3D Polymer Gel Dosimeters with iCBCT 3D Reading and polyGeVero-CT Software Package for Quality Assurance in Radiotherapy
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of 3D Polymer Gel Dosimeters
2.2. Irradiation of 3D Polymer Gel Dosimeters
2.3. iCBCT Scanning of 3D Polymer Gel Dosimeters
2.4. Data Processing
3. Results and Discussion
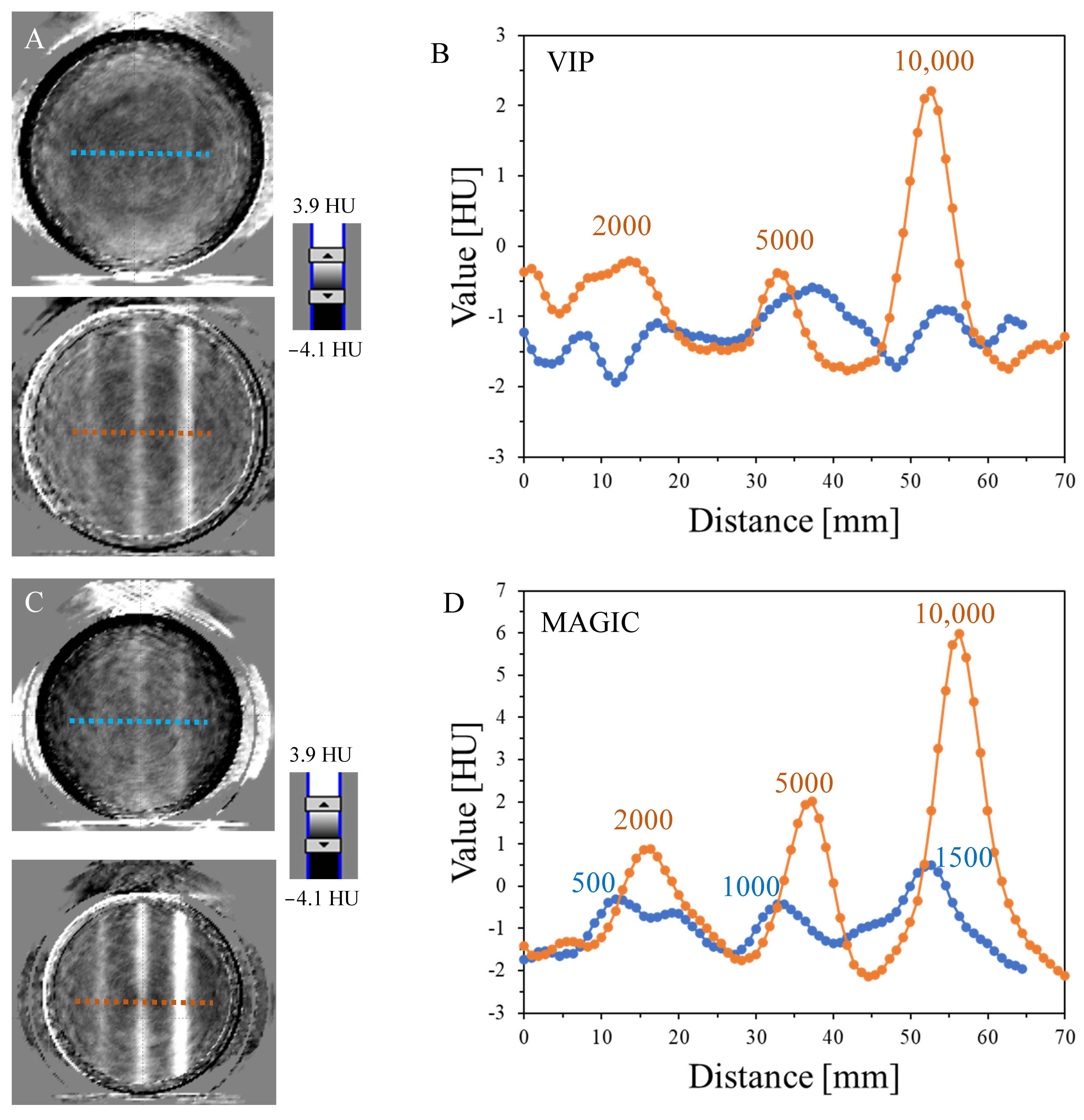
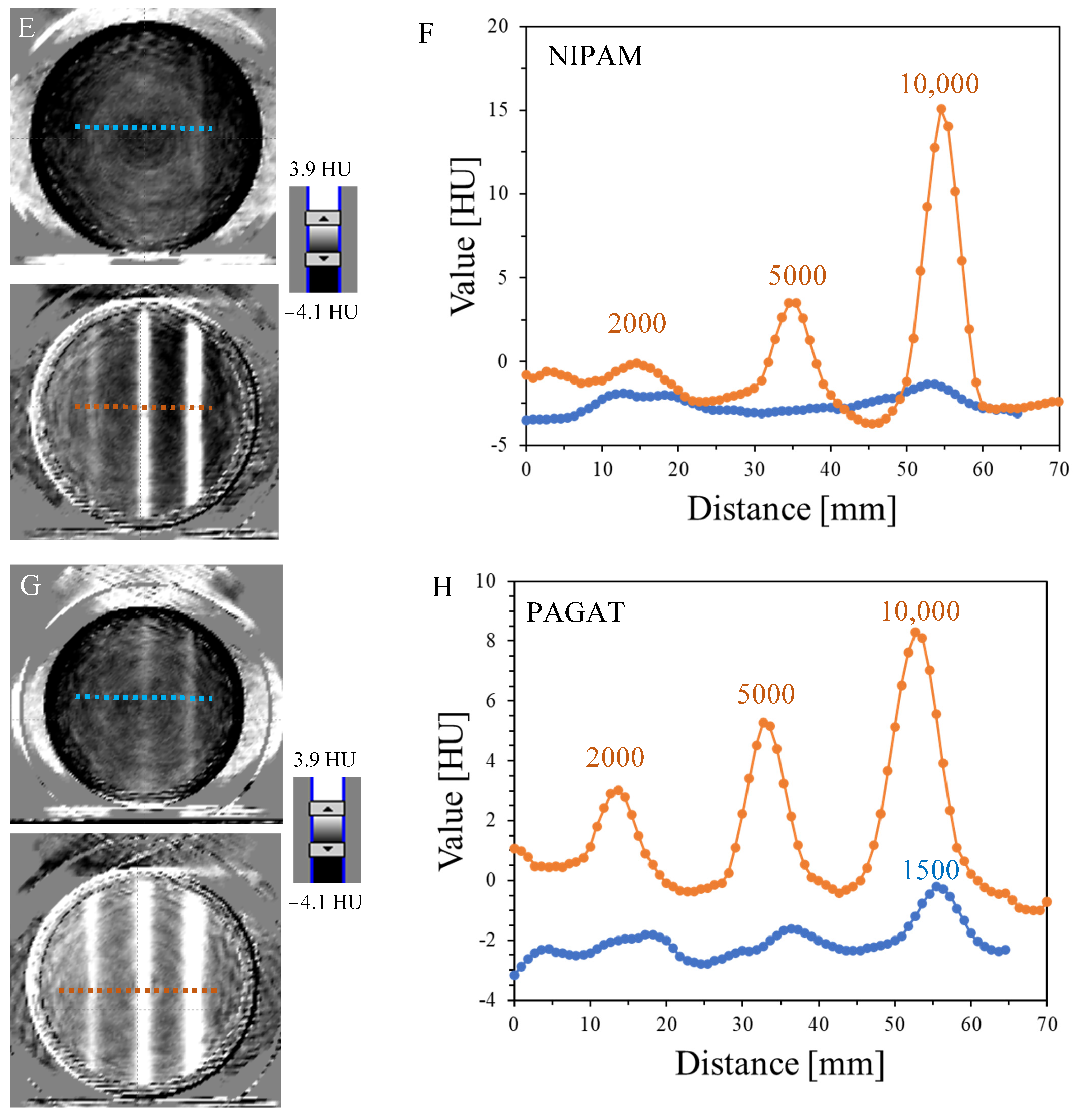
3.1. Impact of MU on the 3D Reading
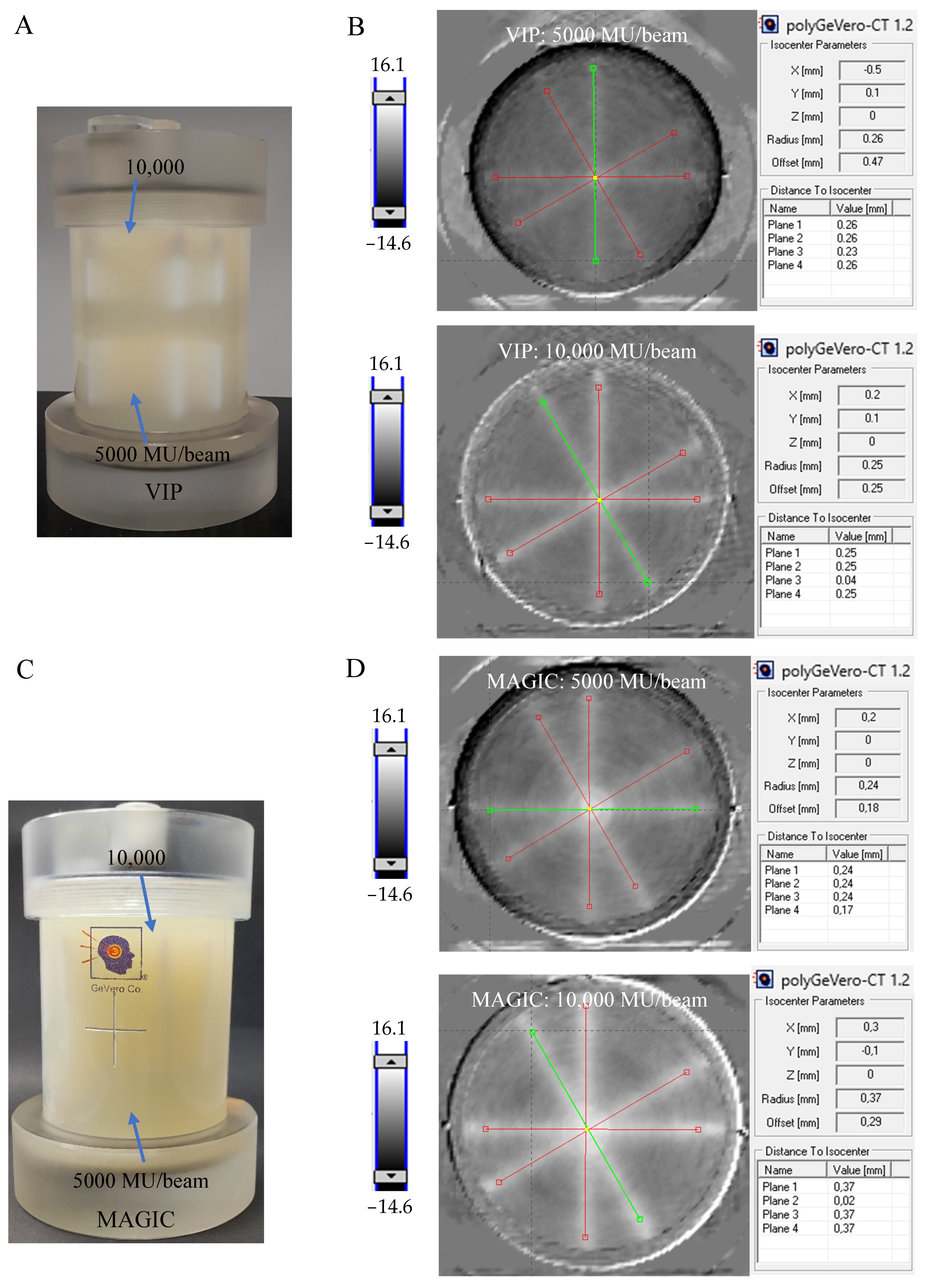
3.2. Radiation Isocenter Determination
3.3. Workload and Cost of Dosimeters
3.4. Uncertainty Budget
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Slotman, B.J.; Solberg, T.D.; Verellen, D. (Eds.) Extracranial Stereotactic Radiotherapy and Radiosurgery; Taylor & Francis: London, UK, 2005. [Google Scholar]
- Trifiletti, D.M.; Chao, S.T.; Sahgal, A.; Sheehan, J.P. (Eds.) Stereotactic Radiosurgery and Stereotactic Body Radiation Therapy. A Comprehensive Guide; Springer Nature: Cham, Switzerland, 2019. [Google Scholar]
- Quality Control of Medical Electron Accelerators. Recommendations Number 11; Swiss Society of Radiobiology and Medical Physics: Baden, Switzerland, 2015; ISBN 3 908 125 57 X.
- Klein, E.E.; Hanley, J.; Bayouth, J.; Yin, F.F.; Simon, W.; Dresser, S.; Serago, C.; Aguirre, F.; Ma, L.; Arjomandy, B.; et al. Task group 142 report: Quality assurance of medical accelerators. Med. Phys. 2009, 36, 4197–4212. [Google Scholar] [CrossRef]
- Zhang, M.; Zhou, S.M.; Qu, T. What Do We Mean When We Talk about the Linac Isocenter? Int. J. Med. Phys. Clin. Eng. Radiat. Oncol. 2015, 4, 233–242. [Google Scholar] [CrossRef]
- Treuer, H.; Hoevels, M.; Luyken, K.; Gierich, A.; Kocher, M.; Muller, R.P.; Sturm, V. On isocentre adjustment and quality control in linear accelerator based radiosurgery with circular collimators and room lasers. Phys. Med. Biol. 2000, 45, 2331–2342. [Google Scholar] [CrossRef]
- Gonzalez, A.; Castro, A.I.; Martinez, J.A. A procedure to determine the radiation isocenter size in a linear accelerator. Med. Phys. 2004, 31, 1489–1493. [Google Scholar] [CrossRef] [PubMed]
- Depuydt, T.; Penne, R.; Verellen, D.; Hrbacek, J.; Lang, S.; Leysen, K.; Vandevondel, I.; Poels, K.; Reynders, T.; Gevaert, T.; et al. Computer-aided analysis of star shot films for high-accuracy radiation therapy treatment units. Phys. Med. Biol. 2012, 57, 2997–3011. [Google Scholar] [CrossRef] [PubMed]
- Fan, Q.; Zhou, S.; Lei, Y.; Li, S.; Zhang, M.A. Quality assurance approach for linear accelerator mechanical isocenters with portal images. Int. J. Med. Phys. Clin. Eng. Radiat. Oncol. 2018, 7, 100–114. [Google Scholar] [CrossRef]
- Peace, T.; Subramanian, B.; Ravindran, P. An experimental study on using a diagnostic computed radiography system as a quality assurance tool in radiotherapy. Australas. Phys. Eng. Sci. Med. 2008, 31, 226–234. [Google Scholar] [CrossRef] [PubMed]
- Liu, G.; van Doorn, T.; Bezak, E. The linear accelerator mechanical and radiation isocentre assessment with an electronic portal imaging device (EPID). Australas. Phys. Eng. Sci. Med. 2004, 27, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Dong, L.; Shiu, A.; Tung, S.; Boyer, A. Verification of radiosurgery target point alignment with an electronic portal imaging device (EPID). Med. Phys. 1997, 24, 263–267. [Google Scholar] [CrossRef]
- Winey, B.; Sharp, G.; Bussière, M.A. Fast double template convolution isocenter evaluation algorithm with subpixel accuracy. Med. Phys. 2011, 38, 223–227. [Google Scholar] [CrossRef]
- Rowshanfarzad, P.; Sabet, M.; O’Connor, D.J.; Greer, P.B. Isocenter verification for linac-based stereotactic radiation therapy: Review of principles and techniques. J. Appl. Clin. Med. Phys. 2011, 12, 185–195. [Google Scholar] [CrossRef]
- Lutz, W.; Winston, K.R.; Maleki, N. A system for stereotactic radiosurgery with a linear accelerator. Int. J. Radiat. Oncol. Biol. Phys. 1988, 14, 373–381. [Google Scholar] [CrossRef]
- Baldock, C.; De Deene, Y.; Doran, S.; Ibbott, G.; Jirasek, A.; Lepage, M.; McAuley, K.B.; Oldham, M.; Schreiner, L.J. Polymer gel dosimetry. Phys. Med. Biol. 2010, 55, R1–R63. [Google Scholar] [CrossRef] [PubMed]
- De Deene, Y. Radiation dosimetry by use of radiosensitive hydrogels and polymers: Mechanisms, state-of-the-art and perspective from 3D to 4D. Gels 2022, 8, 599. [Google Scholar] [CrossRef] [PubMed]
- Maras, P.; Kozicki, M. Fast isocenter determination using 3D polymer gel dosimetry with kilovoltage cone-beam CT reading and the polyGeVero-CT software package for linac quality assurance in radiotherapy. Materials 2022, 15, 6807. [Google Scholar] [CrossRef]
- Gore, J.C.; Kang, Y.S.; Schultz, R.J. Measurement of radiation dose distributions by nuclear magnetic resonance (NMR) imaging. Phys. Med. Biol. 1984, 29, 1189–1197. [Google Scholar] [CrossRef] [PubMed]
- Maryanski, M.J.; Gore, J.C.; Kennan, R.P.; Schulz, R.J. NMR relaxation enhancement in gels polymerized and cross-linked by ionizing radiation: A new approach to 3D dosimetry by MRI. Magn. Reson. Imaging 1993, 11, 253–258. [Google Scholar] [CrossRef]
- Maryanski, M.J.; Audet, C.; Gore, J.C. Effects of crosslinking and temperature on the dose response of a BANG polymer gel dosimeter. Phys. Med. Biol. 1997, 42, 303–311. [Google Scholar] [CrossRef]
- De Deene, Y.; Venning, A.; Hurley, C.; Healy, B.J.; Baldock, C. Dose–response stability and integrity of the dose distribution of various polymer gel dosimeters. Phys. Med. Biol. 2022, 47, 2459–2470. [Google Scholar] [CrossRef]
- Vandecasteele, J.; De Deene, Y. On the validity of 3D polymer gel dosimetry, II: Physico-chemical effects. Phys. Med. Biol. 2013, 58, 43–61. [Google Scholar] [CrossRef]
- De Deene, Y.; Vergote, K.; Claeys, C.; De Wagter, C. The fundamental radiation properties of normoxic polymer gel dosimeters: A comparison between a methacrylic acid based gel and acrylamide based gels. Phys. Med. Biol. 2006, 51, 653–673. [Google Scholar] [CrossRef]
- Fong, P.M.; Keil, D.C.; Does, M.D.; Gore, J.C. Polymer gels for magnetic resonance imaging of radiation dose distributions at normal room atmosphere. Phys. Med. Biol. 2001, 46, 3105–3113. [Google Scholar] [CrossRef]
- Fernandes, J.P.; Pastorello, B.F.; de Araujo, D.B.; Baffa, O. Formaldehyde increases MAGIC gel dosimeter melting point and sensitivity. Phys. Med. Biol. 2008, 53, N53–N58. [Google Scholar] [CrossRef] [PubMed]
- Jirasek, A.; Hilts, M.; McAuley, K.B. Polymer gel dosimeters with enhanced sensitivity for use in X-ray CT polymer gel dosimetry. Phys. Med. Biol. 2010, 55, 5269–5281. [Google Scholar] [CrossRef] [PubMed]
- Johnston, H.; Hilts, M.; Carrick, J.; Jirasek, A. An X-ray CT polymer gel dosimetry prototype: II. Gel characterization and clinical application. Phys. Med. Biol. 2012, 57, 3155–3175. [Google Scholar] [CrossRef] [PubMed]
- Pappas, E.; Maris, T.; Angelopoulos, A.; Paparigopoulou, M.; Sakelliou, L.; Sandilos, P.; Voyiatzi, S.; Vlachos, L. A new polymer gel for magnetic resonance imaging (MRI) radiation dosimetry. Phys. Med. Biol. 1999, 44, 2677. [Google Scholar] [CrossRef]
- Kozicki, M.; Maras, P.; Rybka, K.; Bieganski, T.; Kadłubowski, S.; Petrokokkinos, L. On the development of the VIPAR polymer gel dosimeter for three-dimensional dose measurements. Macromol. Symp. 2007, 254, 345–352. [Google Scholar]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Dudek, M.; Cłapa, M. On the development of a VIPARnd radiotherapy 3D polymer gel dosimeter. Phys. Med. Biol. 2017, 62, 986–1008. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Berg, A.; Maras, P.; Jaszczak, M.; Dudek, M. Clinical radiotherapy application of N-vinylpyrrolidone-containing 3D polymer gel dosimeters with remote external MR-reading. Phys. Med. 2020, 69, 134–146. [Google Scholar] [CrossRef] [PubMed]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Dudek, M. A chemical evolution of NVP-containing VIPAR-family 3D polymer gel dosimeters—A brief overview. J. Phys. Conf. Ser. 2019, 1305, 012067. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Al-Tarawneh, R.E.; Eyadeh, M.M.; Hammoudeh, I.M.E.; Shatnawi, M.T.M. Improved Dose Response of N-(hydroxymethyl)acrylamide Gel Dosimeter with Calcium Chloride for Radiotherapy. Gels 2022, 8, 78. [Google Scholar] [CrossRef]
- Khan, M.; Heilemann, G.; Lechner, W.; Georg, D.; Berg, A. Basic properties of a new polymer gel for 3D-dosimetry at high dose-rates typical for FFF irradiation based on dithiothreitol and methacrylic acid (MAGADIT): Sensitivity, range, reproducibility, accuracy, dose rate effect and impact of oxygen scavenger. Polymers 2019, 11, 1717. [Google Scholar] [CrossRef] [PubMed]
- Moftah, B.; Basfar, A.A.; Almousa, A.A.; Kafi, A.; Rabaeh, K.A. Novel 3D polymer gel dosimeters based on N-(3-Methoxypropyl)acrylamide (NMPAGAT) for quality assurance in radiation oncology. Radiat. Meas. 2020, 135, 106372. [Google Scholar] [CrossRef]
- d’Errico, F.; Lazzeri, L.; Dondi, D.; Mariani, M.; Marrale, M.; Souza, S.O.; Gambarini, S. Novel GTA-PVA Fricke gels for three-dimensional dose mapping in radiotherapy. Radiat. Meas. 2017, 106, 612–617. [Google Scholar] [CrossRef]
- Gallo, S.; Artuso, E.; Brambilla, M.G.; Gambarini, G.; Lenardi, C.; Monti, A.F.; Torresin, A.; Pignoli, E.; Veronese, I. Characterization of radiochromic poly(vinylalcohol)–glutaraldehyde Fricke gels for dosimetry in external X-ray radiation therapy. J. Phys. D Appl. Phys. 2019, 52, 225601. [Google Scholar] [CrossRef]
- Rabaeh, K.A.; Eyadeh, M.M.; Hailat, T.F.; Madas, B.G.; Aldweri, F.M.; Almomani, A.M.; Awad, S.I. Improvement on the performance of chemically cross-linked Fricke methylthymol-blue radiochromic gel dosimeter by addition of dimethyl sulfoxide. Radiat. Meas. 2021, 141, 106540. [Google Scholar] [CrossRef]
- Babic, S.; Battista, J.; Jordan, K. An apparent threshold dose response in ferrous xylenol-orange gel dosimeters when scanned with a yellow light source. Phys. Med. Biol. 2008, 53, 1637–1650. [Google Scholar] [CrossRef]
- Dudek, M.; Piotrowski, M.; Maras, P.; Jaszczak, M.; Kozicki, M. Anisotropic diffusion of Fe ions in Fricke-XO-Pluronic F-127 and Fricke-XO-gelatine 3D radiotherapy dosimeters. Phys. Med. Biol. 2021, 66, 155005. [Google Scholar] [CrossRef]
- Kozicki, M.; Kwiatos, K.; Kadlubowski, S.; Dudek, M. TTC-Pluronic 3D radiochromic gel dosimetry of ionizing radiation. Phys. Med. Biol. 2017, 62, 5668–5690. [Google Scholar] [CrossRef]
- Kwiatos, K.; Maras, P.; Kadlubowski, S.; Stempień, Z.; Dudek, M.; Kozicki, M. Tetrazolium salts-Pluronic F-127 gels for 3D radiotherapy dosimetry. Phys. Med. Biol. 2018, 63, 095012. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak, M.; Kwiatos, K.; Maras, P.; Kadlubowski, S.; Wach, R.; Dudek, M. Three-dimensional radiochromic and polymer gel dosimeters with Pluronic F-127 matrix—A review of current research. J. Phys. Conf. Ser. 2019, 1305, 012035. [Google Scholar] [CrossRef]
- Hayashi, S.-I.; Ono, K.; Fujino, K.; Ikeda, S.; Tanaka, K. Novel radiochromic gel dosimeter based on a polyvinyl alcohol-Iodide complex. Radiat. Meas. 2020, 131, 106226. [Google Scholar] [CrossRef]
- Babic, S.; Battista, J.; Jordan, K. Radiochromic leuco dye micelle hydrogels: II. Low diffusion rate leuco crystal violet gel. Phys. Med. Biol. 2009, 54, 6791–6808. [Google Scholar] [CrossRef]
- Warman, J.M.; de Haas, M.P.; Luthjens, L.H.; Yao, T.; Navarro-Campos, J.; Yuksel, S.; Aarts, J.; Thiele, S.; Houter, J.; In Het Zandt, W. FluoroTome 1: An apparatus for tomographic imaging of radio-fluorogenic (RFG) gels. Polymers 2019, 11, 1729. [Google Scholar] [CrossRef]
- Warman, J.M.; de Haas, M.P.; Luthjens, L.H.; Denkova, A.G.; Yao, T. A radio-fluorogenic polymer-gel makes fixed fluorescent images of complex radiation fields. Polymers 2018, 10, 685. [Google Scholar] [CrossRef] [PubMed]
- Maeyama, T.; Hase, S. Nanoclay gel-based radio-fluorogenic gel dosimeters using various fluorescence probes. Radiat. Phys. Chem. 2018, 151, 42–46. [Google Scholar] [CrossRef]
- Sandwall, P.A.; Bastow, B.P.; Spitz, H.B.; Elson, H.R.; Lamba, M.; Connick, W.B.; Fenichel, H. Radio-fluorogenic gel dosimetry with coumarin. Bioengineering 2018, 5, 53. [Google Scholar] [CrossRef] [PubMed]
- Brown, S.; Venning, A.; De Deene, Y.; Vial, P.; Oliver, L.; Adamovics, J.; Baldock, C. Radiological properties of the PRESAGE and PAGAT polymer dosimeters. Appl. Radiat. Isot. 2008, 66, 1970–1974. [Google Scholar] [CrossRef]
- Tajaldeen, A.; Alghamdi, S. Investigation of dosimetric impact of organ motion in static and dynamic conditions for three stereotactic ablative body radiotherapy techniques: 3d conformal radiotherapy, intensity modulated radiation therapy, and volumetric modulated arc therapy by using presage 3d dosimeters. Exp. Oncol. 2019, 41, 153–159. [Google Scholar] [PubMed]
- Alqathami, M.; Adamovics, J.; Benning, R.; Qiao, G.; Geso, M.; Blencowe, A. Evaluation of ultra-sensitive leucomalachite dye derivatives for use in the PRESAGE® dosimeter. Radiat. Phys. Chem. 2013, 85, 204–209. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Naglik, R.; Dudek, M.; Kadlubowski, S.; Wach, R. Preliminary study on a 3D lung mimicking dosimeter based on Pluronic F-127 matrix. Radiat. Phys. Chem. 2021, 185, 109479. [Google Scholar] [CrossRef]
- De Deene, Y.; Vergote, K.; Claeys, C.; De Wagter, C. Three dimensional radiation dosimetry in lung-equivalent regions by use of a radiation sensitive gel foam: Proof of principle. Med. Phys. 2006, 33, 2586–2597. [Google Scholar] [CrossRef]
- Haraldsson, P.; Karlsson, A.; Wieslander, E.; Gustavsson, H.; Bäck, S.Å.J. Dose response evaluation of a low-density normoxic polymer gel dosimeter using MRI. Phys. Med. Biol. 2006, 51, 919–928. [Google Scholar] [CrossRef] [PubMed]
- Høye, E.M.; Skyt, P.S.; Yates, E.S.; Muren, L.P.; Petersen, J.B.B.; Balling, P. A new dosimeter formulation for deformable 3D dose verification. J. Phys. Conf. Ser. 2015, 573, 012067. [Google Scholar] [CrossRef]
- Jensen, S.V.; Valdetaro, L.B.; Poulsen, P.R.; Balling, P.; Petersen, J.B.B.; Muren, L.P. Dose-response of deformable radiochromic dosimeters for spot scanning proton therapy. Phys. Imaging Radiat. Oncol. 2020, 16, 134–137. [Google Scholar] [CrossRef]
- De Deene, Y.; Skyt, P.S.; Hil, R.; Booth, J.T. FlexyDos3D: A deformable anthropomorphic 3D radiation dosimeter: Radiation properties. Phys. Med. Biol. 2015, 60, 1543–1563. [Google Scholar] [CrossRef]
- Kozicki, M.; Bartosiak, M.; Maras, P.; Wach, R.; Kadlubowski, S. First Combined, Double-Density LCV-Pluronic F-127 Radiochromic Dosimeter Mimicking Lungs and Muscles. Adv. Mater. Technol. 2023, 8, 2201023. [Google Scholar] [CrossRef]
- Maras, P.; Jaszczak, M.; Kozicki, M. Basic features of VIC-T dosimeter with spiral CT readout. CT scanning conditions and data processing with a new polyGeVero-CT software package. Radiat. Phys. Chem. 2021, 189, 109730. [Google Scholar] [CrossRef]
- Gore, J.C.; Ranade, M.; Maryanski, M.J. Radiation dose distributions in three dimensions from tomographic optical density scanning of polymer gels: I. Development of an optical scanner. Phys. Med. Biol. 1996, 41, 2695–2704. [Google Scholar] [CrossRef]
- Doran, S.J. The history and principles of optical computed tomography for scanning 3-D radiation dosimeters: 2008 update. J. Phys. Conf. Ser. 2009, 164, 012020. [Google Scholar] [CrossRef]
- Mather, M.L.; De Deene, Y.; Whittaker, A.K.; Simon, G.P.; Rutgers, R.; Baldock, C. Investigation of ultrasonic properties of PAG and MAGIC polymer gel dosimeters. Phys. Med. Biol. 2002, 47, 4397–4409. [Google Scholar] [CrossRef]
- Mather, M.L.; Whittaker, A.K.; Baldock, C. Ultrasound evaluation of polymer gel dosimeters. Phys. Med. Biol. 2002, 47, 1449–1458. [Google Scholar] [CrossRef] [PubMed]
- Atkins, T.J.; Humphrey, V.F.; Duck, F.A.; Tooley, M.A. Investigation of ultrasonic properties of MAGIC gels for pulse-echo gel dosimetry. J. Phys. Conf. Ser. 2010, 250, 012075. [Google Scholar] [CrossRef]
- Lagendijk, J.J.W.; Raaymakers, B.W.; Raaijmakers, A.J.E.; Overweg, J.; Brown, K.J.; Kerkhof, E.M.; van der Put, R.W.; Hårdemark, B.; van Vulpen, M.; van der Heide, U.A. MRI/linac integration. Radiother. Oncol. 2008, 86, 5–29. [Google Scholar] [CrossRef] [PubMed]
- Fallone, B.G.; Murray, B.; Rathee, S.; Stanescu, T.; Steciw, S.; Vidakovic, S.; Blosser, E.; Tymofichuk, D. First MR images obtained during megavoltage photon irradiation from a prototype integrated linac-MR system. Med. Phys. 2009, 36, 2084–2088. [Google Scholar] [CrossRef] [PubMed]
- Keall, P.J.; Barton, M.; Crozier, S. The Australian magnetic resonance imaging–linac program. Semin. Radiat. Oncol. 2014, 24, 203–206. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Maslowski, A.; Messmer, P.; Lehmann, M.; Strzelecki, A.; Yu, E.; Paysan, P.; Brehm, M.; Munro, P.; Star-Lack, J.; et al. Acuros CTS: A fast, linear Boltzmann transport equation solver for computed tomography scatter—Part II: System modeling, scatter correction, and optimization. Med. Phys. 2018, 45, 1914–1925. [Google Scholar] [CrossRef] [PubMed]
- Jaffray, D.A.; Siewerdsen, J.H.; Wong, J.W.; Martinez, A.A. Flat-Panel Cone-Beam Computed Tomography for image guided radiation therapy. Int. J. Radiat. Oncol. Biol. Phys. 2002, 53, 1337–1349. [Google Scholar] [CrossRef]
- Pant, K.; Umeh, C.; Oldham, M.; Floyd, S.; Giles, W.; Adamson, J. Comprehensive radiation and imaging isocenter verification using NIPAM kV-CBCT dosimetry. Med. Phys. 2020, 47, 927–936. [Google Scholar] [CrossRef]
- Adamson, J.; Carroll, J.; Trager, M.; Yoon, S.W.; Kodra, J.; Maynard, E.; Hilts, M.; Oldham, M.; Jirasek, A. Delivered dose distribution visualized directly with onboard kV-CBCT: Proof of principle. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 1271–1279. [Google Scholar] [CrossRef]
- Dorsch, S.; Mann, P.; Lang, C.; Hearing, P.; Runz, A.; Karger, C.P. Feasibility of polymer gel-based measurements of radiation isocenter accuracy in magnetic fields. Phys. Med. Biol. 2018, 63, 11NT02. [Google Scholar] [CrossRef] [PubMed]
- Dorsch, S.; Mann, P.; Elter, A.; Runz, A.; Kluter, S.; Karger, C.P. Polymer gel-based measurements of the isocenter accuracy in an MR-LINAC. J. Phys. Conf. Ser. 2019, 1305, 012007. [Google Scholar] [CrossRef]
- Dorsch, S.; Mann, P.; Elter, A.; Runz, A.; Spindeldreier, C.K.; Klüter, S.; Karger, C.P. Measurement of isocenter alignment accuracy and image distortion of an 0.35 T MR-Linac system. Phys. Med. Biol. 2019, 64, 205011. [Google Scholar] [CrossRef]
- Kim, J.H.; Kim, B.; Shin, W.G.; Son, J.; Choi, C.H.; Park, J.M.; Hwang, U.J.; Kim, J.-i.; Jung, S. 3D star shot analysis using MAGAT gel dosimeter for integrated imaging and radiation isocenter verification of MR-Linac system. J. Appl. Clin. Med. Phys. 2022, 23, e13615. [Google Scholar] [CrossRef] [PubMed]
- Ono, K.; Fujino, K.; Kurihara, R.; Hayashi, S.-I.; Akagi, Y.; Hirokawa, Y. Three-dimensional Winston–Lutz test using reusable polyvinyl alcohol-iodide (PVA-I) radiochromic gel dosimeter. Phys. Med. Biol. 2021, 66, 205001. [Google Scholar] [CrossRef]
- Kozicki, M.; Jaszczak, M.; Maras, P.; Kadlubowski, S. Measurement of the radiation dose and radiation isocenter of the TrueBeam accelerator using 3D polymer gel dosimeters from the VIPAR family with different chemical history. Measurement 2023, 221, 113452. [Google Scholar] [CrossRef]
- Chain, J.N.M.; Jirasek, A.; Schreiner, L.J.; McAuley, K.B. Cosolvent-free polymer gel dosimeters with improved dose sensitivity and resolution for X-ray CT dose. Phys. Med. Biol. 2011, 56, 2091–2102. [Google Scholar] [CrossRef] [PubMed]
- Brindha, S.; Venning, A.J.; Hill, B.; Baldock, C. Experimental study of attenuation properties of normoxic polymer gel dosimeters. Phys. Med. Biol. 2004, 49, N353–N361. [Google Scholar] [CrossRef]
- Papadakis, A.E.; Maris, T.G.; Zacharopoulou, F.; Pappas, E.; Zacharakis, G.; Damilakis, J. An evaluation of the dosimetric performance characteristics of N-vinylpyrrolidone-based polymer gels. Phys. Med. Biol. 2007, 52, 5069–5083. [Google Scholar] [CrossRef]
- Venning, A.J.; Hill, B.; Brindha, S.; Healy, B.J.; Baldock, C. Investigation of the PAGAT polymer gel dosimeter using magnetic resonance imaging. Phys. Med. Biol. 2005, 50, 3875–3888. [Google Scholar] [CrossRef]
- ISO 1042; Laboratory Glassware—One-Mark Volumetric Flasks. International Organization for Standardization: Genève, Switzerland, 1998.
- ISO 835; Laboratory Glassware—Graduated Pipettes. International Organization for Standardization: Genève, Switzerland, 2007.
- Andreo, P.; Burns, D.T.; Hohlfeld, K.; Huq, M.S.; Kanai, T.; Laitano, F.; Smyth, V.G.; Vynckier, S. Absorbed Dose Determination in External Beam Radiotherapy: An International Code of Practice for Dosimetry Based on Standards of Absorbed Dose to Water; IAEA TRS-398; International Atomic Energy Agency: Vienna, Austria, 2000; ISSN 1011-4289. [Google Scholar]
- JCGM 100:2008; Evaluation of Measurement Data—Guide to the Expression of Uncertainty in Measurement. The Joint Committee for Guides in Metrology (JCGM): Research Triangle Park, NC, USA, 2008.
- ISO. Guide to the Expression of Uncertainty in Measurement; International Organization for Standardization: Geneva, Switzerland, 1995. [Google Scholar]




| Dosimeters Components | VIP (w/v) [30] | NIPAM (w/w) [80] | MAGIC (w/w) [25] | PAGAT (w/w) [81] |
|---|---|---|---|---|
| NVP | 8% | – | – | – |
| NIPAM | – | 15% | – | – |
| MMA | – | – | 9% | – |
| AA | – | – | – | 3% |
| MBA | 4% | 4.5% | – | 3% |
| Gelatine | 7.5% | 5% | 8% | 5% |
| HQ | – | – | 0.2% | – |
| THPC | – | 5 mM | – | 10 mM |
| AsAc | 0.007% | – | 0.0352% | – |
| CuSO4 × 5H2O | 0.0008% | – | 0.002% | – |
| Dosimeter | Irradiation (MU) | |||||
|---|---|---|---|---|---|---|
| 10,000 | 5000 | 2000 | 1500 | 1000 | 500 | |
| Signal values (HU) | ||||||
| VIP | 3.9 | 1.0 | - | - | - | - |
| MAGIC | 8.1 | 3.8 | 2.1 | 1.9 | 1.1 | <1.0 |
| NIPAM | 18.7 | 5.9 | 0.9 | - | - | - |
| PAGAT | 8.5 | 5.6 | 2.5 | 2.0 | - | - |
| PABIGnx * | 5.8 | 3.9 | 1.8 | - | - | - |
| VIP ** | 4.3 | 2.1 | - | - | - | - |
| Dosimeter | The Cost of 1 Litre of Dosimeter (USD) |
|---|---|
| VIP | 75 |
| NIPAM | 124 |
| MAGIC | 49 |
| PAGAT | 42 |
| PABIGnx [18] | 57 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kozicki, M.; Maras, P.; Jaszczak-Kuligowska, M. 3D Polymer Gel Dosimeters with iCBCT 3D Reading and polyGeVero-CT Software Package for Quality Assurance in Radiotherapy. Materials 2024, 17, 1283. https://doi.org/10.3390/ma17061283
Kozicki M, Maras P, Jaszczak-Kuligowska M. 3D Polymer Gel Dosimeters with iCBCT 3D Reading and polyGeVero-CT Software Package for Quality Assurance in Radiotherapy. Materials. 2024; 17(6):1283. https://doi.org/10.3390/ma17061283
Chicago/Turabian StyleKozicki, Marek, Piotr Maras, and Malwina Jaszczak-Kuligowska. 2024. "3D Polymer Gel Dosimeters with iCBCT 3D Reading and polyGeVero-CT Software Package for Quality Assurance in Radiotherapy" Materials 17, no. 6: 1283. https://doi.org/10.3390/ma17061283






