Enhancing the Proton Exchange Membrane in Tubular Air-Cathode Microbial Fuel Cells through a Hydrophobic Polymer Coating on a Hydrogel
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Polymer Coating
2.2. Characterization
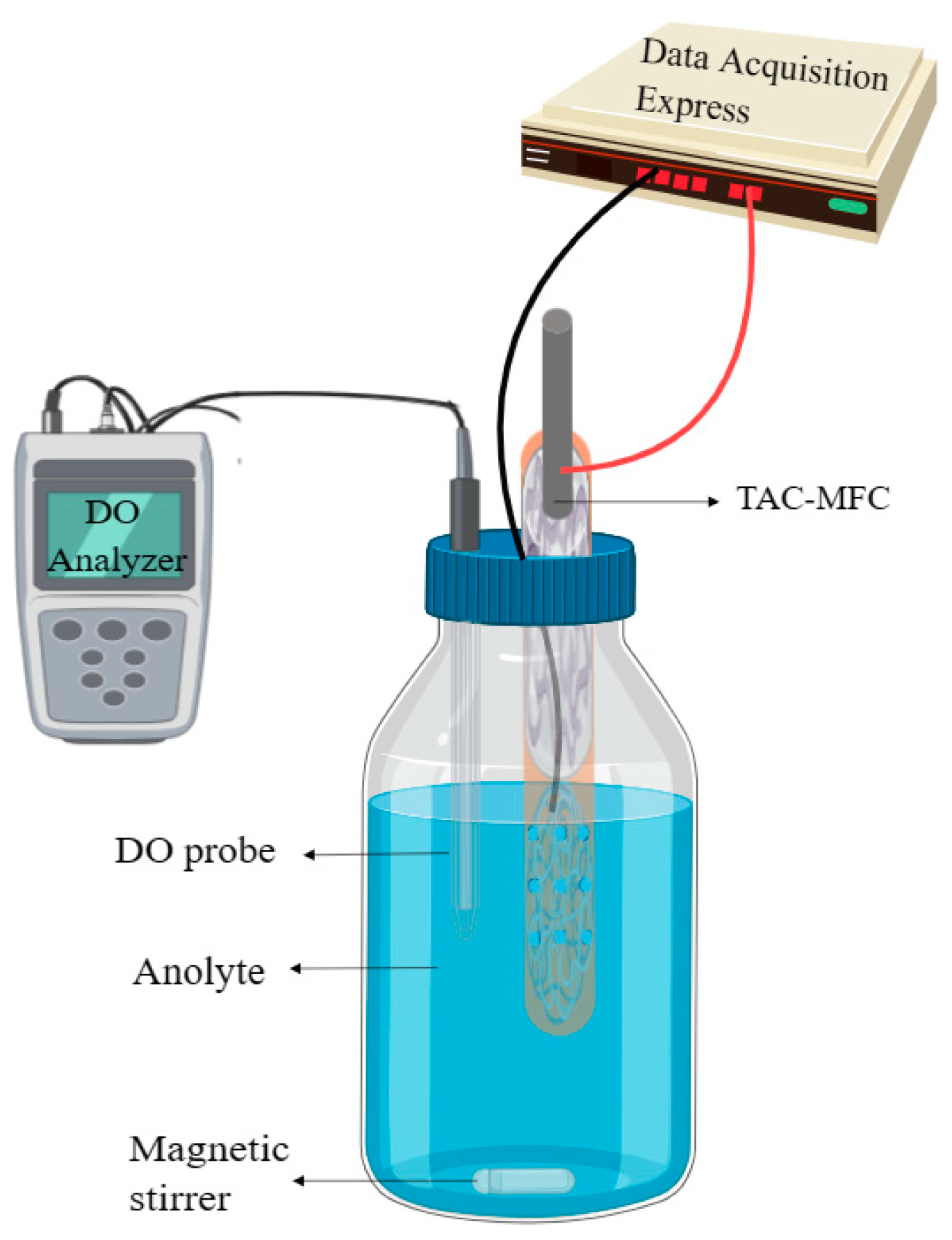
2.3. MFC Setup and Operation
2.4. Measurement and Calculation Method
3. Results and Discussion
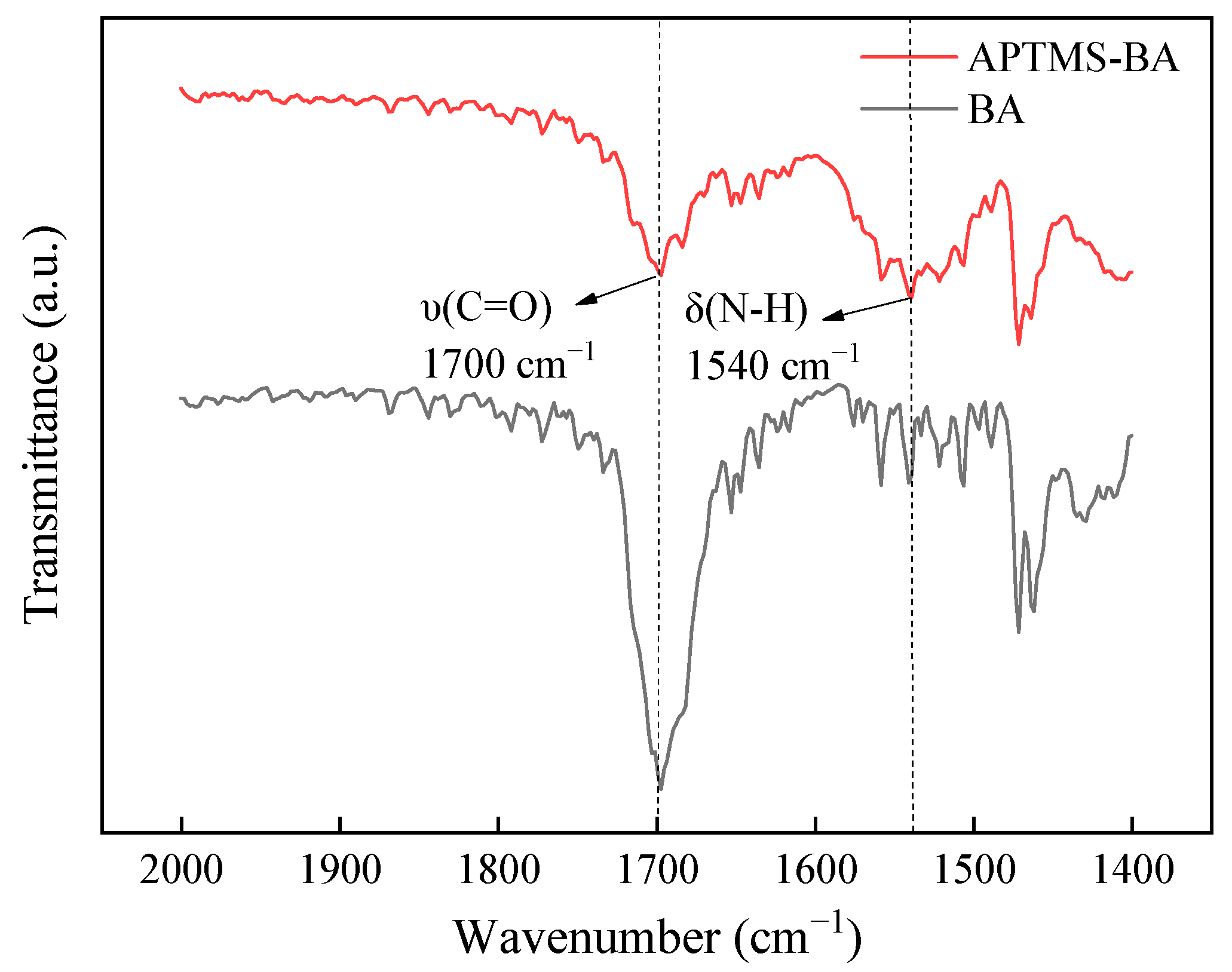
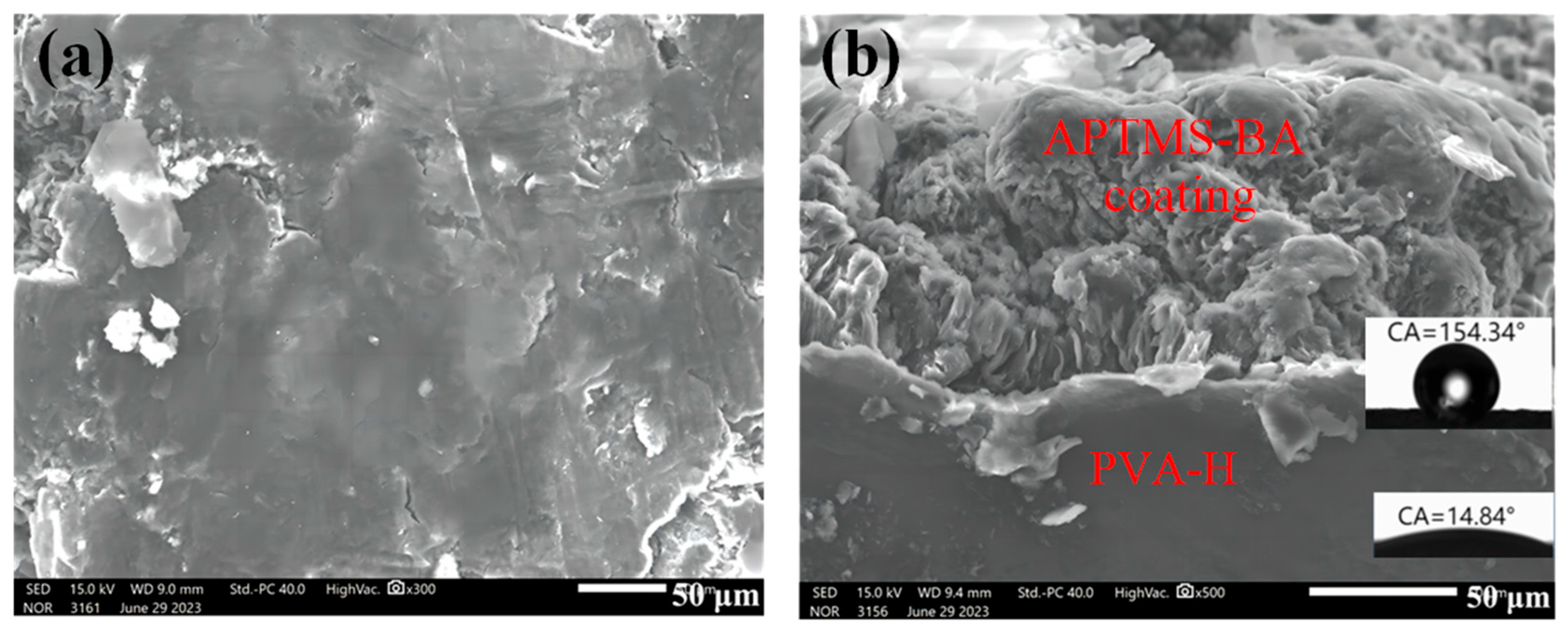
3.1. Structure and Morphology Characterization
3.2. Water Retention Test for Hydrogels
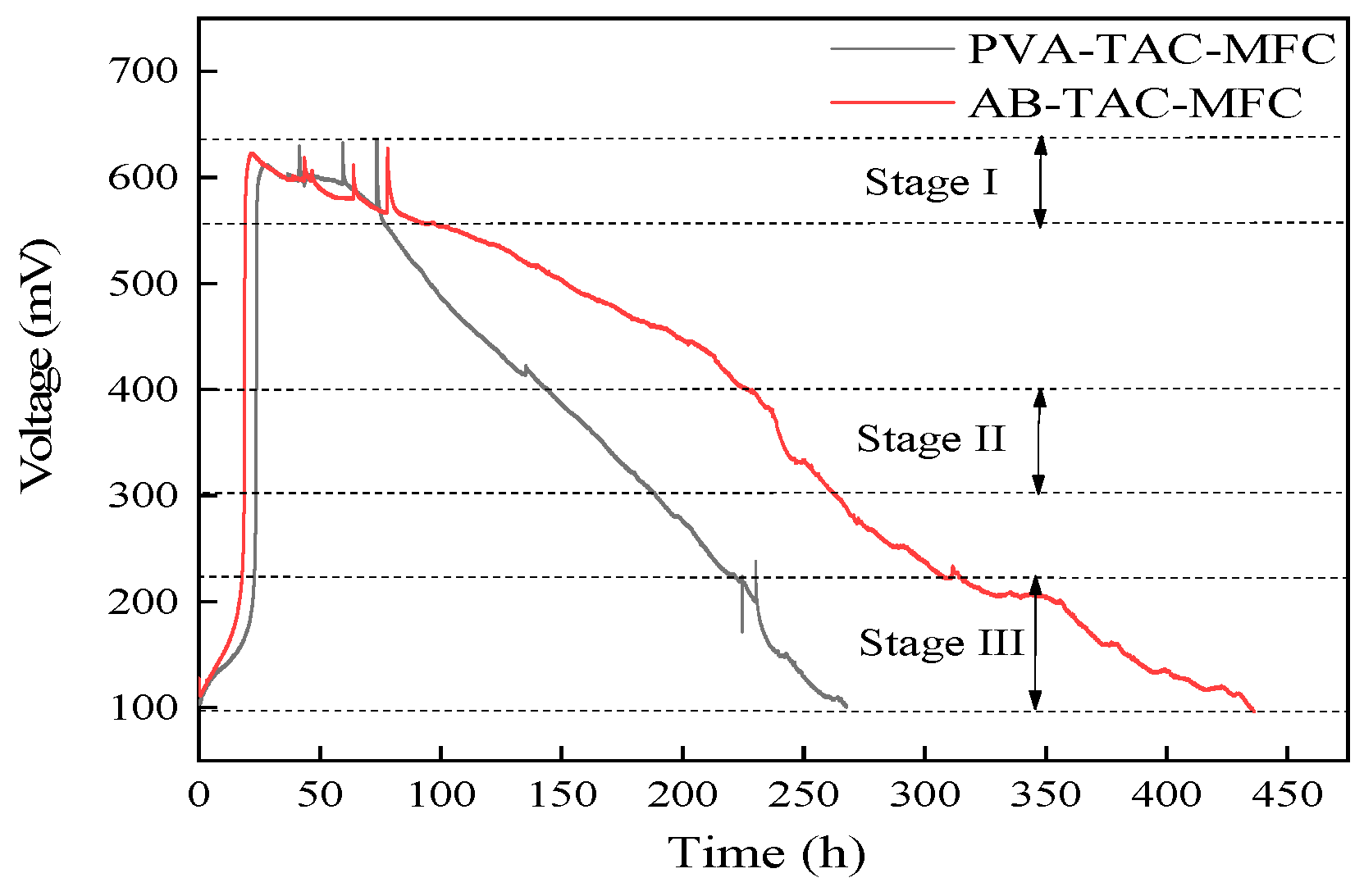
3.3. Voltage Output of Tubular Air-Cathode Microbial Fuel Cells
3.4. Impact of Hydrophobic Polymer Coating on the Kinetics of Cathodic Oxygen Reduction Reaction
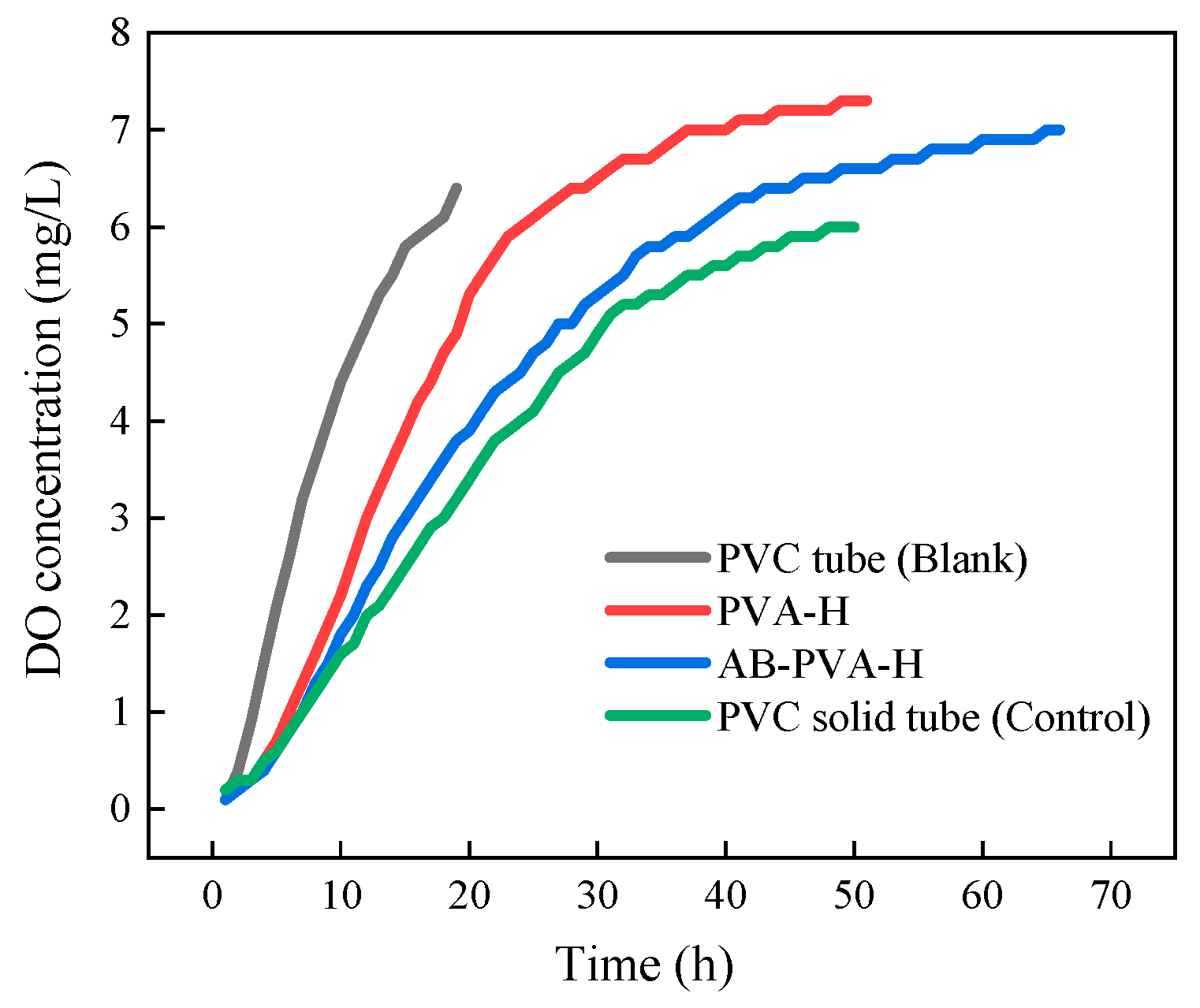
3.5. Transmembrane Diffusion of Oxygen
3.6. Degradation Efficiency of Chemical Oxygen Demand
4. Conclusions
- The polymer coating extends the PEM usage time by three times, increasing MFC runtime from the original 265 h to 440 h, an extension of approximately 66%;
- Hydrophobicity in the polymer coating reduces PEM contamination levels and reduces the internal resistance of the MFC. At the end of the MFC operation, the internal resistance of the MFC is reduced by 45%. The polymer coating prevents the anode liquid from penetrating into the cathode along the PEM surface, reduces the formation of biological fouling on the cathode, and effectively maintains the ORR activity of the cathode;
- The polymer coating reduces the degree of oxygen transmembrane diffusion to the anode chamber. The oxygen permeability coefficient is significantly lower compared with the PEM without the polymer coating. The degradation efficiency of COD from 16% to 35% increased by a factor of one.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ahn, Y.; Logan, B.E. Effectiveness of domestic wastewater treatment using microbial fuel cells at ambient and mesophilic temperatures. Bioresour. Technol. 2010, 101, 469–475. [Google Scholar] [CrossRef]
- Mkilima, T.; Saspugayeva, G.; Tussupova, Z.; Kaliyeva, G.; Dakieva, K.; Kumarbekuly, S.; Tungushbayeva, Z.; Kalelova, G. Electrode material impact on microbial fuel cell and electro-Fenton systems for enhanced slaughterhouse wastewater treatment: A comparative study of graphite and titanium. Water Environ. Res. 2024, 96, e10989. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, R.K.; Sarangi, P.K.; Vivekanand, V.; Pareek, N.; Shaik, K.B.; Subudhi, S. Microbial fuel cells for waste nutrients minimization: Recent process technologies and inputs of electrochemical active microbial system. Microbiol. Res. 2022, 265, 127216. [Google Scholar] [CrossRef] [PubMed]
- Ait-Itto, F.Z.; Behan, J.A.; Martinez, M.; Barrière, F. Development of bioanodes rich in exoelectrogenic bacteria using iron-rich palaeomarine sediment inoculum. Bioelectrochemistry 2024, 156, 108618. [Google Scholar] [CrossRef] [PubMed]
- Neumann, D.; Heuer, A.; Hemkemeyer, M.; Martens, R.; Tebbe, C.C. Importance of soil organic matter for the diversity of microorganisms involved in the degradation of organic pollutants. ISME J. 2014, 8, 1289–1300. [Google Scholar] [CrossRef] [PubMed]
- Ishaq, A.; Said, M.I.M.; Azman, S.B.; Houmsi, M.R.; Isah, A.S.; Jagun, Z.T.; Mohammad, S.J.; Bello, A.A.D.; Abubakar, U.A. The influence of various chemical oxygen demands on microbial fuel cells performance using leachate as a substrate. Environ. Sci. Pollut. Res. 2024, 31. [Google Scholar] [CrossRef] [PubMed]
- Rahimnejad, M.; Bakeri, G.; Najafpour, G.; Ghasemi, M.; Oh, S.-E. A review on the effect of proton exchange membranes in microbial fuel cells. Biofuel Res. J. 2014, 1, 7–15. [Google Scholar] [CrossRef]
- Tai, M.H.; Thiam, H.S.; Tee, S.F.; Lim, Y.S.; Saw, L.H.; Lai, S.O. Self-healing sulfonated poly(ether ether ketone)-based polymer electrolyte membrane for direct methanol fuel cells: Effect of solvent content. Polymers 2023, 15, 4641. [Google Scholar] [CrossRef] [PubMed]
- Li, C.; Yi, K.; Hu, S.; Yang, W. Cathodic biofouling control by microbial separators in air-breathing microbial fuel cells. Environ. Sci. Ecotechnol. 2023, 15, 100251. [Google Scholar] [CrossRef]
- Cheng, S.; Liu, H.; Logan, B.E. Increased performance of single-chamber microbial fuel cells using an improved cathode structure. Electrochem. Commun. 2006, 8, 489–494. [Google Scholar] [CrossRef]
- Tang, X.; Guo, K.; Li, H.; Du, Z.; Tian, J. Microfiltration membrane performance in two-chamber microbial fuel cells. Biochem. Eng. J. 2010, 52, 194–198. [Google Scholar] [CrossRef]
- Zhang, X.; Sun, H.; Liang, P.; Huang, X.; Chen, X.; Logan, B.E. Air-cathode structure optimization in separator-coupled microbial fuel cells. Biosens. Bioelectron. 2011, 30, 267–271. [Google Scholar] [CrossRef]
- Tao, H.C.; Sun, X.N.; Xiong, Y. A novel hybrid anion exchange membrane for high performance microbial fuel cells. RSC Adv. 2015, 5, 4659–4663. [Google Scholar] [CrossRef]
- Arkatkar, A.; Mungray, A.K.; Sharma, P. Biological modification in air-cathode microbial fuel cell: Effect on oxygen diffusion, current generation and wastewater degradation. Chemosphere 2021, 284, 131243. [Google Scholar] [CrossRef] [PubMed]
- Kondaveeti, S.; Moon, J.M.; Min, B. Optimum spacing between electrodes in an air-cathode single chamber microbial fuel cell with a low-cost polypropylene separator. Bioprocess Biosyst. Eng. 2017, 40, 1851–1858. [Google Scholar] [CrossRef]
- Huang, D.; Song, B.Y.; He, Y.L.; Ren, Q.; Yao, S. Cations diffusion in Nafion117 membrane of microbial fuel cells. Electrochim. Acta 2017, 245, 654–663. [Google Scholar] [CrossRef]
- Ochi, S.; Kamishima, O.; Mizusaki, J.; Kawamura, J. Investigation of proton diffusion in Nafion® 117 membrane by electrical conductivity and NMR. Solid State Ion. 2009, 180, 580–584. [Google Scholar] [CrossRef]
- Labus, K.; Radosinski, L.; Kotowski, P. Functional properties of two-component hydrogel systems based on gelatin and polyvinyl alcohol-experimental studies supported by computational analysis. Int. J. Mol. Sci. 2021, 22, 9909. [Google Scholar] [CrossRef]
- Longoni, A.; Major, G.S.; Jiang, S.; Farrugia, B.L.; Kieser, D.C.; Woodfield, T.B.F.; Rnjak-Kovacina, J.; Lim, K.S. Pristine gelatin incorporation as a strategy to enhance the biofunctionality of poly(vinyl alcohol)-based hydrogels for tissue engineering applications. Biomater. Sci. 2023, 12, 134–150. [Google Scholar] [CrossRef]
- Pelin, I.M.; Silion, M.; Popescu, I.; Rîmbu, C.M.; Fundueanu, G.; Constantin, M. Pullulan/poly(vinyl alcohol) hydrogels loaded with calendula officinalis extract: Design and in vitro evaluation for wound healing applications. Pharmaceutics 2023, 15, 1674. [Google Scholar] [CrossRef]
- Gomaa, M.M.; Hugenschmidt, C.; Dickmann, M.; Abdel-Hady, E.E.; Mohamed, H.F.M.; Abdel-Hamed, M.O. Crosslinked PVA/SSA proton exchange membranes: Correlation between physiochemical properties and free volume determined by positron annihilation spectroscopy. Phys. Chem. Chem. Phys. 2018, 20, 28287–28299. [Google Scholar] [CrossRef] [PubMed]
- Abu-Saied, M.A.; Soliman, E.A.; Abualnaj, K.M.; El Desouky, E. Highly conductive polyelectrolyte membranes poly(vinyl alcohol)/poly(2-acrylamido-2-methyl propane sulfonic acid) (PVA/PAMPS) for fuel cell application. Polymers 2021, 13, 2638. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.M.A. PVC based polyvinyl alcohol zinc oxide composite membrane: Synthesis and electrochemical characterization for heavy metal ions. J. Ind. Eng. Chem. 2013, 19, 1365–1370. [Google Scholar] [CrossRef]
- Xu, J.; Sheng, G.P.; Luo, H.W.; Li, W.W.; Wang, L.F.; Yu, H.Q. Fouling of proton exchange membrane (PEM) deteriorates the performance of microbial fuel cell. Water Res. 2012, 46, 1817–1824. [Google Scholar] [CrossRef] [PubMed]
- Simari, C.; Enotiadis, A.; Nicotera, I. Transport properties and mechanical features of sulfonated polyether ether ketone/organosilica layered materials nanocomposite membranes for fuel cell applications. Membranes 2020, 10, 87. [Google Scholar] [CrossRef]
- Mredha, M.T.I.; Le, H.H.; Cui, J.; Jeon, I. Double-hydrophobic-coating through quenching for hydrogels with strong resistance to both drying and swelling. Adv. Sci. 2020, 7, 1903145. [Google Scholar] [CrossRef]
- Zhu, T.; Jiang, C.; Wang, M.; Zhu, C.; Zhao, N.; Xu, J. Skin-inspired double-hydrophobic-coating encapsulated hydrogels with enhanced water retention capacity. Adv. Funct. Mater. 2021, 31, 2102433. [Google Scholar] [CrossRef]
- Gao, W.; Chang, J.; Li, X.; Li, S.; Zhou, Y.; Hou, X.; Long, L.; Zhao, J.; Yuan, X. A quenched double-hydrophilic coating for the enhancement of water retention of hydrogels. Adv. Funct. Mater. 2023, 33, 2303306. [Google Scholar] [CrossRef]
- Asakura, M.; Aimu, K.; Hayashi, T.; Matsubara, M.; Mieki, A.; Ban, S.; Kawai, T. Bonding characteristics of silane coupling agent and MMA-containing primer to various composite CAD/CAM blocks. Polymers 2023, 15, 3396. [Google Scholar] [CrossRef]
- Temp, R.W.; Packaeser, M.G.; Machry, R.V.; Dapieve, K.S.; Rippe, M.P.; Pereira, G.K.R.; Valandro, L.F. Characteristic fatigue strength and reliability of dental glass-ceramics: Effect of distinct surface treatments—Hydrofluoric acid etching and silane treatment vs one-step self-etching ceramic primer. J. Mech. Behav. Biomed. Mater. 2024, 150, 106338. [Google Scholar] [CrossRef]
- Marinescu, L.; Motelica, L.; Ficai, D.; Ficai, A.; Oprea, O.C.; Andronescu, E.; Holban, A.M. A two-step surface modification methodology for the advanced protection of a stone surface. Nanomaterials 2023, 14, 68. [Google Scholar] [CrossRef]
- Si, F.; Zhao, N.; Chen, L.; Xu, J.; Tao, Q.; Li, J.; Ran, C. A superhydrophobic surface with high performance derived from STA-APTES organic–inorganic molecular hybrid. J. Colloid Interface Sci. 2013, 407, 482–487. [Google Scholar] [CrossRef] [PubMed]
- Rao, Z.; Zhu, D.; Xu, Y.; Lan, M.; Jiang, L.; Wang, Z.; Tang, B.; Liu, H. Enhanced proton transfer in proton-exchange membranes with interconnected and zwitterion-functionalized covalent porous material structures. ChemSusChem 2023, 16, e202202279. [Google Scholar] [CrossRef]
- Xu, G.; Zou, J.; Guo, Z.; Li, J.; Ma, L.; Li, Y.; Cai, W. Bi-functional composting the sulfonic acid based proton exchange membrane for high temperature fuel cell application. Polymers 2020, 12, 1000. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Ban, J.Y.; Oh, C.H.; Park, H.K.; Choi, S. A solvent-free microbial-activated air cathode battery paper platform made with pencil-traced graphite electrodes. Sci. Rep. 2016, 6, 28588. [Google Scholar] [CrossRef] [PubMed]




| Type of Proton Exchange Membrane | K (cm s−1) |
|---|---|
| PVC tube (Blank) | 3.145 ± 0.003 |
| PVA-H | 2.402 ± 0.005 |
| AB-PVA-H 1 | 1.859 ± 0.002 |
| PVC solid tube (Control) | 1.564 ± 0.003 |
| MFC Type | COD I (mg L−1) | COD II (mg L−1) | COD III (mg L−1) | ηCOD (%) |
|---|---|---|---|---|
| PVA-TAC-MFC | 5370 | 5008 | 4496 | 16 |
| AB-TAC-MFC | 5472 | 4993 | 3566 | 35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Huang, J.; Wu, C.-H.; Li, F.; Wang, X.; Chen, S.-C. Enhancing the Proton Exchange Membrane in Tubular Air-Cathode Microbial Fuel Cells through a Hydrophobic Polymer Coating on a Hydrogel. Materials 2024, 17, 1286. https://doi.org/10.3390/ma17061286
Huang J, Wu C-H, Li F, Wang X, Chen S-C. Enhancing the Proton Exchange Membrane in Tubular Air-Cathode Microbial Fuel Cells through a Hydrophobic Polymer Coating on a Hydrogel. Materials. 2024; 17(6):1286. https://doi.org/10.3390/ma17061286
Chicago/Turabian StyleHuang, Junlin, Chih-Hung Wu, Fuying Li, Xiang Wang, and Sheng-Chung Chen. 2024. "Enhancing the Proton Exchange Membrane in Tubular Air-Cathode Microbial Fuel Cells through a Hydrophobic Polymer Coating on a Hydrogel" Materials 17, no. 6: 1286. https://doi.org/10.3390/ma17061286






