Influence of Low-Molecular-Weight Esters on Melt Spinning and Structure of Poly(lactic acid) Fibers
Abstract
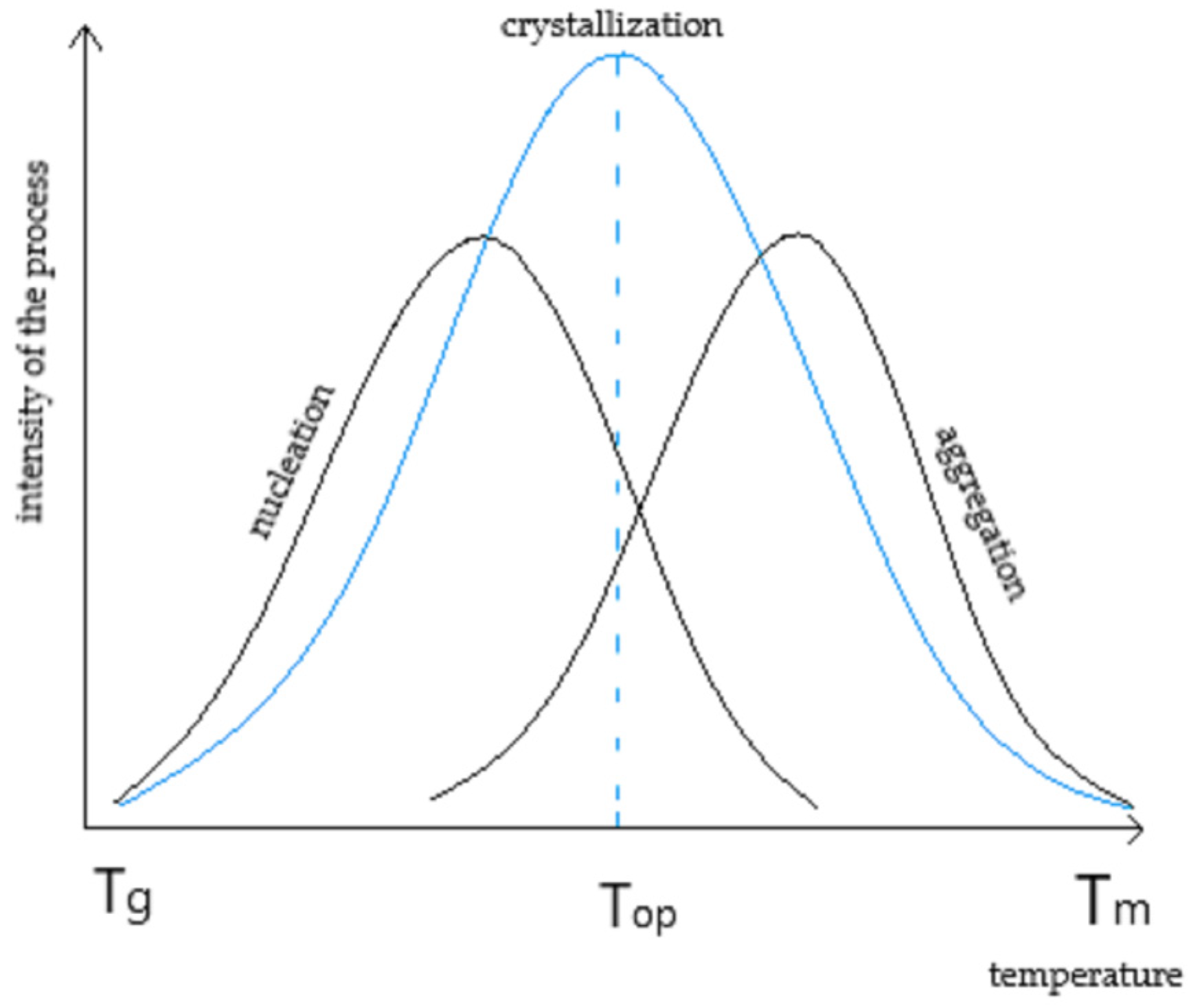
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Technological Processes
2.2.1. Modification of Polymer
2.2.2. Spinning Process
2.3. Methods
3. Results
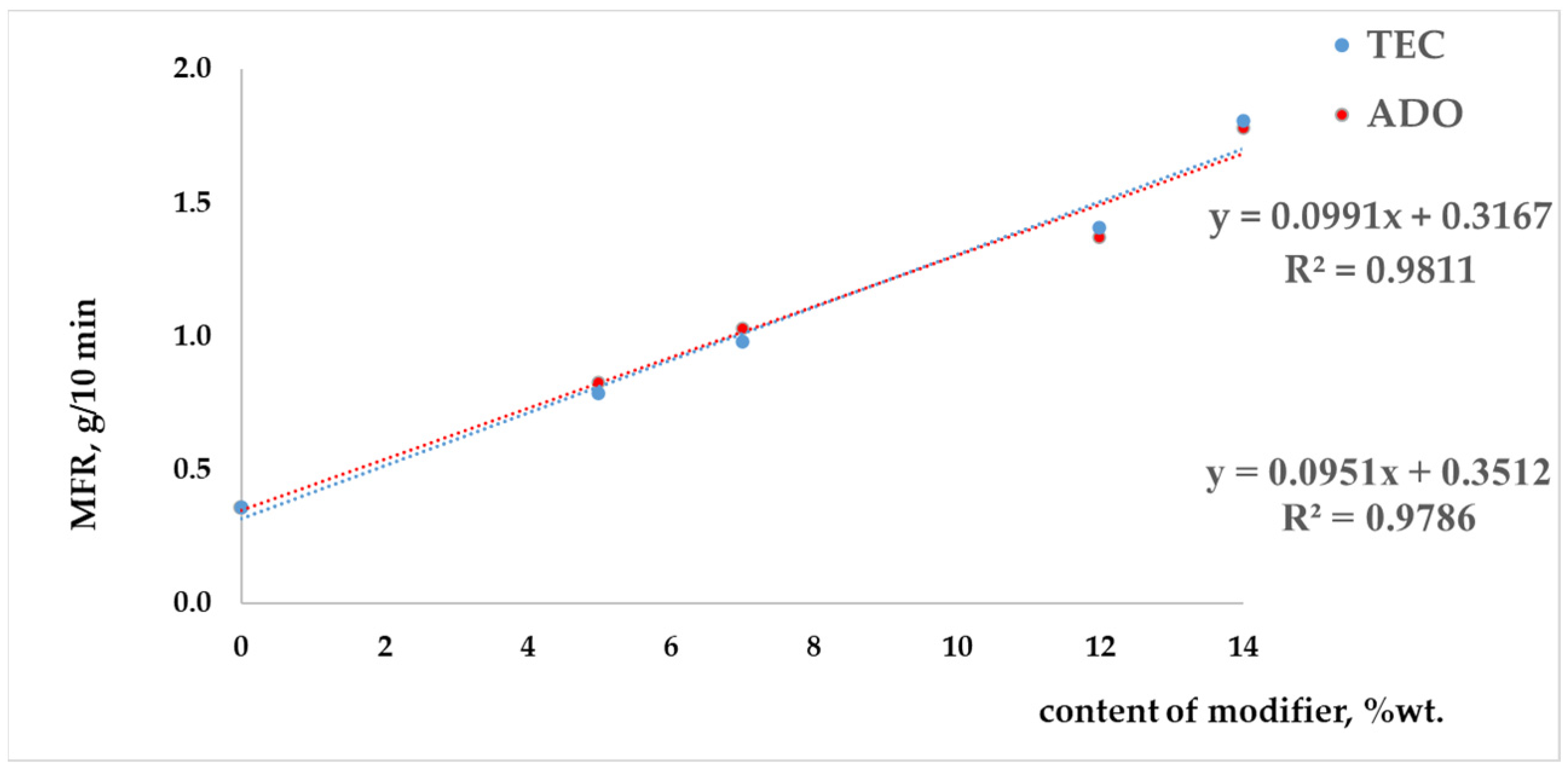
3.1. Analysis of Modified PLA Regranulates
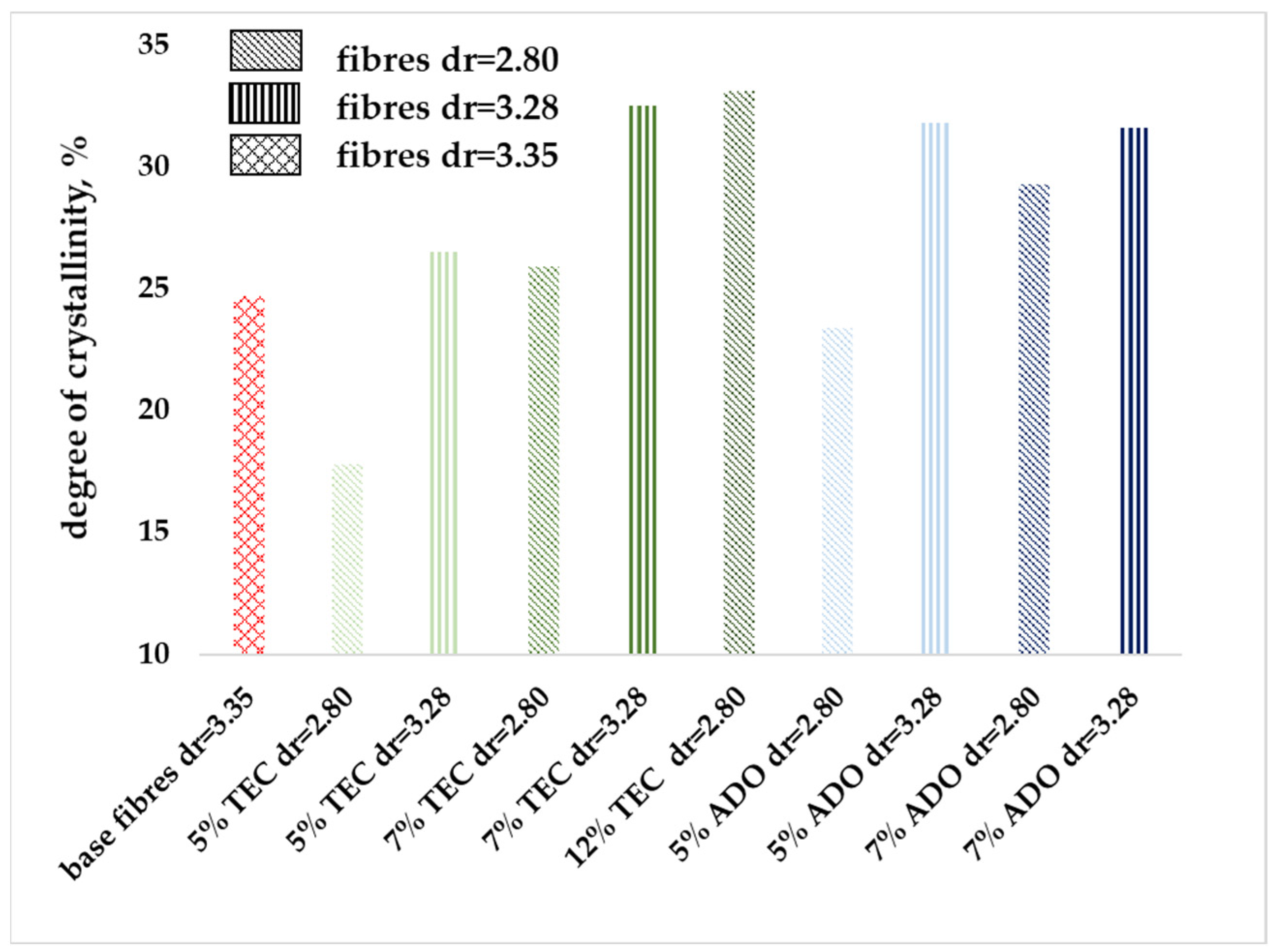
3.2. Analysis of Modified Fibers
- Base fibers from pure PLA dr = 3.35;
- Fibers with TEC at a concentration of 5, 7, 12% wt., two draw ratios—2.80 and 3.28;
- Fibers with ADO at a concentration of 5, 7% wt., two draw ratios—2.80 and 3.28.
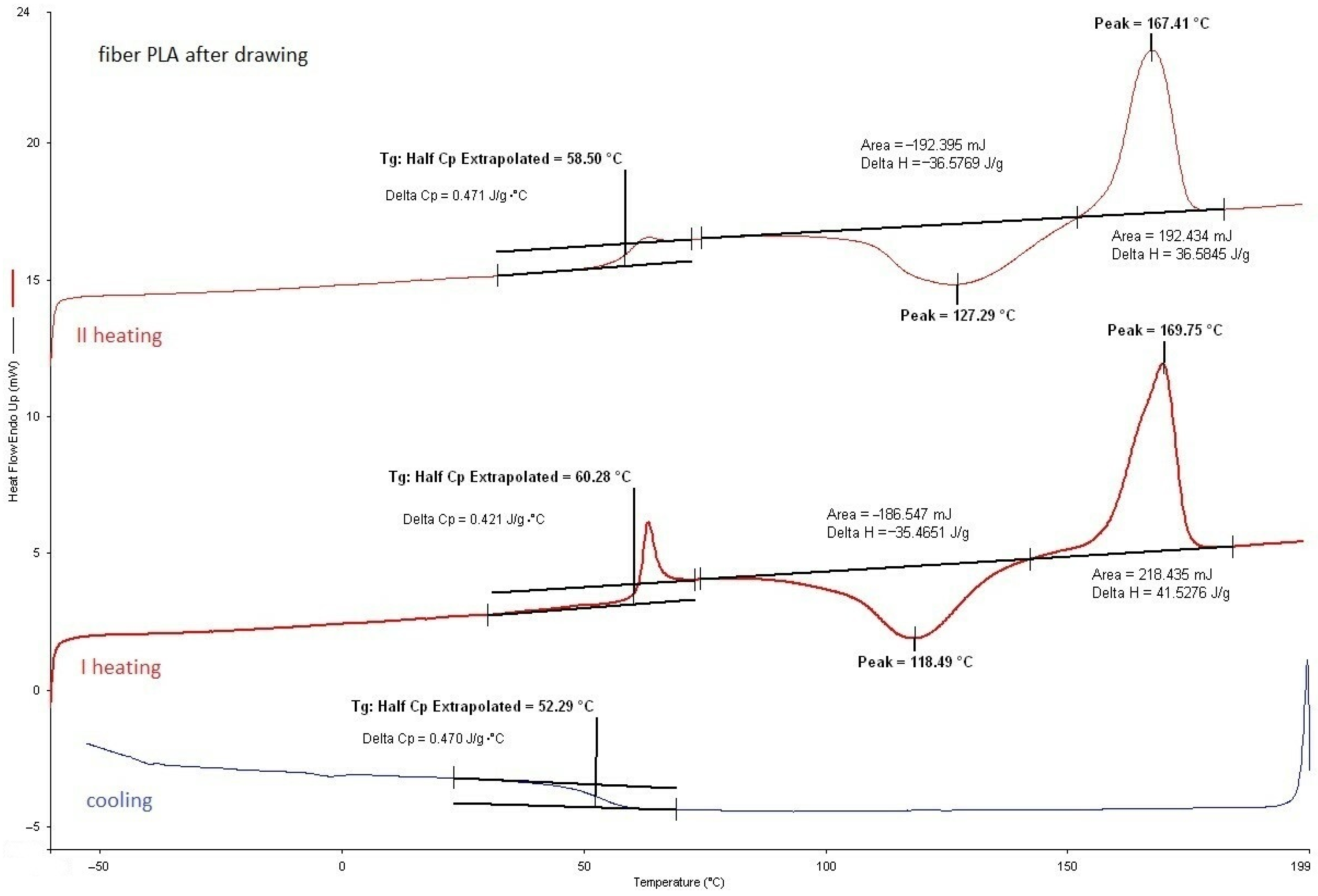
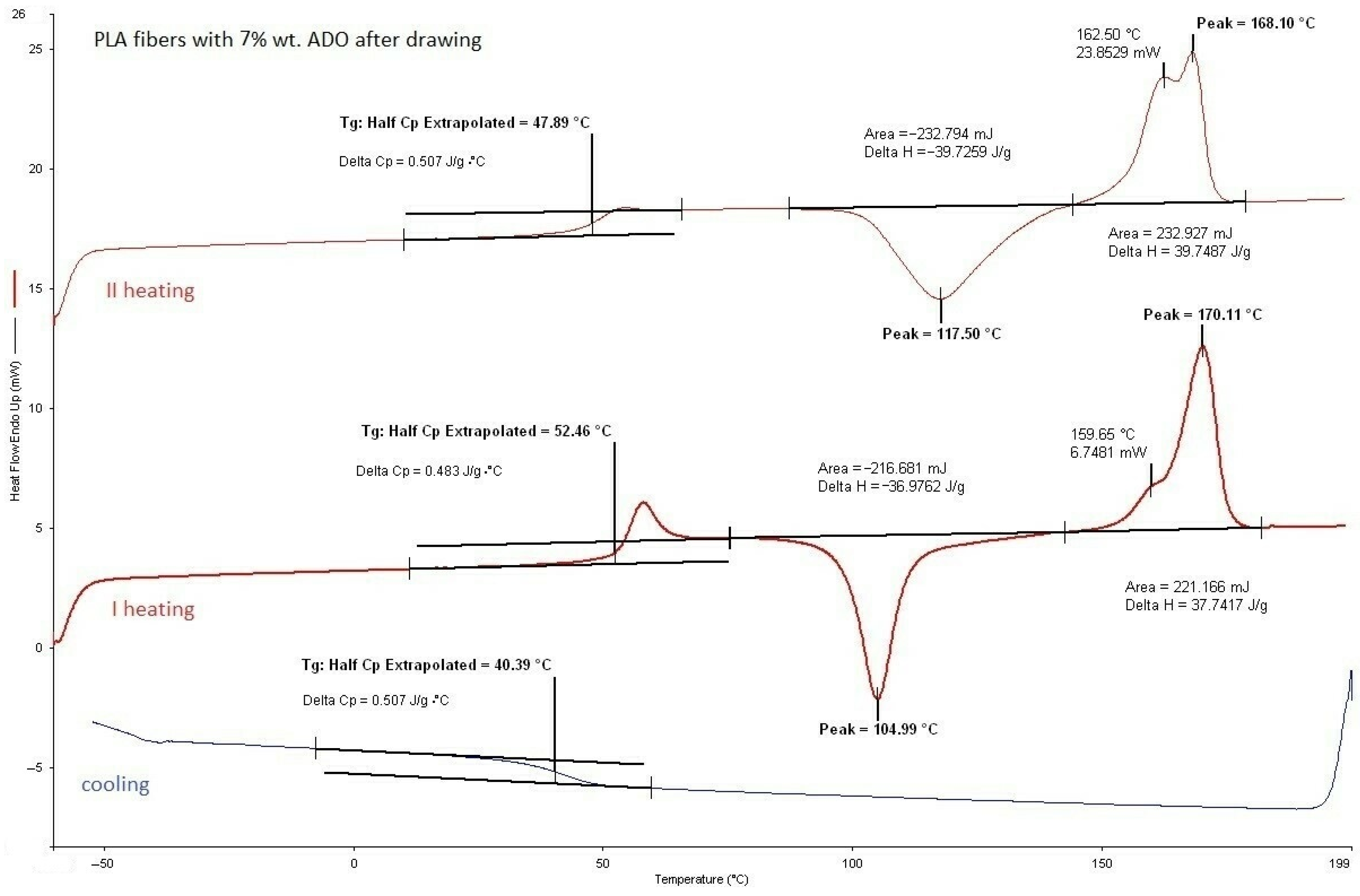
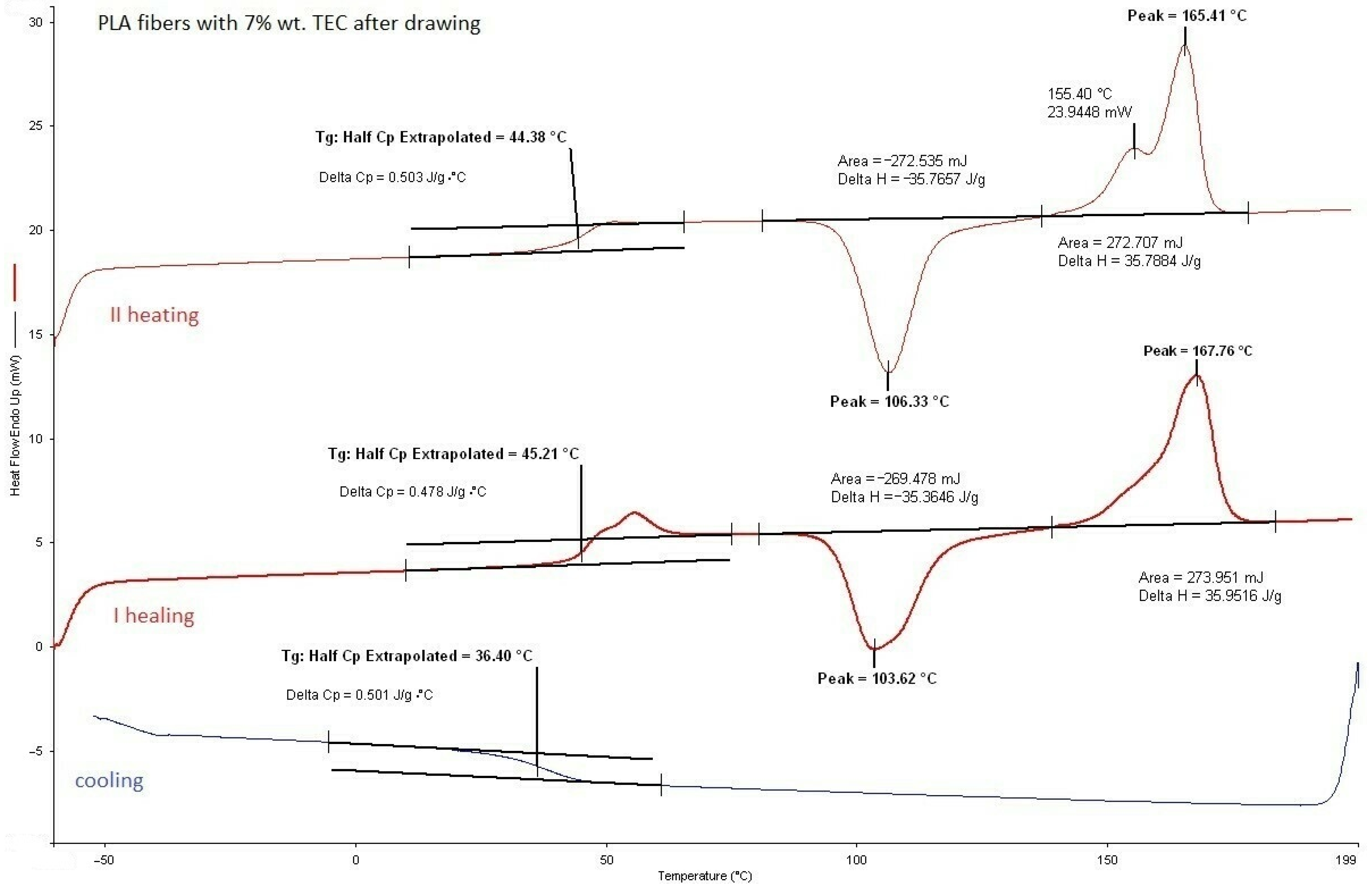
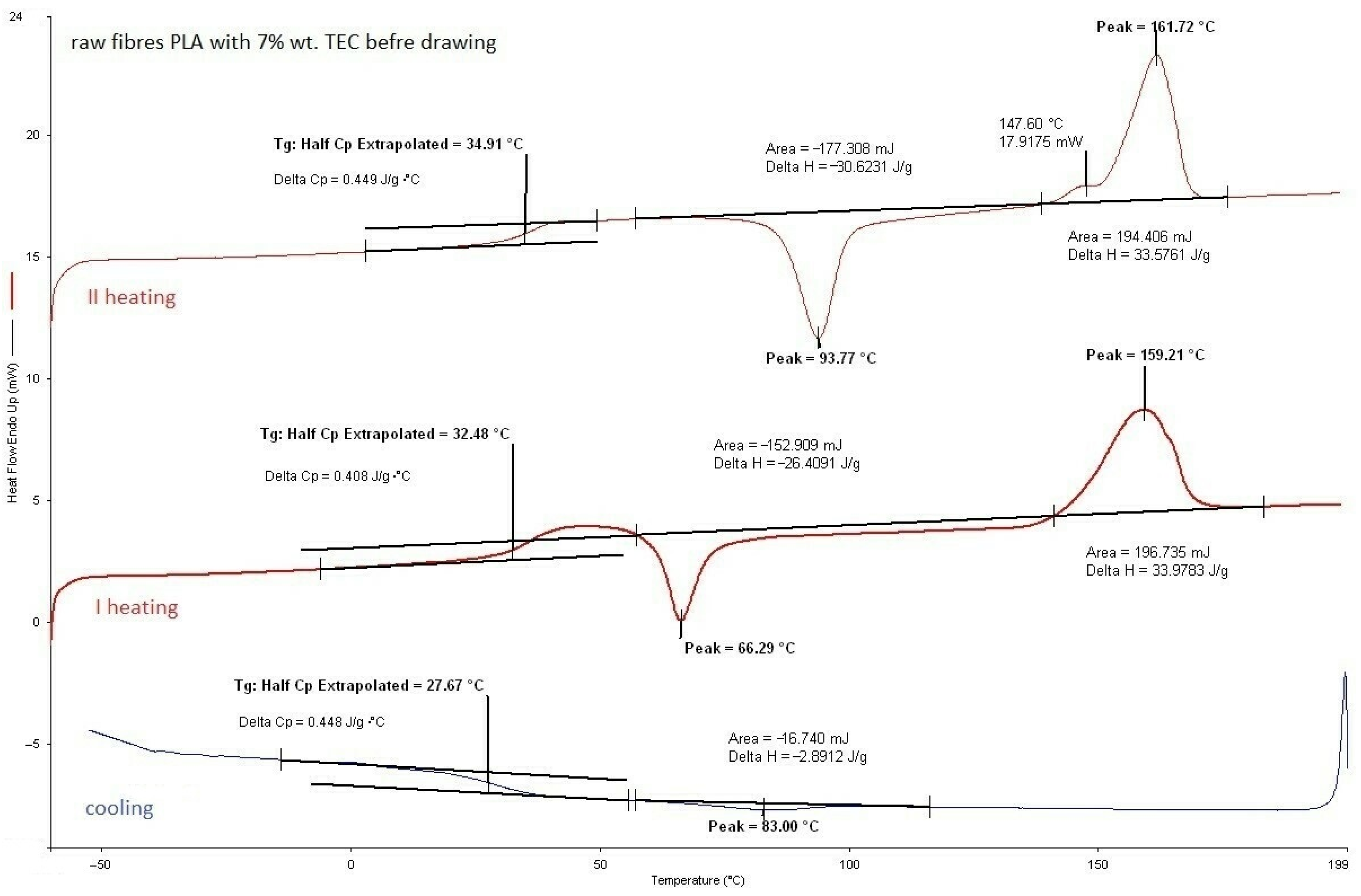
3.2.1. DSC Analysis of Modified Fibers
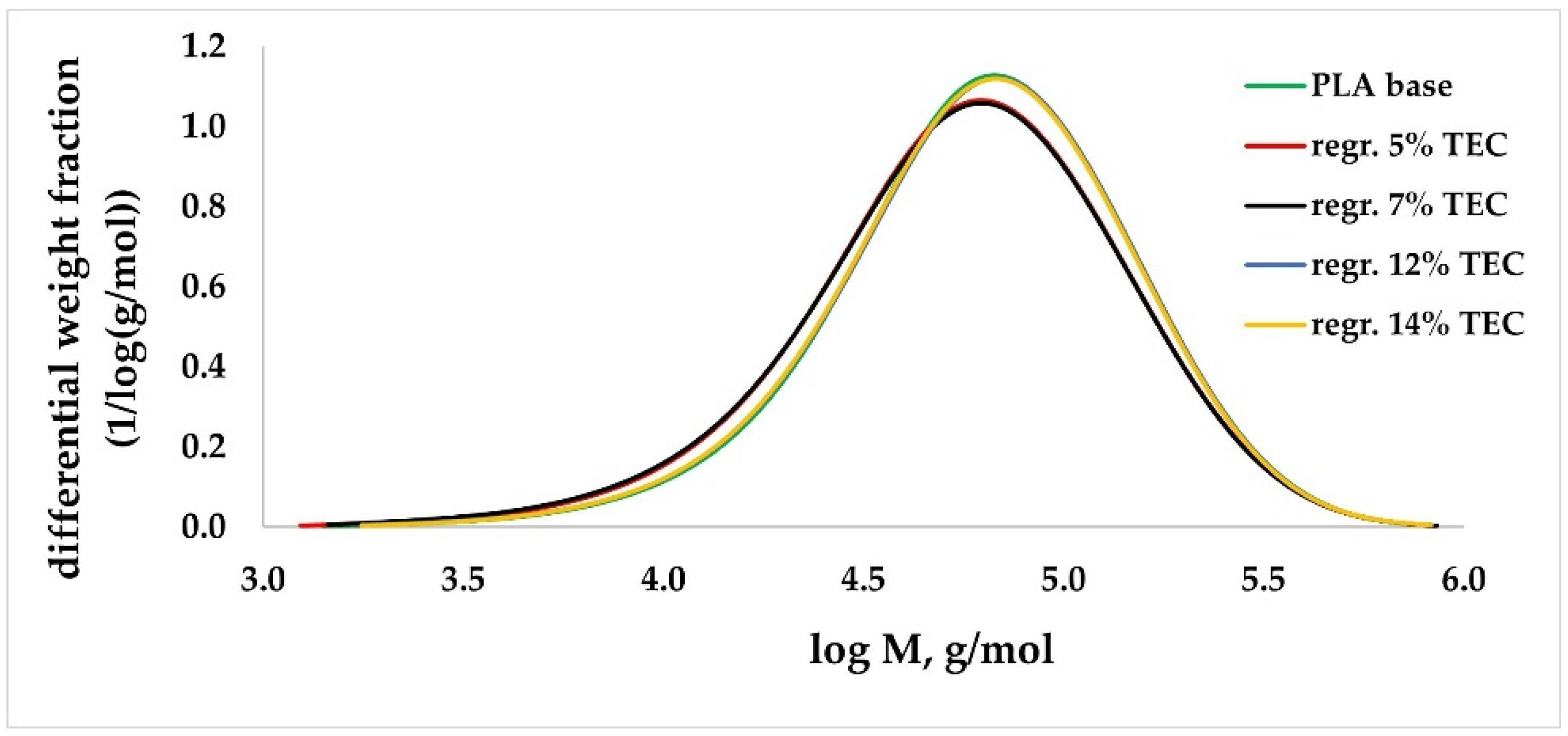
3.2.2. GPC/SEC Analysis
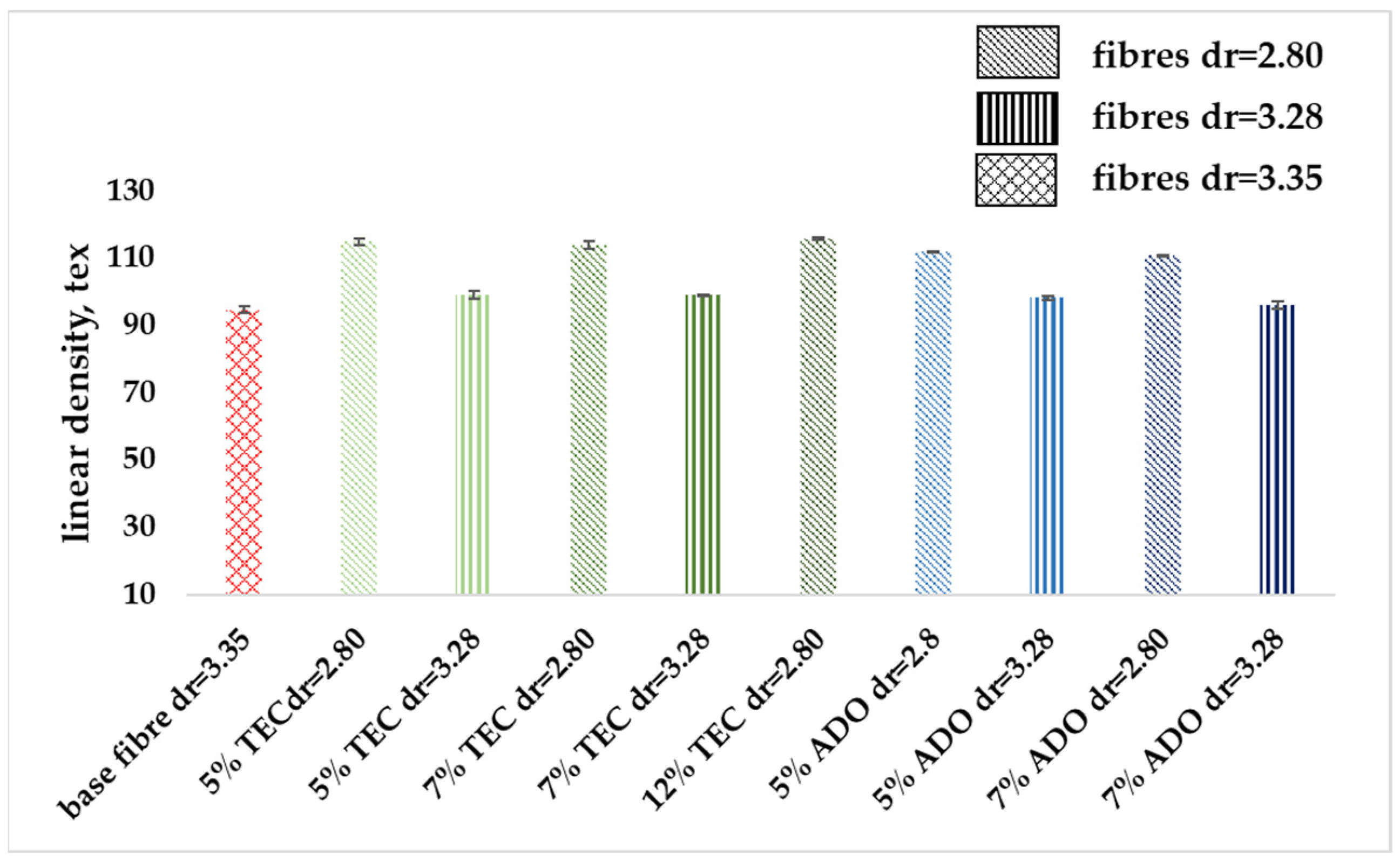
3.2.3. Mechanical Properties
3.2.4. SEM Analysis
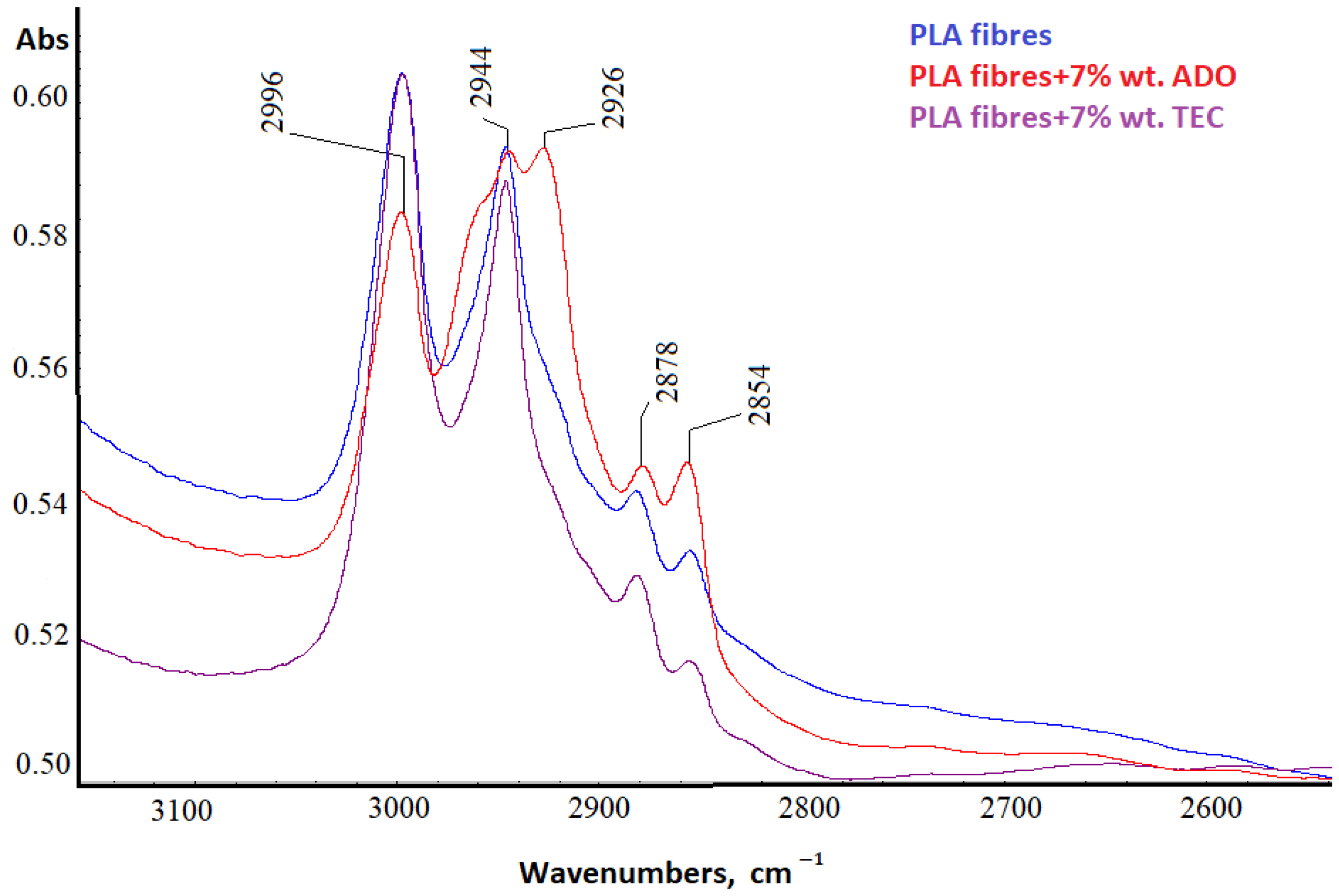
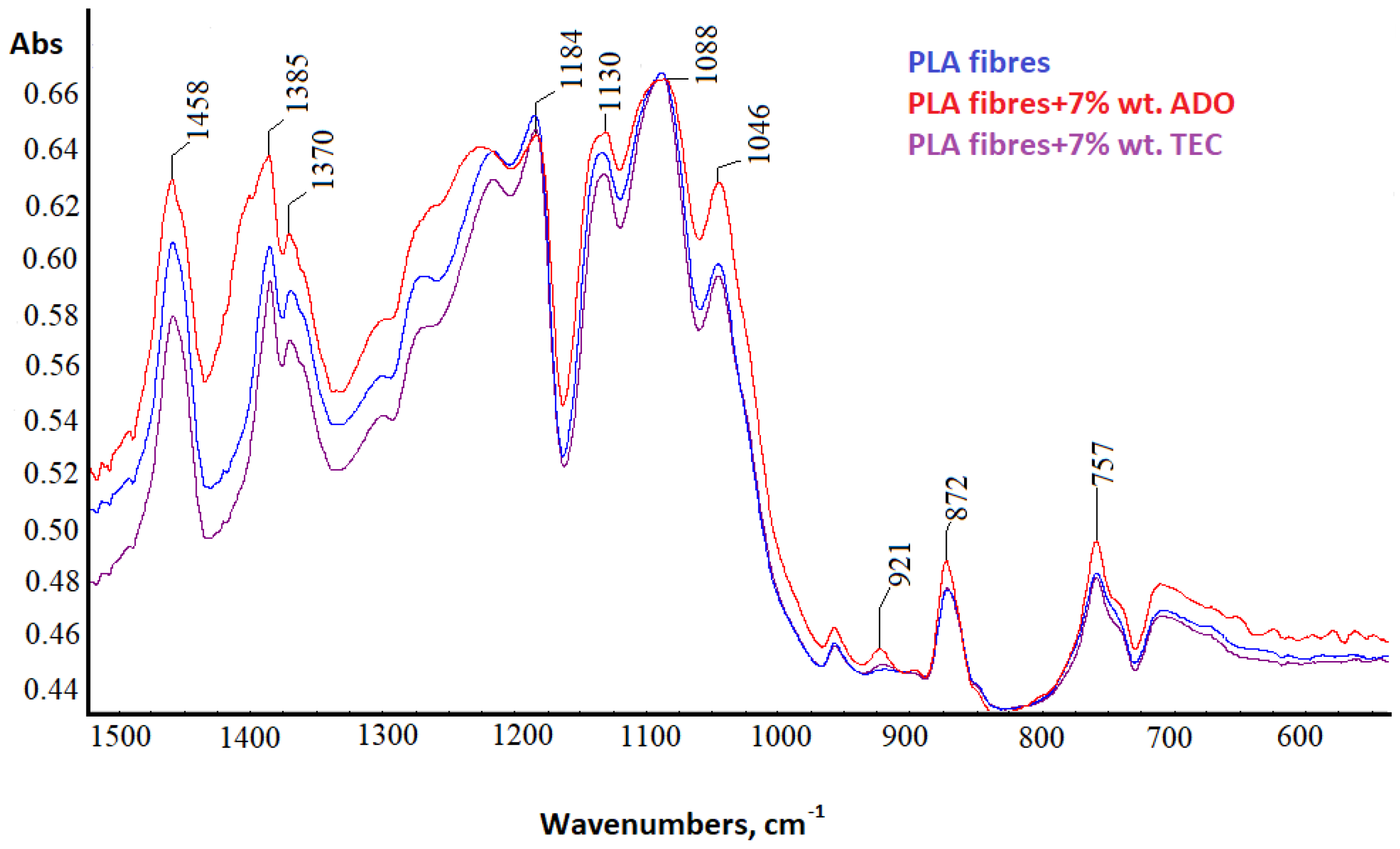
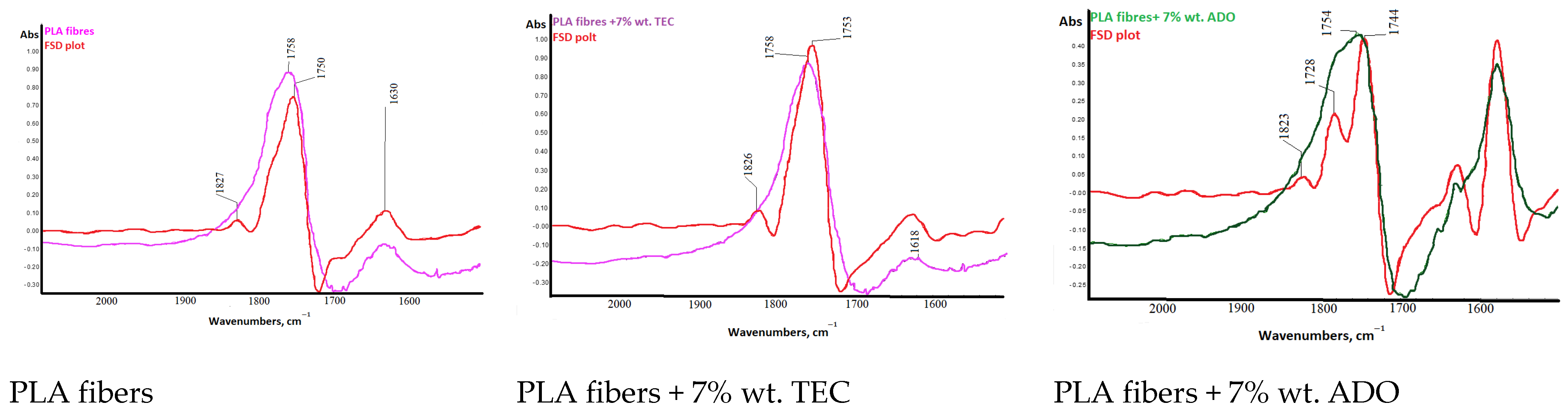
3.2.5. Spectral Analysis
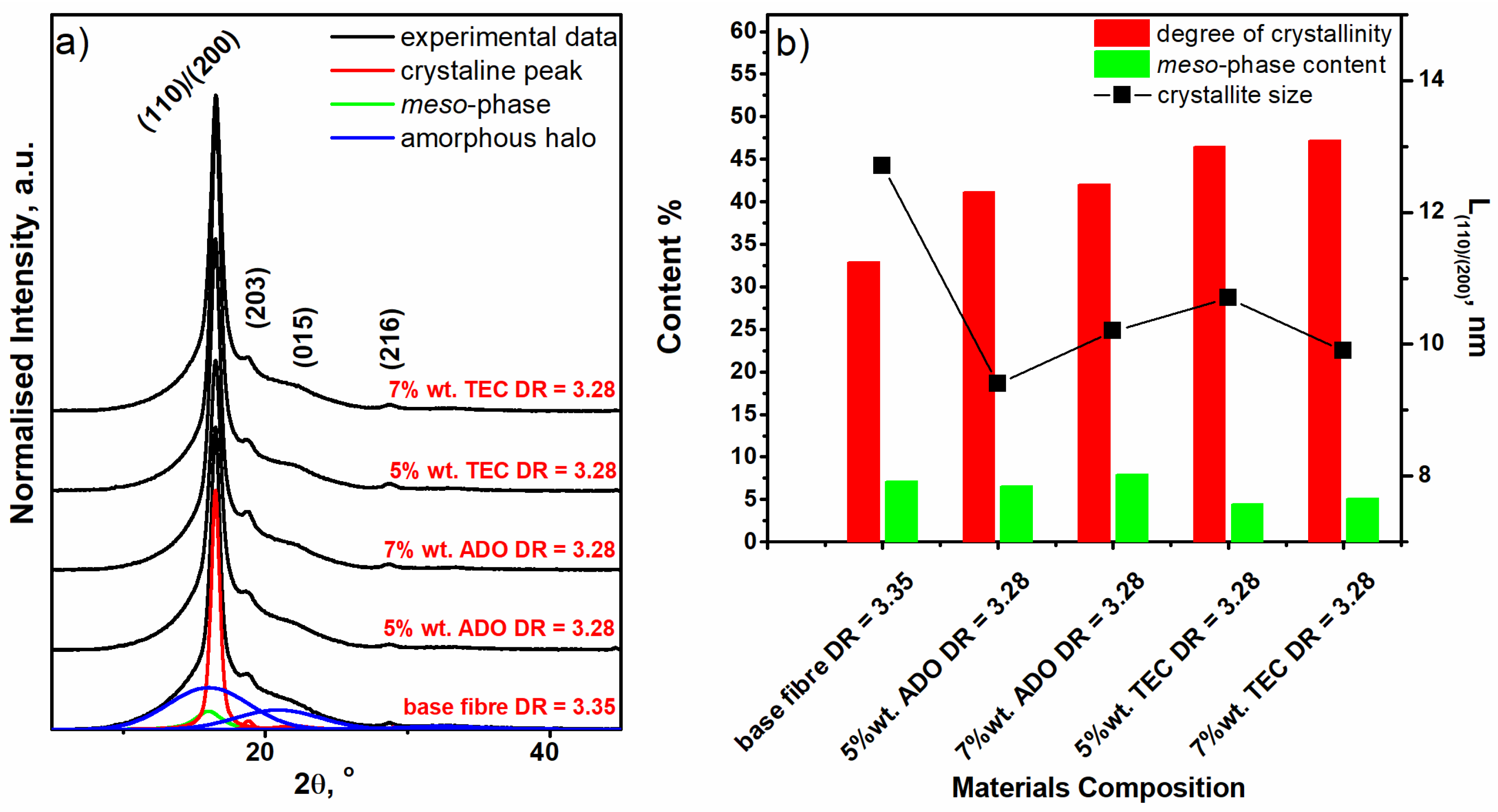
3.2.6. WAXD Analysis
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ioannidou, S.M.; Pateraki, C.; Ladakis, D.; Papapostolou, H.; Tsakona, M.; Vlysidis, A.; Kookos, I.K.; Koutinas, A. Sustainable production of bio-based chemicals and polymers via integrated biomass refining and bioprocessing in a circular bioeconomy context. Bioresour. Technol. 2020, 307, 123093. [Google Scholar] [CrossRef]
- Reddy, M.M.; Vivekanandhana, S.; Misra, M.; Bhatia, S.K.; Mohanty, A.K. Biobased plastics and bionanocomposites: Current status and future opportunities. Prog. Polym. Sci. 2013, 38, 1653–1689. [Google Scholar] [CrossRef]
- Dziuba, R.; Kucharska, M.; Madej-Kiełbik, L.; Sulak, K.; Wiśniewska-Wrona, M. Biopolymers and biomaterials for special applications within the context of the circular economy. Materials 2021, 14, 7704. [Google Scholar] [CrossRef]
- Wiśniewska-Wrona, M.; El Fray, M. Functional three-component polymeric biocomposites for the treatment of bedsores. Prog. Chem. Appl. Chitin Its Deriv. 2018, 23, 185–208. [Google Scholar] [CrossRef]
- Wiśniewska, K.; Rybak, Z.; Wątrobiński, M.; Struszczyk, M.H.; Filipiak, J.; Żywicka, B.; Szymonowicz, M. Bioresorbable polymeric materials—Current state of knowledge. Polimery 2021, 66, 1. [Google Scholar] [CrossRef]
- Bibire, T.; Yilmaz, O.; Ghiciuc, C.M.; Bibire, N.; Dănilă, R. Biopolymers for Surgical Applications. Coatings 2022, 12, 211. [Google Scholar] [CrossRef]
- Senthamaraikannan, C.; Akash, K.; Amanullah, S.; Barath, M.; Manojkumar, R.; Jagadeeshwaran, J. Overview of Polylactic acid and its derivatives in medicinal applications. IOP Conf. Series. Mater. Sci. Eng. 2020, 988, 012003. [Google Scholar] [CrossRef]
- Mohammed, A.S.A.; Naveed, M.; Jost, N. Polysaccharides; Classification, Chemical Properties, and Future Perspective Applications in Fields of Pharmacology and Biological Medicine (A Review of Current Applications and Upcoming Potentialities). J. Polym. Environ. 2021, 29, 2359–2371. [Google Scholar] [CrossRef]
- Reddy, M.; Ponnamma, D.; Choudhary, R.; Sadasivuni, K.K. A comparative review of natural and synthetic biopolymer composite scaffolds. Polymers 2021, 13, 1105. [Google Scholar] [CrossRef] [PubMed]
- Muir, V.G.; Burdick, J.A. Chemically Modified Biopolymers for the Formation of Biomedical Hydrogels. Chem. Rev. 2021, 121, 10908–10949. [Google Scholar] [CrossRef]
- Jiménez, L.; Mena, M.J.; Prendiz, J.; Salas, L.; Vega-Baudrit, J. Polylactic Acid (PLA) as a Bioplastic and its Possible Applications in the Food Industry. J. Food Sci. 2019, 5, 048. [Google Scholar] [CrossRef]
- Shady, F.; Anderson, D.G.; Langer, R. Physical and Mechanical Properties of PLA and Their Functions in Widespread Applications—A Comprehensive Review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar] [CrossRef]
- Menghui-Zhao, G.L.; Bo Yang, F.X.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Rahul, M.R.; Janorkar, A.V.; Hirt, D.E. Poly(lactic acid) modifications. Prog. Polym. Sci. 2010, 35, 338–356. [Google Scholar] [CrossRef]
- Tokiwa, Y.; Calabia, B.P. Biodegradability and biodegradation of poly(lactide). Appl. Microbiol. Biotechnol. 2006, 72, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Gao, W.; Iffelsberger, C.; Pumera, M. Dual polymer engineering enables high-performance 3D printed Zn-organic battery cathodes. Appl. Mater. Today 2022, 28, 101515. [Google Scholar] [CrossRef]
- Le Phuong, H.A.; Ayob, N.A.A.; Blanford, C.F.; Rawi, N.F.M.; Szekely, G. Nonwoven Membrane Supports from Renewable Resources: Bamboo Fiber Reinforced Poly(Lactic Acid) Composites. ACS Sustain. Chem. Eng. 2019, 7, 11885–11893. [Google Scholar] [CrossRef]
- Boarino, A.; Schreier, A.; Leterrier, Y.; Klok, H.A. Uniformly Dispersed Poly(lactic acid)-Grafted Lignin Nanoparticles Enhance Antioxidant Activity and UV-Barrier Properties of Poly(lactic acid) Packaging Films. ACS Appl. Polym. Mater. 2022, 4, 4808–4817. [Google Scholar] [CrossRef] [PubMed]
- Alhulaybi, Z.A. Fabrication and Characterization of Poly(lactic acid)-Based Biopolymer for Surgical Sutures. ChemEngineering 2023, 7, 98. [Google Scholar] [CrossRef]
- Liu, S.B.; Yu, J.; Li, H.; Wang, K.; Wu, G.; Wang, B.; Liu, M.; Zhang, Y.; Wang, P.; Zhang, J.; et al. Controllable Drug Release Behavior of Polylactic Acid (PLA) Surgical Suture Coating with Ciprofloxacin (CPFX)—Polycaprolactone (PCL)/Polyglycolide (PGA). Polymers 2020, 12, 288. [Google Scholar] [CrossRef]
- Liu, S.; Wu, G.; Zhang, X.; Yu, J.; Liu, M.; Zhang, Y.; Wang, P.; Yin, X.; Zhang, J.; Li, F.; et al. Preparation and properties of poly (lactic acid) (PLA) suture loaded with PLA microspheres enclosed drugs (PM-Ds). J. Text. Inst. 2019, 110, 1596–1605. [Google Scholar] [CrossRef]
- Zhang, Z.; Zhu, L.; Hu, W.; Dai, J.; Ren, P.; Shao, X.; Xiong, B.; Zhang, T.; Ji, Z. Polypropylene mesh combined with electrospun poly (L-lactic acid) membrane in situ releasing sirolimus and its anti-adhesion efficiency in rat hernia repair. Colloids Surf. B Biointerface 2022, 218, 112772. [Google Scholar] [CrossRef] [PubMed]
- Ulrich, D.; Le Teuff, I.; Huberlant, S.; Carteron, P.; Letouzey, V.; de Tayrac, R. A preclinical evaluation of polypropylene/polylacticacid hybrid meshes for fascial defect repair using a rat abdominal hernia model. PLoS ONE 2017, 12, e0179246. [Google Scholar] [CrossRef] [PubMed]
- Petre, D.G.; Leeuwenburgh, S.C.G. The Use of Fibers in Bone Tissue Engineering. Tissue Eng. Part B Rev. 2022, 28, 141–159. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Wang, W.; Zhang, X.; Yang, L.; Zhang, J. Research Progress of Biodegradable Polymers in Repairing Achilles Tendon Injury. Front. Mater. 2022, 9, 815930. [Google Scholar] [CrossRef]
- Labrecque, L.V.; Kumar, R.A.; Dave, V.; Gross, R.A.; McCarthy, S.P. Citrate Esters as Plasticizers for Poly(lactic acid). J. Appl. Polym. Sci. 1997, 66, 1507–1513. [Google Scholar] [CrossRef]
- Park, J.W.; Im, S.S.; Km, S.H.; Kim, Y.H. Biodegradable polymer blends of poly(L-lactic acid) and gelatinized starch. Polym. Eng. Sci. 2000, 40, 2539–2550. [Google Scholar] [CrossRef]
- Martin, O.; Averous, L. Poly(lactic acid): Plasticization and properties of biodegradable multiphase systems. Polymer 2001, 42, 6209–6219. [Google Scholar] [CrossRef]
- Eguiburu, J.L.; Iruin, J.J.; Fernan-dez-Berridi, M.J.; Roman, J.S. Blends of amorphous and crystalline polylactides with poly(methyl methacrylate) and poly(methyl acrylate): A miscibility study. Polymer 1998, 39, 6891–6897. [Google Scholar] [CrossRef]
- Nijenhuis, A.J.; Colstee, E.; Grijpma, D.W.; Pennings, A.J. High molecular weight poly(l-lactide) and poly(ethylene oxide) blends: Thermal characterization and physical properties. Polymer 1996, 37, 5849–5857. [Google Scholar] [CrossRef]
- Ljungberg, S.; Wesselen, B.J. The effects of plasticizers on the dynamic mechanical and thermal properties of poly (lactic acid). Appl. Polym. Sci 2020, 86, 1227–1234. [Google Scholar] [CrossRef]
- Jacobsen, S.; Fritz, H.G. Plasticizing Polylactide-The Effect of Different Plasticizers on the Mechanical Properties. Polym. Eng. Sci. 1999, 39, 1303–1310. [Google Scholar] [CrossRef]
- Hu, Y.; Rogunova, M.; Topolkaraev, V.; Hiltner, A.; Baer, E. Aging of poly(lactide)/poly(ethylene glycol) blends. Part 1. Poly(lactide) with low stereoregularity. Polymer 2003, 44, 5701–5710. [Google Scholar] [CrossRef]
- Kulinski, Z.; Piorkowska, E. Crystallization, structure and properties of plasticized poly (L-lactide). Polymer 2005, 46, 10290–10300. [Google Scholar] [CrossRef]
- Piorkowska, E.; Kulinski, Z.; Galeski, A.; Masirek, R. Plasticization of semicrystalline poly(l-lactide) with poly(propylene glycol). Polymer 2006, 47, 7178–7188. [Google Scholar] [CrossRef]
- Zhang, J.; Wang, S.; Zhao, D.; Zhang, Y.; Pang, W.; Zhang, B.; Li, Q. Improved processability and performance of biomedical devices with poly(lactic acid)/poly(ethylene glycol) blends. J. Appl. Polym. Sci. 2017, 137, 45194–45201. [Google Scholar] [CrossRef]
- Tanrattanakul, V.; Bunkaew, P. Effect of different plasticizers on the properties of bio-based thermoplastic elastomer containing poly(lactic acid) and natural rubber. Express Polym. Lett. 2014, 8, 387–396. [Google Scholar] [CrossRef]
- Cadogan, D.F.; Howick, C.J. Plasticizers. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley: Hoboken, NJ, USA, 2000. [Google Scholar] [CrossRef]
- Pearce, E.M. Kirk-Othmer Encyclopedia of Chemical Technology, 3rd ed.; Wiley-Interscience: Hoboken, NY, USA, 1978; Volume 1. [Google Scholar]
- Henton, D.; Gruber, P.; Lunt, J.; Randall, J. Polylactic Acid Technology. In Natural Fibers, Biopolymers and Biocomposites; Mohanty, A.K., Misra, M., Drzal, L.T., Eds.; CRC Press: Boca Raton, FL, USA, 2005; pp. 527–577. [Google Scholar]
- Gzyra-Jagieła, K.; Sulak, K.; Draczyński, Z.; Podzimek, S.; Gałecki, S.; Jagodzińska, S.; Borkowski, D. Modification of Poly(lactic acid) by the Plasticization for Application in the Packaging Industry. Polymers 2021, 13, 3651. [Google Scholar] [CrossRef] [PubMed]
- Mustapa, I.R.; Shanks, R.A.; Kong, I. Poly(lactic acid)-hemp-nanosilica hybrid composites: Thermomechanical, thermal behavior and morphological properties. Int. J. Adv. Sci. Eng. Technol. 2013, 3, 192–199. [Google Scholar]
- Aslan, S.; Calandrelli, L.; Laurienzo, P.; Malinconico, M.; Migliaresi, C. Poly(D,L-lactic acid)/poly(∈-caprolactone) blend membranes: Preparation and morphological characterization. J. Mater. Sci. 2000, 35, 1615–1622. [Google Scholar] [CrossRef]
- Kurata, M.; Tsunashima, Y. Viscosity-Molecular Weight Relationship and Unperturbed Dimensions of Linear Chain Molecules. In Polymer Handbook, 2nd ed.; Wiley: Hoboken, NJ, USA, 1975. [Google Scholar]
- Dorgman, J.; Janzen, J.; Knauss, D.; Hait, S.B.; Limoges, B.R.; Hutchinson, M.H. Fundamental Solution and Single-Chain Properties of Polylactides. J. Polym. Sci. Part B Polym. Phys. 2005, 43, 3100–3111. [Google Scholar] [CrossRef]
- PN-EN ISO 139:2006; Textile—Normal Climates for Acclimatization and Research. PKN: Warszawa, Poland, 2006.
- PN-EN ISO 2062:2010; Textiles—Yarns from Packages—Determination of Single-End Breaking force and Elongation at Break Using Constant Rate of Extension (CRE) Tester. PKN: Warszawa, Poland, 2010.
- De Santis, P.; Kovacs, J. Molecular conformation of poly(S-lactic acid). Biopolymers 1968, 6, 299–306. [Google Scholar] [CrossRef]
- Hoogsteen, W.; Postema, A.R.; Pennings, A.J.; Ten Brinke, G.; Zugenmaier, P. Crystal structure, conformation and morphology of solution-spun poly(L-lactide) fibers. Macromolecules 1990, 23, 634–642. [Google Scholar] [CrossRef]
- Kobayshi, J.; Asahi, T.; Ichiki, M.; Okikawa, A.; Suzuki, H.; Watanabe, T. Structural and optical properties of poly lactic acids. J. Appl. Phys. 1995, 77, 2957–2973. [Google Scholar] [CrossRef]
- Cartier, L.; Okihara, T.; Ikada, Y.; Tsuji, H.; Puiggali, J.; Lotz, B. Epitaxial crystallization and crystalline polymorphism of polylactides. Polymer 2000, 41, 8909–8919. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Sakamo, K.; Ono, Y.; Kawahara, W. Melting behavior of poly(L-lactic acid): X-ray and DSC analyses of the melting process. Polymer 2008, 49, 1943–1951. [Google Scholar] [CrossRef]
- Eling, B.; Gogolewski, S.; Pennings, A.J. Biodegradable materials of poly(l-lactic acid): 1. Melt-spun and solution-spun fibers. Polymer 1982, 23, 1587–1593. [Google Scholar] [CrossRef]
- Le Marec, P.E.; Ferry, L.; Quantin, J.C.; Benezet, J.C.; Bonfils, F. Influence of melt processing conditions on poly(lactic acid) degradation: Molar mass distribution and crystallization. Polym. Degrad. Stab. 2014, 110, 353–363. [Google Scholar] [CrossRef]
- Yasuniwa, M.; Tsubakihara, S.; Iura, K.; Ono, Y.; Dan, Y.; Takahashi, K. Crystallization behavior of poly(L-lactic acid). Polymer 2006, 47, 7554–7563. [Google Scholar] [CrossRef]
- Kowalczyk, M.; Pluta, M.; Piorkowska, E.; Krasnikova, N. Plasticization of Polylactide with Block Copolymers of Ethylene Glycol and Propylene Glycol. J. Appl. Polym. Sci. 2012, 125, 4292–4301. [Google Scholar] [CrossRef]
- Silverstein, R.M. Spectrometric Identification of Organic Compounds; Wiley & Sons, Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Cohn, D.; Younes, H.J. Biodegradable PEO/PLA block copolymers. Biomed. Mater. Res. 1988, 22, 993. [Google Scholar] [CrossRef]
- Lee, J.K.; Lee, K.H.; Jin, B.S. Effect of constrained annealing on the microstructures of extrusion cast polylactic acid films. Eur. Polym. J. 2001, 37, 907. [Google Scholar] [CrossRef]
- Zhang, J.; Tsuji, H.; Noda, I.; Ozaki, Y. Structural Changes and Crystallization Dynamics of Poly(L-lactide) during the Cold-Crystallization Process Investigated by Infrared and Two-Dimensional Infrared Correlation Spectroscopy. Macromolecules 2004, 37, 6433–6439. [Google Scholar] [CrossRef]
- Mohammed, H.; Mustapa, I.; Daud, N.; Jumah, N.; Syafiqah Sudin, N.A.; Majhool, A.; Mahmoudi, E. Thermomechanical Study and Thermal Behavior of Plasticized Poly(Lactic Acid) Nanocomposites. Solid State Phenom. 2021, 317, 333–340. [Google Scholar] [CrossRef]
- Murariu, M.; Da Silva Ferreira, A.; Alexandre, M.; Dubois, P. Polylactide (PLA) designed with desired end-use properties: 1. PLA compositions with low molecular weight ester-like plasticizers and related performances. Polym. Adv. Technol. 2008, 19, 636–646. [Google Scholar] [CrossRef]
- Larrañaga, A.; Guay-Begin, A.A.; Chevallier, P.; Sabbatier, G.; Fernandez, J.; Laroche, G.; Sarasua, J.R. Grafting of a model protein on lactide and caprolactone based biodegradable films for biomedical applications. Biomatter 2014, 4, e27979. [Google Scholar] [CrossRef] [PubMed]
- Larrañaga, A.; Sarasu, J.R. Effect of bioactive glass particles on the thermal degradation behaviour of medical polyesters. Polym. Degrad. Stab. 2013, 98, 751–758. [Google Scholar] [CrossRef]
- Urbańczyk, G.W. Fiber Physics; Lodz University of Technology: Łódż, Poland, 2002; ISBN 83-7283-039-8. [Google Scholar]
- Rabiej, M. A hybrid immune-evolutionary strategy algorithm for the analysis of the wide-angle X-ray diffraction curves of semicrystalline polymers. J. Appl. Crystallogr. 2014, 47, 1502–1511. [Google Scholar] [CrossRef]
- Hindeleh, A.M.; Johnson, D.J. Crystallinity and Crystallite Size Measurement in Polyamide and Polyester Fibers. Polymer 1978, 19, 27–32. [Google Scholar] [CrossRef]
- Stoclet, G.; Seguela, R.; Lefebvre, J.-M.; Rochas, C. New insights on the strain-induced mesophase of poly(d,l-lactide): In situ WAXS and DSC study of the thermo-mechanical stability. Macromolecules 2010, 43, 7228–7237. [Google Scholar] [CrossRef]
- Puchalski, M.; Kwolek, S.; Szparaga, G.; Chrzanowski, M.; Krucinska, I. Investigation of the influence of PLA molecular structure on the crystalline forms (α″ and α) and Mechanical Properties of Wet Spinning Fibres. Polymers 2017, 9, 18. [Google Scholar] [CrossRef] [PubMed]
- Mustapa, I.; Shank, R.; Kong, R. Natural Fibre Reinforced Poly(lactic acid) Nanocomposites Plasticized with Tributyl Citrate. In Proceedings of the Polychar21: World Forum on Advanced Materials, Gwangju, Republic of Korea, 11–15 March 2013; p. 237. [Google Scholar]




| Substrates | Producer | Mw, g/mol | Structure | Contents of D-lactde (%) |
|---|---|---|---|---|
| poly (D, L-lactide) | Nature Works® LLC, Plymouth, MN, USA | 82,700 | 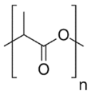 | 1.4 |
| triethyl citrate (TEC) | Gentham Life Sciences Ltd, Corsham, UK | 276 | 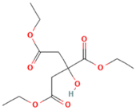 | - |
| bis (2-ethylhexyl) adipate (ADO) | Boryszew S.A., Warszawa, PL, USA | 370 | 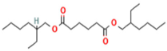 | - |
| Sample | Draw Ratio (dr) | Temperature, °C | Spindle Speed, min−1 × 103 | |
|---|---|---|---|---|
| Goted | Plate | |||
| Base fibers | 3.35 | 70 | 120 | 6800 |
| Modified fibers with 12% wt. TEC | 2.80 | 60 | 110 | 7100 |
| Modified fibers with 5% and 7% wt. of TEC | 3.28 | 70 | 120 | 6600 |
| 2.80 | 70 | 120 | 6848 | |
| Modified fibers with 5% and 7% wt. of ADO | 3.28 | 70 | 120 | 6600 |
| 2.80 | 70 | 120 | 6848 | |
| Sample | DSC | GPC/SEC | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Tg, °C | ∆Cp, J/gK | Tcc, °C | ∆Hcc, J/g | Tm, °C | ∆Hm, J/g | Χc, % | Mw, g/mol | Cobtained, % | ||
| PLA Base | 60.0 | 0.50 | 123.9 | 42.1 | 165.9 | 42.2 | 0.11 | 82,700 | - | |
| Modified Regranulates | ||||||||||
| Modifier | C, % wt. | |||||||||
| TEC | 5.0 | 49.2 | 0.50 | 110.2 | 37.4 | 158.0 166.6 | 37.4 | <0.01 | 77,500 | 4.0 |
| 7.0 | 44.4 | 0.50 | 106.3 | 35.8 | 155.4 165.4 | 35.8 | <0.01 | 77,100 | 6.4 | |
| 12.0 | 34.6 | 0.49 | 98.0 | 34.4 | 147.1 161.4 | 34.4 | <0.01 | 82,700 | 11.9 | |
| 14.0 | 30.1 | 0.47 | 96.3 | 33.2 | 145.0 159.5 | 33.2 | <0.01 | 82,600 | 12.0 | |
| ADO | 5.0 | 47.9 | 0.51 | 117.5 | 39.7 | 162.5 168.1 | 39.7 | <0.01 | 77,200 | 5.0 |
| 7.0 | 42.8 | 0.49 | 95.5 | 35.6 | 154.5 166.3 | 35.6 | <0.01 | 77,600 | 7.0 | |
| 12.0 | 39.9 | 0.47 | 91.6 | 36.2 | 150.5 160.0 | 37.8 | 1.72 | 82,900 | 12.0 | |
| 14.0 | 38.3 | 0.49 | 91.1 | 35.1 | 150.8 162.6 | 37.1 | 2.15 | 83,300 | 14.0 | |
| Sample | DSC | |||||||
|---|---|---|---|---|---|---|---|---|
| Tg, °C | ∆Cp, J/gK | Tcc, °C | ∆Hcc, J/g | Tm, °C | ∆Hm, J/g | |||
| Base Fibers PLA dr = 3.35 | 65.4 | 0.20 | 76.2 | 20.0 | 162.1 | 44.6 | ||
| Modified Fibers | ||||||||
| Modifier | C,% wt. | dr | ||||||
| TEC | 5.0 | 2.80 | 48.5 | 0.29 | 65.1 | 16.1 | 161.2 | 42.5 |
| 3.28 | 48.5 | 0.29 | 65.1 | 16.1 | 161.2 | 42.5 | ||
| 7.0 | 2.80 | 44.5 | 0.26 | 61.3 | 15.5 | 161.4 | 41.4 | |
| 3.28 | 44.6 | 0.21 | 115.3 | 10.0 | 161.1 | 42.5 | ||
| 12.0 | 2.80 | 33.2 | 0.14 | 112.6 | 6.2 | 158.2 | 39.4 | |
| ADO | 5.0 | 2.80 | 49.8 | 0.33 | 62.9 | 20.1 | 154.2; 162.7 | 43.5 |
| 3.28 | 51.0 | 0.20 | 78.4 | 13.4 | 153.9; 162.6 | 45.2 | ||
| 7.0 | 2.80 | 46.5 | 0.21 | 71.2 | 13.6 | 155.3; 163.6 | 42.9 | |
| 3.28 | 49.0 | 0.18 | 110.9 | 11.8 | 154.8; 162.9 | 43.4 | ||
| Sample | GPC/SEC | |||||||
|---|---|---|---|---|---|---|---|---|
| Mn, g/mol | Mw, g/mol | Mw/Mn | Mw Difference to Granules, % | |||||
| Base Fiber PLA Rc = 3.35 | 38,000 | 78,700 | 2.1 | 4.9 | ||||
| Modified Fibers | ||||||||
| Modifier | C, % wt. | dr | Mn, g/mol | Mw, g/mol | Mw/Mn | Mw Difference, % | Cobtained, % wt. | |
| To Regranulates | To Base Fiber | |||||||
| TEC | 5.0 | 2.80 | 35,100 | 76,600 | 2.2 | 1.1 | 2.7 | 4.0 |
| 3.28 | 35,700 | 76,500 | 2.1 | 1.3 | 2.8 | 4.0 | ||
| 7.0 | 2.80 | 36,000 | 76,400 | 2.1 | 0.9 | 2.9 | 6.6 | |
| 3.28 | 36,500 | 74,500 | 2.0 | 3.4 | 5.3 | 6.4 | ||
| 12.0 | 2.80 | 33,500 | 70,400 | 2.1 | 14.9 | 10.5 | 10.6 | |
| ADO | 5.0 | 2.80 | 36,200 | 76,600 | 2.1 | 0.7 | 2.6 | 4.4 |
| 3.28 | 37,700 | 77,500 | 2.1 | −0.4 | 1.5 | 4.7 | ||
| 7.0 | 2.80 | 37,200 | 74,700 | 2.0 | 3.7 | 5.1 | 7.0 | |
| 3.28 | 37,200 | 75,900 | 2.0 | 2.1 | 3.5 | 7.0 | ||
| Sample | 500× | 1000× | |
|---|---|---|---|
| Base fibers PLA dr = 3.35 | 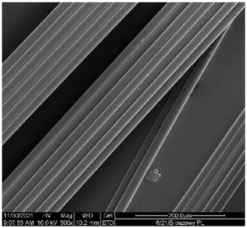 |  | |
| Modified fibers | |||
| TEC, 7% wt. | dr = 3.28 |  | 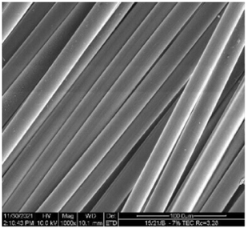 |
| ADO, 7% wt. | dr = 3.28 | 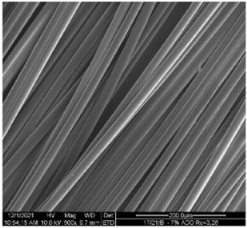 |  |
| Sample | Fiber Diameter, µm | Linear Mass, dtex | ||
|---|---|---|---|---|
| Base Fibers PLA Rc = 3.35 | 20.3 ± 1.8 | 94.8 ± 0.6 | ||
| Modifier | C, % wt. | dr | ||
| TEC | 5.0 | 2.80 | 21.9 ± 1.4 | 115.0 ± 1.0 |
| 3.28 | 19.6± 0.9 | 99.2 ± 1.0 | ||
| 7.0 | 2.80 | 21.1 ± 1.0 | 114.0 ± 1.0 | |
| 3.28 | 18.9 ± 1.4 | 99.0 ± 0.3 | ||
| 12.0 | 2.80 | 21.5 ± 1.0 | 116.1 ± 1.3 | |
| ADO | 5.0 | 2.80 | 20.8 ± 1.3 | 112.0 ± 0.1 |
| 3.28 | 19.9 ± 1.4 | 98.3 ± 0.6 | ||
| 7.0 | 2.80 | 20.5 ± 1.7 | 111.0 ± 0.2 | |
| 3.28 | 18.8 ± 1.5 | 96.1 ± 1.2 | ||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gzyra-Jagieła, K.; Sulak, K.; Draczyński, Z.; Kęska, S.; Puchalski, M.; Madej-Kiełbik, L. Influence of Low-Molecular-Weight Esters on Melt Spinning and Structure of Poly(lactic acid) Fibers. Materials 2024, 17, 1268. https://doi.org/10.3390/ma17061268
Gzyra-Jagieła K, Sulak K, Draczyński Z, Kęska S, Puchalski M, Madej-Kiełbik L. Influence of Low-Molecular-Weight Esters on Melt Spinning and Structure of Poly(lactic acid) Fibers. Materials. 2024; 17(6):1268. https://doi.org/10.3390/ma17061268
Chicago/Turabian StyleGzyra-Jagieła, Karolina, Konrad Sulak, Zbigniew Draczyński, Sławomir Kęska, Michał Puchalski, and Longina Madej-Kiełbik. 2024. "Influence of Low-Molecular-Weight Esters on Melt Spinning and Structure of Poly(lactic acid) Fibers" Materials 17, no. 6: 1268. https://doi.org/10.3390/ma17061268






