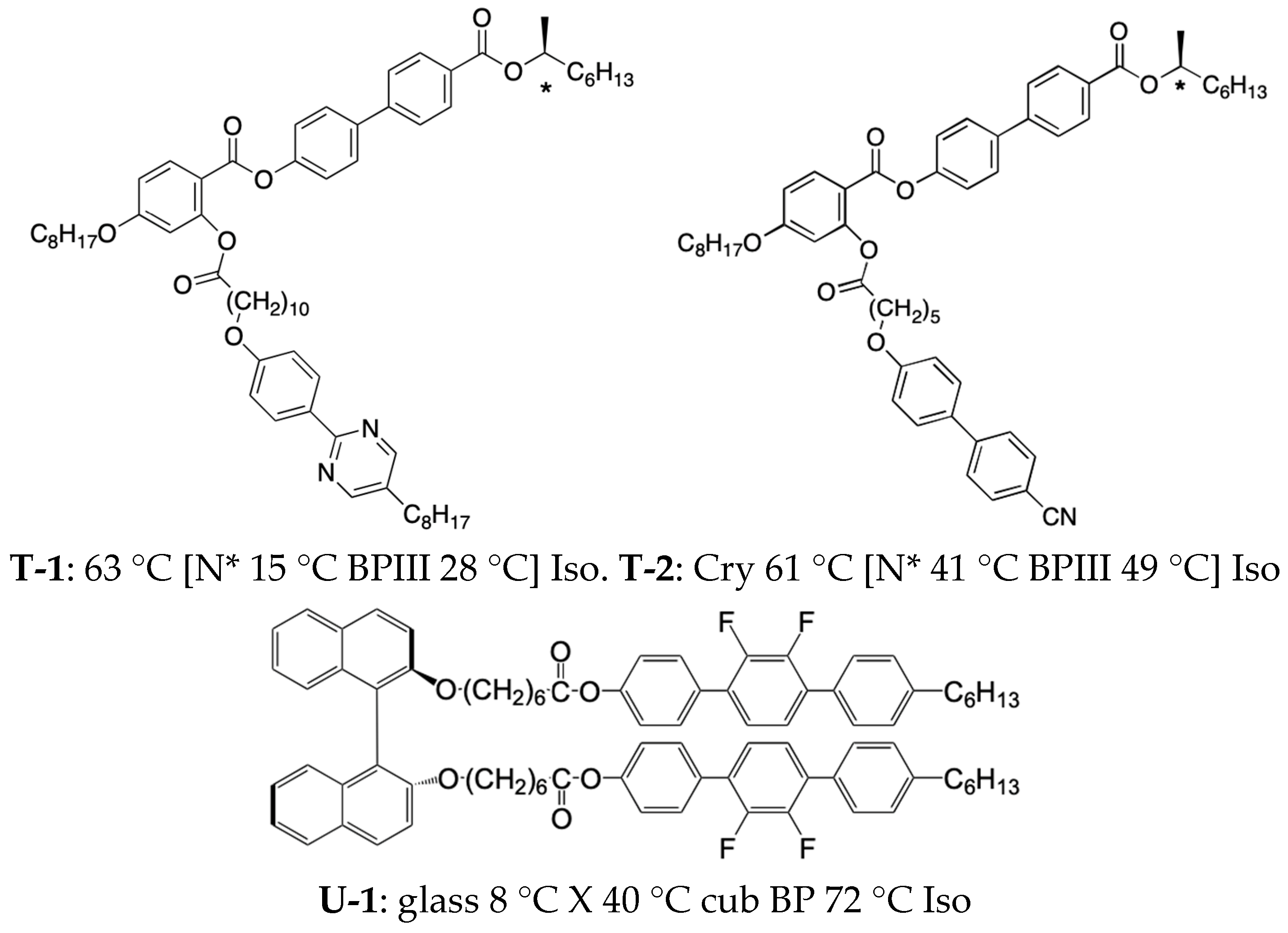
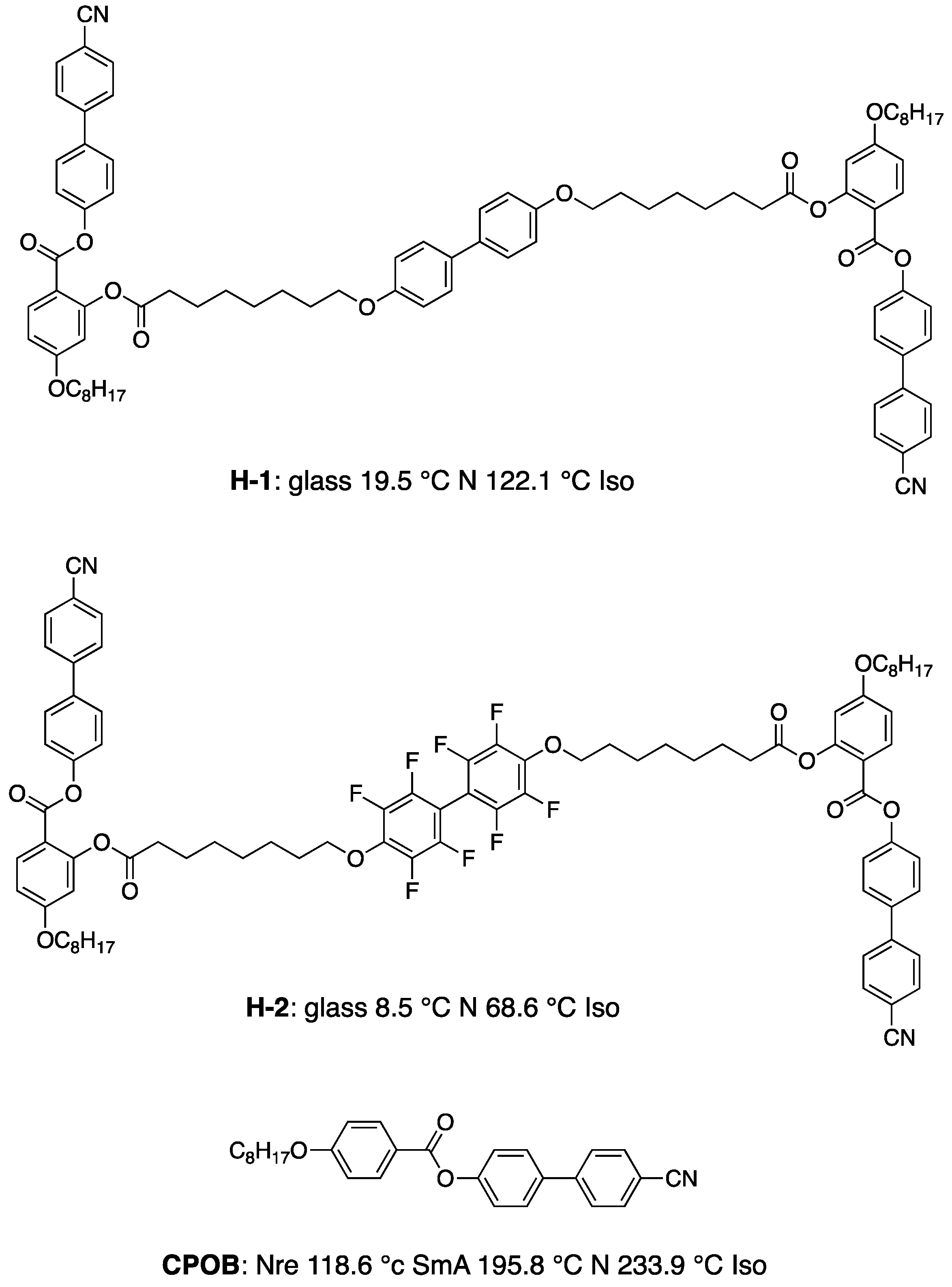
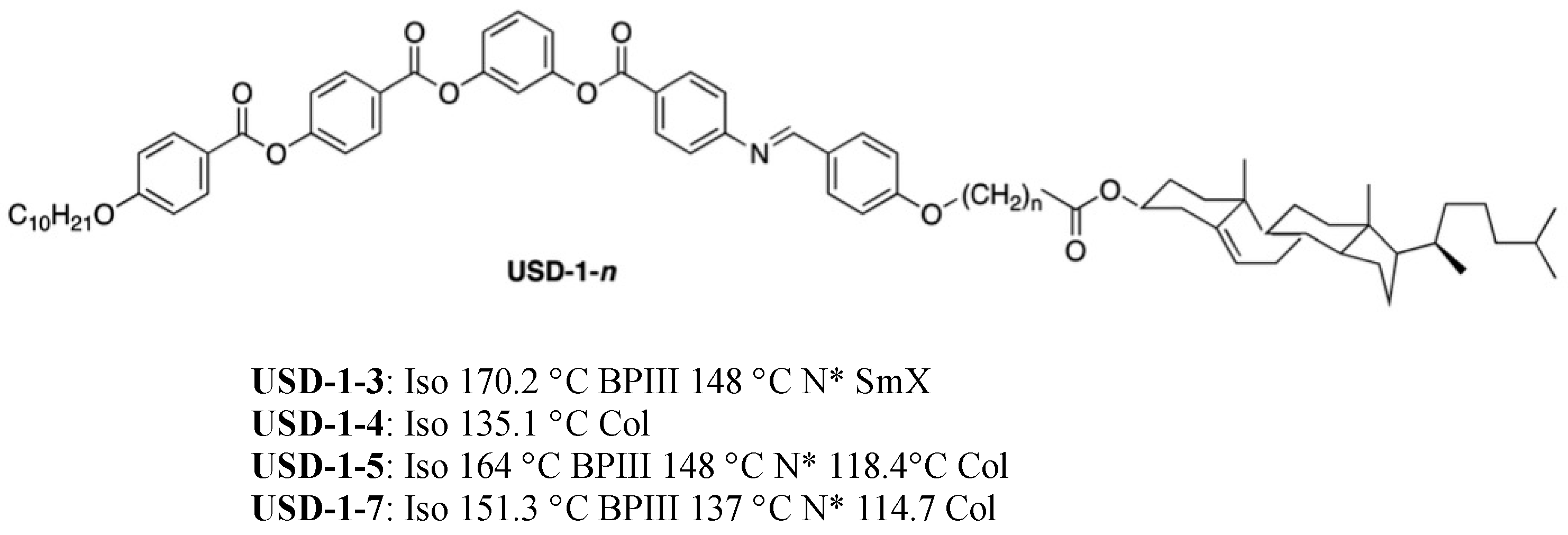
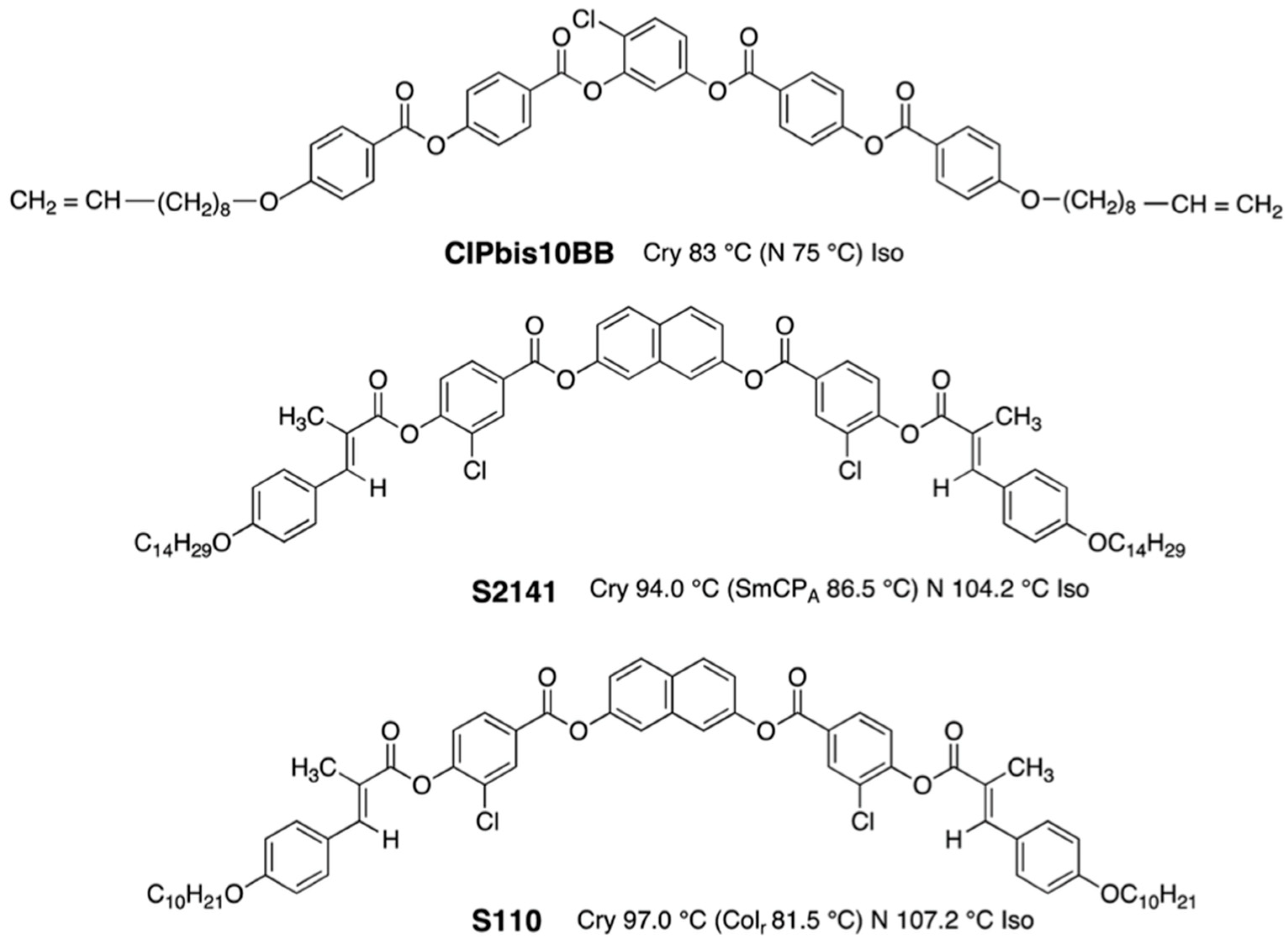
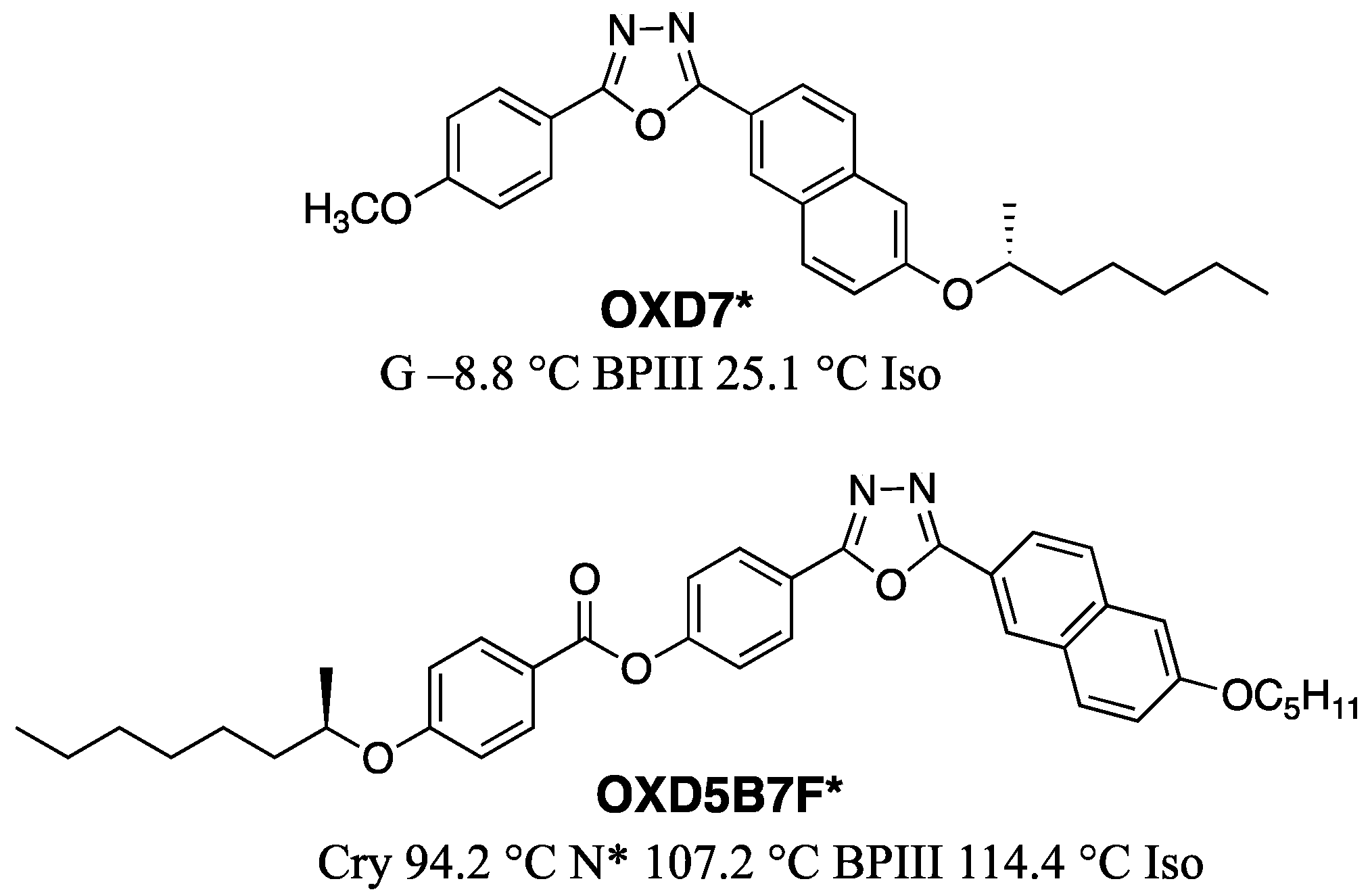
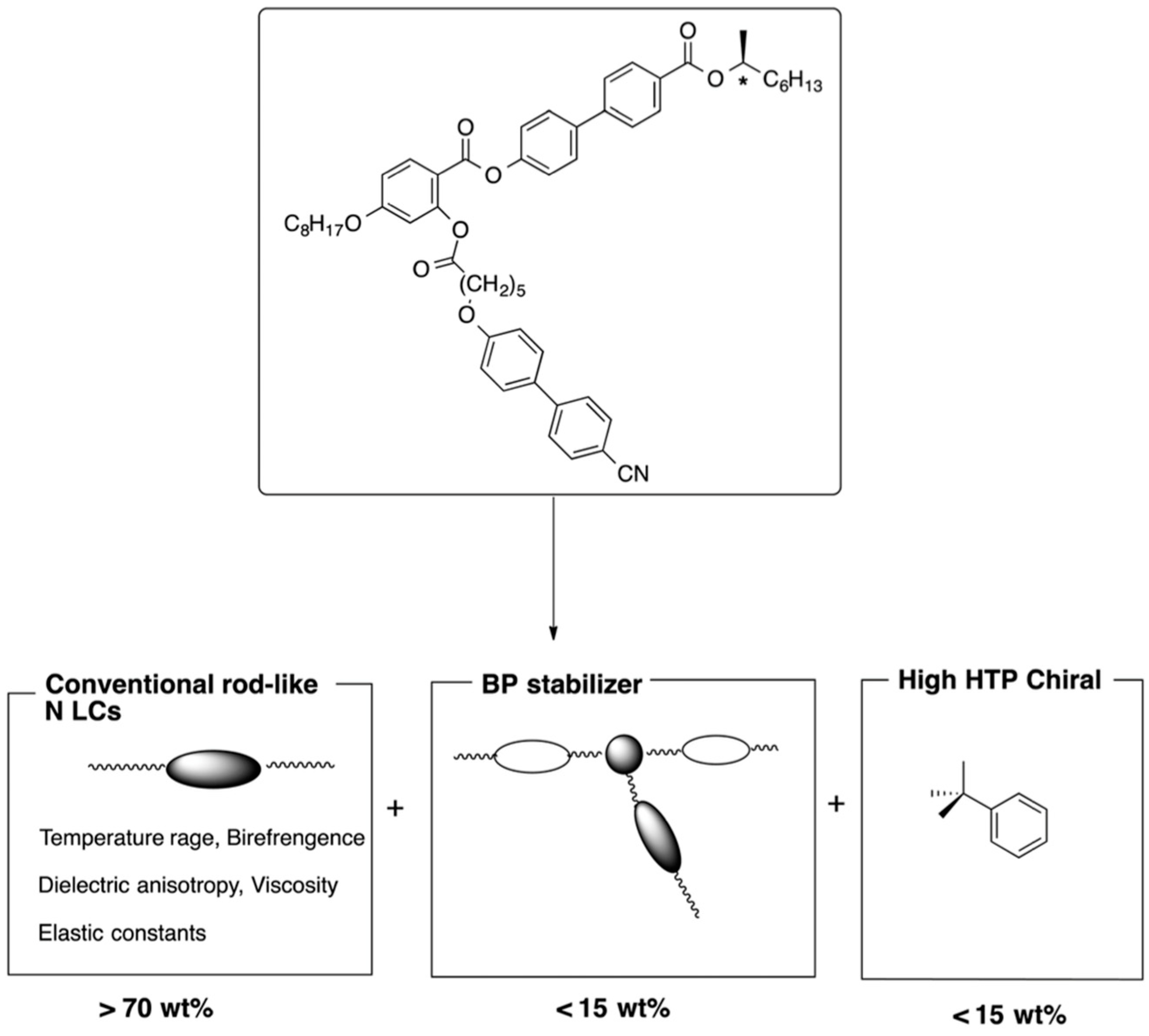
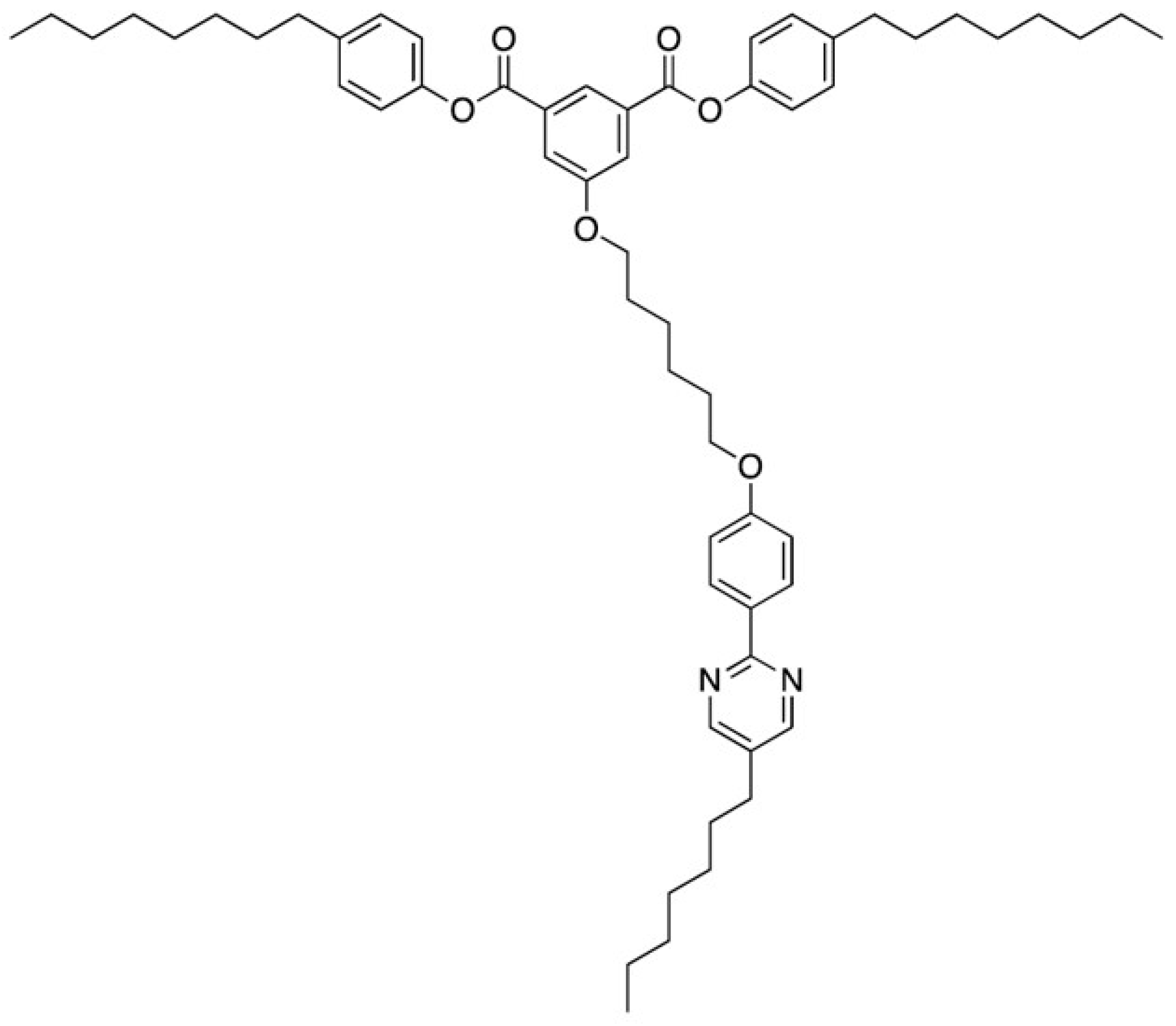
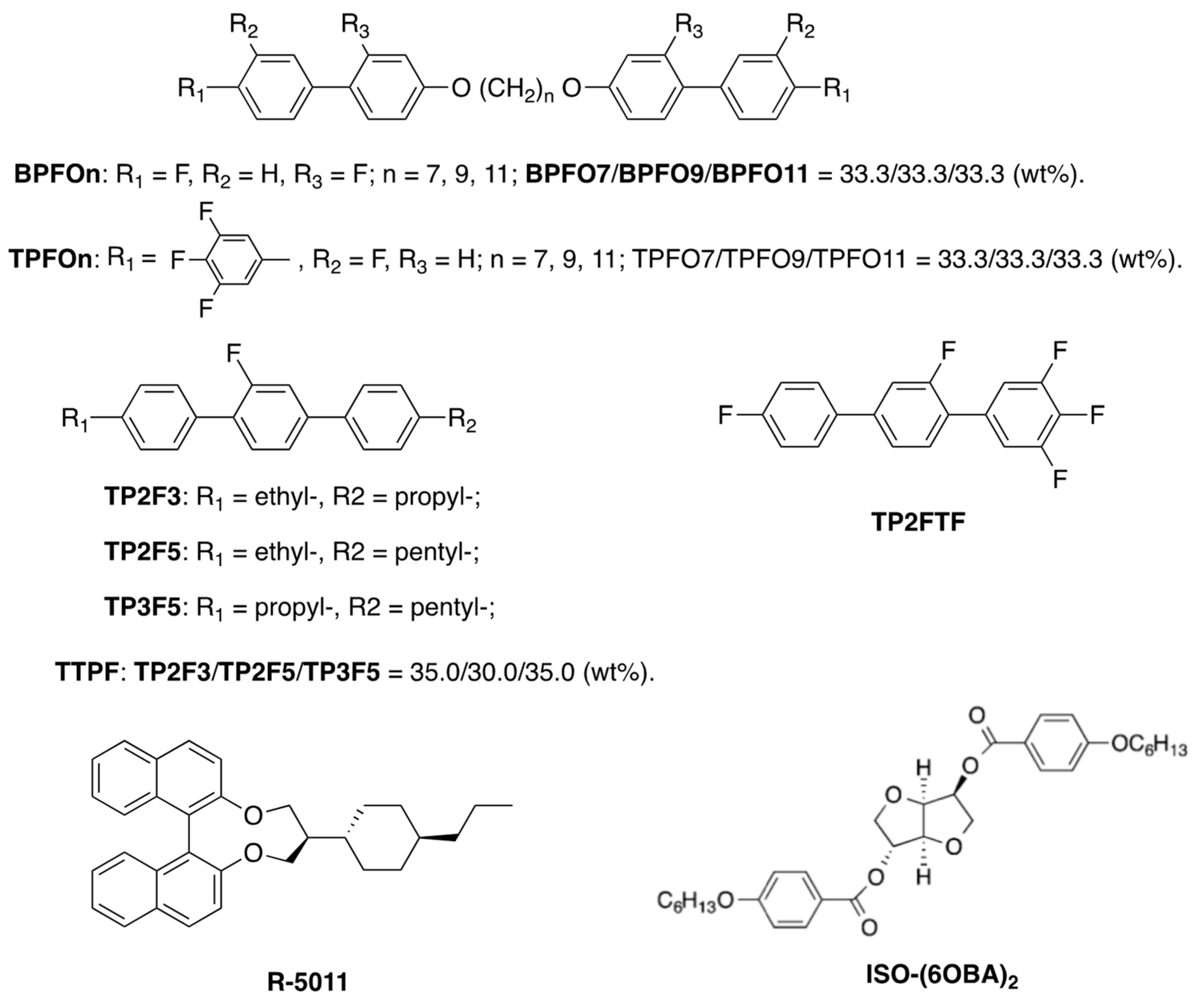
3. Materials Stabilizing BPIII
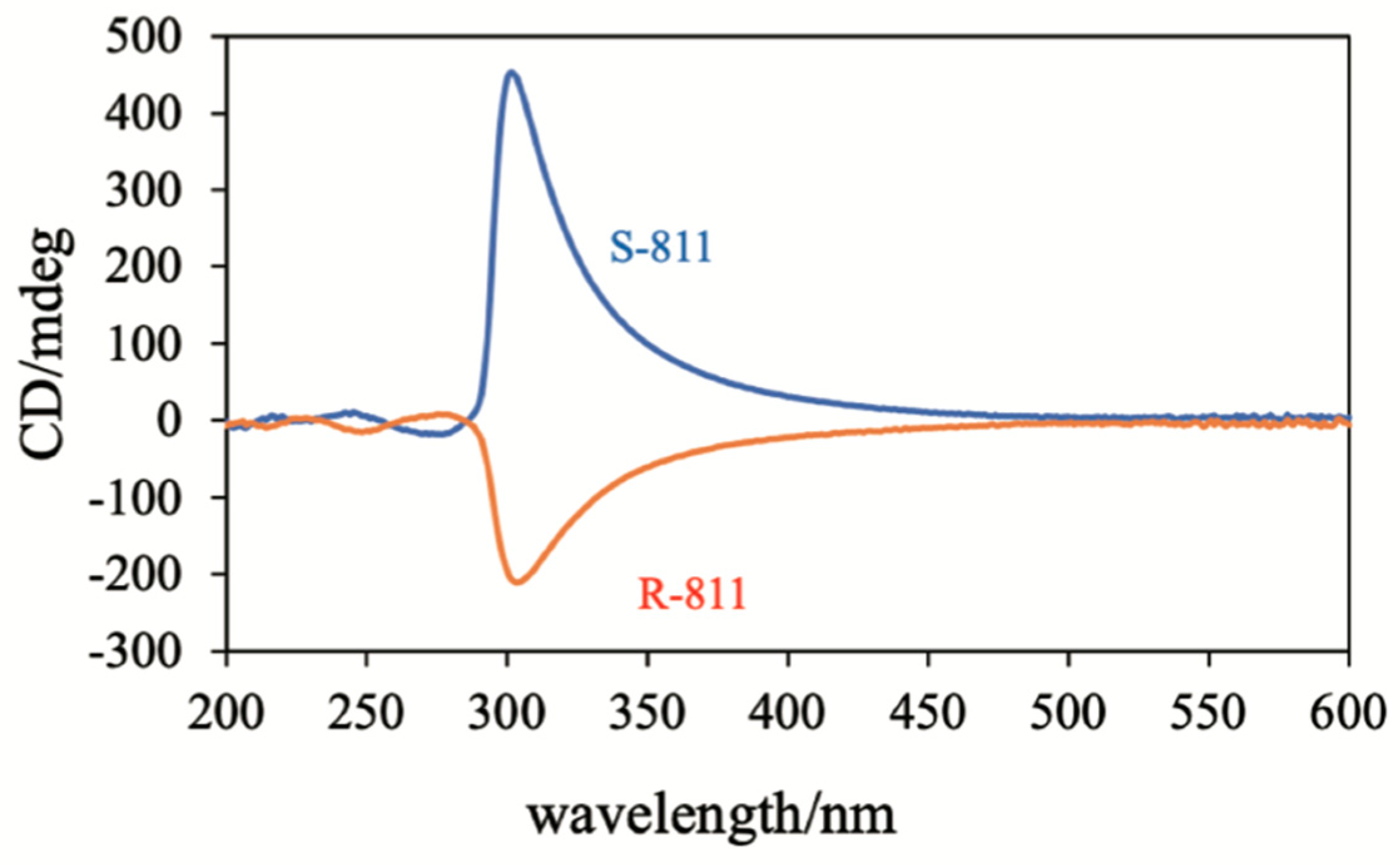
4. Physical Properties in BPIII
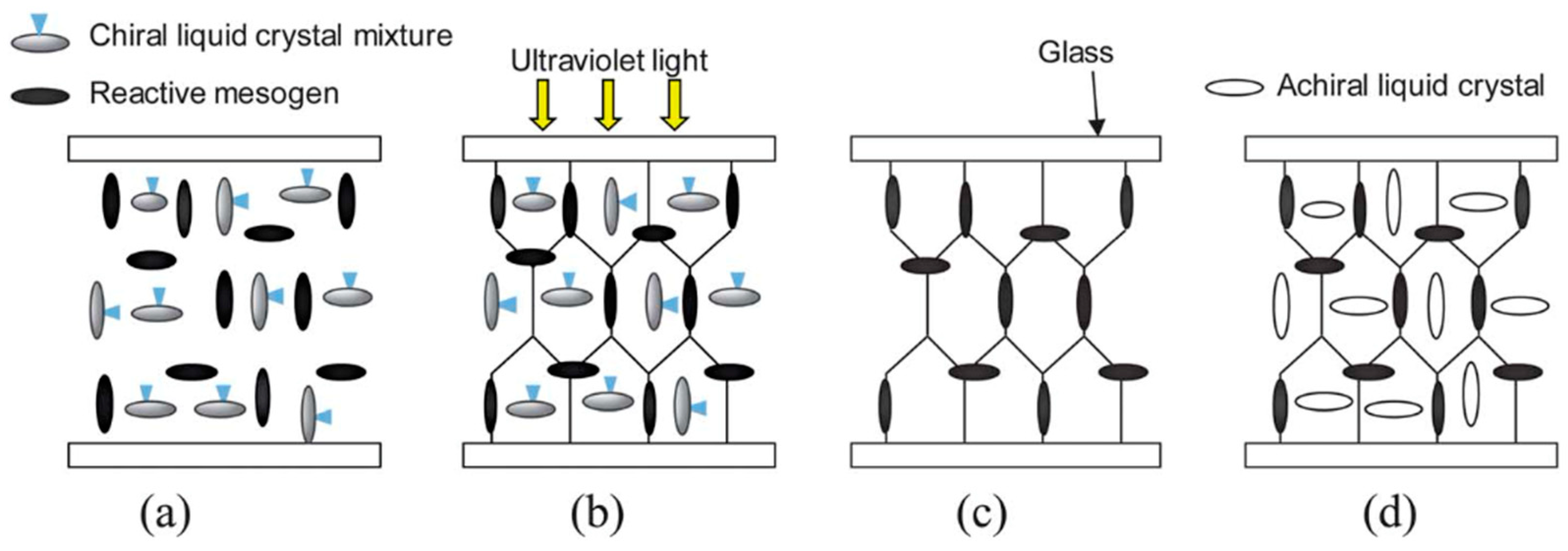
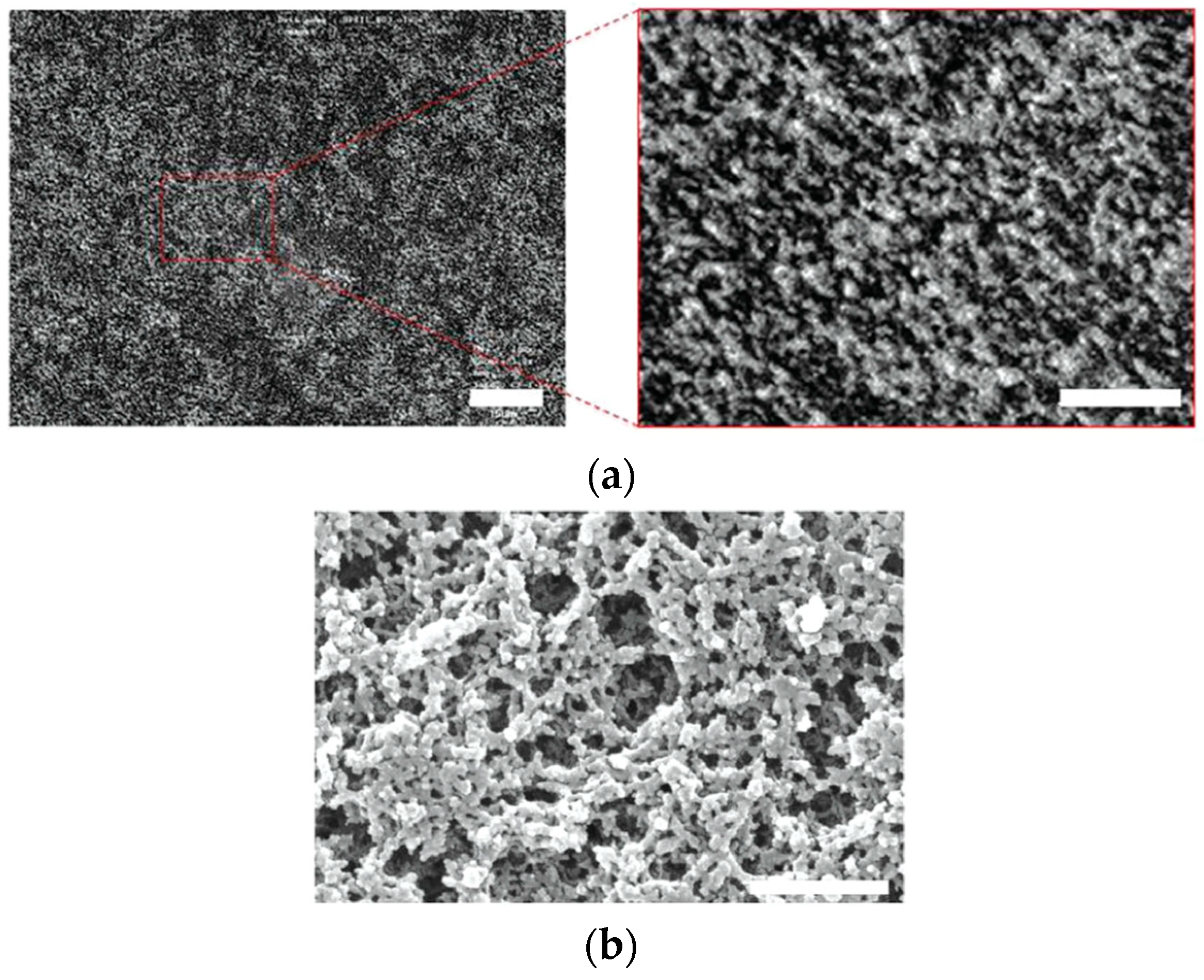
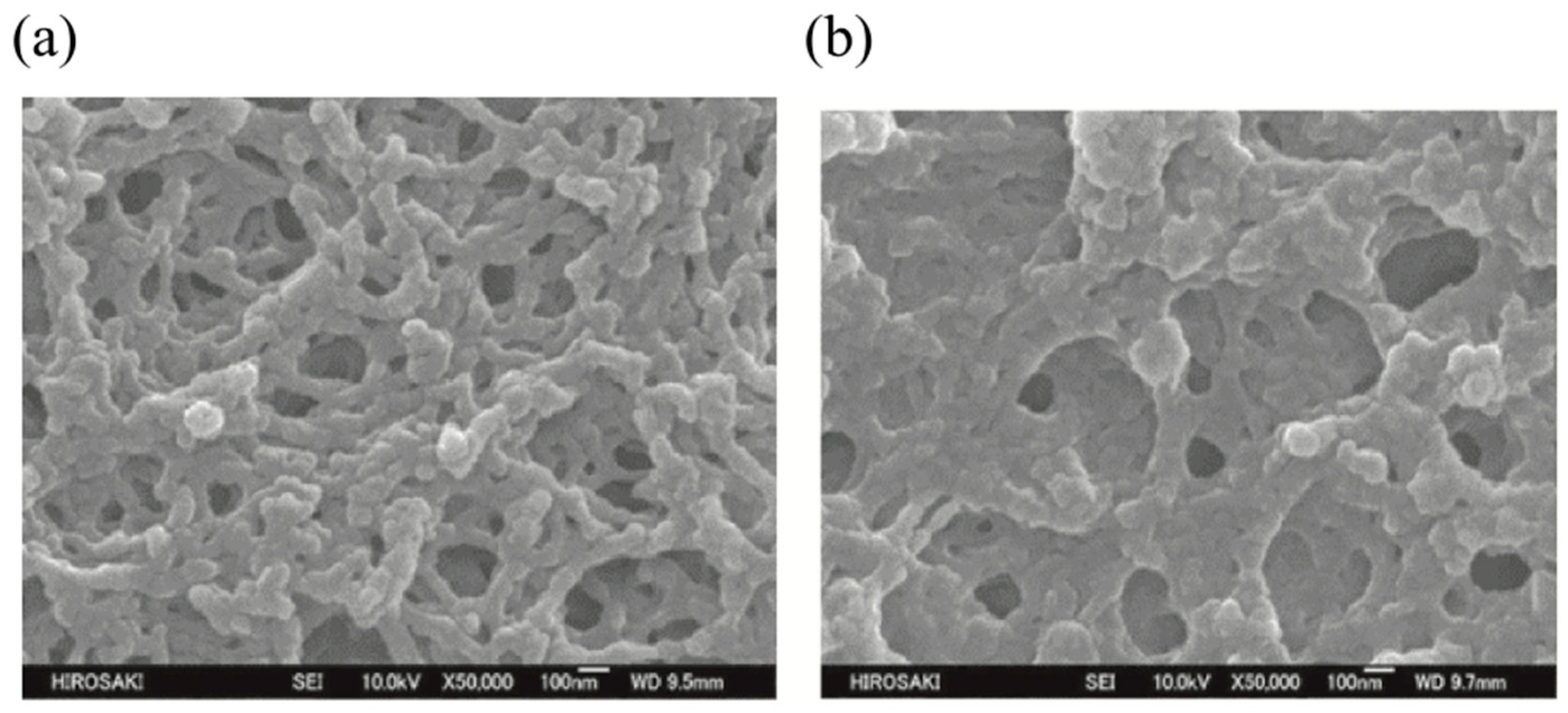
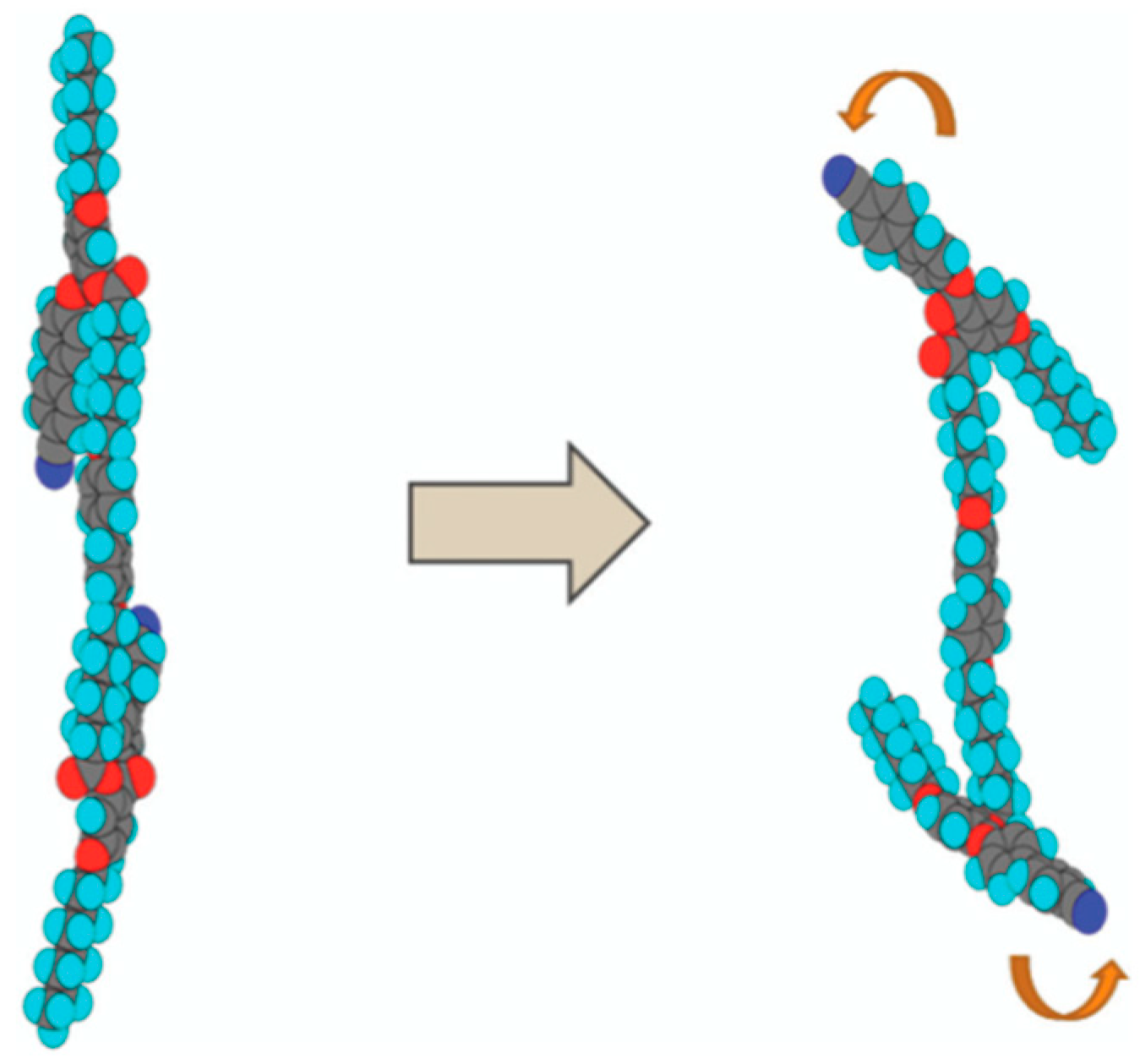
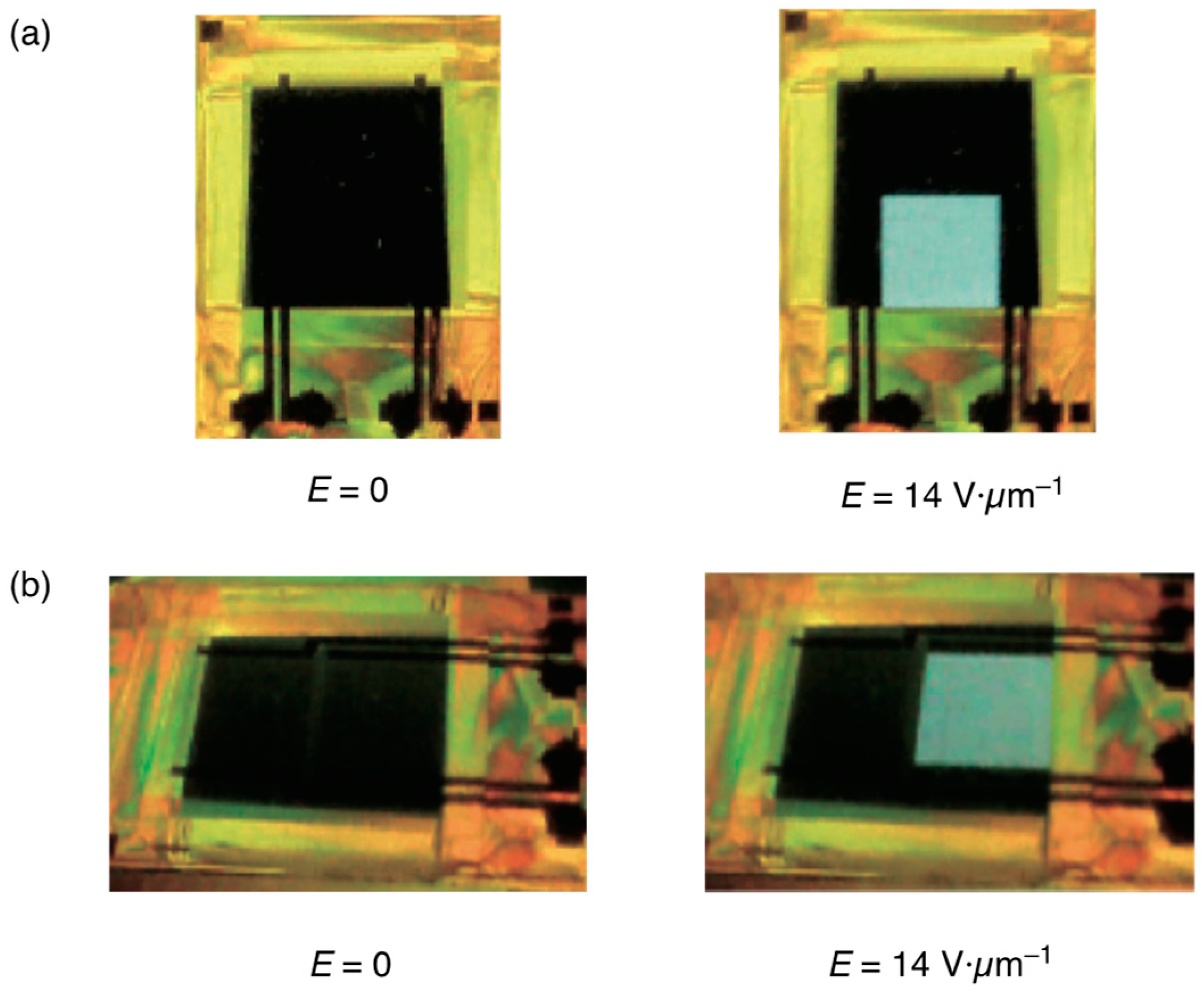
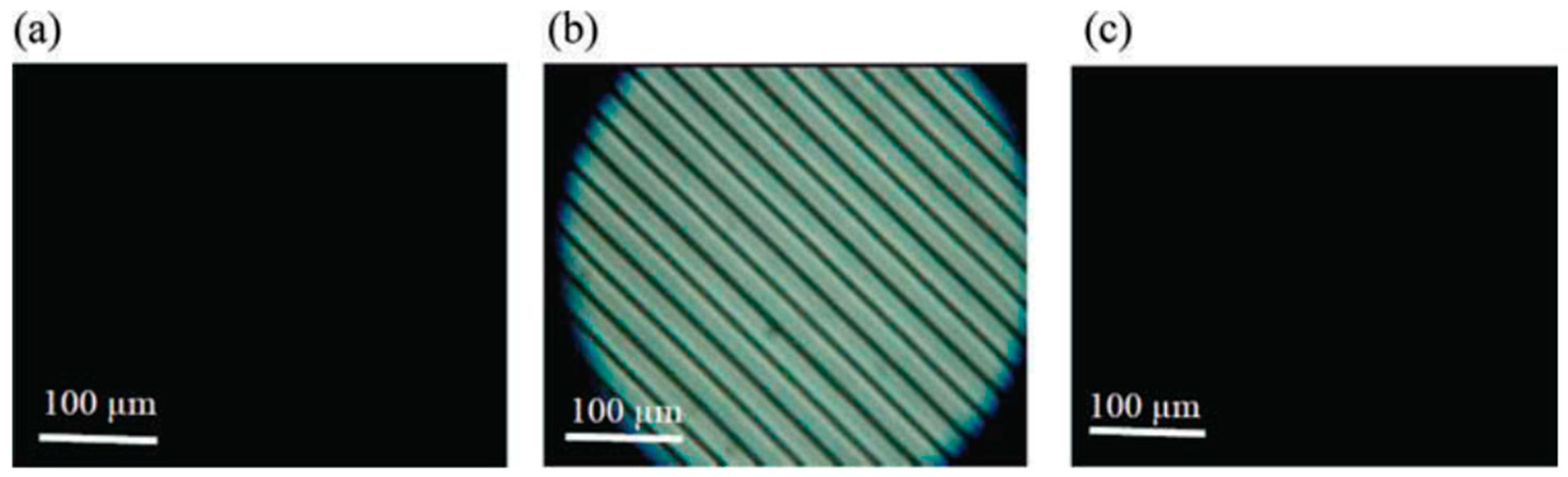
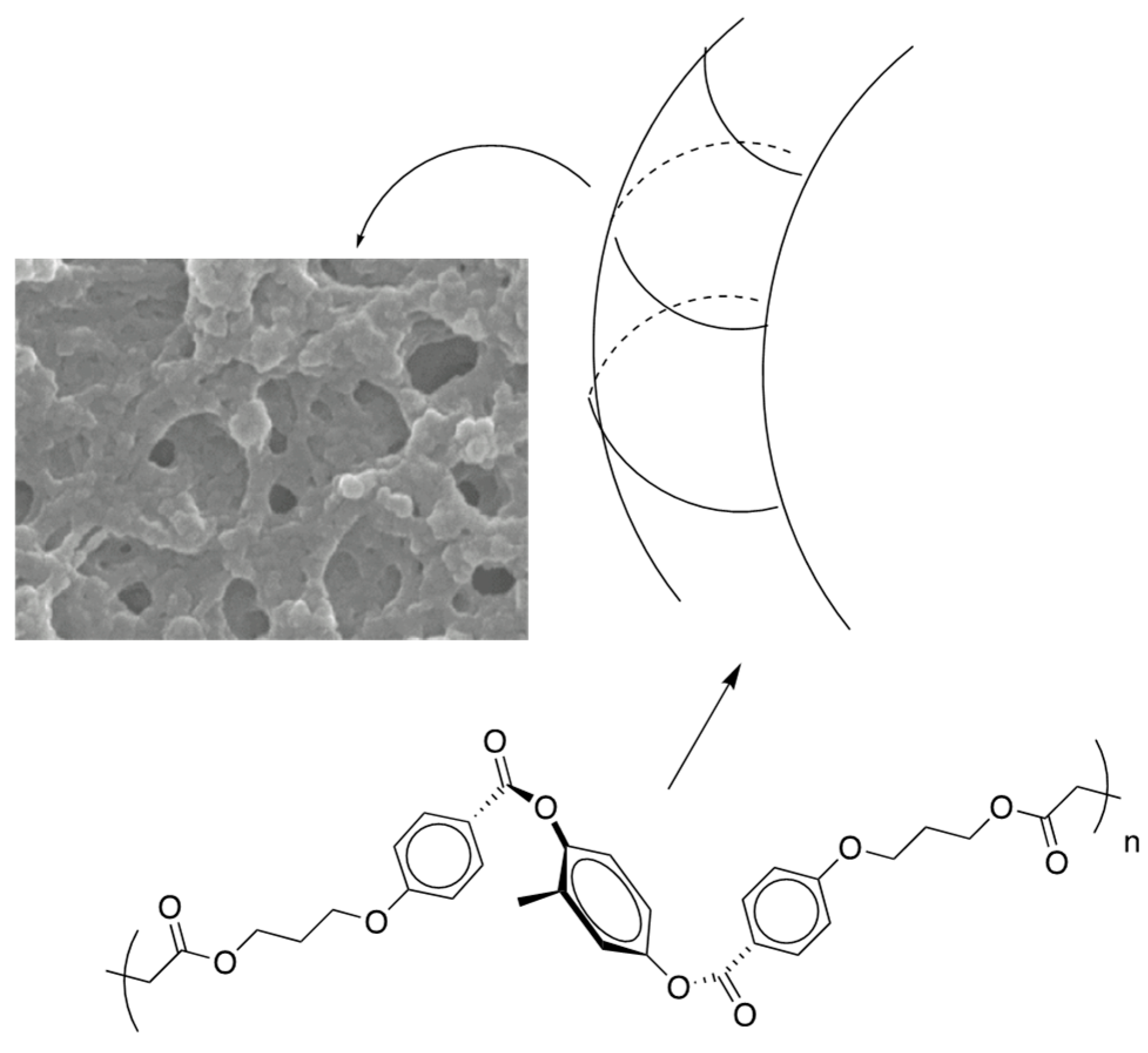
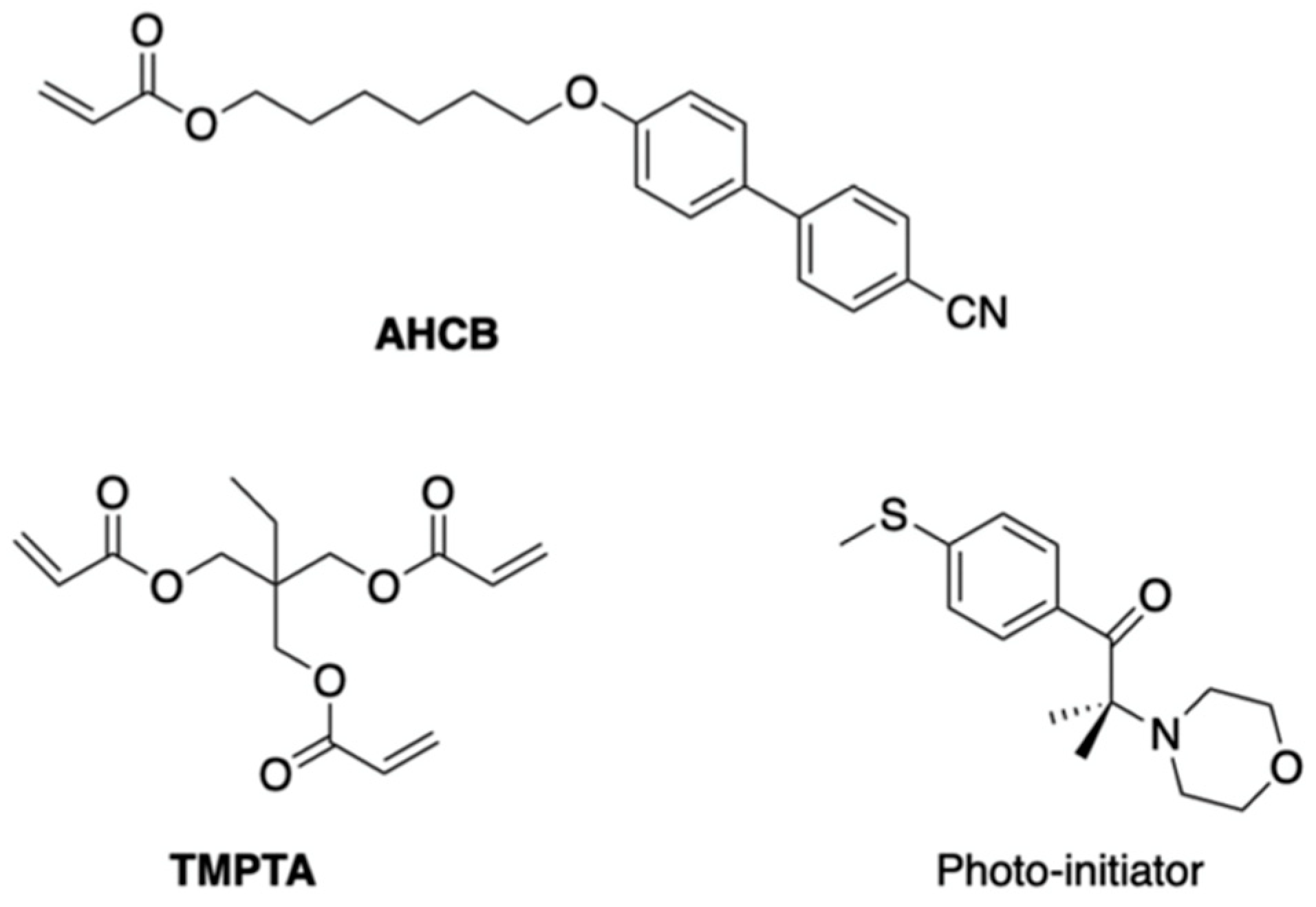
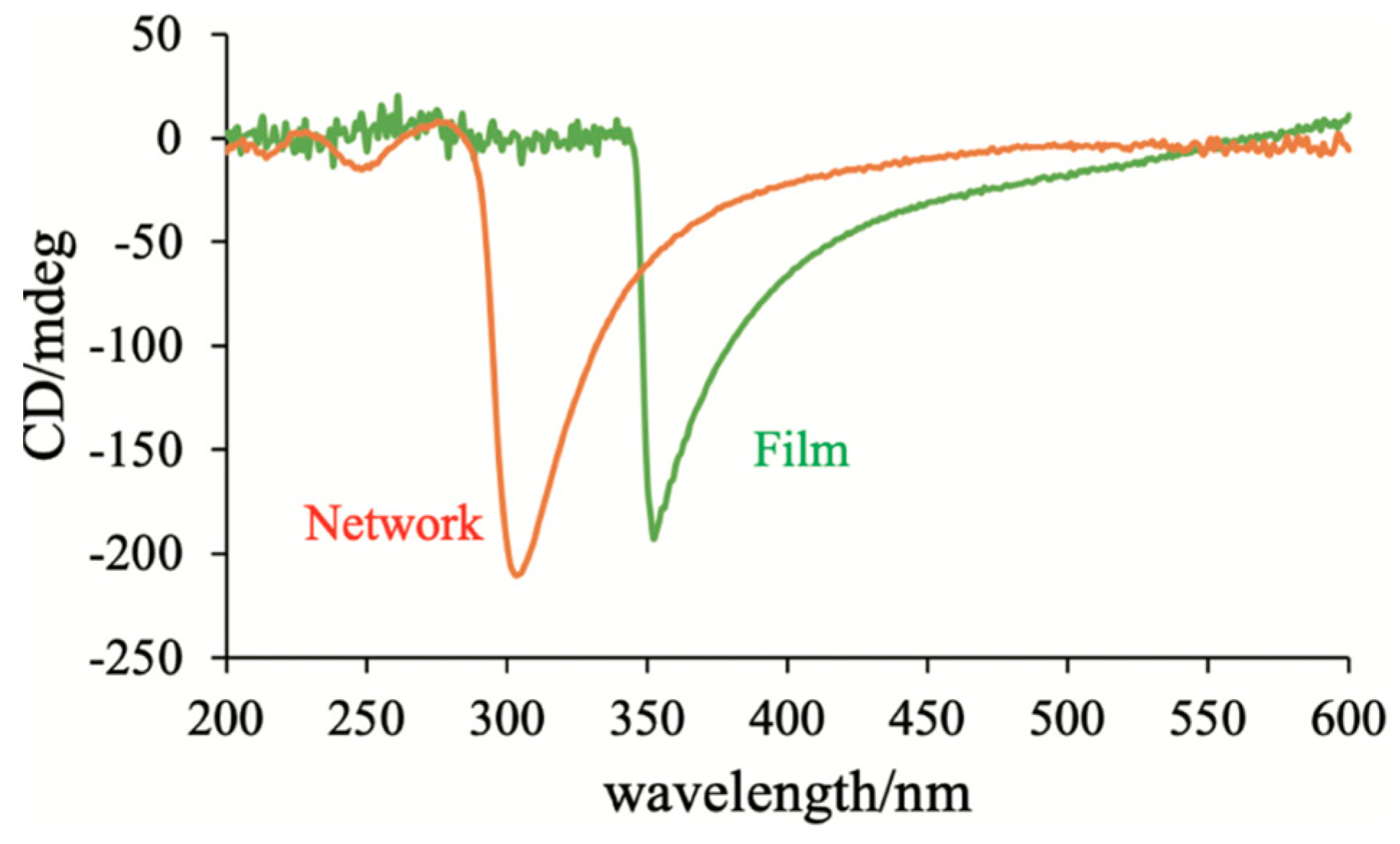
5. The Memory Effect of a Polymer Network Derived from the Defects of BPIII
6. Concluding Remarks
Supplementary Materials
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Stegemeyer, H.; Bergmann, K. Experimental results and problems concerning “Blue Phases”. In Liquid Crystals of One- and Two-Dimensional Order; Helfrich, W., Heppke, G., Eds.; Springer-Verlag: Berlin/Heidelberg, Germany, 1980; pp. 161–175. [Google Scholar]
- Stegemeyer, H.; Blümel, T.; Hiltrop, K.; Onusseit, H.; Porsch, F. Thermodynamic, structural and morphological studies on liquid-crystalline blue phases. Liq. Cryst. 1986, 1, 3–28. [Google Scholar] [CrossRef]
- Wright, D.C.; Mermin, N.D. Crystalline liquids: The blue phases. Rev. Mod. Phys. 1989, 61, 385–432. [Google Scholar] [CrossRef]
- Crooker, P.P. The blue phases. Liq. Cryst. 1989, 5, 751–775. [Google Scholar] [CrossRef]
- Kitzerow, H.-S. The effect of electric fields on blue phases. Mol. Cryst. Liq. Cryst. 1991, 202, 51–83. [Google Scholar] [CrossRef]
- Crooker, P.P. Blue phases. In Chirality in Liquid Crystals; Kitzerow, H.-S., Bahr, C., Eds.; Springer: New York, NY, USA, 2001; pp. 186–222. [Google Scholar]
- Oswald, P.; Pieranski, P. Nematic and Cholesteric Liquid Crystals; Taylor & Francis: Boca Raton, FL, USA, 2005; pp. 493–552. [Google Scholar]
- Kikuchi, H. Liquid crystalline blue phases. Struct. Bond. 2008, 128, 99–117. [Google Scholar]
- Yoshizawa, A. Material design for blue phase liquid crystals and their electro-optical effects. RSC Adv. 2013, 3, 25475–25497. [Google Scholar] [CrossRef]
- Asiqur Rahman, M.D.; Mohd Said, D.; Balamurugan, S. Blue phase liquid crystal: Strategies for phase stabilization and device development. Sci. Technol. Adv. Mater. 2015, 16, 033501. [Google Scholar] [CrossRef]
- Gleeson, H.F.; Miller, R.J.; Tian, L.; Görtz, V.; Goodby, J.W. Liquid crystal blue phases: Stability, field effects and alignment. Liq. Cryst. 2015, 42, 760–771. [Google Scholar] [CrossRef]
- Bagchi, K.; Emeršič, T.; Martínez-González, J.; de Pablo, J.J.; Nealey, P.F. Functional soft materials from blue phase liquid crystals. Sci. Adv. 2023, 9, 9393. [Google Scholar] [CrossRef]
- Dubois-Violette, E.; Pansu, B. Frustration and related topology of blue phases. Mol. Cryst. Liq. Crys. 1988, 165, 151–182. [Google Scholar] [CrossRef]
- Hirose, T.; Yoshizawa, A. Odd-even effects of an asymmetric dimer on the double-twist structure in an amorphous blue phase. J. Mater. Chem. C 2016, 4, 8565–8574. [Google Scholar] [CrossRef]
- Hu, W.; Sun, J.; Wang, Q.; Zhang, L.; Yuan, X.; Chen, F.; Li, K.; Miao, Z.; Yang, D.; Yu, H. Humidity- responsive blue phase liquid-crystalline film with reconfigurable and tailored visual signals. Adv. Funct. Mater. 2020, 30, 2004610. [Google Scholar] [CrossRef]
- Yang, Y.; Kim, Y.-K.; Wang, X.; Tsuei, M.; Abbott, N.L. Structural and optical response of polymer-stabilized blue phase liquid crystal films to volatile organic compounds. ACS. Appl. Mater. Interfaces 2020, 12, 42099–42108. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Wang, L.; Yang, H.; Li, Q. 3D Chiral photonic nanostructures based on blue-phase liquid crystals. Small Sci. 2021, 1, 2100007. [Google Scholar] [CrossRef]
- Finn, P.L.; Cladis, P.E. Cholesteric blue phases in mixtures and in an electric field. Mol. Cryst. Liq. Cryst. 1982, 84, 159–192. [Google Scholar] [CrossRef]
- Hornreich, R.M.; Kugler, M.; Shtrikman, S. Localized instabilities and the order-disorder transition in cholesteric liquid crystals. Phys. Rev. Lett. 1982, 48, 1404–1407. [Google Scholar] [CrossRef]
- Collings, P.J. Optical rotatory dispersion measurements in the third cholesteric blue phase. Phys. Rev. 1983, A30, 1990. [Google Scholar] [CrossRef]
- Hornreich, R.M.; Shtrikman, S. Broken icosahedral symmetry: A quasicrystalline structure for cholesteric blue phase III. Phys. Rev. Lett. 1986, 56, 1723–1726. [Google Scholar] [CrossRef]
- Rokhsar, D.S.; Sethna, J.P. Quasicrystalline textures of cholesteric liquid crystals: Blue phase III? Phys. Rev. Lett. 1986, 56, 1727–1730. [Google Scholar] [CrossRef] [PubMed]
- Henrich, O.; Stratford, K.; Cates, M.E.; Marenduzzo, D. Structure of blue phase III of cholesteric liquid crystals. Phys. Rev. Lett. 2011, 106, 107801. [Google Scholar] [CrossRef]
- Paul, T.; Saha, J. Origin and structure of liquid crystalline blue phase III. Sci. Rep. 2020, 10, 16016. [Google Scholar] [CrossRef] [PubMed]
- Kikuchi, H.; Yokota, M.; Hisakado, Y.; Yang, H.; Kajiyama, T. Polymer-stabilized liquid crystal blue phases. Nat. Mater. 2002, 1, 64–68. [Google Scholar] [CrossRef] [PubMed]
- Coles, H.J.; Pivnenko, M.N. Liqui crystal ‘blue phases’ with a wide temperature range. Nature 2005, 436, 997–1000. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Pan, G.; Yang, Z.; Zhao, D.; Niu, G.; Huang, W.; Yuan, X.; Guo, J.; Cao, H.; Yang, H. Wide blue phase range in a hydrogen-bonded self-assembled complex of chiral fluoro-substituted benzoic acid and pyrimidine derivative. Adv. Mater. 2009, 21, 2050–2053. [Google Scholar] [CrossRef]
- Wang, J.; Shi, Y.; Yang, K.; Wei, J.; Guo, J. Stabilization and optical switching of liquid crystal blue phase doped with azobenzene-based bent-shaped hydrogen-bonded assemblies. RSC Adv. 2015, 5, 67357–67364. [Google Scholar] [CrossRef]
- Cao, W.; Muñoz, A.; Palffy-Muhoray, P.; Taheri, B. Lasing in a three-dimensional photonic crystal of the liquid crystal blue phase II. Nat. Mater. 2002, 1, 111–113. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, S.; Mashiko, S.; Kikuchi, H.; Uchida, K.; Nagamura, T. Laser emission from a polymer-stabilized liquid-crystalline blue phase. Adv. Mater. 2006, 18, 48–51. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Chiou, J.-Y.; Yang, K.-X. Reversible and fast shift in reduction band of a cubic blue phase in a vertical electric field. Appl. Phys. Lett. 2011, 99, 181119. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lai, J.-L.; Chan, C.-C.; Tseng, C.-H. Fast tunable reflection in amorphous blue phase III liquid crystal. J. Appl. Phys. 2013, 113, 123103. [Google Scholar] [CrossRef]
- Rao, L.; Yan, J.; Wu, S.-T. Prospects of emerging polymer-stabilized blue-phase liquid-crystal displays. J. Soc. Inf. Disp. 2010, 18, 954–959. [Google Scholar] [CrossRef]
- Yan, L.; Rao, L.; Jiao, M.; Li, Y.; Cheng, H.-C.; Wu, S.-T. Polymer-stabilized optically isotropic liquid crystals for next-generation display and photonics. J. Mater. Chem. 2011, 21, 7870–7877. [Google Scholar] [CrossRef]
- Lee, H.; Park, H.-J.; Kwon, O.-J.; Yun, S.J.; Park, J.H.; Hong, S.; Shin, S.-T. The world’s first blue phase liquid crystal display. Soc. Inform. Disp. Symp. Dig. Tech. Pap. 2011, 42, 121–124. [Google Scholar] [CrossRef]
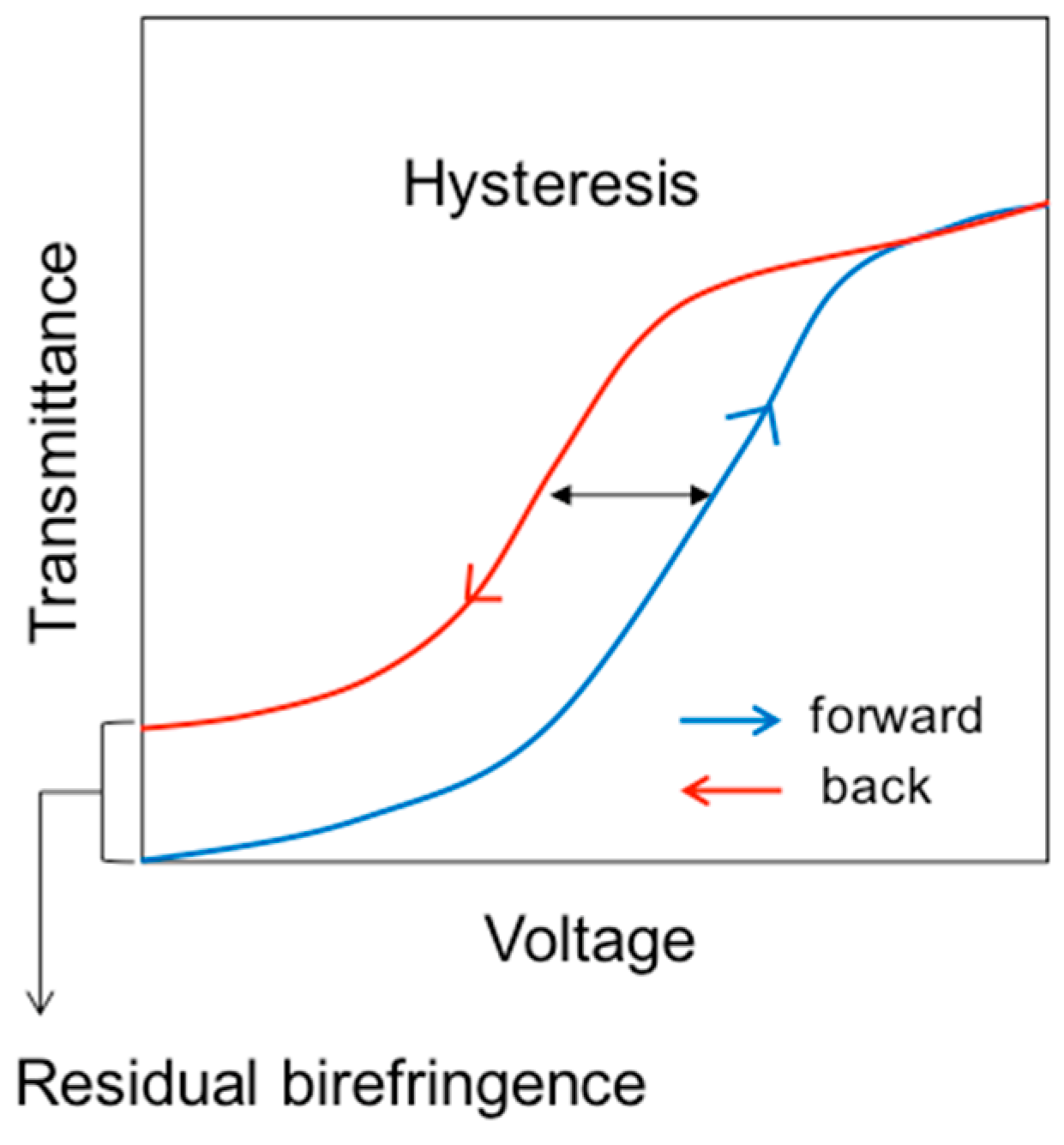
- Chen, K.-M.; Gauza, S.; Xianyu, H.; Wu, S.-T. Hysteresis effects in blue-phase liquid crystals. J. Disp. Technol. 2010, 6, 318–322. [Google Scholar] [CrossRef]
- Sato, M.; Yoshizawa, A. Electro-optical switching in a blue phase III exhibited by a chiral liquid crystal oligomer. Adv. Mater. 2007, 19, 4145–4148. [Google Scholar] [CrossRef]
- Yoshizawa, A.; Kamiyama, M.; Hirose, T. Amorphous Blue Phase III Exhibiting Submillisecond Response and Hysteresis-Free Switching at Room Temperature. Appl. Phys. Express 2011, 4, 101701. [Google Scholar] [CrossRef]
- Cladis, P.E.; Garel, T.; Pieranski, P. Kossel diagrams show electric-field-induced cubic-tetragonal structural transition in frustrated liquid-crystal blue phases. Phys. Rev. Lett. 1986, 57, 2841–2844. [Google Scholar] [CrossRef]
- Jéròme, B.; Pieranski, P. Kossel diagrams of blue phases. Liq. Cryst. 1989, 5, 799–812. [Google Scholar] [CrossRef]
- Meiboom, S.; Sammon, M. Structure of the blue phase of a cholesteric liquid crystal. Phys. Rev. Lett. 1980, 44, 882–885. [Google Scholar] [CrossRef]
- Meiboom, S.; Sammon, M. Blue phases f cholesteryl nonanoate. Phys. Rev. 1981, A24, 468–475. [Google Scholar] [CrossRef]
- Thoen, J. Adiabatic scanning calorimetric results for the blue phases of cholesteryl nonanoate. Phys. Rev. 1988, A37, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Kleiman, R.N.; Bishop, D.J.; Pindak, R.; Taborek, P. Shear modulus and specific heat of the liquid-crystal blue phases. Phys. Rev. Lett. 1984, 53, 2137–2140. [Google Scholar] [CrossRef]
- Zasadzinski, J.A.N.; Meiboom, S.; Sammon, M.J.; Berreman, D.W. Freeze-fracture electron-microscope observations of the blue phase III. Phys. Rev. Lett. 1996, 57, 364–367. [Google Scholar] [CrossRef]
- Pišljar, J.; Ghosh, S.; Turlapati, S.; Rao, N.V.S.; Škarabot, M.; Mertelj, A.; Petelin, A.; Nych, A.; Marinčič, M.; Pusovnik, A.; et al. Blue phase III: Topological fluid of skyrmions. Phys. Rev. 2022, X12, 011003. [Google Scholar] [CrossRef]
- Castles, F.; Day, F.V.; Morris, S.M.; Ko, D.-H.; Gardiner, D.J.; Qasim, M.M.; Nosheen, S.; Hands, P.J.W.; Choi, S.S.; Coles, H.J.; et al. Blue-phase templated fabrication of three-dimensional nanostructures for photonic applications. Nat. Mater. 2012, 11, 599–603. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Wu, C.-K.; Chen, F.-C.; Chen, C.-S. Investigation on morphology of polymer structure in polymer-stabilized blue phase. Liq. Cryst. 2016, 43, 1351–1358. [Google Scholar] [CrossRef]
- Gandhi, S.S.; Kim, M.S.; Hwang, J.-Y.; Chien, L.-C. Electro-optical memory of a nanoengineered amorphous blue-phase-III polymer scaffold. Adv. Mater. 2016, 28, 8998–9005. [Google Scholar] [CrossRef]
- Yoshizawa, A.; Kagami, T.; Kato, K.; Abe, K.; Hamada, K.; Uemura, T.; Yamaguchi, M.; Sagisaka, M. Helical polymerization using a polymer network derived from a blue phase as a template for chirality transfer. Liq. Cryst. 2023, 50, 2268–2279. [Google Scholar] [CrossRef]
- Yoshizawa, A.; Iwamochi, H.; Segawa, S.; Sato, M. The role of a liquid crystal oligomer in stabilizing blue phases. Liq. Cryst. 2007, 34, 1039–1044. [Google Scholar] [CrossRef]
- Ye, Y.; Guo, L.; Zhong, T. A review of developments in polymer stabilized liquid crystals. Polymers 2023, 15, 2962. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, H.; Tanaka, Y.; Kawamoto, K.; Kubo, H.; Tsuda, T.; Fujii, A.; Kuwabata, S.; Kikuchi, H.; Ozaki, M. Nanoparticles-stabilized cholesteric blue phases. Appl. Phys. Express 2009, 2, 121501. [Google Scholar] [CrossRef]
- Kitzerow, H.-S.; Schmid, H.; Ranft, A.; Heppke, G.; Hikmet, R.A.M.; Lub, J. Observation of blue phases in chiral networks. Liq. Cryst. 1993, 14, 911–916. [Google Scholar] [CrossRef]
- Hirose, T.; Yoshizawa, A. Comparison of electro-optical switching between polymer-stabilized cubic and amorphous blue phases. Liq. Cryst. 2015, 42, 1290–1297. [Google Scholar] [CrossRef]
- Kim, M.S.; Chien, L.-C. Topology-mediated electro-optical behaviour of a wide-temperature liquid crystalline amorphous blue phase. Soft Matter 2015, 11, 8013–8018. [Google Scholar] [CrossRef]
- Karatairi, E.; Rozic, B.; Kutnjak, Z.; Tzitzios, V.; Nounesis, G.; Cordoyiannis, G.; Thoen, J.; Glorieux, C.; Kralj, S. Nanoparticle-induced widening of the temperature range of liquid-crystalline blue phases. Phys. Rev. 2010, E81, 041703. [Google Scholar]
- Yoshizawa, A.; Sato, M.; Rokunohe, J. A blue phase observed for a novel chiral compound possessing molecular biaxiality. J. Mater. Chem. 2005, 15, 3285–3290. [Google Scholar] [CrossRef]
- Marik, M.; Mukherjee, A.; Jana, D.; Yoshizawa, A.; Chaudhuri, B.K. Dielectric spectroscopy of T-shaped blue-phase-III liquid crystal. Phys. Rev. 2013, A38, 012502. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, A.; Kogawa, Y.; Kobayashi, K.; Takanishi, Y.; Yamamoto, J. A binaphthyl derivative with a wide temperature range of a blue phase. J. Mater. Chem. 2009, 19, 5759–5762. [Google Scholar] [CrossRef]
- Sayama, S.; Yoshizawa, A. Achiral H-shaped liquid crystals exhibiting an electric-field-induced chiral nematic phase. J. Mater. Chem. C 2019, 43, 8865–8868. [Google Scholar] [CrossRef]
- Yelamaggad, C.V.; Shashikala, S.; Liao, G.; Rao, D.S.S.; Prasad, S.K.; Li, Q.; Jakli, A. Blue phase, smectic fluids and unprecedented sequences in liquid crystal dimers. Chem. Mater. 2006, 18, 6100–6102. [Google Scholar] [CrossRef]
- Taushanoff, S.; Le, K.V.; Williams, J.; Twieg, R.J.; Sadashiva, B.K.; Takezoe, H.; Jakli, A. Stable amorphous blue phase of bent-core nematic liquid crystals doped with a chiral material. J. Mater. Chem. 2010, 20, 5893–5898. [Google Scholar] [CrossRef]
- Chiang, I.-H.; Long, C.-J.; Lin, H.-C.; Chuang, W.-T.; Lee, J.-J.; Lin, H.-C. Broad ranges and fast responses of single-component blue-phase liquid crystals containing banana-shaped 1,3,4-oxadiazole cores. ACS Appl. Mater. Interfaces 2014, 6, 228–235. [Google Scholar] [CrossRef]
- Cestari, M.; Diez-Berart, S.; Dunmur, D.A.; Ferrarini, M.R.; de la Fuente, M.R.; Jackson, D.J.B.; Lopez, D.O.; Luckhurst, G.R.; Perez-Jubindo, M.A.; Richardson, R.M.; et al. Phase behavior and properties of the liquid-crystal dimer 1″,7″-bis(4-cyanobiphenyl-4′-yl) heptane: A twist-bend nematic liquid crystal. Phys. Rev. E 2011, 84, 031704. [Google Scholar] [CrossRef]
- Archbold, C.T.; Davis, E.J.; Mandle, R.J.; Cowling, S.J.; Goodby, J.W. Chiral dopants and the twist-bend nematic phase-induction of novel mesomorphic behaviour in an apolar bimesogen. Soft Matter 2015, 11, 7547–7557. [Google Scholar] [CrossRef]
- Cordoyiannis, G.; Lavrič, M.; Trček, M.; Tzitzios, V.; Lelidis, I.; Nounesis, G.; Daniel, M.; Kutnjak, Z. Quantum dot-driven stabilization of liquid-crystalline blue phases. Front. Phys. 2020, 8, 315. [Google Scholar] [CrossRef]
- Khatun, N.; Sridurai, V.; Csorba, K.F.; Nair, G.G. Topological defects stabilized by a soft twist-bend dimer and quantum dots lead to a wide thermal range and ultra-fast electro-optic response in a liquid crystalline amorphous blue phase. J. Mater. Chem. C 2023, 11, 9686–9694. [Google Scholar] [CrossRef]
- Rao, L.; Ge, Z.; Gauza, S.; Chen, K.-M.; Wu, S.-T. Emerging liquid crystal displays based on the Kerr effect. Mol. Cryts. Liq. Cryst. 2010, 527, 186–198. [Google Scholar] [CrossRef]
- Cheng, H.-C.; Yan, J.; Ishinabe, T.; Wu, S.-T. Vertical field switching for blue-phase liquid crystal devices. Appl. Phys. Lett. 2011, 98, 261102. [Google Scholar] [CrossRef]
- Lee, S.H.; Bhattacharya, S.S.; Jin, H.S.; Jeong, K.-U. Devices and materials for high-performance mobile liquid crystal displays. J. Mater. Chem. 2012, 22, 11893–11903. [Google Scholar] [CrossRef]
- Kitzerow, H.-S.; Crooker, P.P.; Heppke, G. Line shapes of field-induced blue-phase-III selective reflections. Phys. Rev. Lett. 1991, 67, 2151–2154. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.K.; Crooker, P.P. Blue phase III of chiral liquid crystals in an electric field. Phys. Rev. 1988, A37, 4001. [Google Scholar] [CrossRef] [PubMed]
- Yoshizawa, A. Molecular design for stabilizing a blue phase III and electro-optical switching in the blue phase. J. Soc. Inf. Disp. 2008, 16, 1189–1194. [Google Scholar] [CrossRef]
- Chen, H.-Y.; Lu, S.-F.; Hsieh, Y.-C. Unusual electro-optical behavior in a wide-temperature BPIII cell. Opt. Express 2013, 21, 9774–9779. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.-Y.; Lu, S.-F.; Wu, P.-H.; Wang, C.-S. Transflective BPIII mode with no internal reflector. Liq. Cryst. 2017, 44, 473–478. [Google Scholar] [CrossRef]
- Khan, R.K.; Mohiuddin, G.; Begum, N.; Turlapati, S.; Namdiraju, R.V.S.; Debbarma, B.K.; Ghosh, S. Extremely high Kerr constant and low operating voltage in a stable room-temperature blue phase III derived from three-ring-based bent-core molecules. ACS Appl. Mater. Interfaces 2022, 14, 42628–42634. [Google Scholar] [CrossRef]
- Hu, W.; Wang, L.; Wang, M.; Zhong, T.; Wang, Q.; Zhang, L.; Chen, F.; Li, K.; Miao, Z.; Yang, D.; et al. Ultrastable liquid crystalline blue phase from molecular synergistic self-assembly. Nat. Commun. 2021, 12, 1440. [Google Scholar] [CrossRef]










Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yoshizawa, A. Amorphous Blue Phase III: Structure, Materials, and Properties. Materials 2024, 17, 1291. https://doi.org/10.3390/ma17061291
Yoshizawa A. Amorphous Blue Phase III: Structure, Materials, and Properties. Materials. 2024; 17(6):1291. https://doi.org/10.3390/ma17061291
Chicago/Turabian StyleYoshizawa, Atsushi. 2024. "Amorphous Blue Phase III: Structure, Materials, and Properties" Materials 17, no. 6: 1291. https://doi.org/10.3390/ma17061291




