Study on the Re-Aging Behavior of Cu-Rich Precipitates in a FeCu Alloy under Electropulsing
Abstract
:1. Introduction
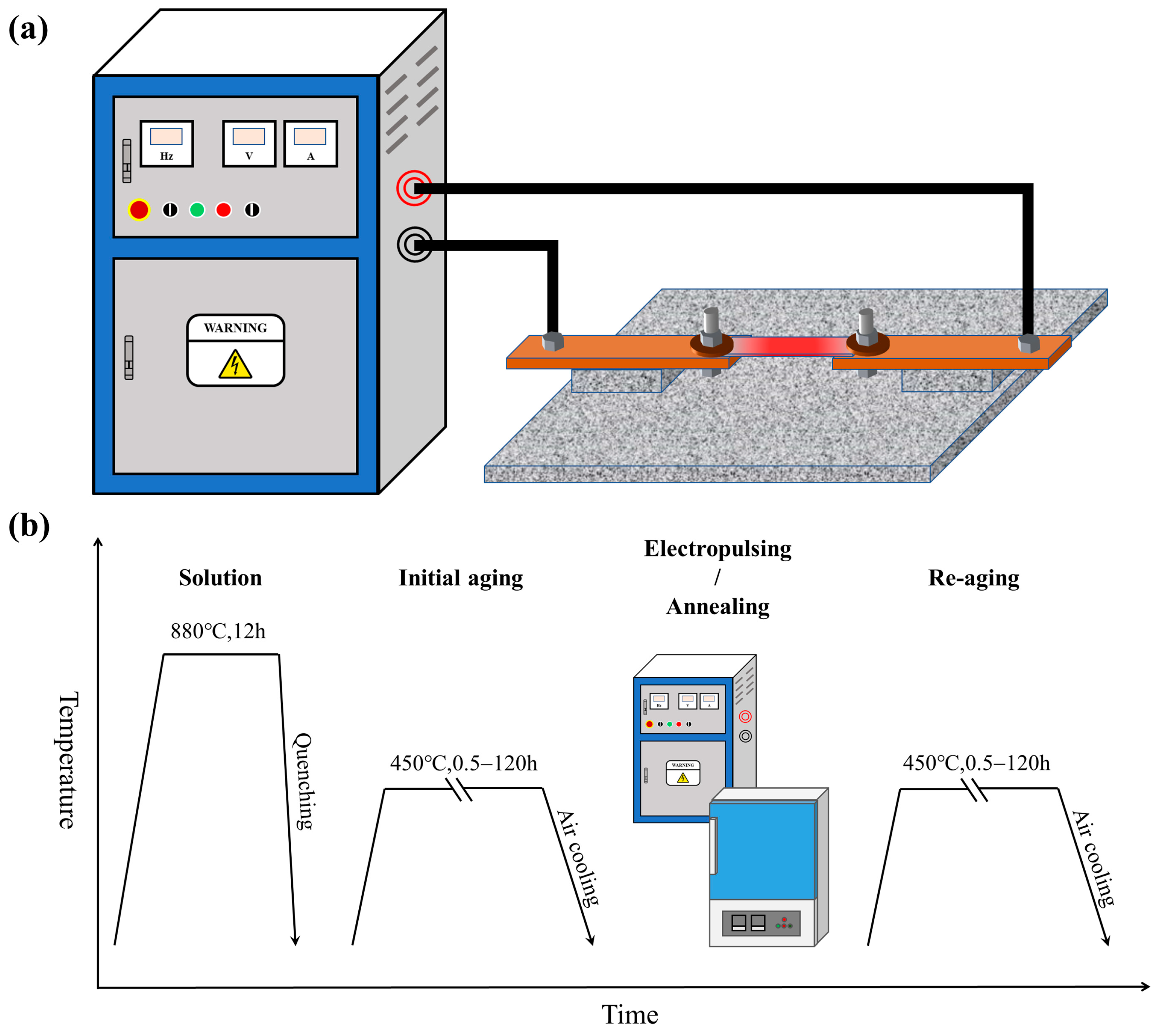
2. Materials and Methods
3. Results
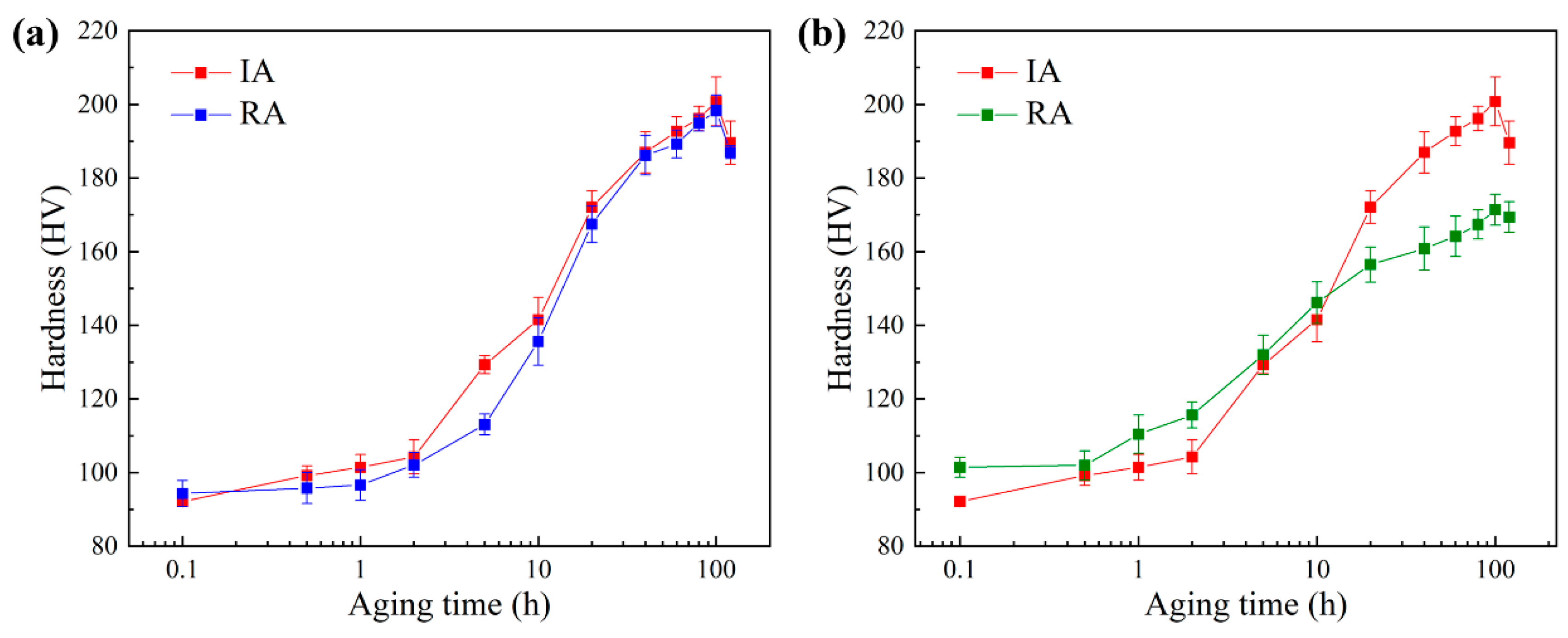
3.1. Hardening Behavior
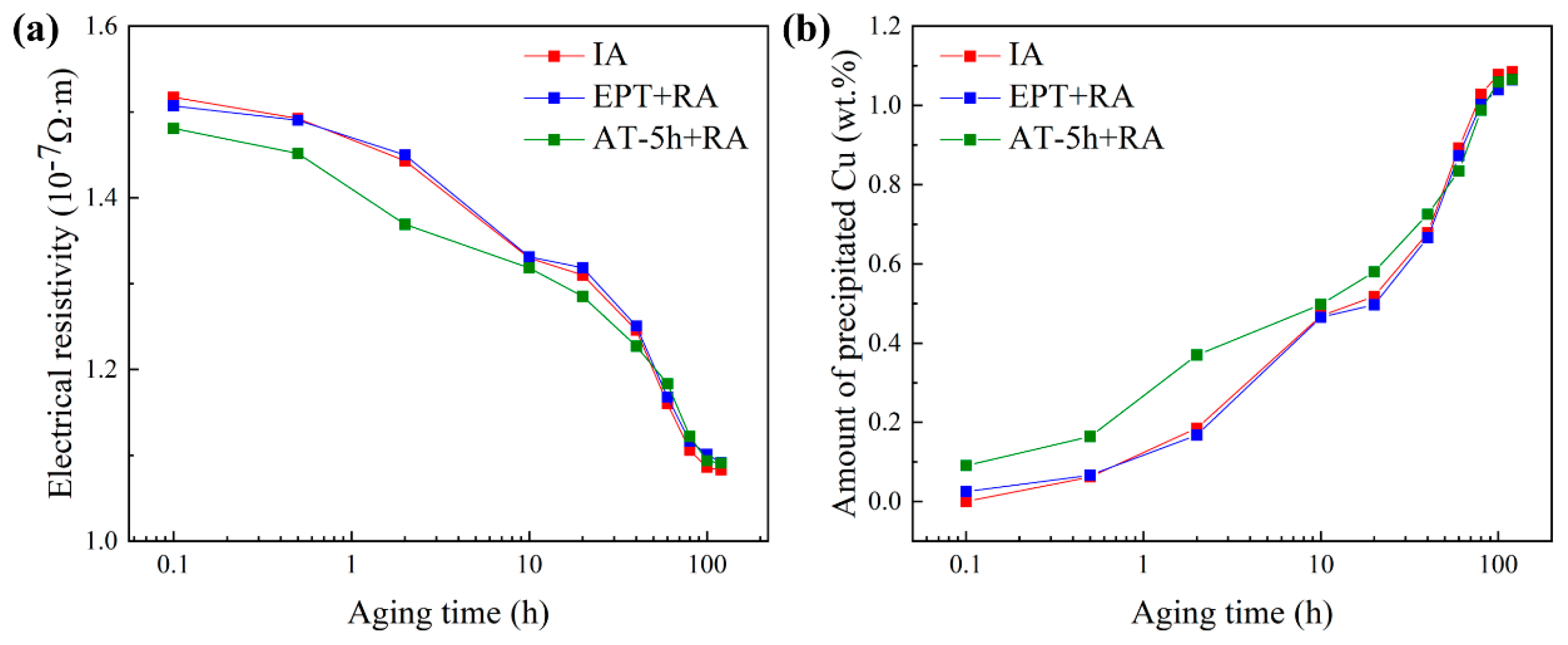
3.2. Cu Precipitation Behavior
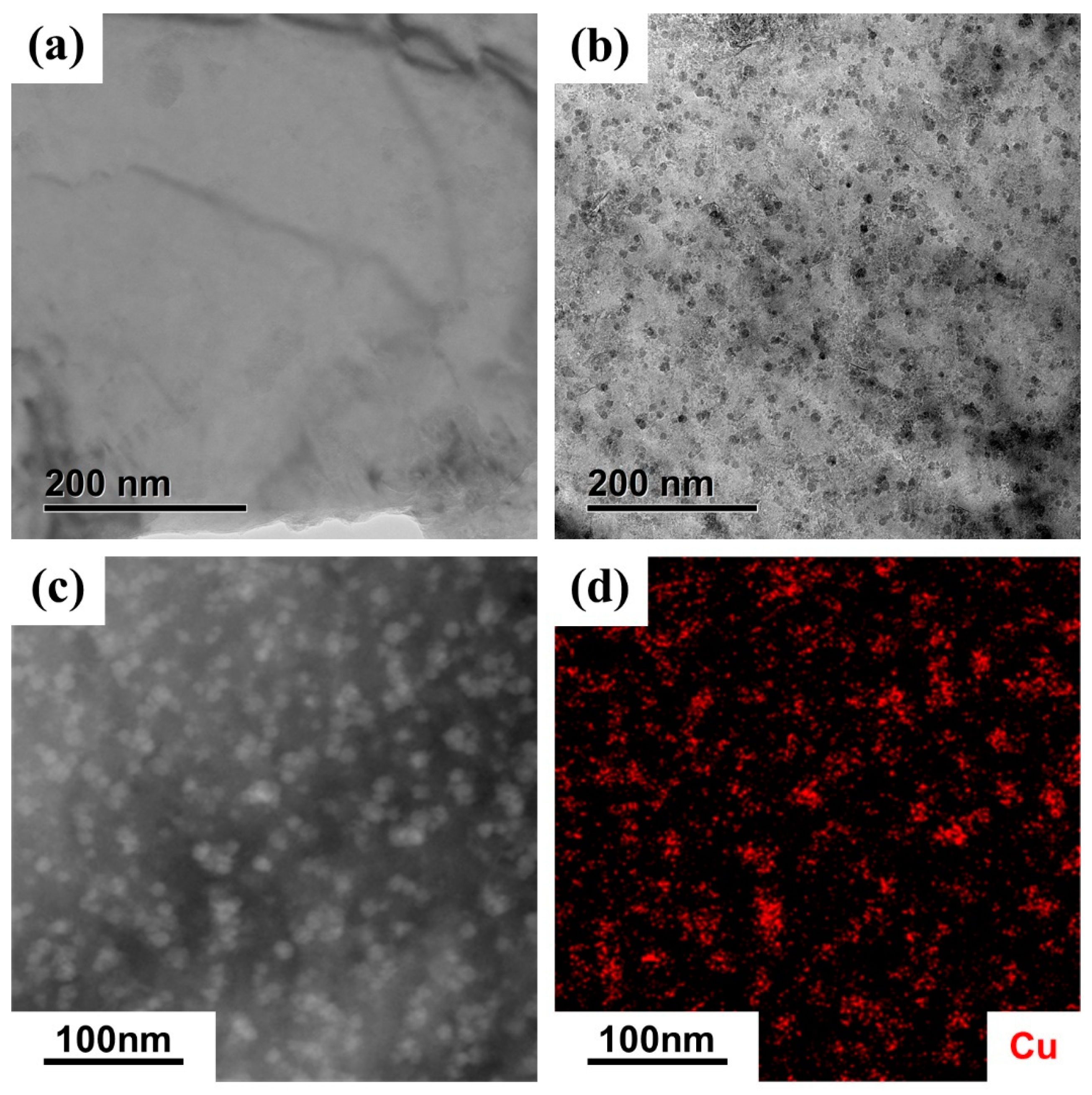
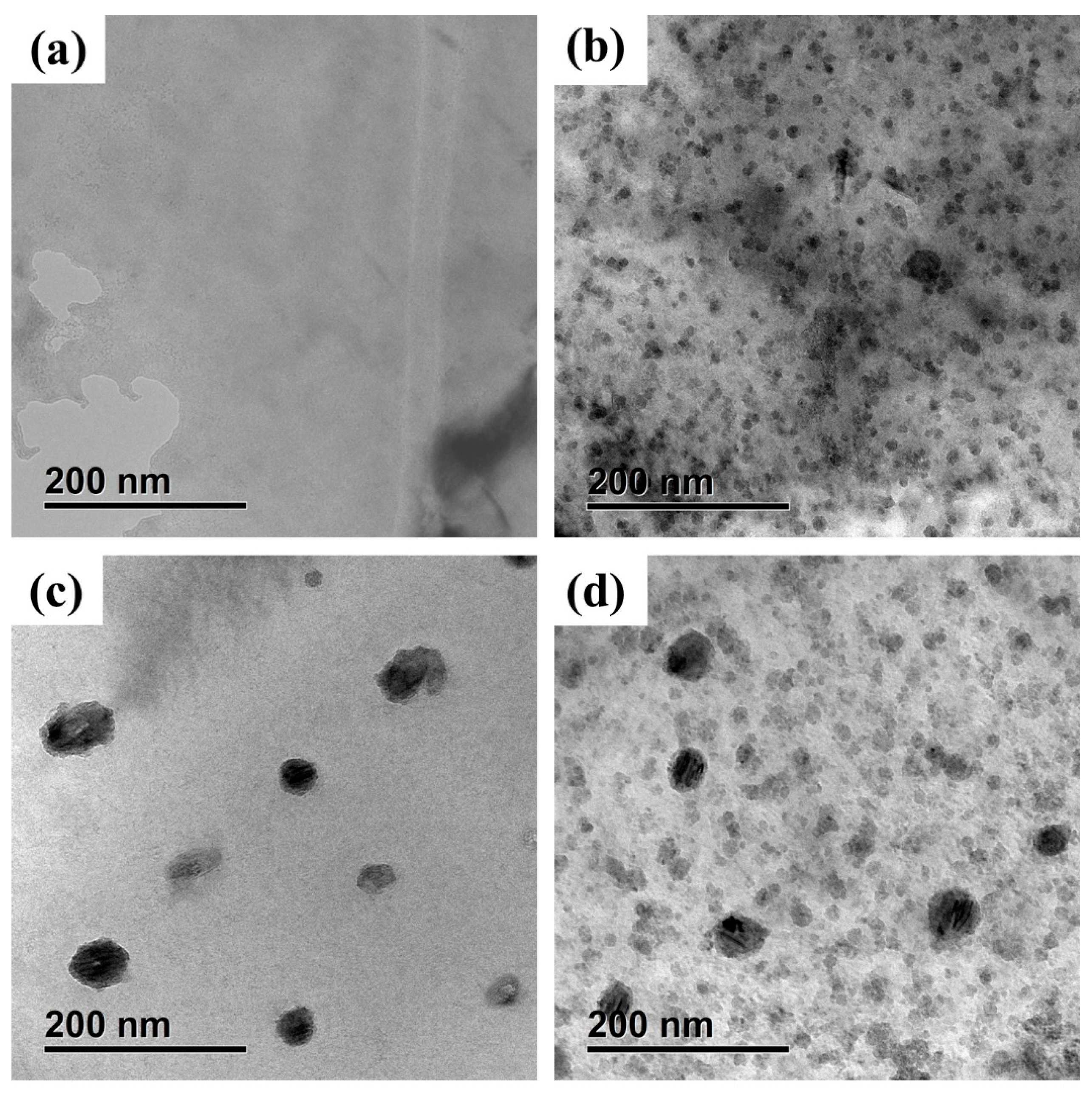
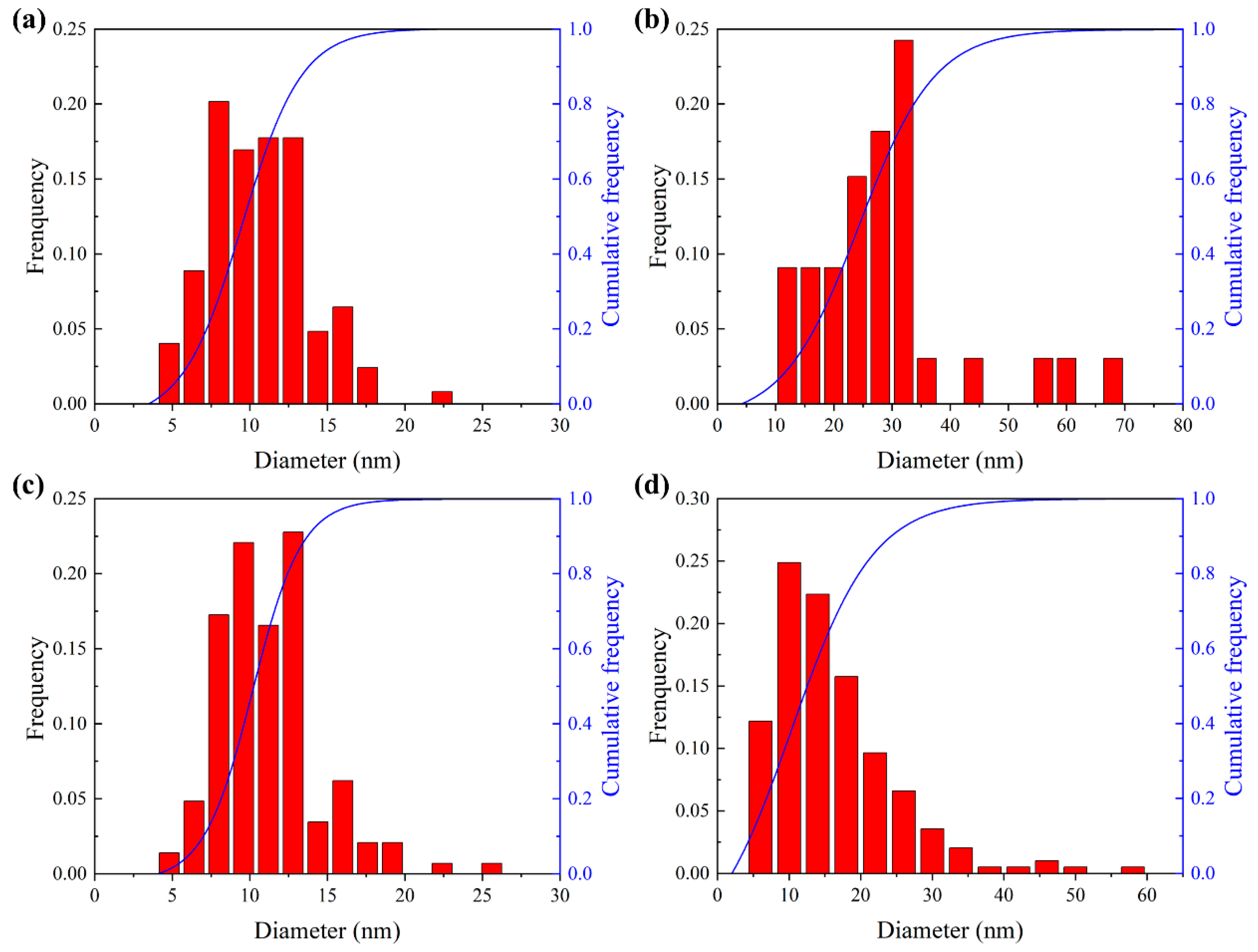
3.3. Observation of CRPs
4. Discussion
5. Conclusions
- (1)
- The evolution behavior of CRPs and the changes in the hardness of the alloy during the re-aging process after EPT with 15.4 A/mm2 were consistent with the initial aging process. The peak hardness of initial aging and re-aging was 201 HV and 198 HV, respectively.
- (2)
- The evolution behavior of CRPs and the changes in the hardness of the alloy during the re-aging process after annealing for 5 h showed a faster trend than the initial aging process, especially in the early stage. The peak hardness of initial aging and re-aging was 201 HV and 170 HV, respectively.
- (3)
- The dissolution of CRPs in the EPT samples was more thorough than that in the AT samples. The residual CRPs in the AT samples accelerated the nucleation and ripening of new generated CRPs, causing an accelerated aging process and a decrease in peak hardness.
- (4)
- From the perspective of hardness and CRP evolution, the degradation rate of the FeCu alloy after EPT during re-aging was not faster than that of initial aging.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Odette, G.R.; Nanstad, R.K. Predictive reactor pressure vessel steel irradiation embrittlement models: Issues and opportunities. JOM 2009, 61, 17–23. [Google Scholar] [CrossRef]
- Odette, G.; Yamamoto, T.; Williams, T.; Nanstad, R.; English, C. On the history and status of reactor pressure vessel steel ductile to brittle transition temperature shift prediction models. J. Nucl. Mater. 2019, 526, 151863. [Google Scholar] [CrossRef]
- Van Duysen, J.; de Bellefon, G.M. 60th Anniversary of electricity production from light water reactors: Historical review of the contribution of materials science to the safety of the pressure vessel. J. Nucl. Mater. 2017, 484, 209–227. [Google Scholar]
- Shi, L.; Zou, J.; Sun, L.; Wang, Y.; Ni, L.; Liang, S. Effect of electropulsing treatment on microstructure and mechanical properties of Cu–20Ni–20Mn alloy. Mater. Sci. Eng. A 2022, 855, 143847. [Google Scholar] [CrossRef]
- Shimodaira, M.; Toyama, T.; Yoshida, K.; Inoue, K.; Ebisawa, N.; Tomura, K.; Yoshiie, T.; Konstantinović, M.J.; Gérard, R.; Nagai, Y. Contribution of irradiation-induced defects to hardening of a low-copper reactor pressure vessel steel. Acta Mater. 2018, 155, 402–409. [Google Scholar] [CrossRef]
- Vassilaros, M.G.; Mayfield, M.E.; Wichman, K.R. Annealing of nuclear reactor pressure vessels. Nucl. Eng. Des. 1998, 181, 61–69. [Google Scholar] [CrossRef]
- Gurovich, B.; Kuleshova, E.; Shtrombakh, Y.; Fedotova, S.; Zabusov, O.; Prikhodko, K.; Zhurko, D. Evolution of weld metals nanostructure and properties under irradiation and recovery annealing of VVER-type reactors. J. Nucl. Mater. 2013, 434, 72–84. [Google Scholar] [CrossRef]
- Almirall, N.; Wells, P.; Ke, H.; Edmondson, P.; Morgan, D.; Yamamoto, T.; Odette, G. On the elevated temperature thermal stability of nanoscale Mn-Ni-Si precipitates formed at lower temperature in highly irradiated reactor pressure vessel steels. Sci. Rep. 2019, 9, 9587. [Google Scholar] [CrossRef] [PubMed]
- Ulbricht, A.; Dykas, J.; Chekhonin, P.; Altstadt, E.; Bergner, F. Small-angle neutron scattering study of neutron-irradiated and post-irradiation annealed VVER-1000 reactor pressure vessel weld material. Front. Nucl. Eng. 2023, 2, 1176288. [Google Scholar] [CrossRef]
- Kuleshova, E.A.; Gurovich, B.A.; Maltsev, D.A.; Frolov, A.S.; Bukina, Z.V.; Fedotova, S.V.; Saltykov, M.A.; Krikun, E.V.; Erak, D.Y.; Zhurko, D.A.; et al. Phase and structural transformations in VVER-440 RPV base metal after long-term operation and recovery annealing. J. Nucl. Mater. 2018, 501, 261–274. [Google Scholar] [CrossRef]
- Shi, J.J.; Zhao, W.Z.; Wu, Y.C.; Liu, X.B.; Jiang, J.; Cao, X.Z.; Wang, B.Y. Evolution of microstructures and hardening property of initial irradiated, post-irradiation annealed and re-irradiated Chinese-type low-Cu reactor pressure vessel steel. J. Nucl. Mater. 2019, 523, 333–341. [Google Scholar] [CrossRef]
- Kuleshova, E.; Gurovich, B.; Fedotova, S.; Zhuchkov, G.; Frolov, A.; Maltsev, D.; Saltykov, M.; Dementyeva, M. Comparison of the high Ni VVER-1000 weld microstructure under the primary irradiation and re-irradiation. J. Nucl. Mater. 2020, 540, 152384. [Google Scholar] [CrossRef]
- Kim, M.-J.; Lee, K.; Oh, K.H.; Choi, I.-S.; Yu, H.-H.; Hong, S.-T.; Han, H.N. Electric current-induced annealing during uniaxial tension of aluminum alloy. Scr. Mater. 2014, 75, 58–61. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Zhu, J.; Ye, X.; Tang, G. Microstructure and texture evolution of cold-rolled Mg-3Al-1Zn alloy by electropulse treatment stimulating recrystallization. Scr. Mater. 2016, 112, 23–27. [Google Scholar] [CrossRef]
- Park, J.-W.; Jeong, H.-J.; Jin, S.-W.; Kim, M.-J.; Lee, K.; Kim, J.J.; Hong, S.-T.; Han, H.N. Effect of electric current on recrystallization kinetics in interstitial free steel and AZ31 magnesium alloy. Mater. Charact. 2017, 133, 70–76. [Google Scholar] [CrossRef]
- Kim, M.-J.; Yoon, S.; Park, S.; Jeong, H.-J.; Park, J.-W.; Kim, K.; Jo, J.; Heo, T.; Hong, S.-T.; Cho, S.H. Elucidating the origin of electroplasticity in metallic materials. Appl. Mater. Today 2020, 21, 100874. [Google Scholar] [CrossRef]
- Samuel, E.I.; Bhowmik, A.; Qin, R. Accelerated spheroidization induced by high intensity electric pulse in a severely deformed eutectoid steel. J. Mater. Res. 2011, 25, 1020–1024. [Google Scholar] [CrossRef]
- Rahnama, A.; Qin, R.S. Electropulse-induced microstructural evolution in a ferritic-pearlitic 0.14% C steel. Scr. Mater. 2015, 96, 17–20. [Google Scholar] [CrossRef]
- Rahnama, A.; Qin, R.S. The effect of electropulsing on the interlamellar spacing and mechanical properties of a hot-rolled 0.14% carbon steel. Mater. Sci. Eng. A 2015, 627, 145–152. [Google Scholar] [CrossRef]
- Bhagurkar, A.G.; Qin, R. Effect of electropulsing on the solidification of mould flux. J. Mater. Res. Technol. 2022, 19, 2146–2155. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, G.; Shek, C.; Zhu, Y.; Xu, Z. On the thermodynamics and kinetics of electropulsing induced dissolution of β-Mg17Al12 phase in an aged Mg–9Al–1Zn alloy. Acta Mater. 2009, 57, 4797–4808. [Google Scholar] [CrossRef]
- Kapoor, R.; Sunil, S.; Reddy, G.B.; Nagaraju, S.; Kolge, T.S.; Sarkar, S.K.; Biswas, A.; Sharma, A. Electric current induced precipitation in maraging steel. Scr. Mater. 2018, 154, 16–19. [Google Scholar] [CrossRef]
- Jeong, H.-J.; Kim, M.-J.; Park, J.-W.; Yim, C.D.; Kim, J.J.; Kwon, O.D.; Madakashira, P.P.; Han, H.N. Effect of pulsed electric current on dissolution of Mg17Al12 phases in as-extruded AZ91 magnesium alloy. Mater. Sci. Eng. A 2017, 684, 668–676. [Google Scholar] [CrossRef]
- Onodera, Y.; Hirano, K. The effect of ac frequency on precipitation in Al-5.6 at% Zn. J. Mater. Sci. 1984, 19, 3935–3939. [Google Scholar] [CrossRef]
- Zhao, Y.; He, B.Y.; Saillet, S.; Domain, C.; Le Delliou, P.; Perez, M.; Qin, R.S. Anti-aging treatment of nuclear power plant steel. Mater. Sci. Eng. A 2018, 735, 73–80. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, X. Correlation of ferrite-phase reconfiguration and mechanical properties in thermally aged duplex stainless steel. Mater. Charact. 2022, 183, 111573. [Google Scholar] [CrossRef]
- Gao, M.L.; Liu, H.L.; Xu, B.; Liu, W.; Li, Q.L. Reverse evolution in nanoscale Cu-rich precipitates of an aged Fe-Cu alloy under electropulsing. Philos. Mag. Lett. 2019, 99, 39–47. [Google Scholar] [CrossRef]
- Qin, S.; Ba, X.; Zhang, X. Accelerated cluster dissolution using electropulsing for ultrafast performance regeneration. Scr. Mater. 2020, 178, 24–28. [Google Scholar] [CrossRef]
- Barbu, A.; Mathon, M.H.; Maury, F.; Belliard, J.F.; Novion, C.H.D. A comparison of the effect of electron irradiation and of thermal aging on the hardness of FeCu binary alloys. J. Nucl. Mater. 1998, 257, 206–211. [Google Scholar] [CrossRef]
- Liu, L.; Nishida, K.; Dohi, K.; Nomoto, A.; Soneda, N.; Murakami, K.; Li, Z.; Chen, D.; Sekimura, N. Effects of solute elements on hardening and microstructural evolution in neutron-irradiated and thermally-aged reactor pressure vessel model alloys. J. Nucl. Sci. Technol. 2016, 53, 1546–1553. [Google Scholar] [CrossRef]
- Fujii, K.; Fukuya, K. Effects of cold work on solute atom clustering during thermal aging in RPV model alloy. J. Nucl. Sci. Technol. 2021, 58, 527–532. [Google Scholar] [CrossRef]
- Xiao, H.; Jiang, S.S.; Shi, C.C.; Zhang, K.F.; Lu, Z.; Jiang, J.F. Study on the microstructure evolution and mechanical properties of an Al-Mg-Li alloy aged by electropulsing assisted ageing processing. Mater. Sci. Eng. A 2019, 756, 442–454. [Google Scholar] [CrossRef]
- Jung, J.G.; Jung, M.; Lee, S.M.; Shin, E.; Shin, H.C.; Lee, Y.K. Cu precipitation kinetics during martensite tempering in a medium C steel. J. Alloys Compd. 2013, 553, 299–307. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Deng, S.; Xu, B.; Li, Q.; Shu, G.; Liu, W. Changes in the magnetic and mechanical properties of thermally aged Fe–Cu alloys due to nano-sized precipitates. J. Phys. D Appl. Phys. 2015, 49, 035006. [Google Scholar] [CrossRef]
- Xiong, Z.P.; Timokhina, I.; Pereloma, E. Clustering, nano-scale precipitation and strengthening of steels. Prog. Mater Sci. 2021, 118, 100764. [Google Scholar] [CrossRef]
- Bhagat, A.N.; Pabi, S.K.; Ranganathan, S.; Mohanty, O.N. Study on copper precipitation during continuous heating and cooling of HSLA steels using electrical resistivity. Mater. Sci. Technol. 2007, 23, 158–164. [Google Scholar] [CrossRef]
- Russell, K.C.; Brown, L. A dispersion strengthening model based on differing elastic moduli applied to the iron-copper system. Acta Metall. 1972, 20, 969–974. [Google Scholar] [CrossRef]
- Sun, H.; Li, D.; Diao, Y.; He, Y.; Yan, L.; Pang, X.; Gao, K. Nanoscale Cu particle evolution and its impact on the mechanical properties and strengthening mechanism in precipitation-hardening stainless steel. Mater. Charact. 2022, 188, 111885. [Google Scholar] [CrossRef]
- Markus, T.; Watson, A. Cu-Fe Binary System: Data Extracted from the Landolt-Börnstein Book Series and Associated Databases; Springer: Berlin/Heidelberg, Germany, 2015; Available online: https://materials.springer.com/sgte-phase-diagram/sm_sgte_R_Cu_Fe (accessed on 2 February 2024).
- Voorhees, P.W. The theory of Ostwald ripening. J. Stat. Phys. 1985, 38, 231–252. [Google Scholar] [CrossRef]
- Xiao, H.; Zhang, K.F.; Shi, C.C.; Lu, Z.; Jiang, J.F. Influence of electropulsing treatment combined with pre-deformation on ageing behavior and mechanical properties of 5A90 Al-Li alloy. J. Alloys Compd. 2019, 784, 1234–1247. [Google Scholar] [CrossRef]
- Monzen, R.; Iguchi, M.; Jenkins, M.L. Structural changes of 9R copper precipitates in an aged Fe-Cu alloy. Philos. Mag. Lett. 2000, 80, 137–148. [Google Scholar] [CrossRef]
- Lu, W.J.; Zhang, X.F.; Qin, R.S. Stability of precipitates under electropulsing in 316L stainless steel. Mater. Sci. Technol. 2015, 31, 1530–1535. [Google Scholar] [CrossRef]
- Jiang, Y.; Tang, G.; Guan, L.; Wang, S.; Xu, Z.; Shek, C.; Zhu, Y. Effect of electropulsing treatment on solid solution behavior of an aged Mg alloy AZ61 strip. J. Mater. Res. 2008, 23, 2685–2691. [Google Scholar] [CrossRef]
- Zhang, D.; Zhang, Z.; Pan, Y.L.; Jiang, Y.B.; Zhuang, L.Z.; Zhang, J.S.; Zhang, X.F. Current-driving intergranular corrosion performance regeneration below the precipitates solvus temperature in Al-Mg alloy. J. Mater. Sci. Technol. 2020, 53, 132–139. [Google Scholar] [CrossRef]
- Dolinsky, Y.; Elperin, T. Thermodynamics of phase transitions in current-carrying conductors. Phys. Rev. B 1993, 47, 14778–14785. [Google Scholar] [CrossRef]
- Dolinsky, Y.; Elperin, T. Peculiarities of coexistence of phases with different electric conductivities under the influence of electric current. Mater. Sci. Eng. A 2000, 287, 219–226. [Google Scholar] [CrossRef]
- Dolinsky, Y.; Elperin, T. Peculiarities of coexistence of phases with different electric conductivities under the influence of an electric current. Phys. Rev. B 1998, 58, 3008–3014. [Google Scholar] [CrossRef]
- Hillel, A.; Rossiter, P. Resistivity mechanisms during clustering in alloys. Philos. Mag. B 1981, 44, 383–388. [Google Scholar] [CrossRef]
- Ishino, S.; Chimi, Y.; Tobita, T.; Ishikawa, N.; Suzuki, M.; Iwase, A. Radiation enhanced copper clustering processes in Fe-Cu alloys during electron and ion irradiations as measured by electrical resistivity. J. Nucl. Mater. 2003, 323, 354–359. [Google Scholar] [CrossRef]


| Element (wt. %) | Fe | Cu | C | ELSE |
|---|---|---|---|---|
| FeCu | Bal. | 1.1 | <0.005 | <0.05 |
| Parameters | Temperature (°C) | Processing Time | Electric Parameters |
|---|---|---|---|
| IA or RA | 450 | 0.5 h/1 h/2 h/5 h/ 10 h/20 h/40 h/60 h/ 80 h/100 h/120 h | - |
| EPT | 700 | 150 s | 200 Hz/75 V/ 15.4 A/mm2 |
| AT | 700 | 150 s/1 h/2 h/3 h/ 4 h/5 h/6 h | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xia, S.; Yang, T.; Gao, M.; Hu, X.; Li, Q. Study on the Re-Aging Behavior of Cu-Rich Precipitates in a FeCu Alloy under Electropulsing. Materials 2024, 17, 1287. https://doi.org/10.3390/ma17061287
Xia S, Yang T, Gao M, Hu X, Li Q. Study on the Re-Aging Behavior of Cu-Rich Precipitates in a FeCu Alloy under Electropulsing. Materials. 2024; 17(6):1287. https://doi.org/10.3390/ma17061287
Chicago/Turabian StyleXia, Shengjun, Tinghe Yang, Menglin Gao, Xing Hu, and Qiulin Li. 2024. "Study on the Re-Aging Behavior of Cu-Rich Precipitates in a FeCu Alloy under Electropulsing" Materials 17, no. 6: 1287. https://doi.org/10.3390/ma17061287




