Physical Properties of Paste Synthesized from Wet- and Dry-Processed Silver Powders
Abstract
:1. Introduction
2. Materials and Methods
2.1. Synthesis Method for Ag Powder
2.2. Preparation of Ag Paste
2.3. Evaluation of Rheology and Conductivity
2.4. Evaluation of Surface
3. Results and Discussion
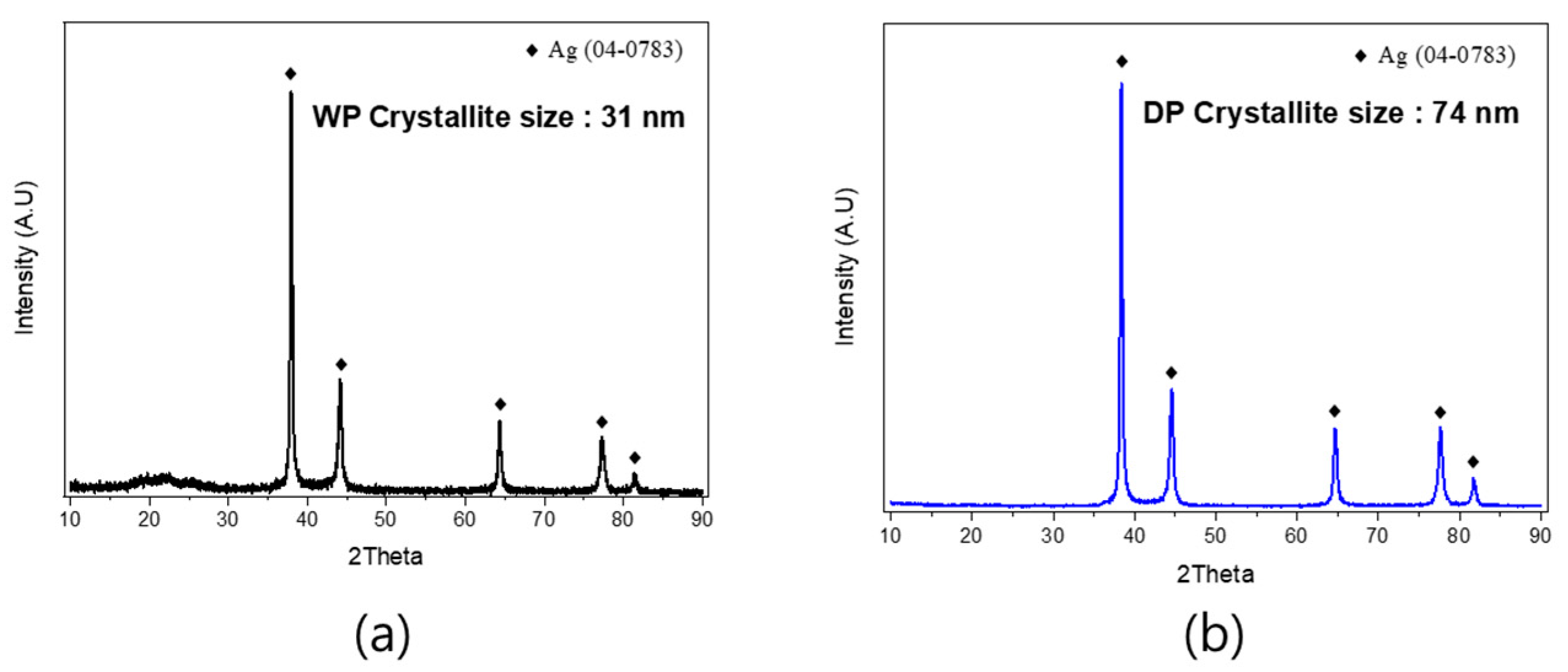
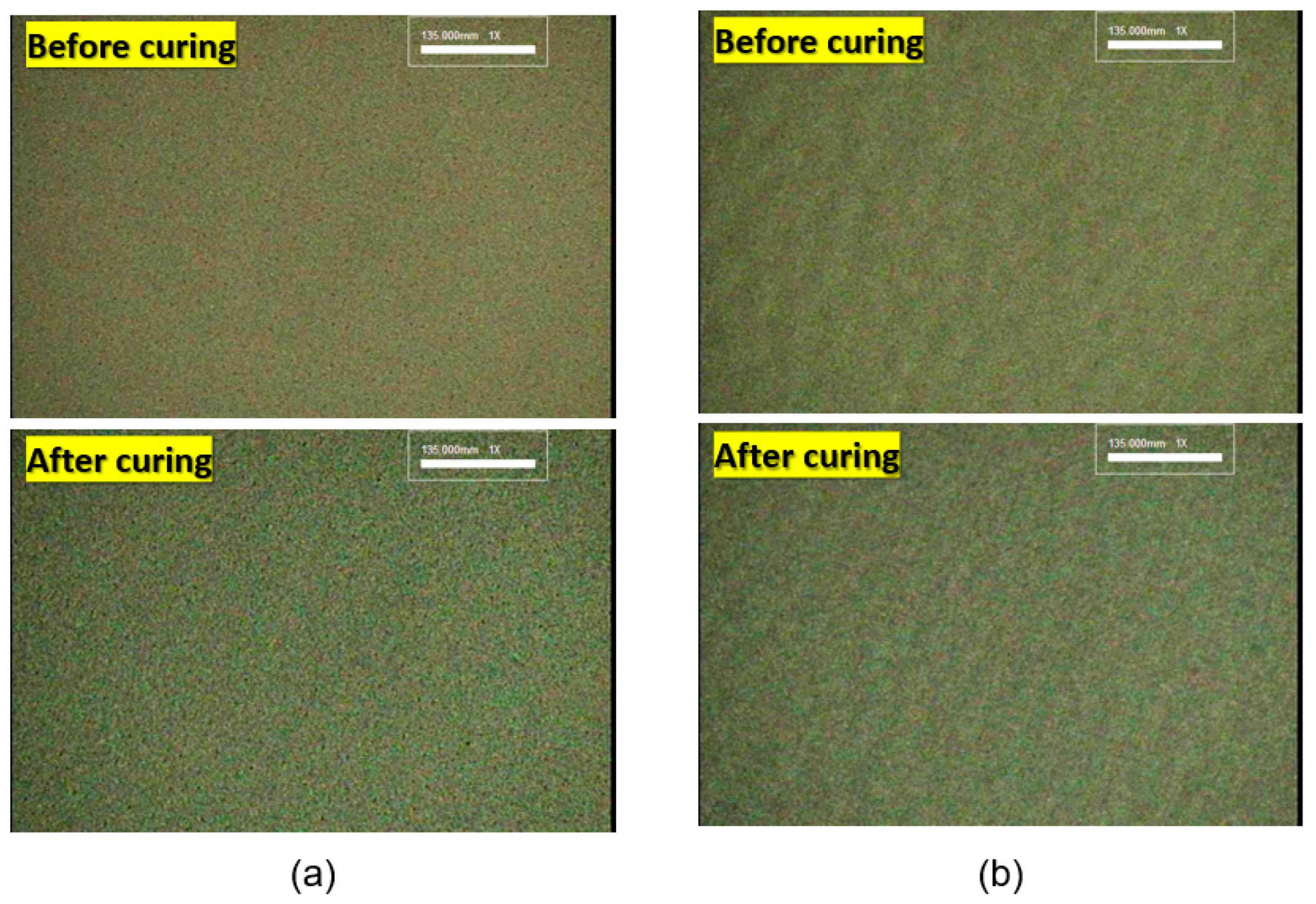
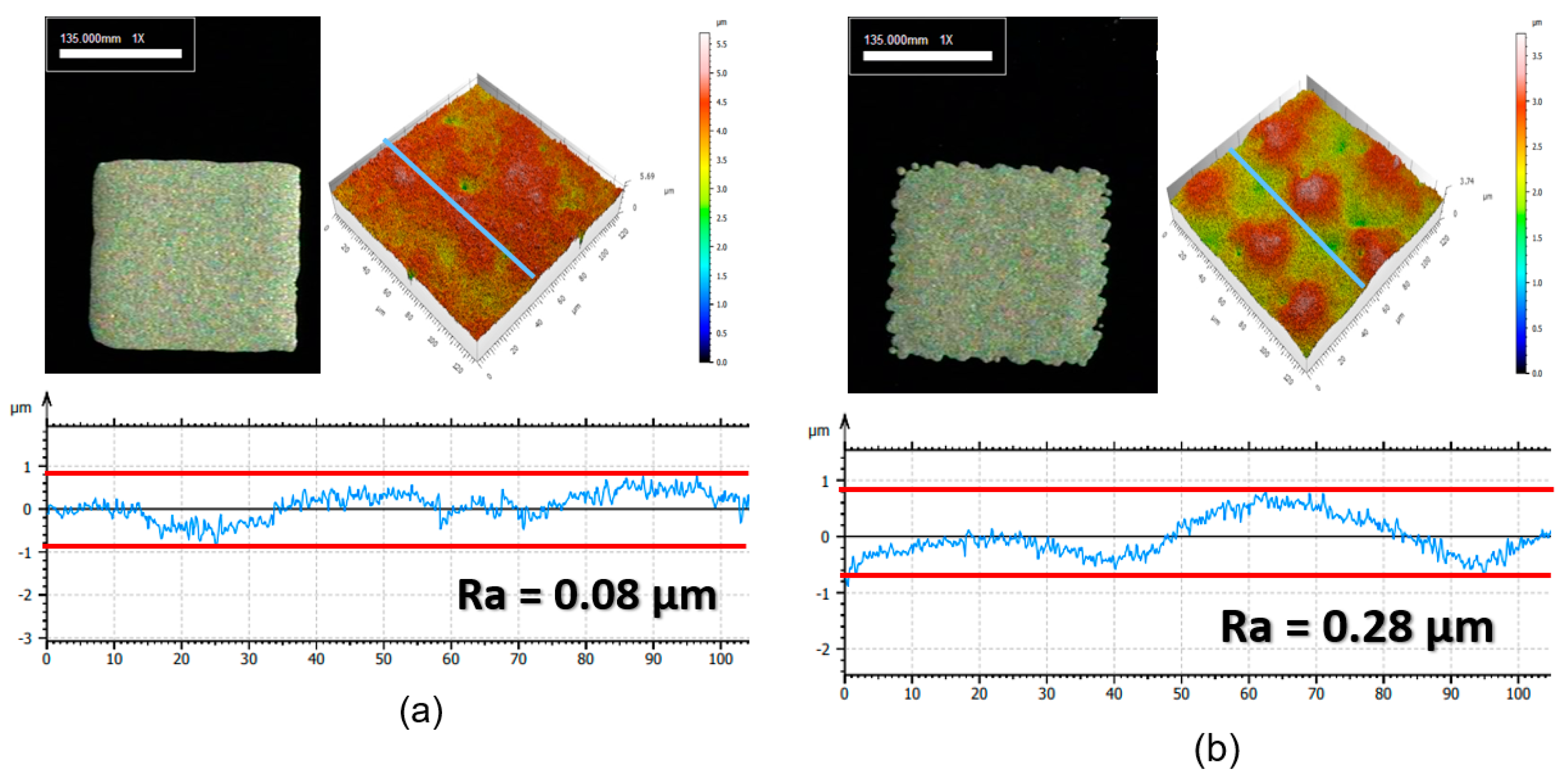
3.1. Characteristics of Ag Particles
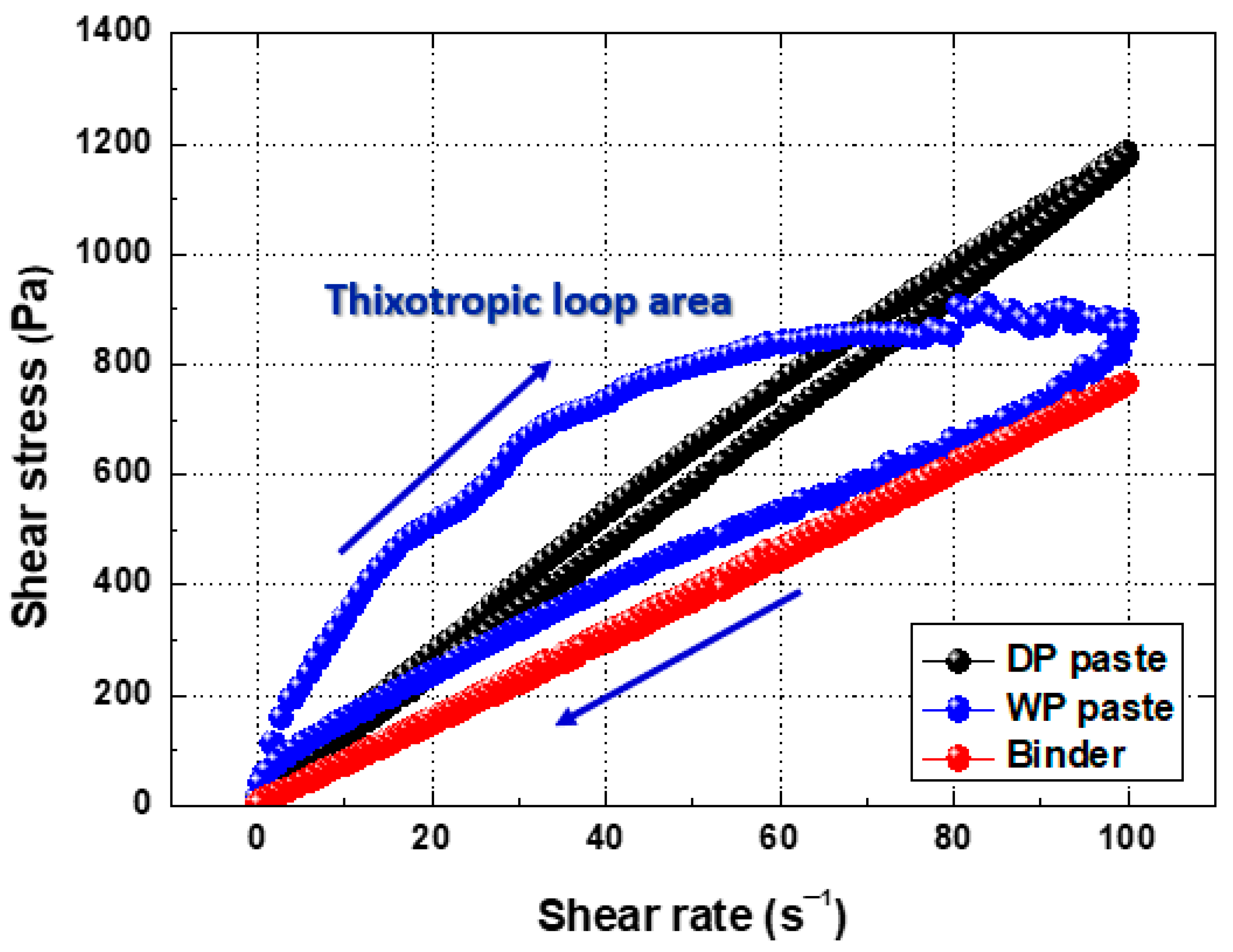
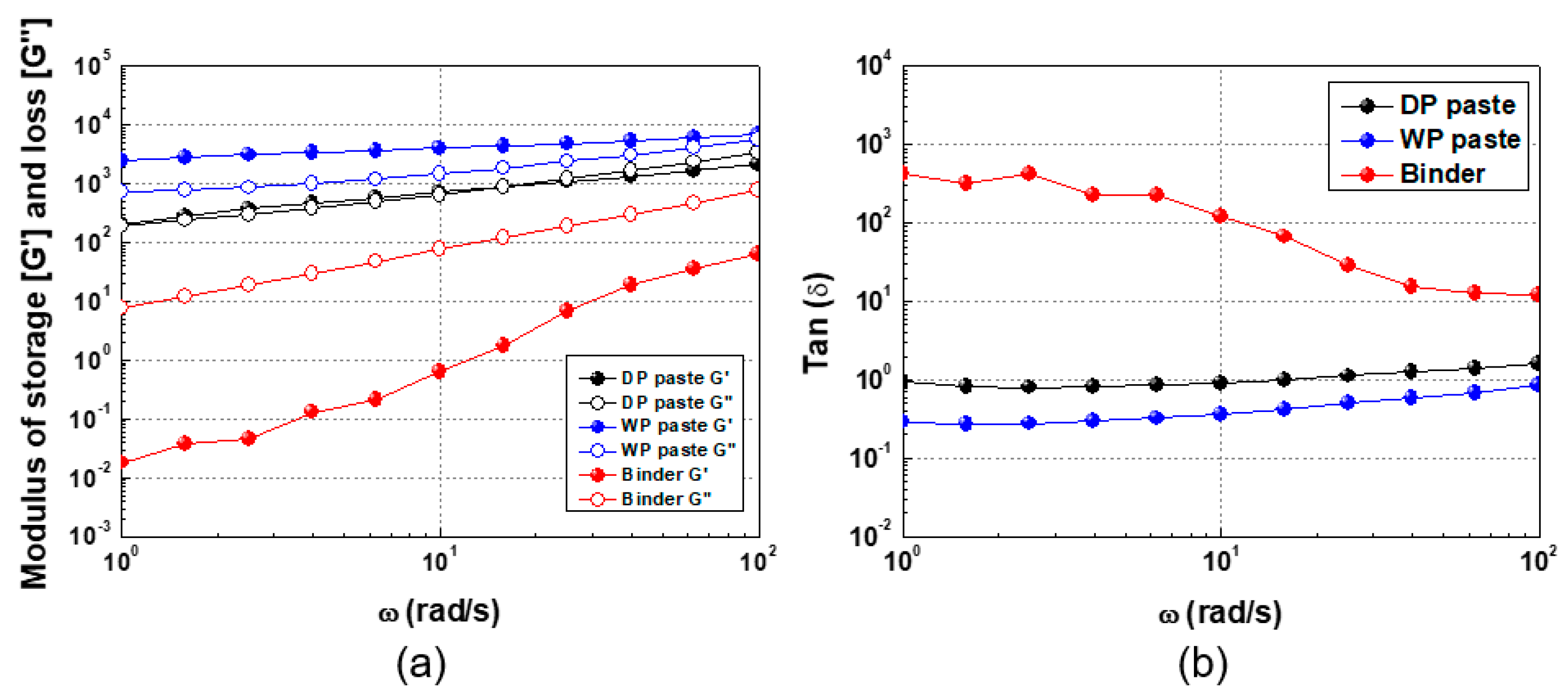
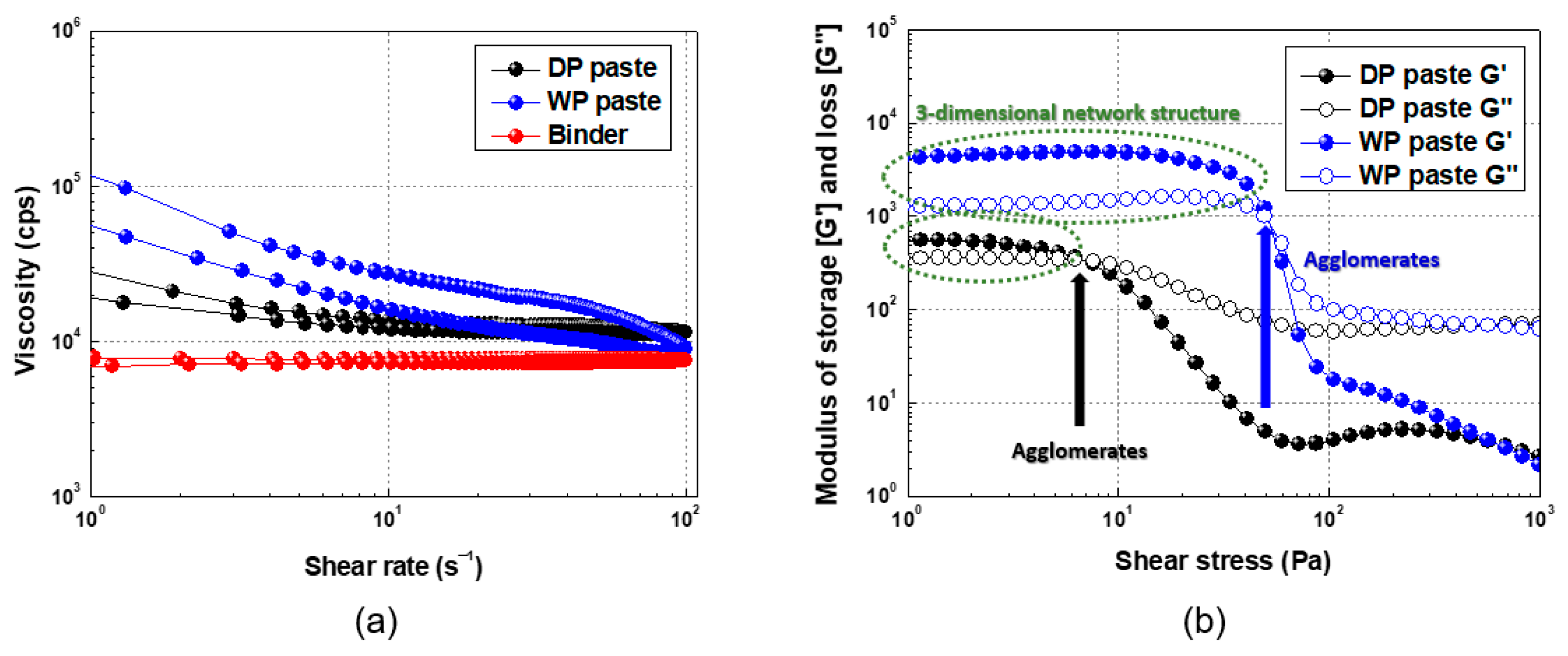
3.2. Rheological Characteristics and Screen Printing of Ag Paste
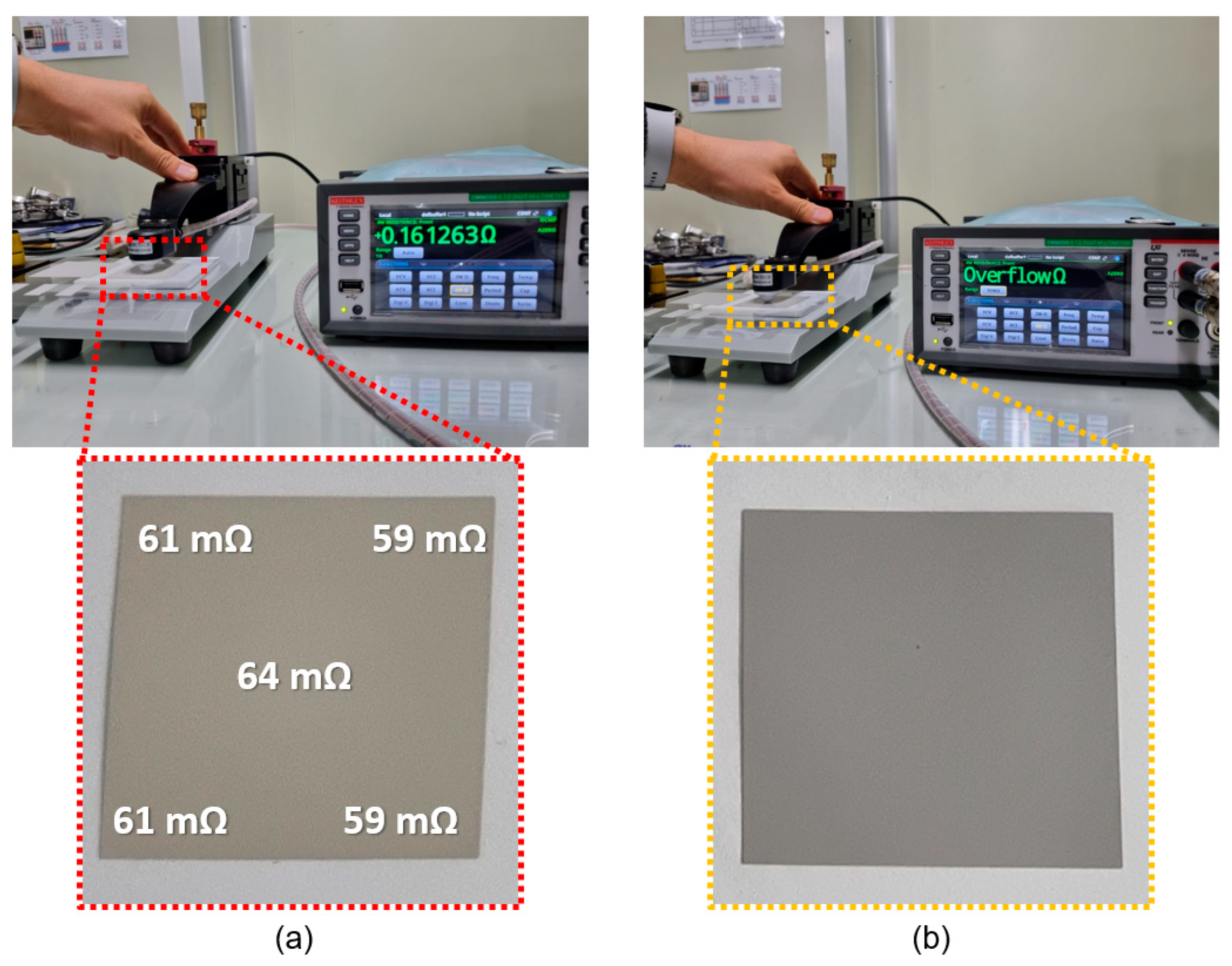
3.3. Electrical Characteristics of Cured Electrode Film
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rane, S.B.; Seth, T.; Phatak, G.J.; Amalnerkar, D.P.; Das, B.K. Influence of surfactants treatment on silver powder and its thick films. Mater. Lett. 2000, 57, 3096–3100. [Google Scholar] [CrossRef]
- Provance, J.D.; Allison, K. Particle size effects on viscosity of silver pastes: A manufacturer’s view. Proc. Int. Symp. Hybrid Microelectron. 1983, 6, 60. [Google Scholar]
- Jiang, Y.; Chen, Y.; Zhang, M.; Qiu, Y.; Lin, Y.; Pan, F. 3D-printing Ag-line of front-electrodes with optimized size and interface to enhance performance of Si solar cells. RSC Adv. 2016, 6, 51871–51876. [Google Scholar] [CrossRef]
- Yamamoto, Y.; Ogihara, T. Electrical Properties of Conductive Paste with Silver Nanoparticles and its Application to Flexible Substrates. Key Eng. Mater. 2010, 421, 297–300. [Google Scholar] [CrossRef]
- Iravani, S.; Korbekandi, H.; Mirmohammadi, S.V.; Zolfaghari, B. Synthesis of silver nanoparticles: Chemical, physical and biological methods. Res. Pharm. Sci. 2014, 9, 385–406. [Google Scholar] [CrossRef] [PubMed]
- Yan, S.; Jiang, C.; Guo, J.; Fan, Y.; Zhang, Y. Synthesis of silver nanoparticles loaded onto polymer-inorganic composite materials and their regulated catalytic activity. Polymers 2019, 11, 401. [Google Scholar] [CrossRef] [PubMed]
- Khodashenas, B.; Ghorbani, H.R. Synthesis of silver nanoparticles with different shapes. Arab. J. Chem. 2019, 12, 1823–1838. [Google Scholar] [CrossRef]
- Guilger-Casagrande, M.; de Lima, R.D. Synthesis of silver nanoparticles mediated by fungi: A review. Front. Bioeng. Biotechnol. 2019, 7, 287. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, T.; Misni, N.; Ithnin, N.R.; Daskum, A.M.; Unyah, N.Z. A review on plants and microorganisms mediated synthesis of silver nanoparticles, role of plants metabolites and applications. Int. J. Environ. Res. Public Health 2022, 19, 674. [Google Scholar] [CrossRef] [PubMed]
- Shanmuganathan, R.; Karuppusamy, I.; Saravanan, M.; Muthukumar, H.; Ponnuchamy, K.; Ramkumar, V.S.; Pugazhendhi, A. Synthesis of silver nanoparticles and their biomedical applications-a comprehensive review. Curr. Pharm. Des. 2019, 25, 2650–2660. [Google Scholar] [CrossRef] [PubMed]
- Garibo, D.; Borbón-Nuñez, H.A.; de León, J.N.D.; García Mendoza, E.; Estrada, I.; Toledano-Magaña, Y.; Tiznado, H.; Ovalle-Marroquin, M.; Soto-Ramos, A.G.; Blanco, A.; et al. Green synthesis of silver nanoparticles using Lysiloma acapulcensis exhibit high-antimicrobial activity. Sci. Rep. 2020, 10, 12805. [Google Scholar] [CrossRef]
- Kim, D.H.; Xiao, J.; Song, J.; Huang, Y.; Rogers, J.A. Stretchable, curvilinear electronics based on inorganic materials. Adv. Mater. 2010, 22, 2108–2124. [Google Scholar] [CrossRef]
- Ko, E.H.; Kim, H.J.; Lee, S.M.; Kim, T.W.; Kim, H.K. Stretchable Ag electrodes with mechanically tunable optical transmittance on wavy-patterned PDMS substrates. Sci. Rep. 2017, 7, 46739. [Google Scholar] [CrossRef] [PubMed]
- Salleh, M.S.N.; Ali, R.R.; Shameli, K.; Hamzah, M.Y.; Kasmani, R.M.; Nasef, M.M. Interaction insight of pullulan-mediated gamma-irradiated silver nanoparticle synthesis and its antibacterial activity. Polymers 2021, 13, 3578. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Yuan, X.; Yang, H.; Chao, Y.; Guo, S.; Wang, C. One-step synthesis of silver nanowires with ultra-long length and thin diameter to make flexible transparent conductive films. Materials 2019, 12, 401. [Google Scholar] [CrossRef] [PubMed]
- Jenkhongkarn, R.; Phisalaphong, M. Effect of reduction methods on the properties of composite films of bacterial cellulose-silver nanoparticles. Polymers 2023, 15, 2996. [Google Scholar] [CrossRef] [PubMed]
- Koo, H.Y.; Park, D. Conductive powder synthesis technology for improving electrical conductivity by one-pot ultrasonic spray pyrolysis process. In New Advances in Powder Technology; IntechOpen: Rijeka, Croatia, 2022. [Google Scholar]
- Jung, D.S.; Lee, H.M.; Kang, Y.C.; Park, S.B. Air-stable silver-coated copper particles of sub-micrometer size. J. Colloid Interface Sci. 2011, 364, 574–581. [Google Scholar] [CrossRef]
- Jung, D.S.; Koo, H.Y.; Wang, S.E.; Park, S.B.; Kang, Y.C. Ultrasonic spray pyrolysis for air-stable copper particles and their conductive films. Acta Mater. 2021, 206, 116569. [Google Scholar] [CrossRef]
- Emil Kaya, E.; Kaya, O.; Alkan, G.; Gürmen, S.; Stopic, S.; Friedrich, B. New proposal for size and size-distribution evaluation of nanoparticles synthesized via ultrasonic spray pyrolysis using search algorithm based on image-processing technique. Materials 2019, 13, 38. [Google Scholar] [CrossRef] [PubMed]
- Zhan, H.; Guo, J.; Shen, J.; Wang, X.; Fan, Z.; Guo, B.; Liu, W.; Shen, H. Synthesis of silver flakes and their application as conductive filler for low-curing-temperature silver pastes. J. Electron. Mater. 2019, 48, 2745–2753. [Google Scholar] [CrossRef]
- Zhan, H.; Guo, J.; Yang, X.; Guo, B.; Liu, W.; Shen, H.; Wang, X.; Tang, W.; Chen, F. Silver frameworks based on self-sintering silver micro-flakes and its application in low temperature curing conductive pastes. J. Mater. Sci. Mater. Electron. 2019, 30, 21343–21354. [Google Scholar] [CrossRef]
- Hsu, S.L.C.; Chen, Y.T.; Chen, M.L.; Chen, I.G. Low sintering temperature nano-silver pastes with high bonding strength by adding silver 2-ethylhexanoate. Materials 2021, 14, 5941. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, D.; Zhang, C.; Chen, W.; Meng, Q.; Yuan, H.; Yang, S. A Fluorinated Polyimide Based Nano Silver Paste with High Thermal Resistance and Outstanding Thixotropic Performance. Polymers 2023, 15, 1150. [Google Scholar] [CrossRef]
- Li, C.; Sun, Y.; Sun, M.; Lin, B.; Zhang, X. Novel cocklebur-like nano silver oxide for low-temperature curing pastes with dense conductive paths. J. Mater. Sci. Mater. Electron. 2023, 34, 1856. [Google Scholar] [CrossRef]
- Li, W.; Yu, C.; Wang, Y.; Yao, Y.; Yu, X.; Zuo, C.; Yu, Y. Experimental Investigation of Effect of Flake Silver Powder Content on Sintering Structure and Properties of Front Silver Paste of Silicon Solar Cell. Materials 2022, 15, 7142. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.; Sun, H.; Qian, Z.; Li, W.; Li, W.; Huang, F.; Li, J.; Gan, G. Effect of Silver Powder Microstructure on the Performance of Silver Powder and Front-Side Solar Silver Paste. Materials 2024, 17, 445. [Google Scholar] [CrossRef] [PubMed]
- Vandevenne, G.; Marchal, W.; Verboven, I.; Drijkoningen, J.; D’Haen, J.; Van Bael, M.K.; Hardy, A.; Deferme, W. A study on the thermal sintering process of silver nanoparticle inkjet inks to achieve smooth and highly conducting silver layers. Phys. Status Solidi A 2016, 213, 1403–1409. [Google Scholar] [CrossRef]
- Amjadi, M.; Yoon, Y.J.; Park, I. Ultra-stretchable and skin-mountable strain sensors using carbon nanotubes–Ecoflex nanocomposites. Nanotechnology 2015, 26, 375501. [Google Scholar] [CrossRef] [PubMed]
- Trung, T.Q.; Lee, N.E. Flexible and stretchable physical sensor integrated platforms for wearable human-activity monitoringand personal healthcare. Adv. Mater. 2016, 28, 4338–4372. [Google Scholar] [CrossRef] [PubMed]
- Amjadi, M.; Kyung, K.U.; Park, I.; Sitti, M. Stretchable, skin-mountable, and wearable strain sensors and their potential applications: A review. Adv. Funct. Mater. 2016, 26, 1678–1698. [Google Scholar] [CrossRef]
- Yang, S.; Yoon, J.C.; Yun, J.Y.; Lim, T.S.; Kim, Y.J.; Yu, J.H. Preparation of conductive metal patterns using Cu nano-colloids prepared by electrical wire explosion process. Res. Chem. Intermed. 2014, 40, 2457–2461. [Google Scholar] [CrossRef]
- Phung, T.H.; Gafurov, A.N.; Kim, I.; Kim, S.Y.; Kim, K.M.; Lee, T.M. IoT device fabrication using roll-to-roll printing process. Sci. Rep. 2021, 11, 19982. [Google Scholar] [CrossRef]
- Son, M.J.; Kim, I.; Yang, S.; Lee, T.M.; Lee, H.J. Employment of roll-offset printing for fabrication of solder bump arrays: Harnessing the rheological properties of lead-free solder pastes using particle size distribution. Microelectron. Eng. 2016, 164, 128–134. [Google Scholar] [CrossRef]
- Wu, C.; Zhang, J.; Fang, B.; Cui, Y.; Xing, Z.; Li, Z.; Zhou, W. Self-floating biomass charcoal supported flower-like plasmon silver/carbon, nitrogen co-doped defective TiO2 as robust visible light photocatalysts. J. Clean. Prod. 2021, 329, 129723. [Google Scholar] [CrossRef]
- Voigt, O.; Peuker, U.A. Suitability of eroded particles from die-sink electro discharge machining for additive manufacturing—Review, characterization and processing. Metals 2022, 12, 1447. [Google Scholar] [CrossRef]
- Bykkam, S.; Ahmadipour, M.; Narisngam, S.; Kalagadda, V.R.; Chidurala, S.C. Extensive studies on X-ray diffraction of green synthesized silver nanoparticles. Adv. Nanopart. 2015, 4, 1–10. [Google Scholar] [CrossRef]
- Siakavella, I.K.; Lamari, F.; Papoulis, D.; Orkoula, M.; Gkolfi, P.; Lykouras, M.; Avgoustakis, K.; Hatziantoniou, S. Effect of plant extracts on the characteristics of silver nanoparticles for topical application. Pharmaceutics 2020, 12, 1244. [Google Scholar] [CrossRef] [PubMed]
- Aziz, S.B.; Abdulwahid, R.T.; Rasheed, M.A.; Abdullah, O.G.; Ahmed, H.M. Polymer blending as a novel approach for tuning the SPR peaks of silver nanoparticles. Polymers 2017, 9, 486. [Google Scholar] [CrossRef] [PubMed]
- Mirda, E.; Idroes, R.; Khairan, K.; Tallei, T.E.; Ramli, M.; Earlia, N.; Maulana, A.; Idroes, G.M.; Muslem, M.; Jalil, Z. Synthesis of chitosan-silver nanoparticle composite spheres and their antimicrobial activities. Polymers 2021, 13, 3990. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.; Karuppiah, P.; Alkhathlan, H.Z.; Kuniyil, M.; Khan, M.; Adil, S.F.; Shaik, M.R. Green synthesis of silver nanoparticles using Juniperus procera extract: Their characterization, and biological activity. Crystals 2022, 12, 420. [Google Scholar] [CrossRef]
- Rahman, M.S.; Hasan, M.S.; Nitai, A.S.; Nam, S.; Karmakar, A.K.; Ahsan, M.S.; Shiddiky, M.J.A.; Ahmed, M.B. Recent developments of carboxymethyl cellulose. Polymers 2021, 13, 1345. [Google Scholar] [CrossRef] [PubMed]
- Silva, Â.D.M.; Silva, M.M.; Figueiredo, H.; Delgado, I.; Lopes, P.E.; Paiva, M.C.; Hilliou, L. Rheologically assisted design of conductive adhesives for stencil printing on PCB. Materials 2021, 14, 7734. [Google Scholar] [CrossRef] [PubMed]
- Koltsov, S.I.; Statsenko, T.G.; Morozova, S.M. Modification of commercial 3D fused deposition modeling printer for extrusion printing of hydrogels. Polymers 2022, 14, 5539. [Google Scholar] [CrossRef] [PubMed]
- Yüce, C.; König, M.; Willenbacher, N. Rheology and screen-printing performance of model silver pastes for metallization of Si-solar cells. Coatings 2018, 8, 406. [Google Scholar] [CrossRef]
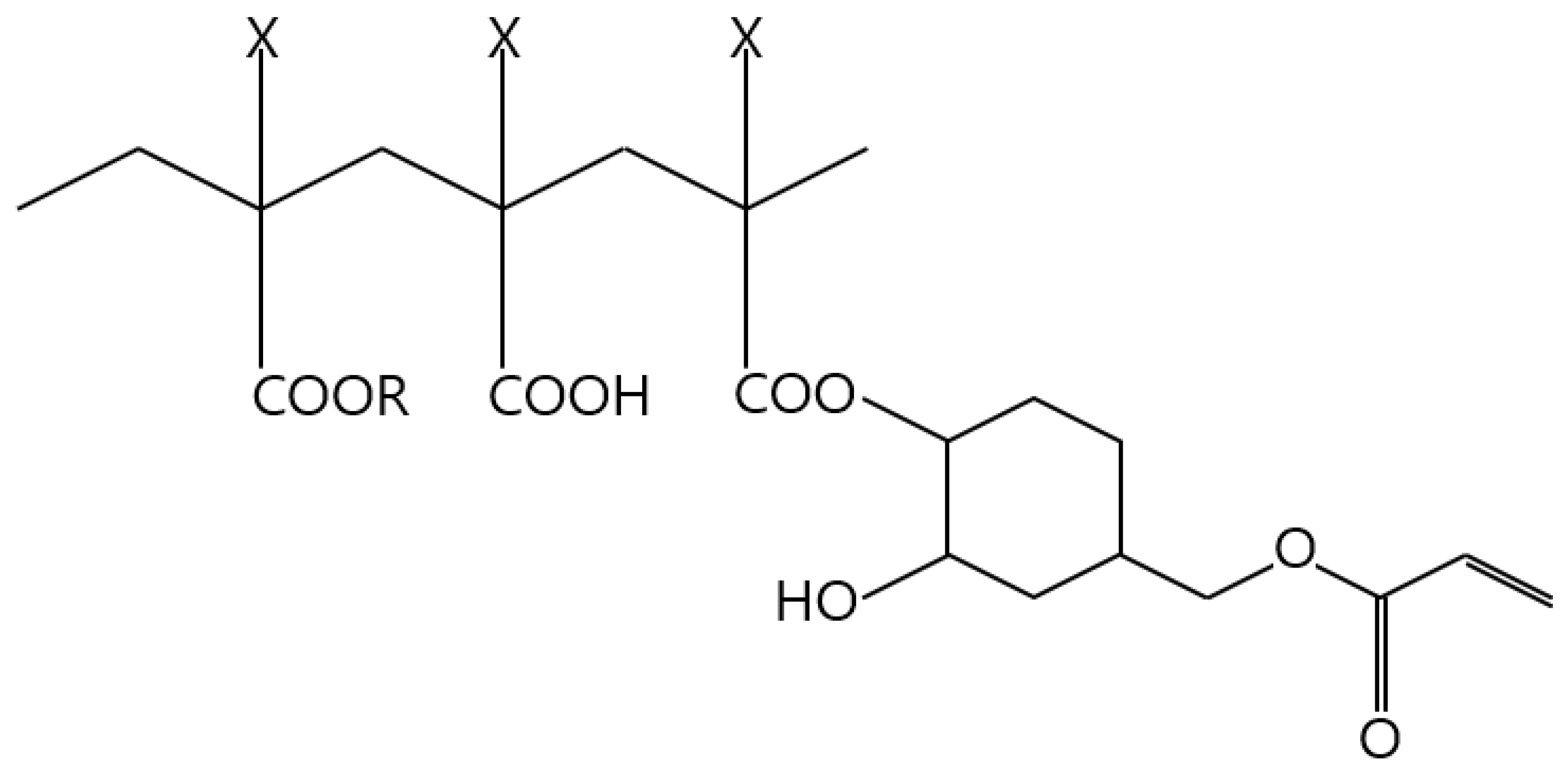
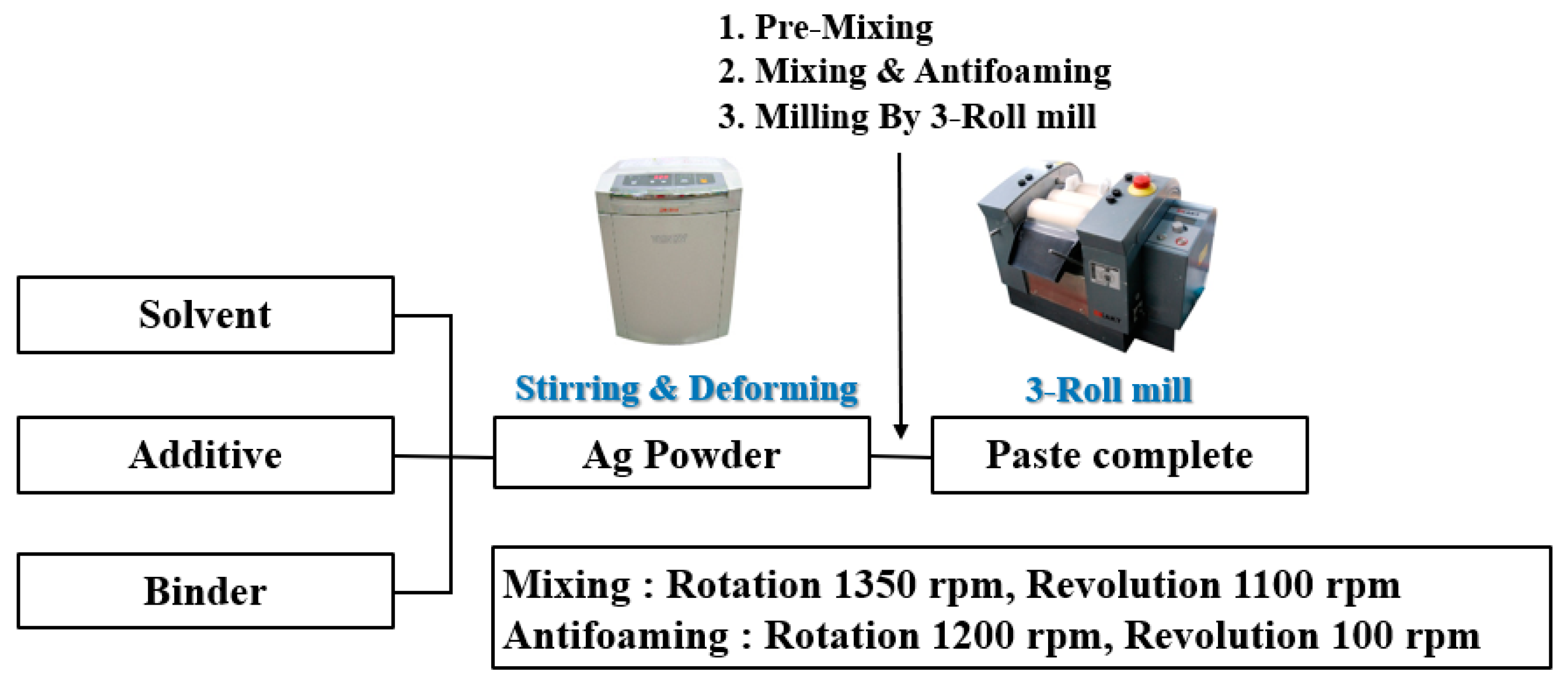
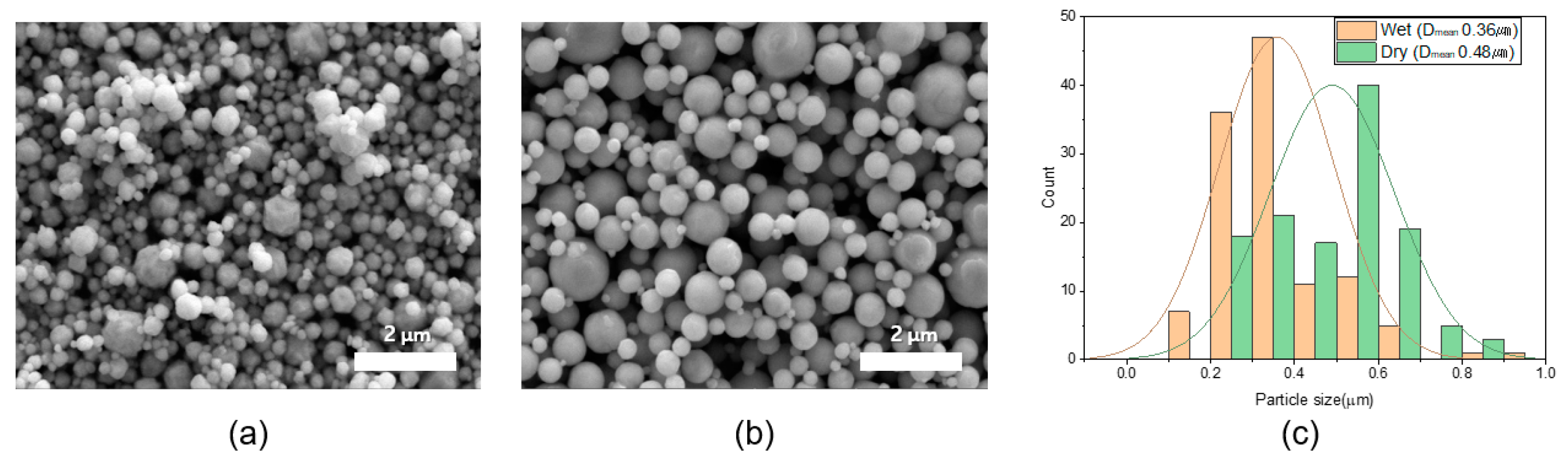
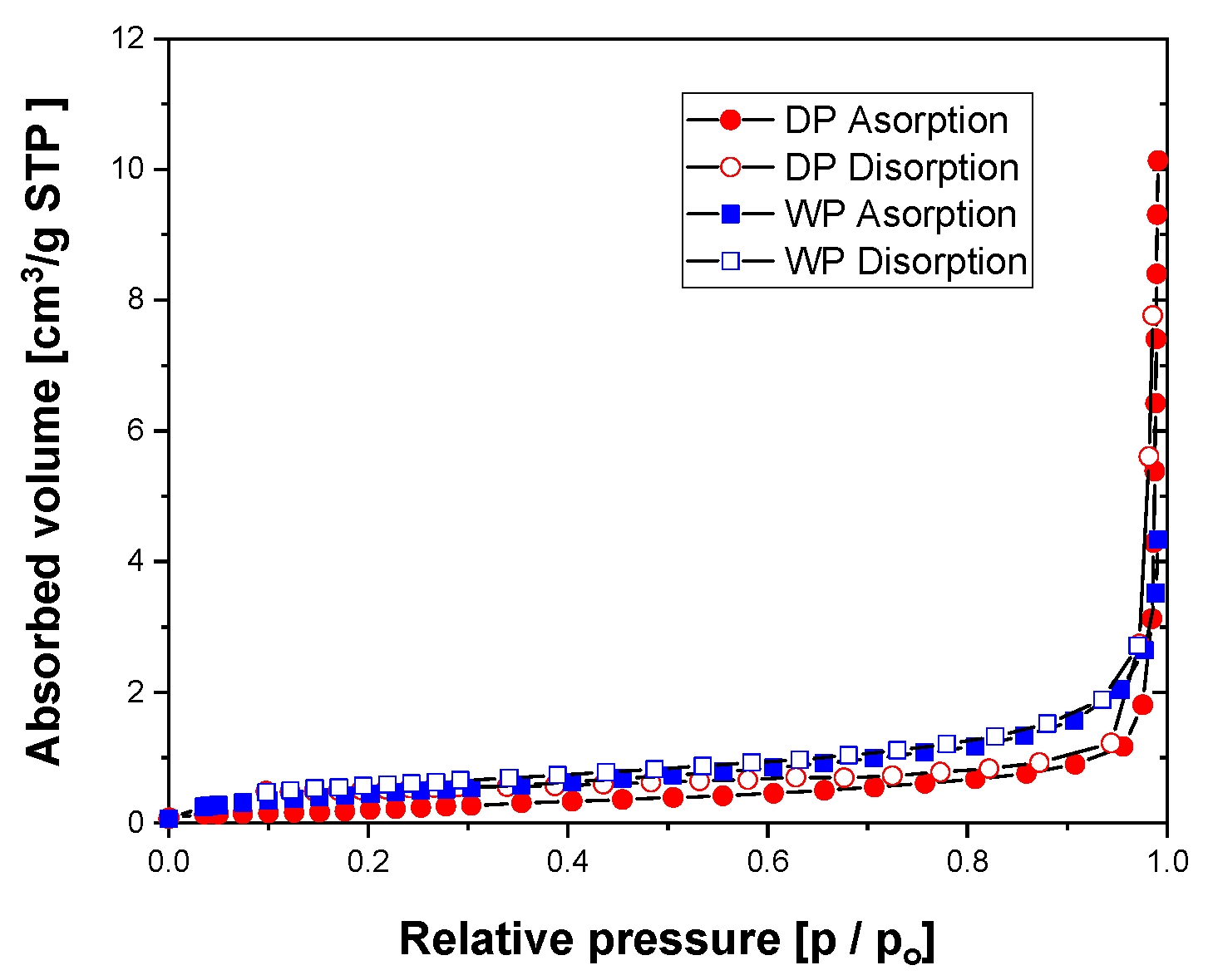



| Wt% | Filler | Binder | BYK-180 | Carbitol | Total |
|---|---|---|---|---|---|
| WP | 86.49% | 10.81% | 0.54% | 2.16% | 100% |
| DP | 86.49% | 10.81% | 0.54% | 2.16% | 100% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nam, H.J.; Shin, M.; Koo, H.Y.; Park, S.-H.; Nam, H.M.; Nam, S.-Y. Physical Properties of Paste Synthesized from Wet- and Dry-Processed Silver Powders. Materials 2024, 17, 1273. https://doi.org/10.3390/ma17061273
Nam HJ, Shin M, Koo HY, Park S-H, Nam HM, Nam S-Y. Physical Properties of Paste Synthesized from Wet- and Dry-Processed Silver Powders. Materials. 2024; 17(6):1273. https://doi.org/10.3390/ma17061273
Chicago/Turabian StyleNam, Hyun Jin, Minkyung Shin, Hye Young Koo, Se-Hoon Park, Hyun Min Nam, and Su-Yong Nam. 2024. "Physical Properties of Paste Synthesized from Wet- and Dry-Processed Silver Powders" Materials 17, no. 6: 1273. https://doi.org/10.3390/ma17061273





