Interfacial Segregation of Sn during the Continuous Annealing and Selective Oxidation of Fe-Mn-Sn Alloys
Abstract
:1. Introduction
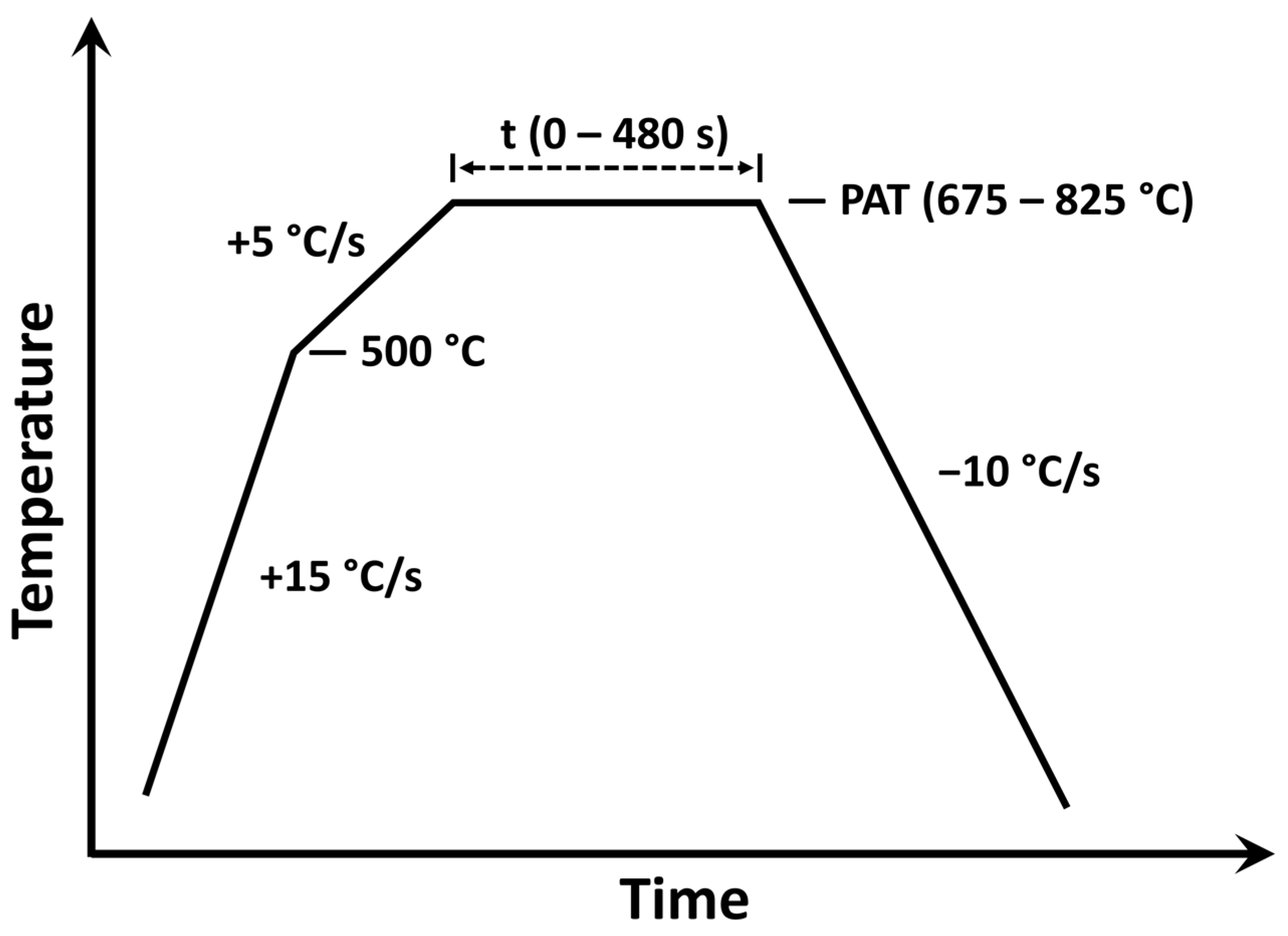
2. Materials and Methods
3. Results
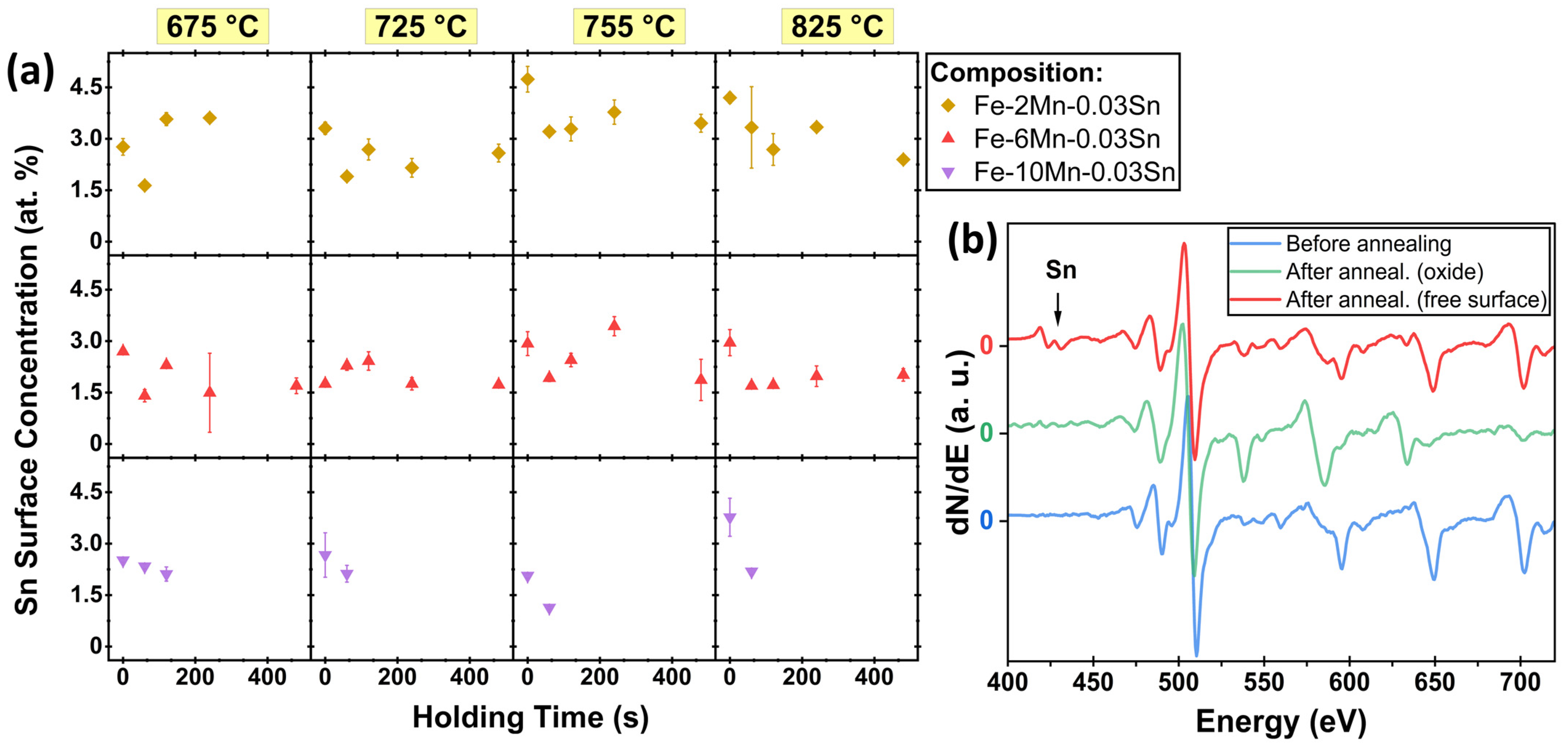
3.1. Sn Segregation to the Free (External) Surface
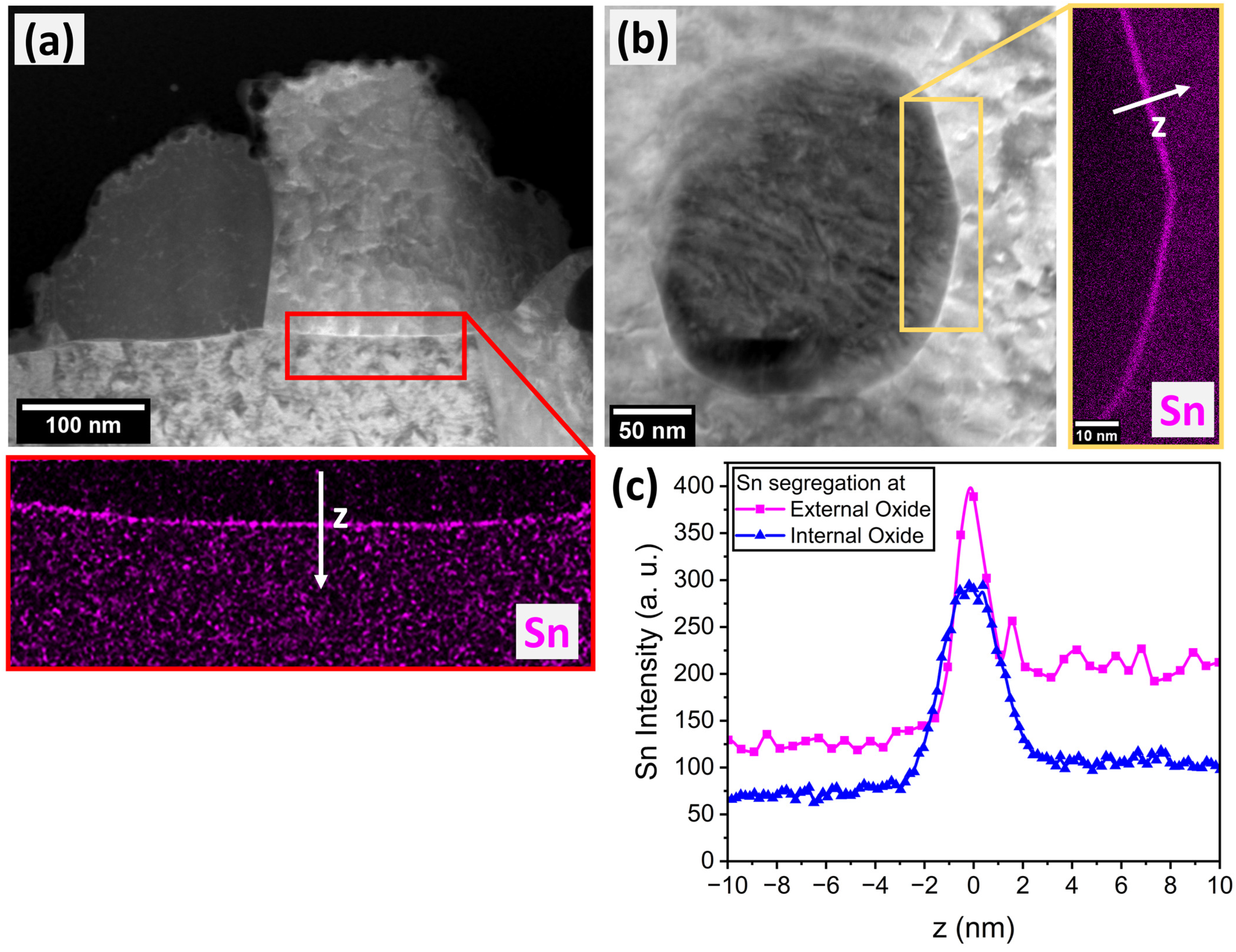
3.2. Sn Segregation to the Metal/Oxide Interfaces
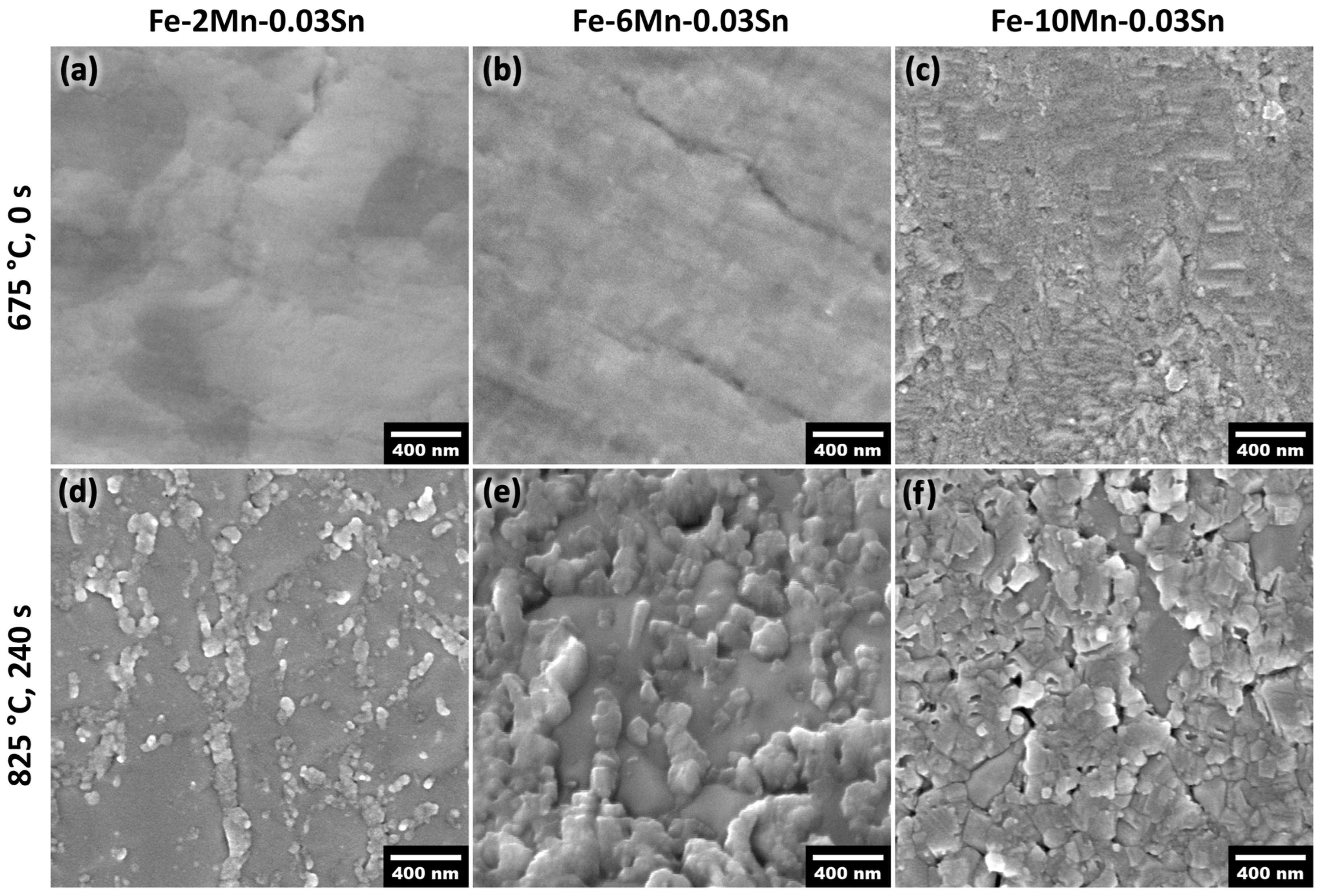
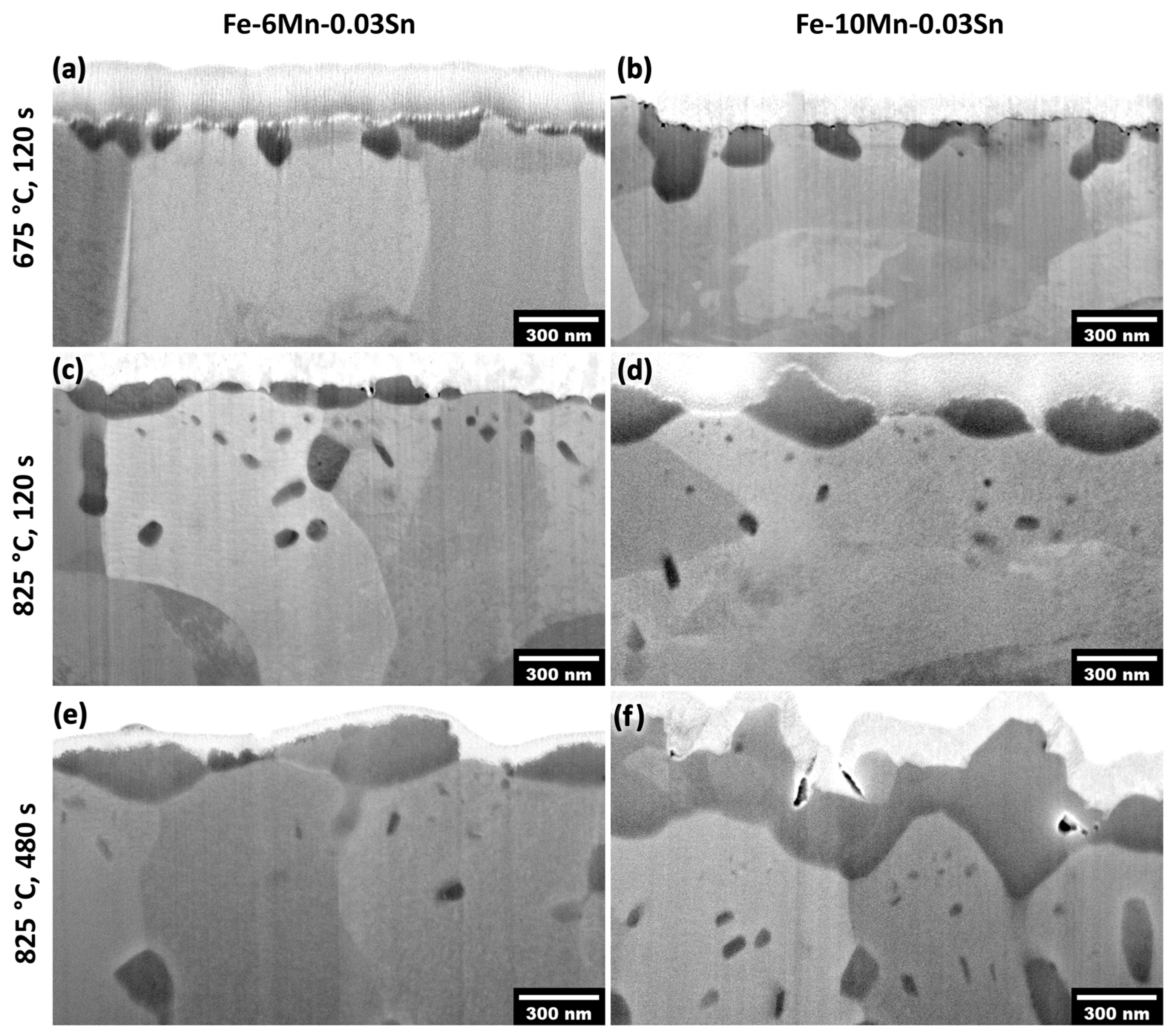
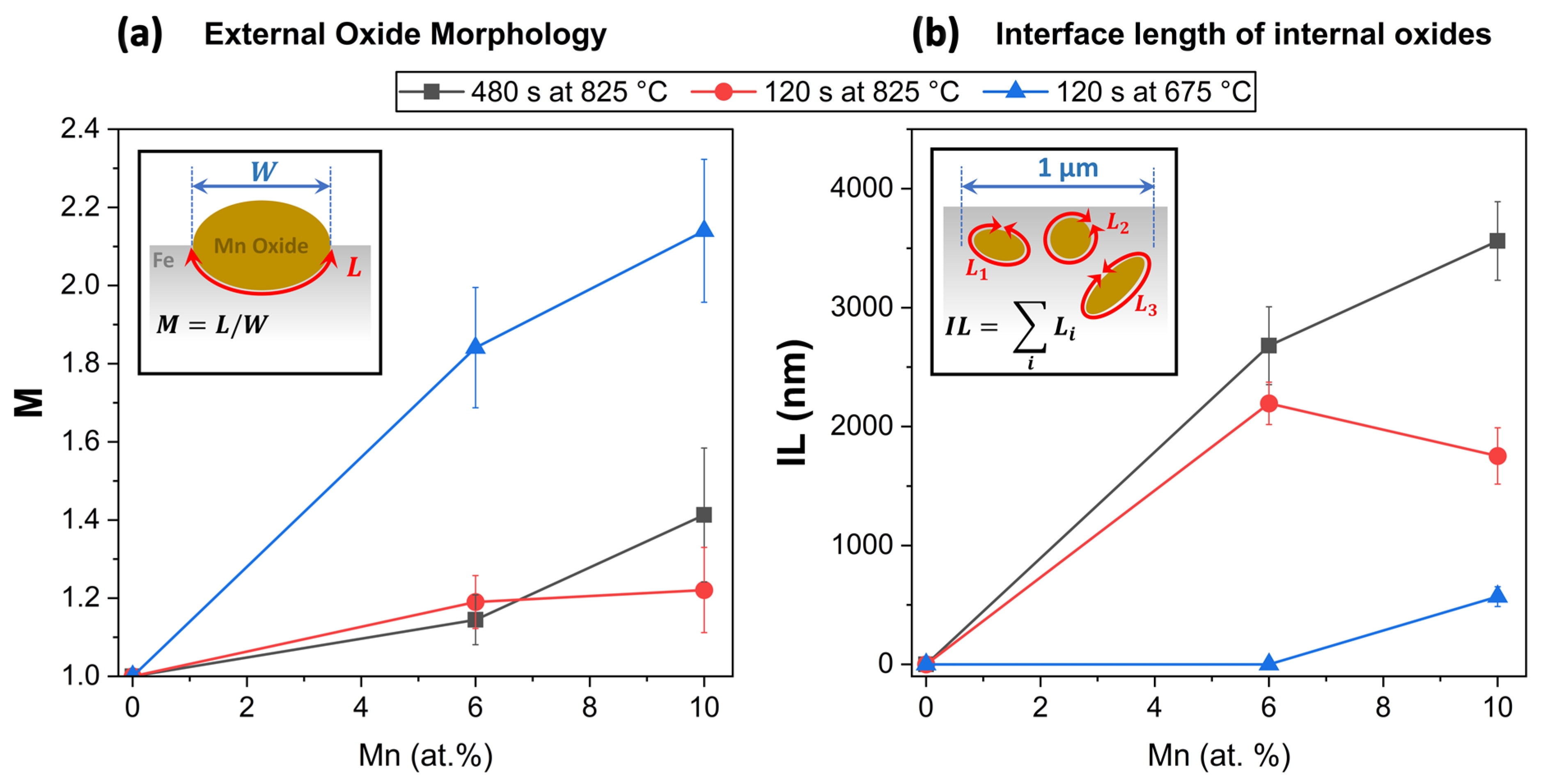
3.3. Thermodynamic and Oxide Morphology Effects on Sn Segregation
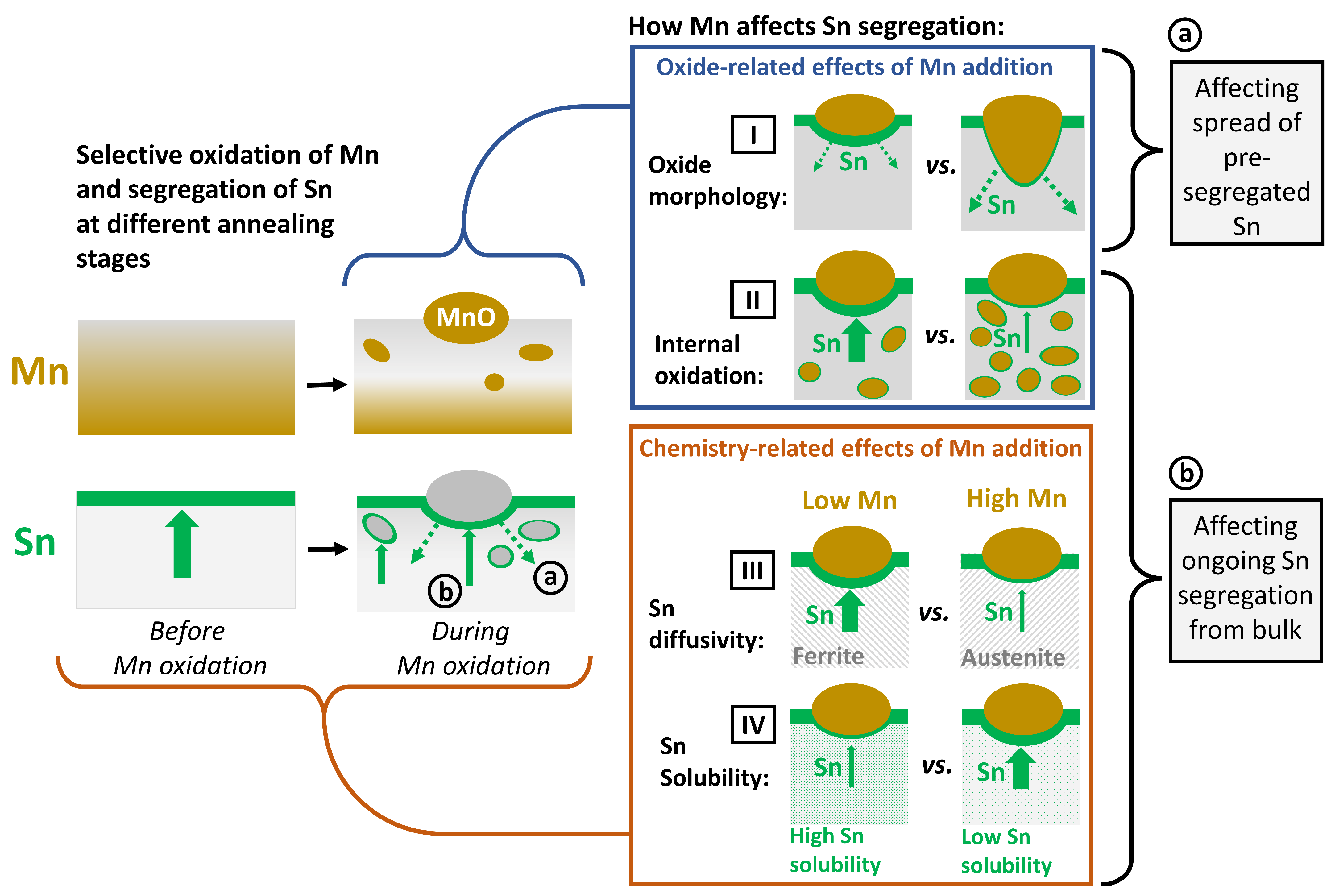
4. Discussion
5. Conclusions
- Significant Sn enrichment at the external substrate surface and at internal metal/oxide interfaces was observed for isothermal holding at 675–825 °C for 0–480 s and for Mn alloy contents of 0–10 at.%.
- Sn segregation to the free surface between oxide nodules was not significantly affected by the Mn concentration of the substrate. Equilibrium segregation models predicted an ambivalent chemistry-related effect of Mn concentration on Sn segregation, contributing to the insensitivity of Sn segregation to the Mn content of the alloy. In the present case, Mn reduces the Sn solubility in Fe and, therefore, increases the driving force for Sn segregation. However, Mn also increases the volume fraction of austenite, thereby reducing the effective Sn diffusivity and decreasing the segregation kinetics.
- Selective oxidation led to a depletion of solute Mn near the metal/external oxide interface, making the subsurface chemically similar between the different alloys. The global composition of the substrate is, therefore, not an adequate proxy to describe the local chemistry of the region in which Sn segregation occurs.
- Sn enrichment at the metal/external oxide interface was lower than at the free surface and decreased with increasing alloy Mn concentration. This is attributed to differences in the subsurface morphologies of external oxides and the interfaces of internal oxide particles acting as Sn sinks.
- The observed Sn segregation is promising for a potential industrial application of Sn microalloying to improve galvanized coatings in a wide range of compositions and processing conditions typical of CGL-compatible AHSSs. Suitability for individual steel grades cannot be evaluated based on global composition alone but must consider all factors determining oxide characteristics, such as the annealing atmosphere and temperature, which will be investigated in detail in future work.
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Melford, D.A. The Influence of Residual and Trace Elements on Hot Shortness and High Temperature Embrittlement. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1980, 295, 89–103. [Google Scholar] [CrossRef]
- Hondros, E.D. Residuals and Properties. Philos. Trans. R. Soc. A Math. Phys. Eng. Sci. 1980, 295, 9–23. [Google Scholar] [CrossRef]
- Tyson, W.R. Kinetics of Temper Embrittlement. Acta Metall. 1978, 26, 1471–1478. [Google Scholar] [CrossRef]
- Guttmann, M. Temper Embrittlement and Ternary Equilibrium Segregation. Mater. Sci. Eng. 1980, 42, 227–232. [Google Scholar] [CrossRef]
- Guttmann, M. Equilibrium Segregation in a Ternary Solution: A Model for Temper Embrittlement. Surf. Sci. 1975, 53, 213–227. [Google Scholar] [CrossRef]
- Raabe, D.; Herbig, M.; Sandlöbes, S.; Li, Y.; Tytko, D.; Kuzmina, M.; Ponge, D.; Choi, P.-P. Grain Boundary Segregation Engineering in Metallic Alloys: A Pathway to the Design of Interfaces. Curr. Opin. Solid State Mater. Sci. 2014, 18, 253–261. [Google Scholar] [CrossRef]
- Raabe, D.; Ponge, D.; Wang, M.M.; Herbig, M.; Belde, M.; Springer, H. 1 Billion Tons of Nanostructure—Segregation Engineering Enables Confined Transformation Effects at Lattice Defects in Steels. IOP Conf. Ser. Mater. Sci. Eng. 2017, 219, 012006. [Google Scholar] [CrossRef]
- Cho, L.; Kim, M.S.; Kim, Y.H.; De Cooman, B.C. Influence of Minor Alloying Elements on Selective Oxidation and Reactive Wetting of CMnSi TRIP Steel during Hot Dip Galvanizing. Metall. Mater. Trans. A 2014, 45, 4484–4498. [Google Scholar] [CrossRef]
- Cho, L.; Seo, E.J.; Jung, G.S.; Suh, D.W.; De Cooman, B.C. Surface Selective Oxidation of Sn-Added CMnSi TRIP Steel. Metall. Mater. Trans. A 2016, 47, 1705–1719. [Google Scholar] [CrossRef]
- Pourmajidian, M.; Langelier, B.; McDermid, J.R. Effect of Process Atmosphere Dew Point and Tin Addition on Oxide Morphology and Growth for a Medium-Mn Third Generation Advanced Steel During Intercritical Annealing. Metall. Mater. Trans. A 2018, 49, 5561–5573. [Google Scholar] [CrossRef]
- Seyed Mousavi, G.; Langelier, B.; McDermid, J.R. Effect of Sn Addition, Process Atmosphere pO2, and Annealing Time on the Selective Oxidation of a C-2Mn-1.7Si (Wt Pct) Advanced High-Strength Steel During Continuous Galvanizing. Metall. Mater. Trans. A 2019, 50, 2898–2911. [Google Scholar] [CrossRef]
- Marder, A.R. The Metallurgy of Zinc-Coated Steel. Prog. Mater. Sci. 2000, 45, 191–271. [Google Scholar] [CrossRef]
- Oh, J.; Cho, L.; Kim, M.; Kang, K.; De Cooman, B.C. The Effect of Bi on the Selective Oxide Formation on CMnSi TRIP Steel. Metall. Mater. Trans. A 2016, 47, 5474–5486. [Google Scholar] [CrossRef]
- Pourbahari, B.; McDermid, J.R. Oxidation Kinetics of Fe-(2-10)Mn-XSb Alloys during Annealing. Materialia 2023, 27, 101698. [Google Scholar] [CrossRef]
- Ruck, A.; Monceau, D.; Grabke, H.J. Effects of Tramp Elements Cu, P, Pb, Sb and Sn on the Kinetics of Carburization of Case Hardening Steels. Steel Res. 1996, 67, 240–246. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Han, J. Current Opinion in Medium Manganese Steel. Mater. Sci. Technol. 2015, 31, 843–856. [Google Scholar] [CrossRef]
- Speer, J.; Rana, R.; Matlock, D.; Glover, A.; Thomas, G.; De Moor, E. Processing Variants in Medium-Mn Steels. Metals 2019, 9, 771. [Google Scholar] [CrossRef]
- McDermid, J.R.; Chakraborty, A. Project Report ZCO-53-1: Identification of Steel Chemistries and Galvanizing Process Design; IZA-GAP: Hamilton, ON, Canada, 2012. [Google Scholar]
- Bellhouse, E.M.; Mcdermid, J.R. Selective Oxidation and Reactive Wetting during Hot-Dip Galvanizing of a 1.0 Pct Al-0.5 Pct Si TRIP-Assisted Steel. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2012, 43, 2426–2441. [Google Scholar] [CrossRef]
- Morris, E. FREED Thermodynamic Database; Thermart Software: San Diego, CA, USA, 2019. [Google Scholar]
- Thompson, K.; Lawrence, D.; Larson, D.J.; Olson, J.D.; Kelly, T.F.; Gorman, B. In Situ Site-Specific Specimen Preparation for Atom Probe Tomography. Ultramicroscopy 2007, 107, 131–139. [Google Scholar] [CrossRef]
- Cahn, J. Thermodynamics of Solid and Fluid Surfaces. In Interfacial Segregation; Johnson, W.C., Blakely, J.M., Eds.; American Society for Metals: Metals Park, OH, USA, 1979; pp. 3–23. [Google Scholar]
- Maugis, P.; Hoummada, K. A Methodology for the Measurement of the Interfacial Excess of Solute at a Grain Boundary. Scr. Mater. 2016, 120, 90–93. [Google Scholar] [CrossRef]
- Dyck, O.; Leonard, D.N.; Edge, L.F.; Jackson, C.A.; Pritchett, E.J.; Deelman, P.W.; Poplawsky, J.D. Accurate Quantification of Si/SiGe Interface Profiles via Atom Probe Tomography. Adv. Mater. Interfaces 2017, 4, 1700622. [Google Scholar] [CrossRef]
- Araullo-Peters, V.; Gault, B.; De Geuser, F.; Deschamps, A.; Cairney, J.M. Microstructural Evolution during Ageing of Al-Cu-Li-x Alloys. Acta Mater. 2014, 66, 199–208. [Google Scholar] [CrossRef]
- Maruyama, N.; Smith, G.D.W.; Cerezo, A. Interaction of the Solute Niobium or Molybdenum with Grain Boundaries in α-Iron. Mater. Sci. Eng. A 2003, 353, 126–132. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Danoix, F.; Gouné, M.; Bagot, P.A.J.; Peng, Z.; Moody, M.P.; Gault, B. Reflections on the Analysis of Interfaces and Grain Boundaries by Atom Probe Tomography. Microsc. Microanal. 2020, 26, 247–257. [Google Scholar] [CrossRef]
- Seidman, D.N.; Krakauer, B.W.; Udler, D. Atomic Scale Studies of Solute-Atom Segregation at Grain Boundaries: Experiments and Simulations. J. Phys. Chem. Solids 1994, 55, 1035–1057. [Google Scholar] [CrossRef]
- Huang, Y.; Mao, Z.; Noebe, R.D.; Seidman, D.N. The Effects of Refractory Elements on Ni-Excesses and Ni-Depletions at γ(f.c.c.)/Γ′(L12) Interfaces in Model Ni-Based Superalloys: Atom-Probe Tomographic Experiments and First-Principles Calculations. Acta Mater. 2016, 121, 288–298. [Google Scholar] [CrossRef]
- De Laeter, J.R.; Böhlke, J.K.; De Bièvre, P.; Hidaka, H.; Peiser, H.S.; Rosman, K.J.R.; Taylor, P.D.P. Atomic Weights of the Elements: Review 2000 (IUPAC Technical Report). Pure Appl. Chem. 2003, 75, 683–800. [Google Scholar] [CrossRef]
- Wagner, J.; McDermid, J.R. On the Surface Segregation of Sn in Cold-Rolled Fe under Continuous Annealing Conditions. Materialia 2024, 33, 102019. [Google Scholar] [CrossRef]
- Carter, C.B.; Williams, D.B. Transmission Electron Microscopy; Carter, C.B., Williams, D.B., Eds.; Springer International Publishing: Cham, Switzerland, 2016; ISBN 978-3-319-26649-7. [Google Scholar]
- Moore, K.T.; Stach, E.A.; Howe, J.M.; Elbert, D.C.; Veblen, D.R. A Tilting Procedure to Enhance Compositional Contrast and Reduce Residual Diffraction Contrast in Energy-Filtered TEM Imaging of Planar Interfaces. Micron 2002, 33, 39–51. [Google Scholar] [CrossRef]
- Gault, B.; Klaes, B.; Morgado, F.F.; Freysoldt, C.; Li, Y.; De Geuser, F.; Stephenson, L.T.; Vurpillot, F. Reflections on the Spatial Performance of Atom Probe Tomography in the Analysis of Atomic Neighborhoods. Microsc. Microanal. 2022, 28, 1116–1126. [Google Scholar] [CrossRef]
- Hondros, E.D.; Seah, M.R.; Hofmann, S.; Lejček, P. Interfacial and Surface Microchemistry. In Physical Metallurgy; Cahn, R., Haasen, P., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1996; pp. 1201–1289. [Google Scholar]
- Seah, M.P. Quantitative Prediction of Surface Segregation. J. Catal. 1979, 57, 450–457. [Google Scholar] [CrossRef]
- Hollomon, J.H.; Turnbull, D. Nucleation. Prog. Met. Phys. 1953, 4, 333–388. [Google Scholar] [CrossRef]
- Thanh, N.T.K.; Maclean, N.; Mahiddine, S. Mechanisms of Nucleation and Growth of Nanoparticles in Solution. Chem. Rev. 2014, 114, 7610–7630. [Google Scholar] [CrossRef]
- Genève, D.; Rouxel, D.; Weber, B.; Confente, M. Segregation across the Metal/Oxide Interface Occurring during Oxidation at High Temperatures of Diluted Iron Based Alloys. Mater. Sci. Eng. A 2006, 435–436, 1–11. [Google Scholar] [CrossRef]
- Genève, D.; Rouxel, D.; Pigeat, P.; Weber, B.; Confente, M. Surface Composition Modification of High-Carbon Low-Alloy Steels Oxidized at High Temperature in Air. Appl. Surf. Sci. 2008, 254, 5348–5358. [Google Scholar] [CrossRef]
- Yoon, K.E.; Noebe, R.D.; Hellman, O.C.; Seidman, D.N. Dependence of Interfacial Excess on the Threshold Value of the Isoconcentration Surface. Surf. Interface Anal. 2004, 36, 594–597. [Google Scholar] [CrossRef]
- Baik, S.I.; Mao, Z.; Ren, Q.; Xue, F.; Campbell, C.E.; Zhang, C.; Zhou, B.; Noebe, R.D.; Seidman, D.N. The Effects of Diffusional Couplings on Compositional Trajectories and Interfacial Free Energies during Phase Separation in a Quaternary Ni-Al-Cr-Re Model Superalloy. Acta Mater. 2022, 234, 118020. [Google Scholar] [CrossRef]
- Jain, D.; Isheim, D.; Seidman, D.N. Carbon Redistribution and Carbide Precipitation in a High-Strength Low-Carbon HSLA-115 Steel Studied on a Nanoscale by Atom Probe Tomography. Metall. Mater. Trans. A Phys. Metall. Mater. Sci. 2017, 48, 3205–3219. [Google Scholar] [CrossRef]
- Miller, M.K.; Forbes, R.G. Atom-Probe Tomography; Springer: Boston, MA, USA, 2014; Volume 10, ISBN 978-1-4899-7429-7. [Google Scholar]
- Pourbahari, B.; McDermid, J.R. On the Interaction between Mn, Sb and O during the Annealing and Selective Oxidation of Fe-Mn-Sb Alloys. Scr. Mater. 2023, 223, 115096. [Google Scholar] [CrossRef]
- Yang, W.; Qu, P.; Zhang, R.; Qin, J.; Liu, C.; Zhang, J.; Liu, L. The Element Segregation Between γ/Γ′ Phases in a Ni-Based Single Crystal Superalloy Studied by 3D-APT and Its Potential Impact on Local Interfacial Misfit Strain. Met. Mater. Int. 2021, 27, 1892–1896. [Google Scholar] [CrossRef]
- Thermo-Calc. TCFE Steels/Fe-Alloys Database Version 12; 2021. Available online: https://web.archive.org/web/20230605065506/https://thermocalc.com/products/databases/steel-and-fe-alloys/ (accessed on 5 June 2023).
- Lea, C.; Seah, M.P. Kinetics of Surface Segregation. Philos. Mag. 1977, 35, 213–228. [Google Scholar] [CrossRef]
- Lejček, P.; Šandera, P.; Horníková, J.; Pokluda, J.; Godec, M. On the Segregation Behavior of Tin and Antimony at Grain Boundaries of Polycrystalline Bcc Iron. Appl. Surf. Sci. 2016, 363, 140–144. [Google Scholar] [CrossRef]
- Seah, M.P.; Lea, C. Surface Segregation and Its Relation to Grain Boundary Segregation. Philos. Mag. 1975, 31, 627–645. [Google Scholar] [CrossRef]
- Grabke, H.J.; Leroy, V.; Viefhaus, H. Segregation on the Surface of Steels in Heat Treatment and Oxidation. ISIJ Int. 1995, 35, 95–113. [Google Scholar] [CrossRef]
- Briant, C.L. On the Chemistry of Grain Boundary Segregation and Grain Boundary Fracture. Metall. Trans. A 1990, 21, 2339–2354. [Google Scholar] [CrossRef]
- Briant, C.L. Grain Boundary Structure, Chemistry, and Failure. Mater. Sci. Technol. 2001, 17, 1317–1323. [Google Scholar] [CrossRef]
- Hondros, E.D.; Seah, M.P. Grain Boundary Activity Measurements by Auger Electron Spectroscopy. Scr. Metall. 1972, 6, 1007–1012. [Google Scholar] [CrossRef]
- Seah, M.P.; Hondros, E.D. Use of a “BET” Analogue Equation to Describe Grain Boundary Segregation. Scr. Metall. 1973, 7, 735–737. [Google Scholar] [CrossRef]
- Hondros, E.D.; Seah, M.P. Segregation to Interfaces. Int. Met. Rev. 1977, 22, 262–301. [Google Scholar] [CrossRef]
- Guttmann, M. The Link between Equilibrium Segregation and Precipitation in Ternary Solutions Exhibiting Temper Embrittlement. Met. Sci. 1976, 10, 337–341. [Google Scholar] [CrossRef]
- Kučera, J.; Stránský, K. Diffusion in Iron, Iron Solid Solutions and Steels. Mater. Sci. Eng. 1982, 52, 1–38. [Google Scholar] [CrossRef]
- Kimura, K.; Iijima, Y.; Hirano, K. Diffusion of Tin in Gamma-Iron. Trans. Jpn. Inst. Met. 1986, 27, 1–4. [Google Scholar] [CrossRef]
- Treheux, D.; Marchive, D.; Delagrange, J.; Guiraldenq, P. Determinacion Des Coefficients d’heterodiffusion a Dilution Infinie de l’etain Dans Le Fer Alpha. C. R. Acad. Sc. Paris Ser. C 1972, 274, 1260–1262. [Google Scholar]
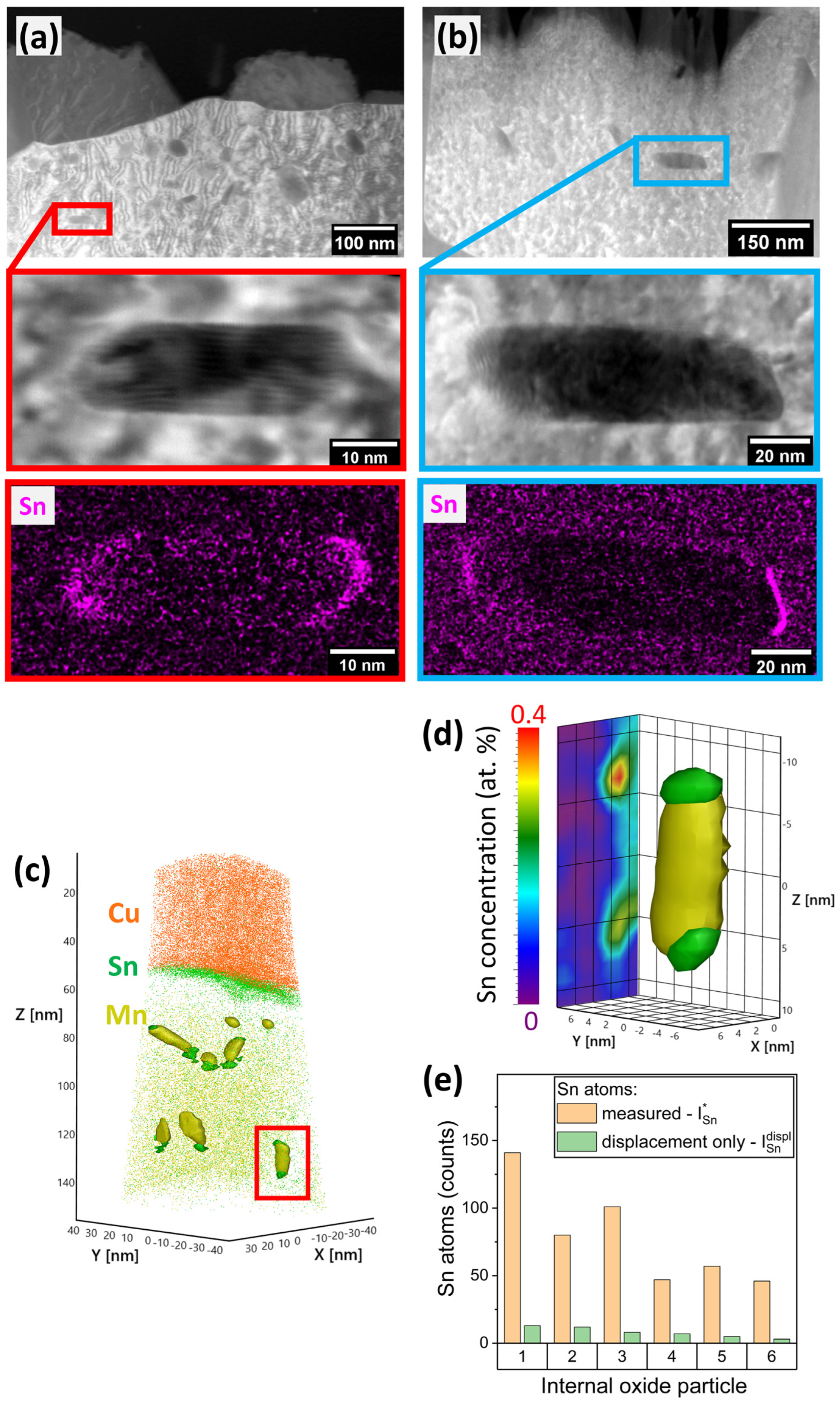
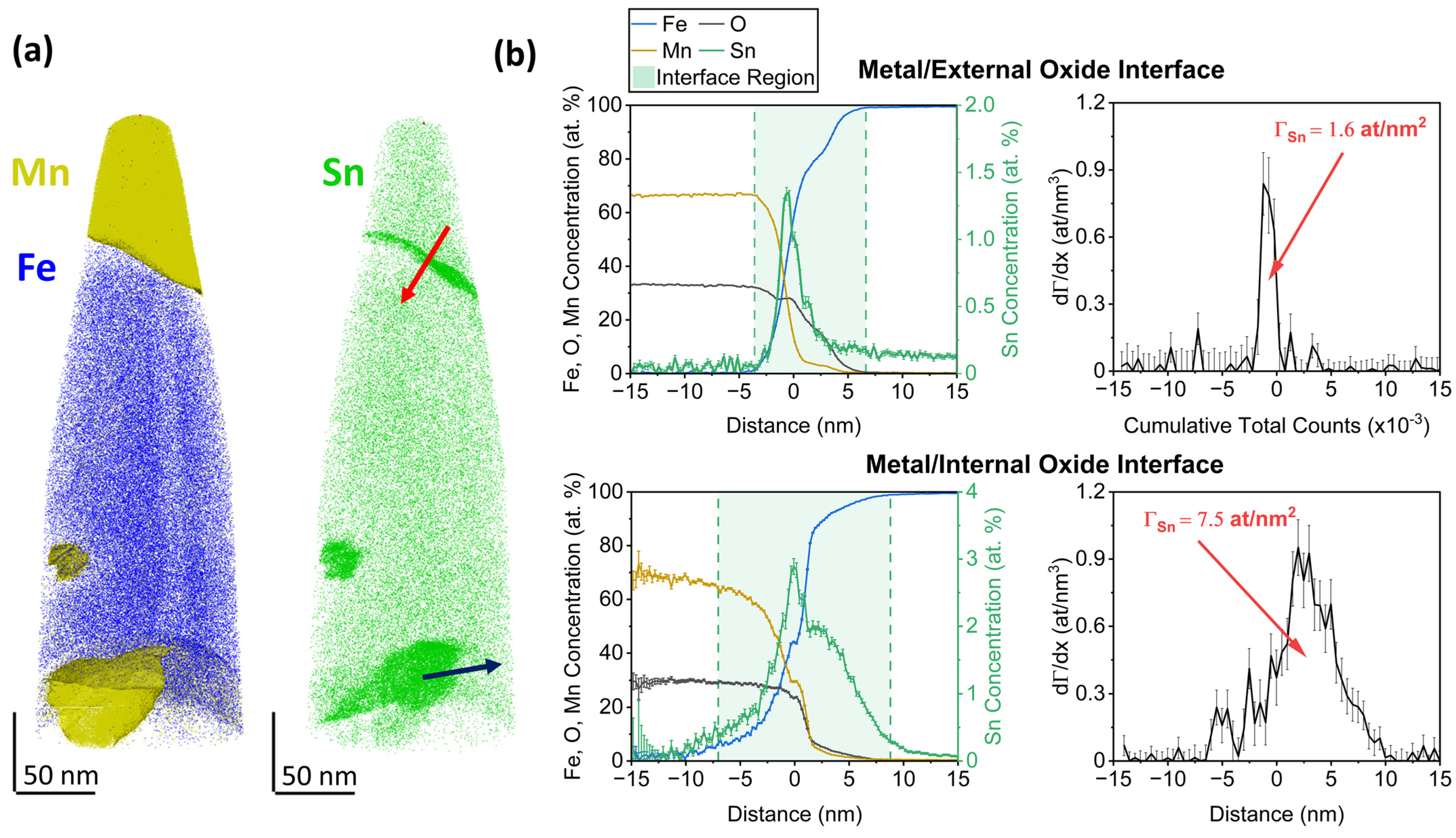



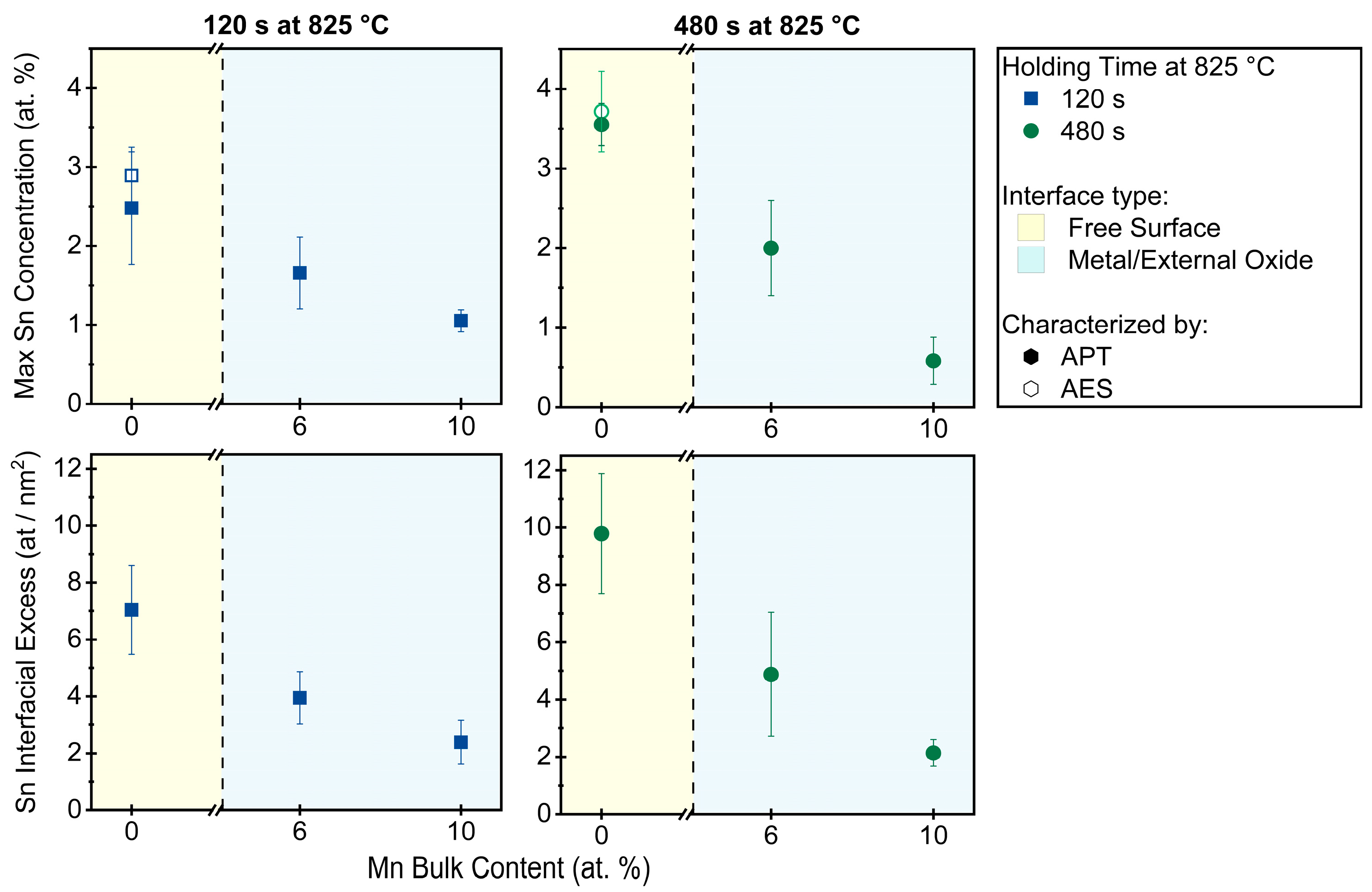
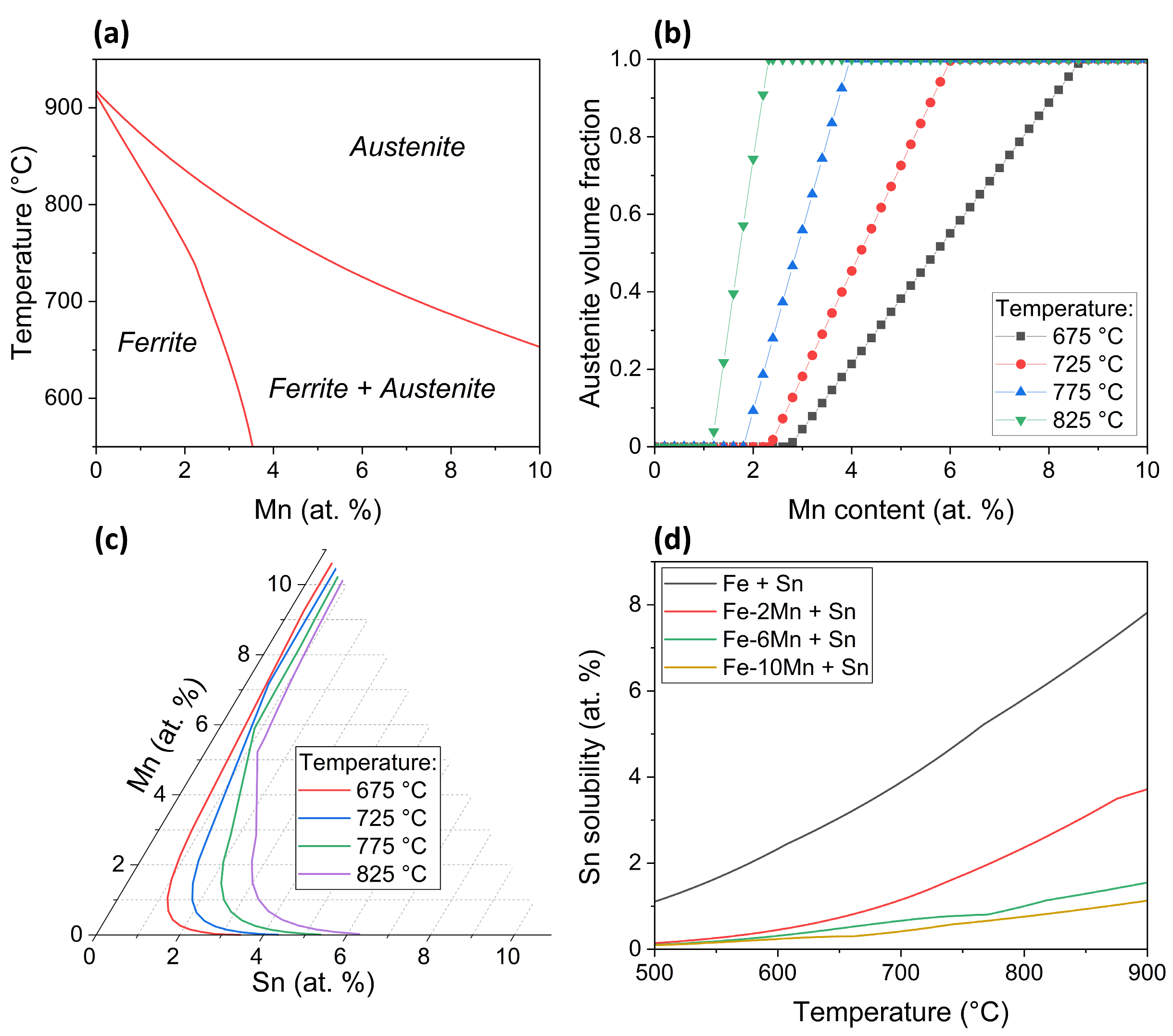
| Alloy System | Mn Concentration (at.%) | Sn Concentration (at.%) |
|---|---|---|
| Fe-0.03Sn | 0.01 | 0.034 |
| Fe-2Mn-0.03Sn | 1.97 | 0.035 |
| Fe-6Mn-0.03Sn | 6.05 | 0.034 |
| Fe-10Mn-0.03Sn | 9.85 | 0.034 |
| Peak Annealing Temperature—PAT (°C) | Oxygen Partial Pressure—pO2 (atm) | Isothermal Holding Times—t (s) | |
|---|---|---|---|
| Surface Segregation Quantified by AES | Interfacial Segregation Quantified by APT | ||
| 675 | 9.2 × 10−25 | 0, 60, 120, 240, 480 | 120 |
| 725 | 2.2 × 10−23 | 0, 60, 120, 240, 480 | |
| 775 | 3.7 × 10−22 | 0, 60, 120, 240, 480 | |
| 825 | 5.0 × 10−21 | 0, 60, 120, 240, 480 | 0, 120, 480 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wagner, J.; McDermid, J.R. Interfacial Segregation of Sn during the Continuous Annealing and Selective Oxidation of Fe-Mn-Sn Alloys. Materials 2024, 17, 1257. https://doi.org/10.3390/ma17061257
Wagner J, McDermid JR. Interfacial Segregation of Sn during the Continuous Annealing and Selective Oxidation of Fe-Mn-Sn Alloys. Materials. 2024; 17(6):1257. https://doi.org/10.3390/ma17061257
Chicago/Turabian StyleWagner, Jonas, and Joseph R. McDermid. 2024. "Interfacial Segregation of Sn during the Continuous Annealing and Selective Oxidation of Fe-Mn-Sn Alloys" Materials 17, no. 6: 1257. https://doi.org/10.3390/ma17061257





