Methanol Oxidation Reaction in Alkaline Media Using Gold Nanoparticles Recovered from Electronic Waste
Abstract
:1. Introduction
2. Materials and Methods
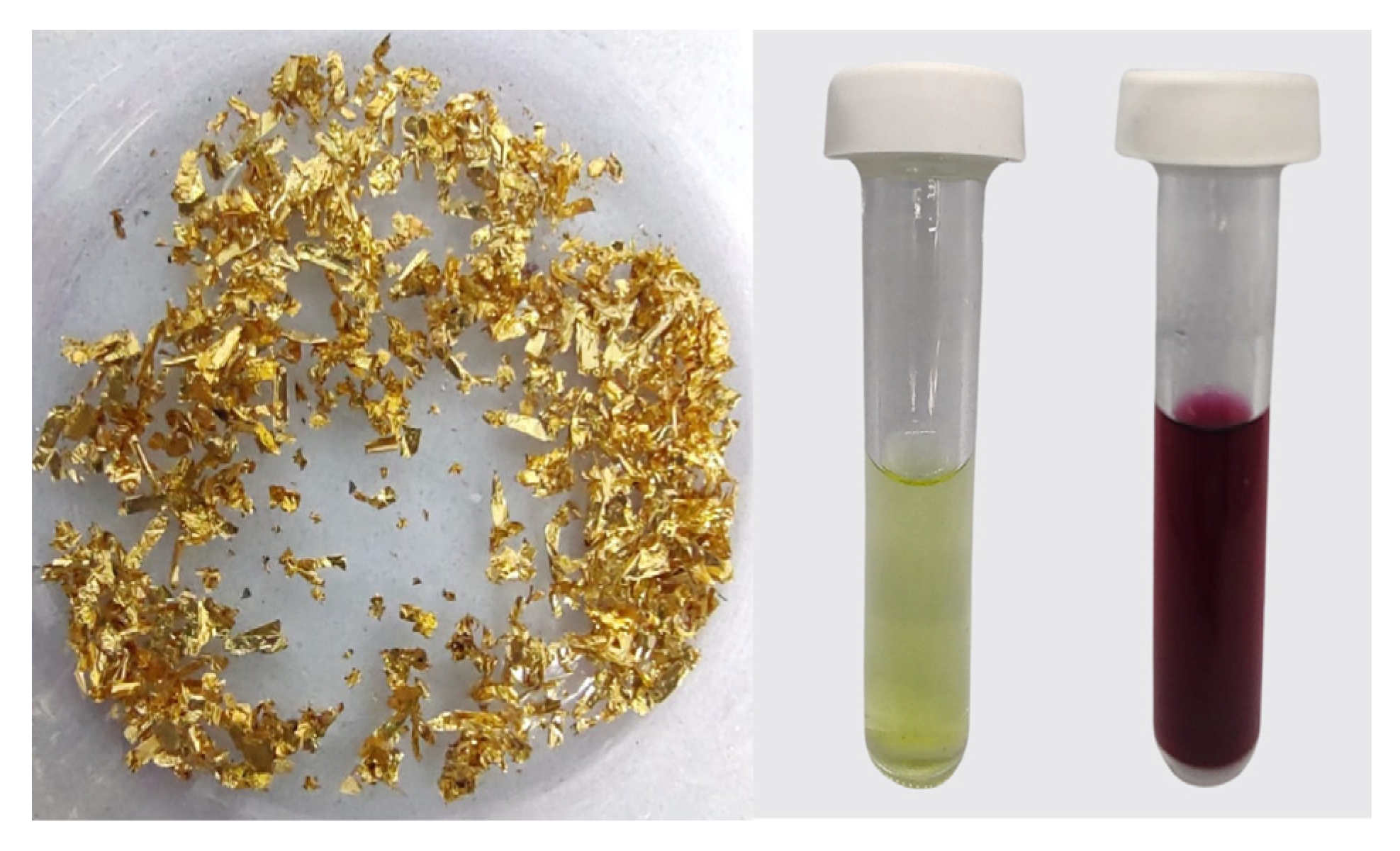
2.1. Synthesis of Au/C NPs
2.2. Structural Characterization of Au NPs and Au/C NPs
2.3. Electrochemical Characterization of Au/C NPs
3. Results and Discussion
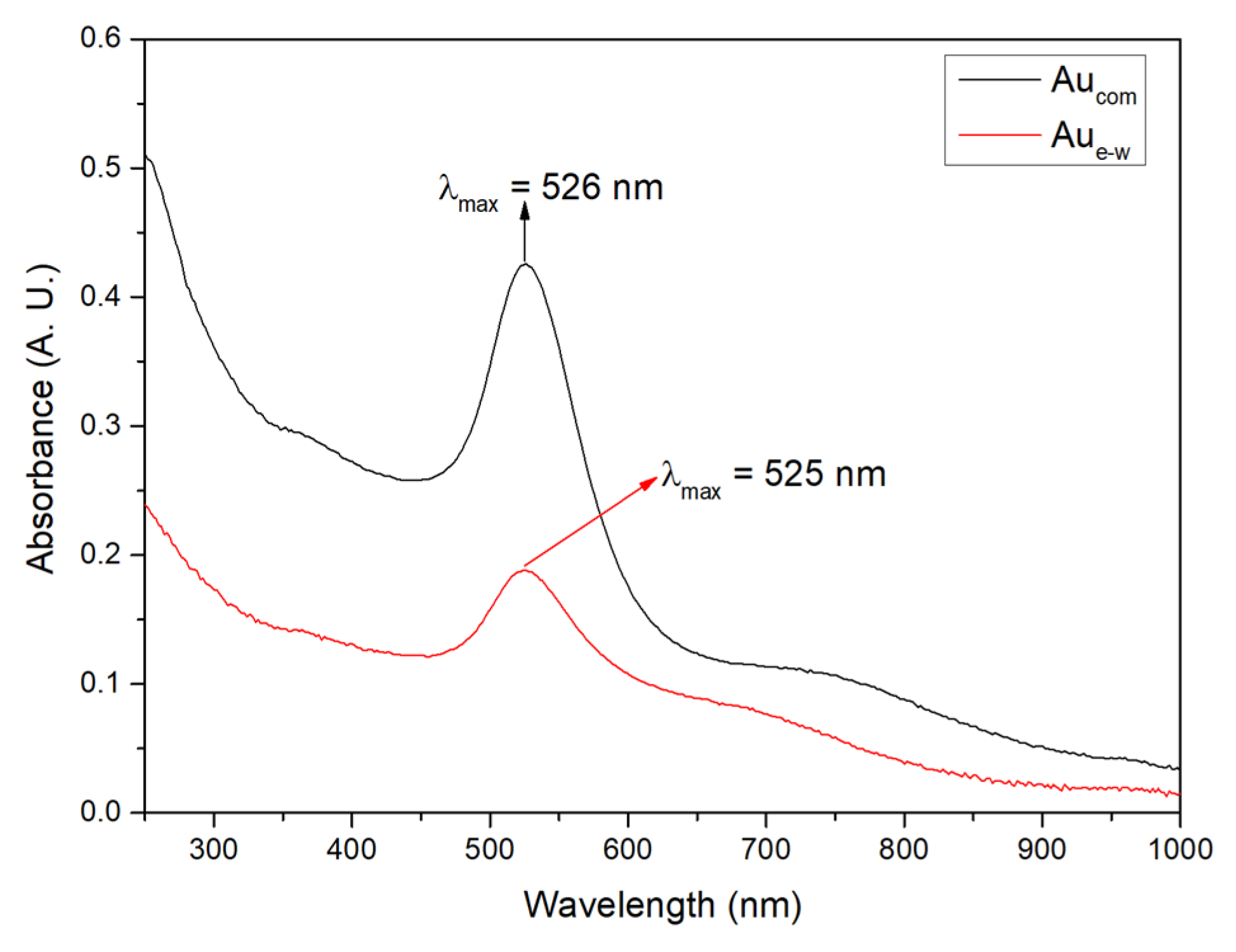
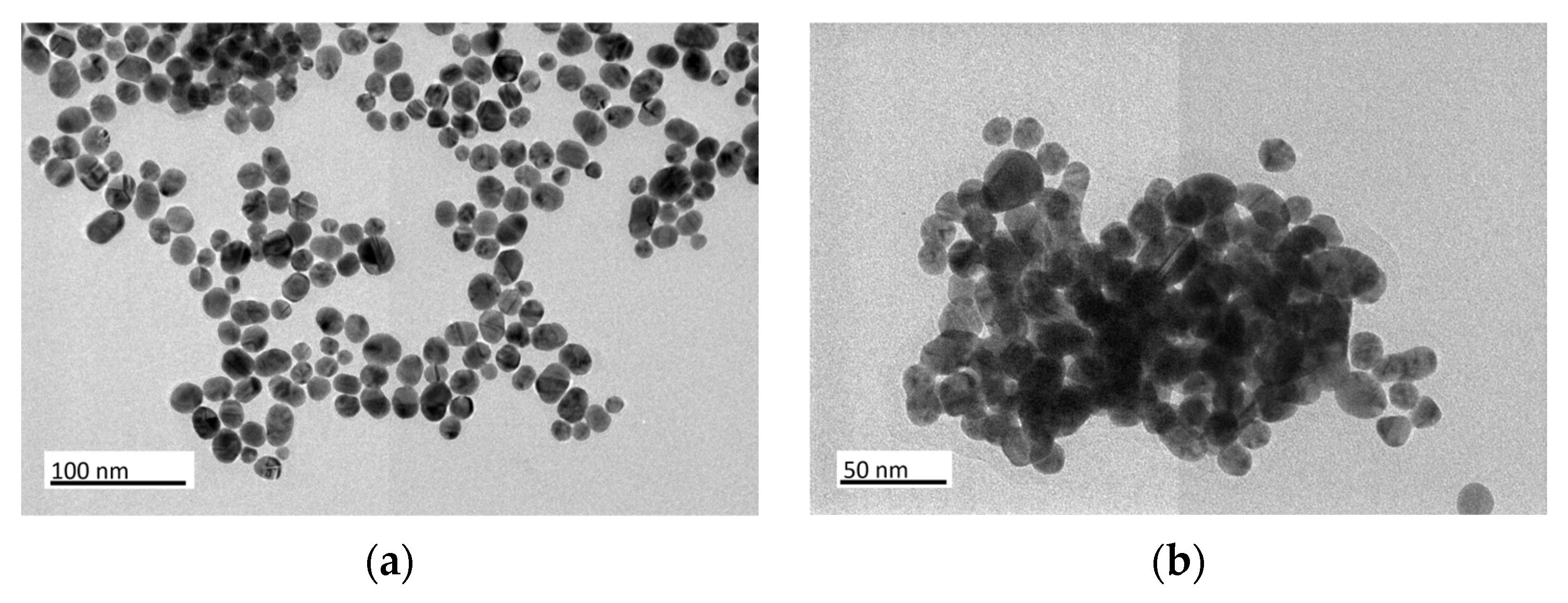
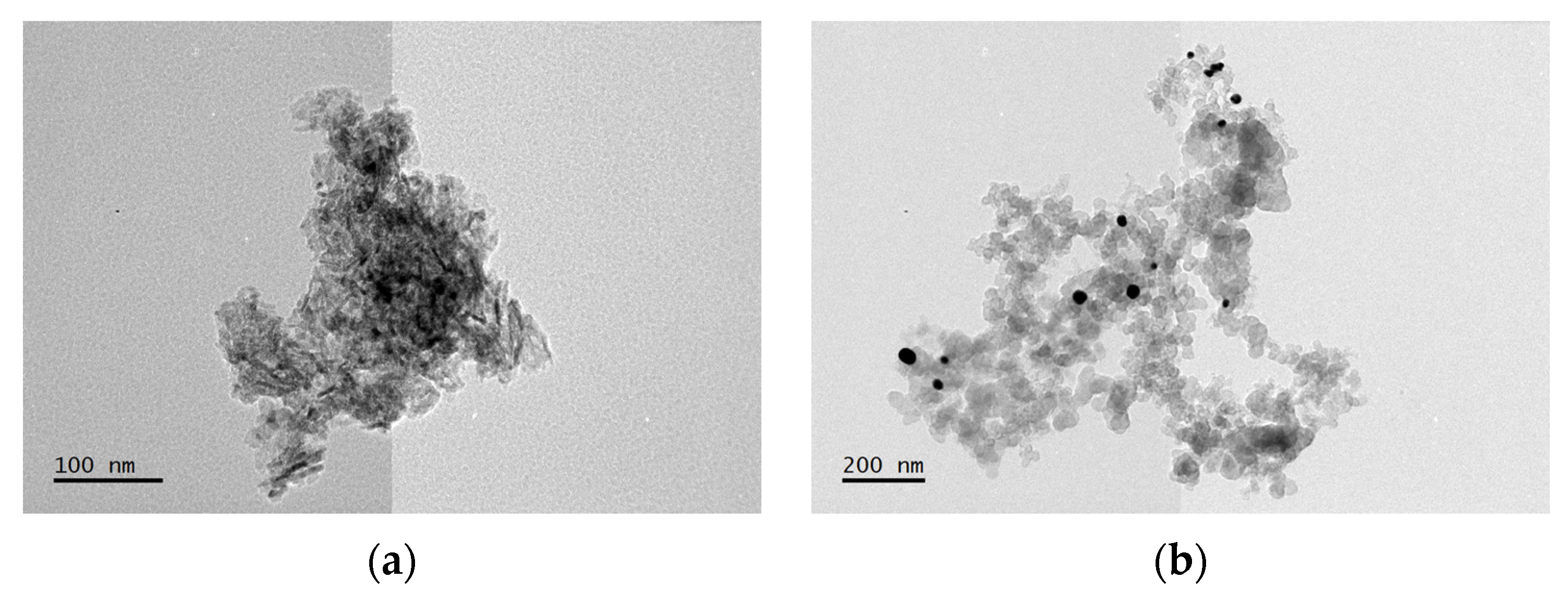
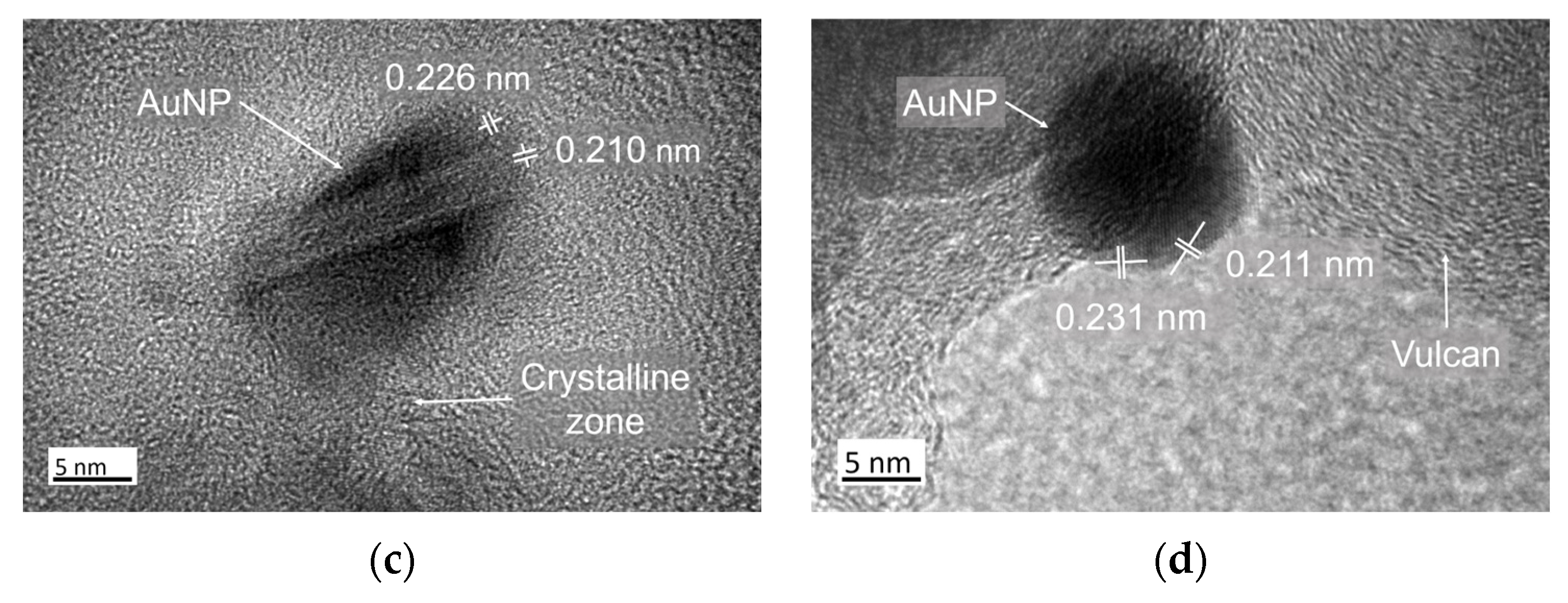
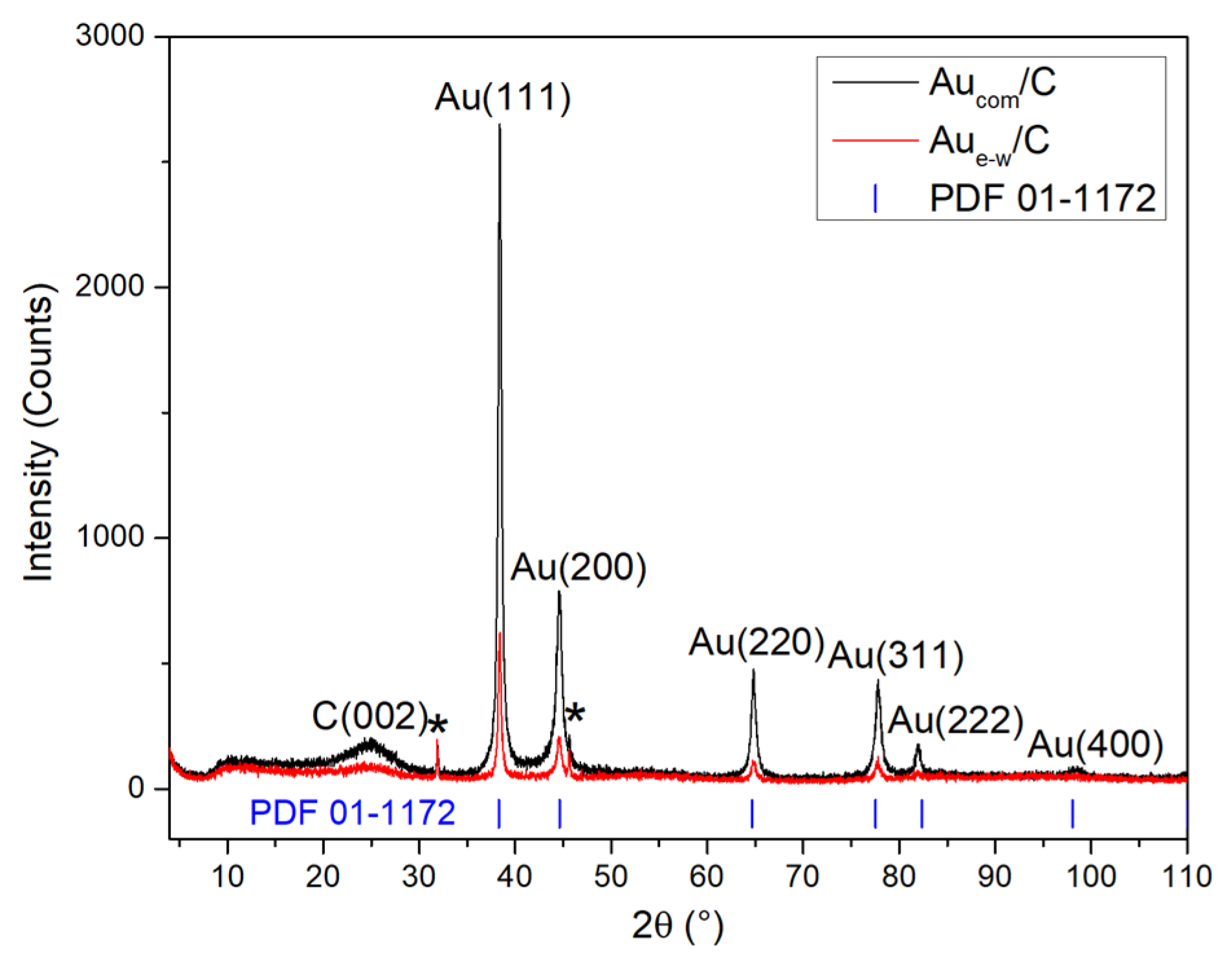
3.1. Structural Characterization of Au NPs and Au/C NPs
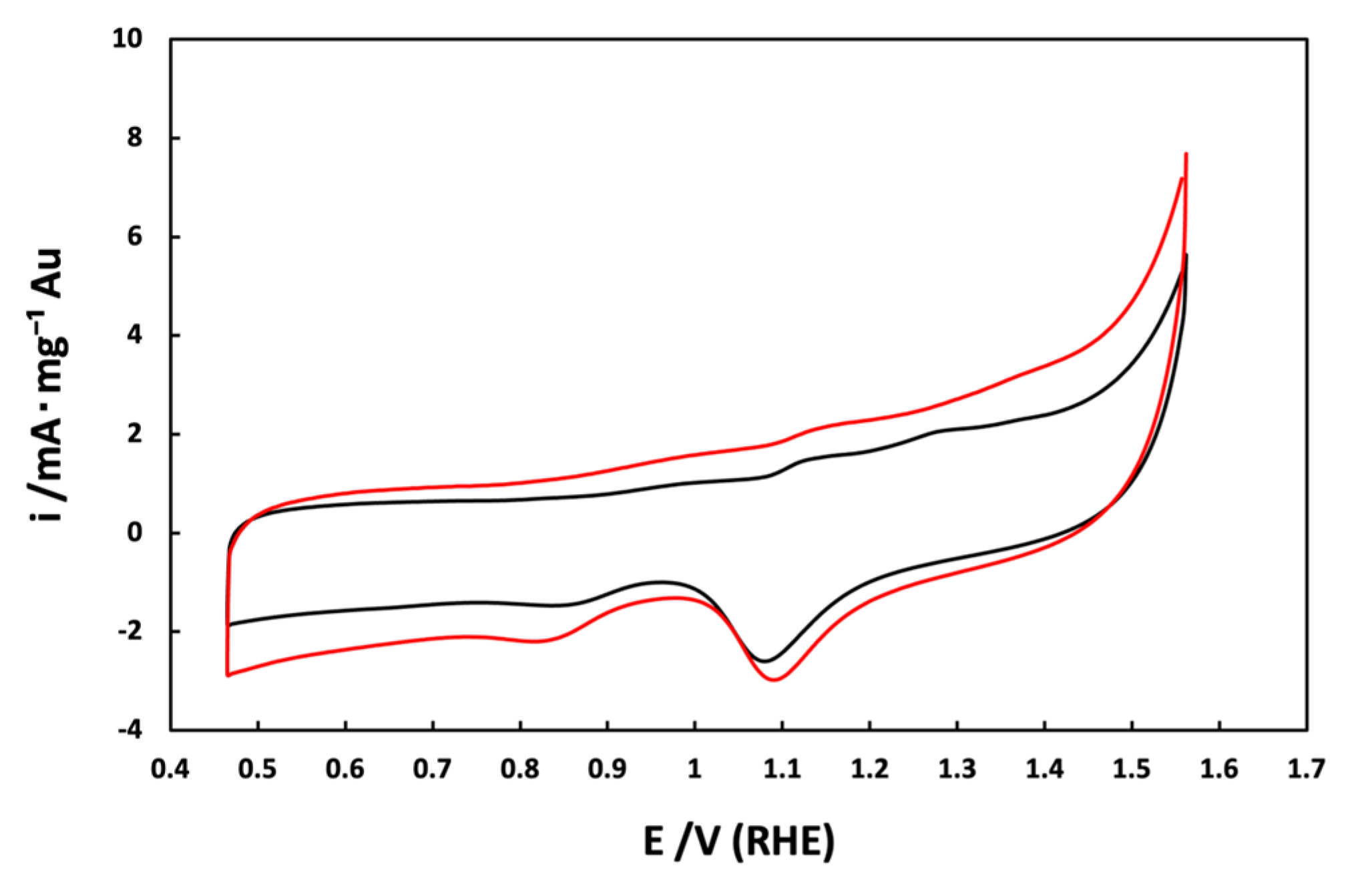
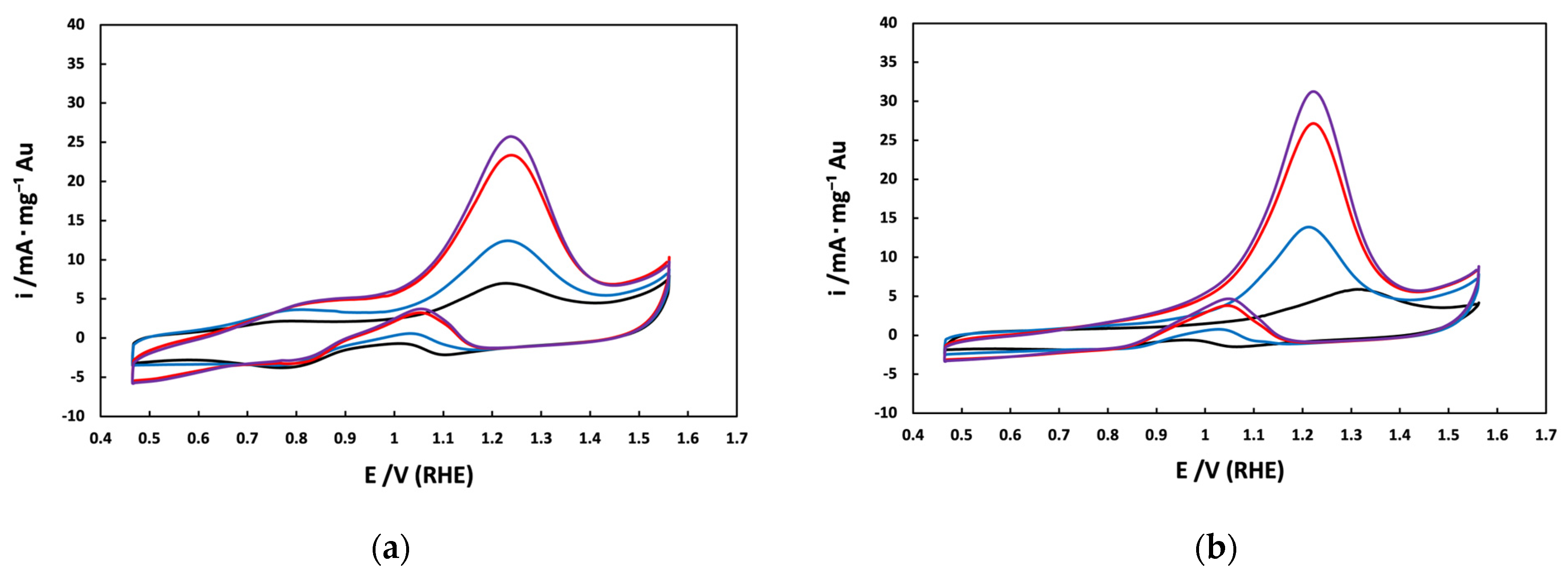
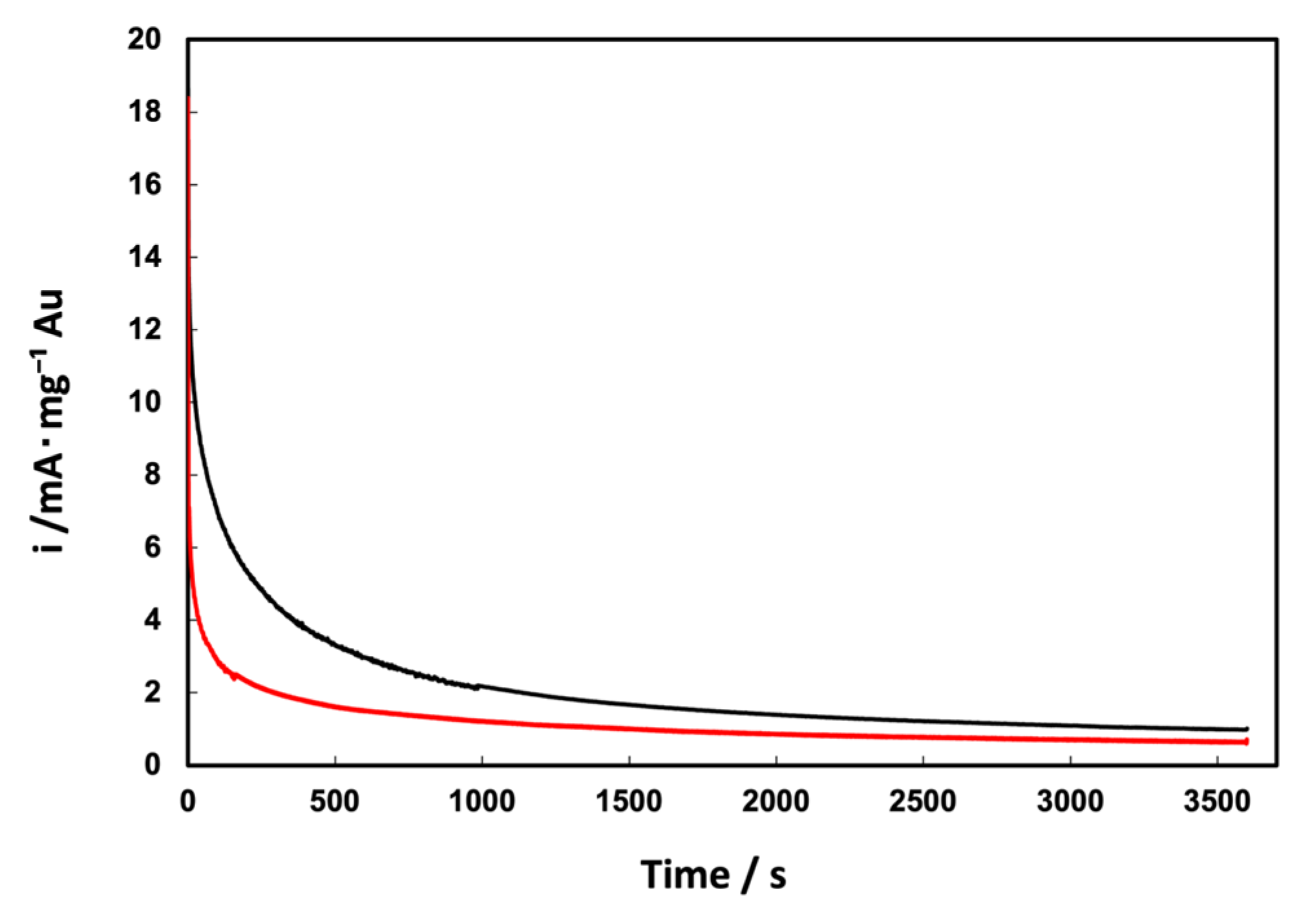
3.2. Electrochemical Characterization of Au/C NPs
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awasthi, A.K.; Iacovidou, E.; Awasthi, M.K.; Johnson, M.; Parajuly, K.; Zhao, M.; Mishra, S.; Pandey, A.K. Assessing Strategic Management of E-Waste in Developing Countries. Sustainability 2023, 15, 7263. [Google Scholar]
- Shawpnil, K.; Nayeem, S.; Hossain, F.; Dayan, A.; Islam, M.M. Easy E-Waste: A Novel Approach Toward Efficient and Sustainable E-Waste Management. In Intelligent Sustainable Systems, 1st ed.; Jennifer, S.R., Isidoros, P., Valentina, E.B., Eds.; Springer: Singapore, 2023; Volume 665, pp. 557–571. [Google Scholar]
- Pant, D.; Dolker, T.; Bajar, S.; Singh, A. Electronic Waste Management: Challenges and Opportunities. In Environmental Microbiology and Biotechnology, 1st ed.; Anoop, S., Shaili, S., Dheeraj, R., Deepak, P., Eds.; Springer: Singapore, 2020; Volume 1, pp. 69–90. [Google Scholar]
- Liou, T.H.; Liu, S.M.; Chen, G.W. Utilization of e-wastes as a sustainable silica source in synthesis of ordered mesostructured titania nanocomposites with high adsorption and photoactivity. Miner. Eng. 2023, 191, 107977. [Google Scholar] [CrossRef]
- Gangwar, C.; Choudhari, R.; Chauhan, A.; Kumar, A.; Singh, A.; Tripathi, A. Assessment of air pollution caused by illegal e-waste burning to evaluate the human health risk. Environ. Int. 2019, 125, 191. [Google Scholar]
- Rajesh, R.; Kanakadhurga, D.; Prabaharan, N. Electronic waste: A critical assessment on the unimaginable growing pollutant, legislations and environmental impacts. Environ. Challenges 2022, 7, 100507. [Google Scholar]
- Ahirwar, R.; Tripathi, A.K. E-Waste Management: A Review of Recycling Process, Environmental and Occupational Health Hazards, and Potential Solutions. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100409. [Google Scholar] [CrossRef]
- Ilankoon, I.M.S.K.; Ghorbani, Y.; Chong, M.N.; Herath, G.; Moyo, T.; Petersen, J. E-Waste in the International Context—A Review of Trade Flows, Regulations, Hazards, Waste Management Strategies and Technologies for Value Recovery. Waste Manag. 2018, 82, 258–275. [Google Scholar] [CrossRef] [PubMed]
- Abdelbasir, S.M.; El-Sheltawy, C.T.; Abdo, D.M. Green Processes for Electronic Waste Recycling: A Review. J. Sustain. Metall. 2018, 4, 295–311. [Google Scholar]
- The United Nations Sustainable Development Goals. Available online: https://www.undp.org/sustainable-development-goals (accessed on 18 February 2024).
- Huy Do, M.; Tien Nguyen, G.; Dong Thach, U.; Lee, Y.; Huu Bui, T. Advances in hydrometallurgical approaches for gold recovery from E-waste: A comprehensive review and perspectives. J. Environ. Chem. Eng. 2022, 10, 107283. [Google Scholar]
- Cecchi, T.; Gao, Z.; Clement, C.; Camus, A.; Karim, A.; Girard, O.; Santato, C. Recovery of gold from e-waste via food waste by products. Nanotechnology 2023, 34, 065203. [Google Scholar]
- Xu, Q.; Du, X.H.; Luo, D.; Strømme, M.; Zhang, Q.F.; Xu, C. Gold recovery from E-waste using freestanding nanopapers of cellulose and ionic covalent organic frameworks. Chem. Eng. J. 2023, 458, 141498. [Google Scholar]
- Ponghiran, W.; Charoensaeng, A.; Khaodhiar, S. The environmental impact assessment of gold extraction processes for discarded computer RAM: A comparative study of two leaching chemicals. J. Mater. Cycles Waste Manag. 2021, 23, 1412. [Google Scholar]
- Wu, H.; Pantaleo, G.; Venezia, A.M.; Liotta, L.F. Mesoporous Silica Based Gold Catalysts: Novel Synthesis and Application in Catalytic Oxidation of CO and Volatile Organic Compounds (VOCs). Catalysts 2013, 3, 774. [Google Scholar] [CrossRef]
- Shcherbakov, V.; Denisov, S.A.; Mostafavi, M. Selective Oxidation of Transient Organic Radicals in the Presence of Gold Nanoparticles. Nanomaterials 2021, 11, 727. [Google Scholar]
- Hu, X.; Zhang, Y.; Ding, T.; Liu, J.; Zhao, H. Multifunctional Gold Nanoparticles: A Novel Nanomaterial for Various Medical Applications and Biological Activities. Front. Bioeng. Biotechnol. 2020, 8, 1. [Google Scholar]
- Sztandera, K.; Gorzkiewicz, M.; Klajnert-Maculewicz, B. Gold Nanoparticles in Cancer Treatment. Mol. Pharm. 2019, 16, 1. [Google Scholar]
- Naeem, M.; Irfan, A.; Begum, R.; Farooqi, Z.H. One step biogenic sugarcane bagasse mediated synthesis of gold nanoparticles and their catalytic applications in removing environmental pollutants. Z. Phys. Chem. 2023, 237, 675. [Google Scholar]
- Qian, H.; Pretzer, L.A.; Velazquez, J.C.; Zhao, Z.; Wong, M.S. Gold nanoparticles for cleaning contaminated water. J. Chem. Technol. Biotechnol. 2013, 88, 735. [Google Scholar]
- Lee, M.J.; Choi, J.H.; Shin, J.H.; Yun, J.; Kim, T.; Kim, Y.J.; Oh, B.K. Gold Nanoclusters with Two Sets of Embedded Enzyme Nanoparticles for Applications as Electrochemical Sensors for Glucose. ACS Appl. Nano Mater. 2023, 6, 12567. [Google Scholar] [CrossRef]
- Steven, J.T.; Golovko, V.B.; Johannessen, B.; Marshall, A.T. Electrochemical stability of carbon-supported gold nanoparticles in acidic electrolyte during cyclic voltammetry. Electrochim. Acta 2016, 187, 593. [Google Scholar]
- Renjith, A.; Lakshminarayanan, V. One step preparation of ‘ready to use’ Au@Pd nanoparticle modified surface using deep eutectic solvents and a study of its electrocatalytic properties in methanol oxidation reaction. J. Mater. Chem. A 2015, 3, 3019–3028. [Google Scholar]
- Sidharthan, K.A.; Joseph, S. Performance evaluation of passive alkaline direct methanol fuel cell-the effect of inorganic salt and TiO2 nano fillers. Sustain. Energy Technol. Assess. 2022, 53, 102673. [Google Scholar] [CrossRef]
- Lo Vecchio, C.; Lyu, X.; Gatto, I.; Zulevi, B.; Serov, A.; Baglio, V. Performance investigation of alkaline direct methanol fuel cell with commercial PGM-free cathodic materials. J. Power Sources 2023, 561, 232732. [Google Scholar]
- Liu, J.; Wang, Q.; Li, T.; Wang, Y.; Li, H.; Cabot, A. PdMoSb trimetallene as high-performance alcohol oxidation electrocatalyst. Nano Res. 2023, 16, 2041. [Google Scholar]
- Li, J.; Li, L.; Wang, J.; Cabot, A.; Zhu, Y. Boosting Hydrogen Evolution by Methanol Reaction on Ni-Based Electrocatalysts: From Fundamental Electrochemistry to Perspectives. ACS Energy Lett. 2024, 9, 853. [Google Scholar]
- Dieterich, E.; Kinkelin, S.J.; Bron, M. Comparative Study of the Synthesis of sub-10 nm Carbon-Supported Gold Nanoparticles and their Suitability for Methanol Electrooxidation in Alkaline Media. ChemNanoMat 2022, 8, e202200098. [Google Scholar]
- Yan, S.; Zhang, S.; Lin, Y.; Liu, G. Electrocatalytic Performance of Gold Nanoparticles Supported on Activated Carbon for Methanol Oxidation in Alkaline Solution. J. Phys. Chem. C 2011, 115, 6986. [Google Scholar]
- Luo, J.; Njoki, P.N.; Lin, Y.; Mott, D.; Wang, L.; Zhong, C.J. Characterization of Carbon-Supported AuPt Nanoparticles for Electrocatalytic Methanol Oxidation Reaction. Langmuir 2006, 22, 2892. [Google Scholar]
- Su-Gallegos, J.; Magallón-Cacho, L.; Ramírez-Aparicio, J.; Borja-Arco, E. Synthesis of Gold Nanoparticles from Gold Coatings Recovered from E-Waste Processors. Materials 2022, 5, 7307. [Google Scholar]
- Yeh, Y.C.; Creran, B.; Rotello, V.M. Gold nanoparticles: Preparation, properties, and applications in bionanotechnology. Nanoscale 2012, 4, 1871. [Google Scholar] [CrossRef]
- Pluchery, O.; Prado, Y.; Watkins, W. A complete explanation of the plasmonic colours of gold nanoparticles and of the bichromatic effect. J. Mater. Chem. C 2023, 11, 15824. [Google Scholar]
- Gold Nanoparticles: Properties and Applications. Available online: https://www.sigmaaldrich.com/MX/es/technicaldocuments/technical-article/materials-science-and-engineering/biosensors-and-imaging/gold-nanoparticles (accessed on 20 December 2023).
- Logeshwaran, N.; Panneerselvam, I.R.; Ramakrishnan, S.; Kumar, R.S.; Kim, A.R.; Wang, Y.; Yoo, D.J. Quasihexagonal Platinum Nanodendrites Decorated over CoS2-N-Doped Reduced Graphene Oxide for Electro-Oxidation of C1-, C2-, and C3-Type Alcohols. Adv. Sci. 2022, 9, 2105344. [Google Scholar]
- Bapat, S.; Giehl, C.; Kohsakowski, S.; Peinecke, V.; Schäffler, M.; Segets, D. On the state and stability of fuel cell catalyst inks. Adv. Powder Technol. 2021, 32, 3845. [Google Scholar]
- Hernández, J.; Solla-Gullón, J.; Herrero, E.; Aldaz, A.; Feliu, J.M. Methanol oxidation on gold nanoparticles in alkaline media: Unusual electrocatalytic activity. Electrochem. Acta 2006, 52, 1662. [Google Scholar]


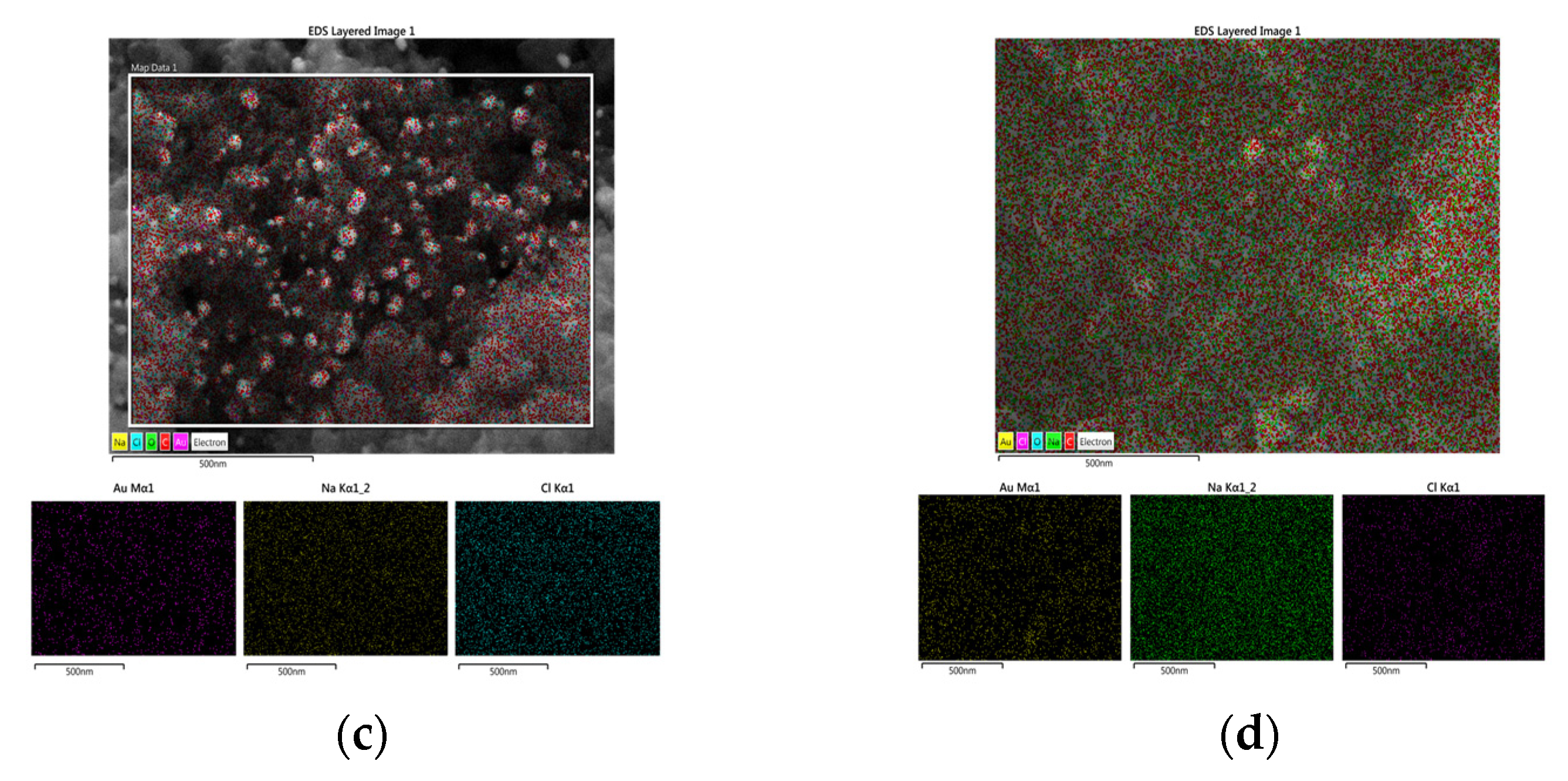
| Element | wt% (Aue-w/C NPs) | wt% (Aucom/C NPs) |
|---|---|---|
| Au | 16.40 | 27.29 |
| Na | 31.84 | 19.95 |
| Cl | 43.55 | 18.12 |
| O | 8.21 | 34.64 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Baruch-Soto, M.; Magallón-Cacho, L.; Ramírez-Aparicio, J.; Ortega-Guzmán, J.; Borja-Arco, E. Methanol Oxidation Reaction in Alkaline Media Using Gold Nanoparticles Recovered from Electronic Waste. Materials 2024, 17, 1267. https://doi.org/10.3390/ma17061267
Baruch-Soto M, Magallón-Cacho L, Ramírez-Aparicio J, Ortega-Guzmán J, Borja-Arco E. Methanol Oxidation Reaction in Alkaline Media Using Gold Nanoparticles Recovered from Electronic Waste. Materials. 2024; 17(6):1267. https://doi.org/10.3390/ma17061267
Chicago/Turabian StyleBaruch-Soto, Mariana, Lorena Magallón-Cacho, Jeannete Ramírez-Aparicio, Jesús Ortega-Guzmán, and Edgar Borja-Arco. 2024. "Methanol Oxidation Reaction in Alkaline Media Using Gold Nanoparticles Recovered from Electronic Waste" Materials 17, no. 6: 1267. https://doi.org/10.3390/ma17061267






