Mechanical Behavior and Microstructure Evaluation of Quicklime-Activated Cement Kiln Dust-Slag Binder Pastes
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Preparation of the Specimens
2.3. Experimental Methods
2.3.1. Flowability and Setting Time
2.3.2. Unconfined Compression Test
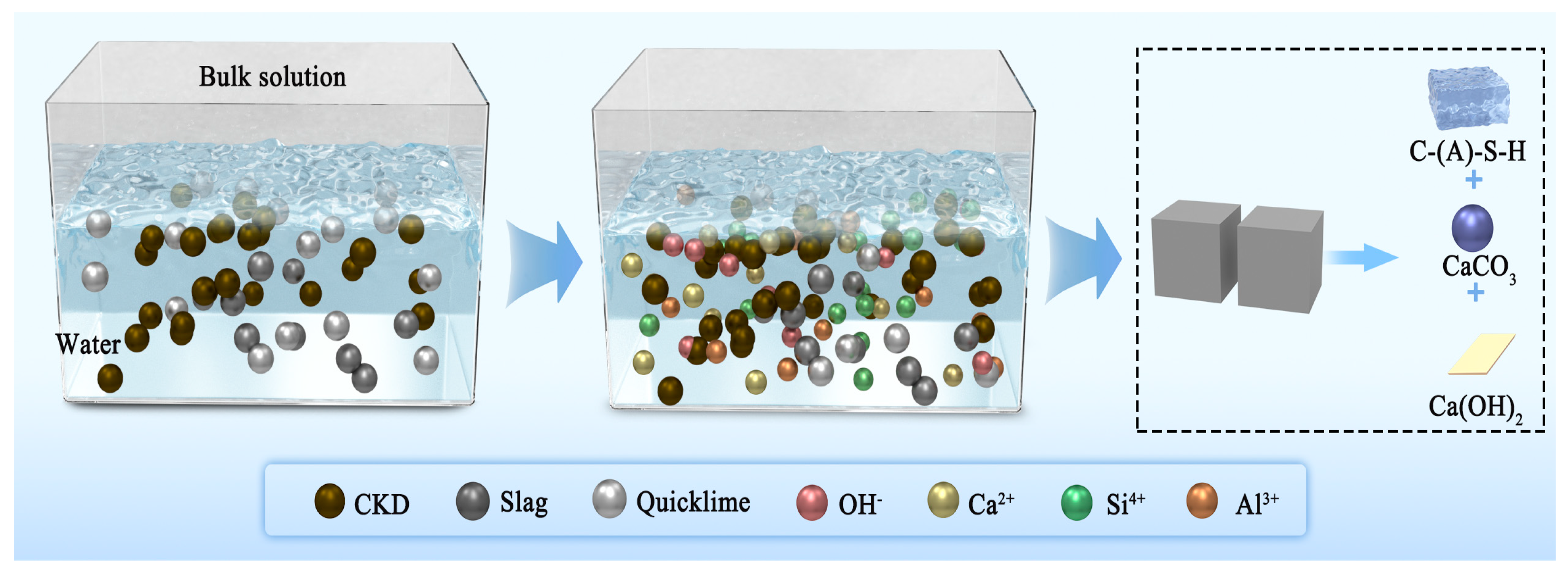
2.3.3. Hydration Product Tests
- The mineral composition of the CKD and binder pastes were analyzed using XRD (XRD instrument model: Rigaku Ultima IV and 1.5418 Å wavelength). The parameters set during the tests had a 0.02 °C step size with a test interval ranging from 10° to 80° and a scan speed of 2°/min.
- The mass changes of reaction products at various temperatures were measured using the TGA (TGA instrument model: TGA/DSC-1, Mettler Toledo, Greifensee, Switzerland) method. The heating temperatures were increased from 30 °C to 1000 °C at a rate of 10 °C/min, and the heating atmosphere was nitrogen. The DTG results were derived from the first derivative of the weight loss data.
- FTIR was used to obtain information regarding the molecular structure and chemical bonds of the materials, and the wavenumber range for sample collection was 400 cm−1 to 4000 cm−1 (FTIR instrument model: Nicolet 10, Thermo Fisher Scientific, Dreieich, Germany).
- The morphology of the hydration product was captured by SEM (SU-70, Hitachi High-tech Corporation, Tokyo, Japan) after 5000 times magnification, and their basic composition was studied by EDS (AZtec, Oxford instruments, Abingdon, UK).
3. Results
3.1. Flowability and Setting Time
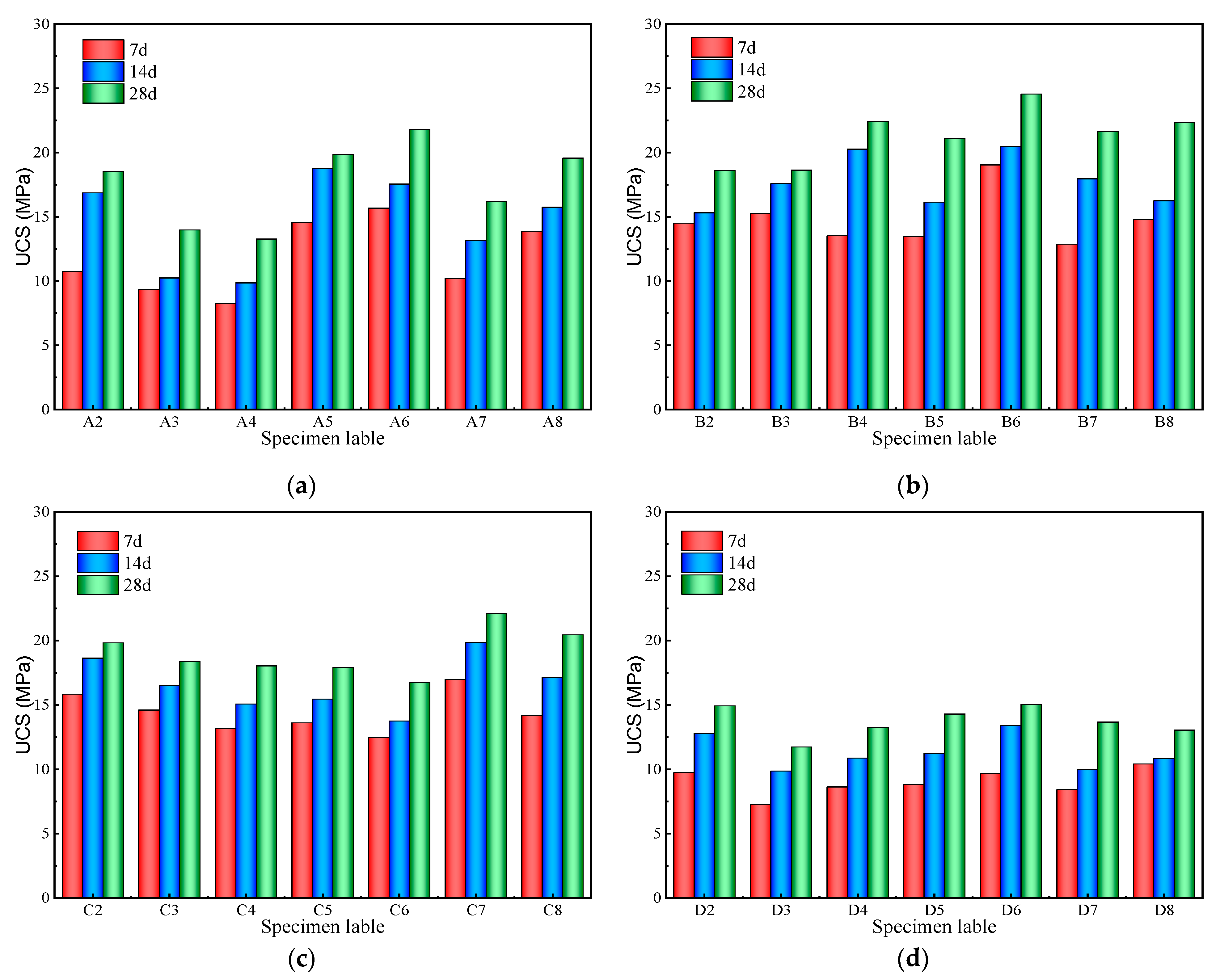
3.2. Unconfined Compressive Strength
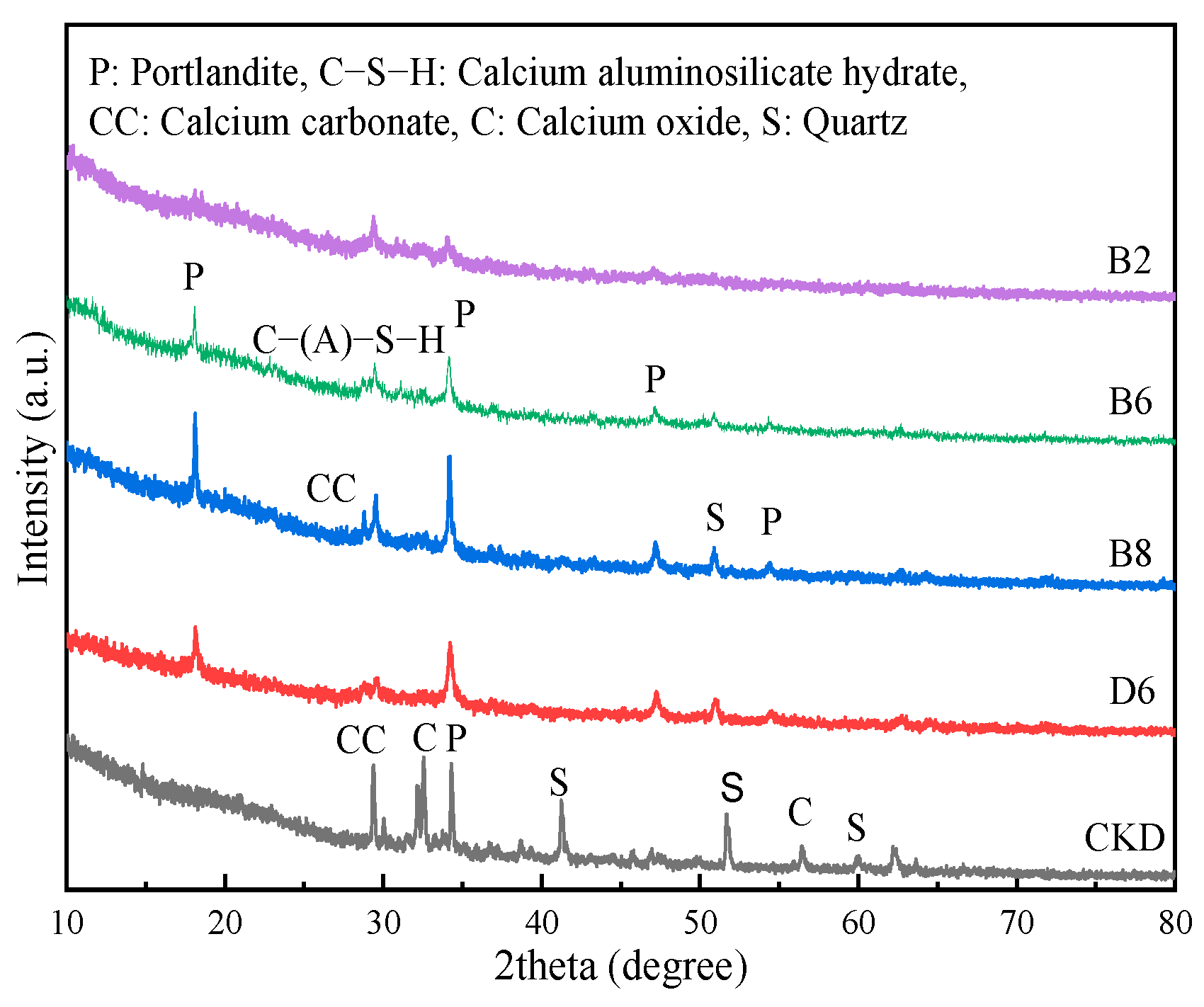
3.3. XRD Analysis
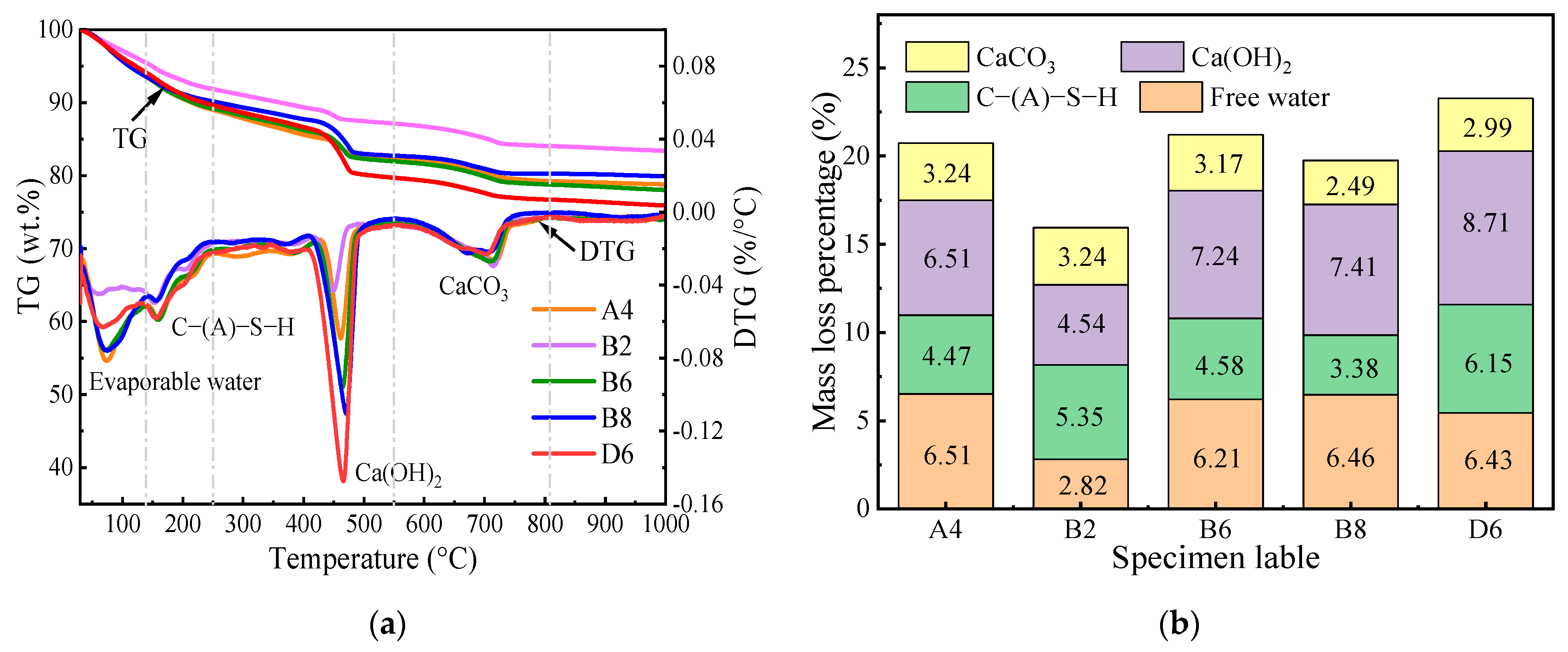
3.4. TG Analysis
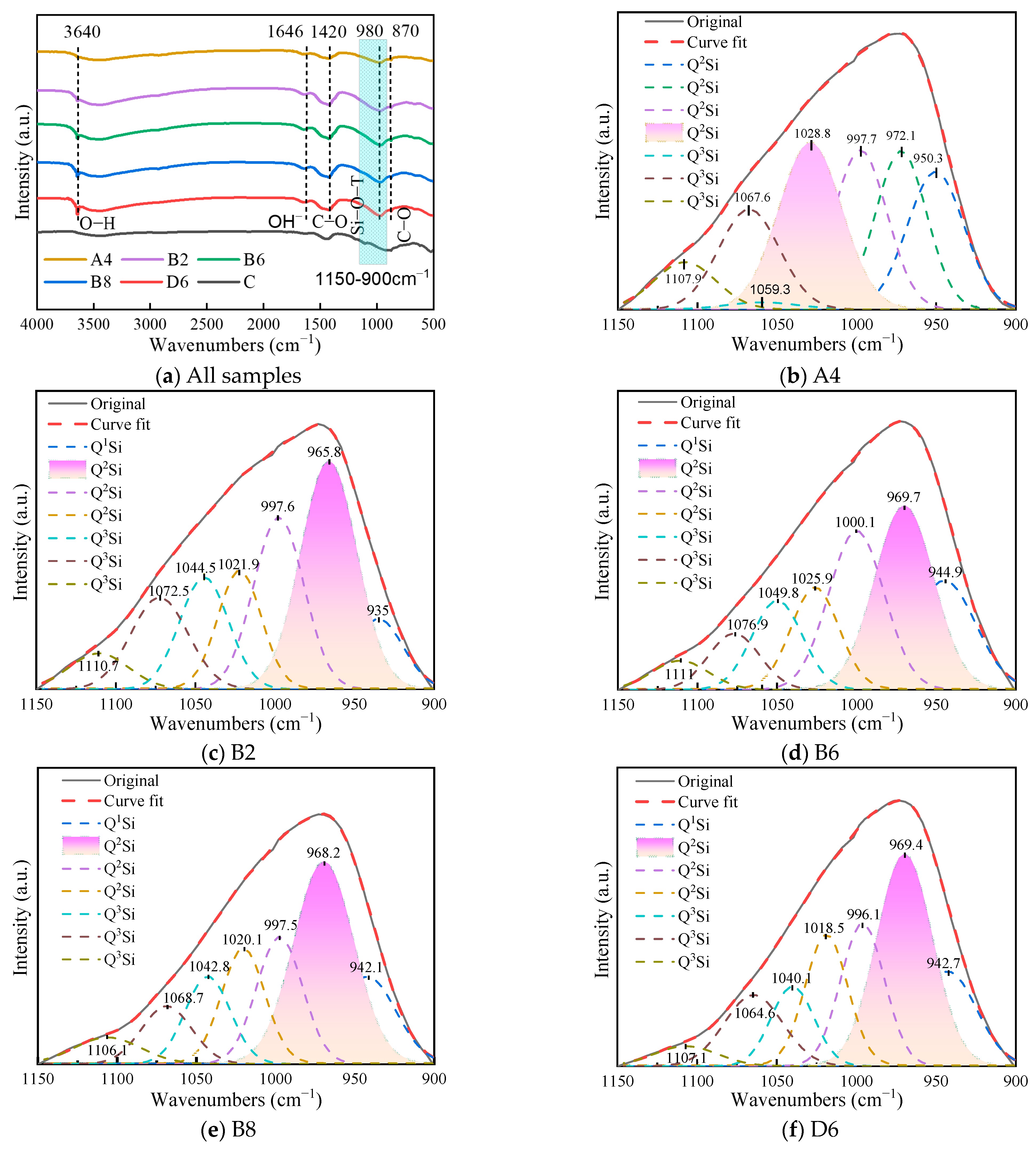
3.5. FTIR Analysis
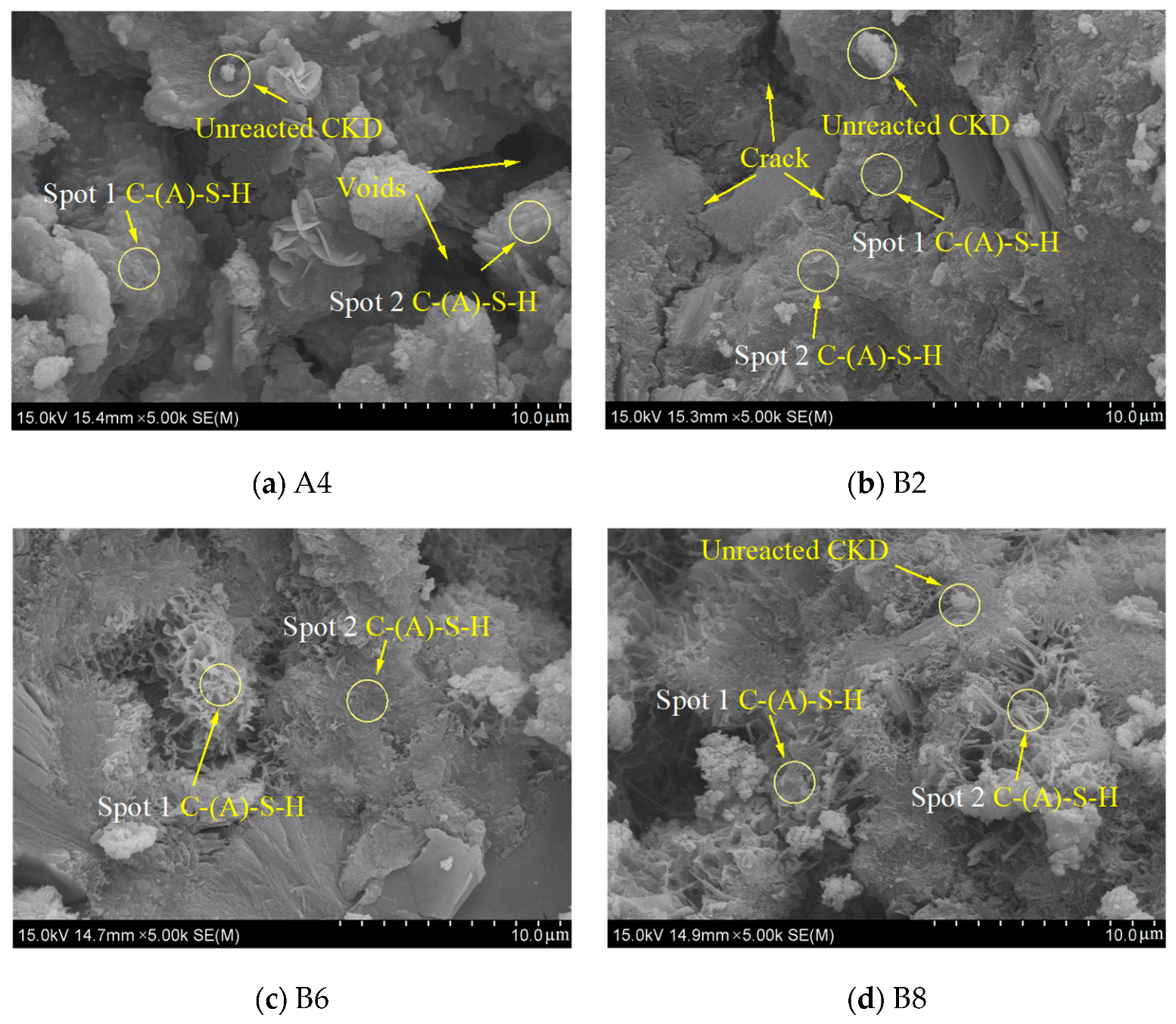
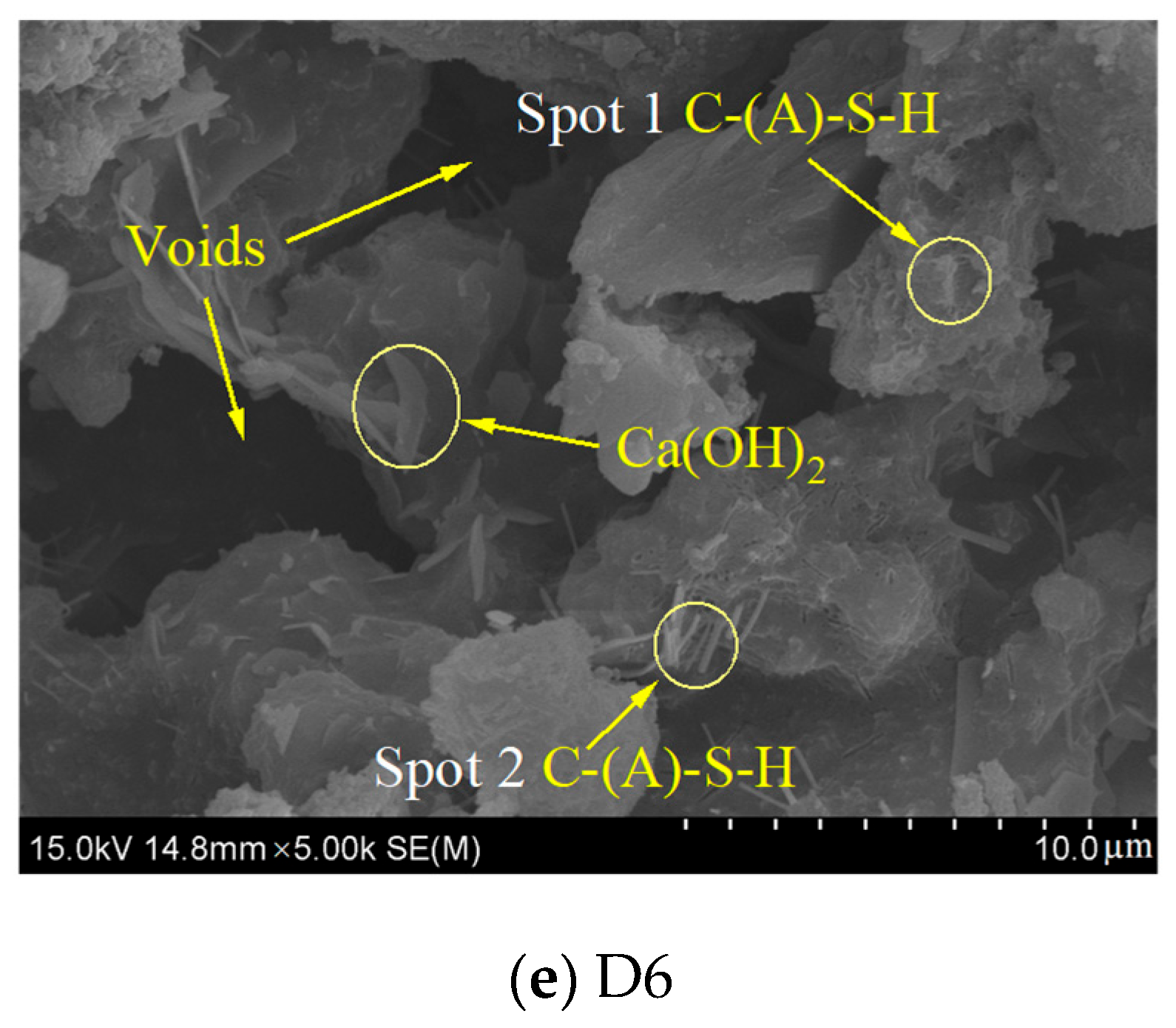
3.6. SEM-EDS
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Saleh, H.M.; El-Saied, F.A.; Salaheldin, T.A.; Hezo, A.A. Influence of Severe Climatic Variability on the Structural, Mechanical and Chemical Stability of Cement Kiln Dust-Slag-Nanosilica Composite Used for Radwaste Solidification. Constr. Build. Mater. 2019, 218, 556–567. [Google Scholar] [CrossRef]
- Bagheri, S.M.; Koushkbaghi, M.; Mohseni, E.; Koushkbaghi, S.; Tahmouresi, B. Evaluation of environment and economy viable recycling cement kiln dust for use in green concrete. J. Build. Eng. 2020, 32, 101809. [Google Scholar] [CrossRef]
- Mebarkia, R.; Bouzeroura, M.; Chelouah, N. Study of the Effect of Cement Kiln Dust on the Mechanical, Thermal and Durability Properties of Compressed Earth Blocks. Constr. Build. Mater. 2022, 349, 128707. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Heikal, M.; Mohammed, M.S.; Abd El-Aleem, S.; Hassan, H.S.; García, S.V.; Alomayri, T. Sustainable Disposal of Cement Kiln Dust in the Production of Cementitious Materials. J. Clean. Prod. 2019, 232, 1218–1229. [Google Scholar] [CrossRef]
- Alharthi, Y.M.; Elamary, A.S.; Abo-El-Wafa, W. Performance of Plain Concrete and Cement Blocks with Cement Partially Replaced by Cement Kiln Dust. Materials 2021, 14, 5647. [Google Scholar] [CrossRef]
- Abdalla, A.A.; Mohammed, A.S. Theoretical models to evaluate the effect of SiO2 and CaO contents on the long-term compressive strength of cement mortar modified with cement kiln dust (CKD). Arch. Civ. Mech. Eng. 2022, 22, 105. [Google Scholar] [CrossRef]
- Abd Ali, Z.T.; Naji, L.A.; Almuktar, S.A.; Faisal, A.A.H.; Abed, S.N.; Scholz, M.; Naushad, M.; Ahamad, T. Predominant mechanisms for the removal of nickel metal ion from aqueous solution using cement kiln dust. J. Water Process. Eng. 2020, 33, 101033. [Google Scholar] [CrossRef]
- Chaunsali, P.; Peethamparan, S. Influence of the Composition of Cement Kiln Dust on Its Interaction with Fly Ash and Slag. Cem. Concr. Res. 2013, 54, 106–113. [Google Scholar] [CrossRef]
- Kaliyavaradhan, S.K.; Ling, T.C.; Mo, K.H. Valorization of Waste Powders from Cement-Concrete Life Cycle: A Pathway to Circular Future. J. Clean. Prod. 2020, 268, 122358. [Google Scholar] [CrossRef]
- Omrani, M.A.; Modarres, A. Emulsified Cold Recycled Mixtures Using Cement Kiln Dust and Coal Waste Ash-Mechanical-Environmental Impacts. J. Clean. Prod. 2018, 199, 101–111. [Google Scholar] [CrossRef]
- Kunal; Siddique, R.; Rajor, A. Use of Cement Kiln Dust in Cement Concrete and Its Leachate Characteristics. Resour. Conserv. Recycl. 2012, 61, 59–68. [Google Scholar] [CrossRef]
- Maslehuddin, M.; Al-Amoudi, O.; Shameem, M.; Rehman, M.; Ibrahim, M. Usage of Cement Kiln Dust in Cement Products–Research Review and Preliminary Investigations. Constr. Build. Mater. 2008, 22, 2369–2375. [Google Scholar] [CrossRef]
- El-Attar, M.M.; Sadek, D.M.; Salah, A.M. Recycling of High Volumes of Cement Kiln Dust in Bricks Industry. J. Clean. Prod. 2017, 143, 506–515. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Khalil, K.A. Application of Thermal Treatment on Cement Kiln Dust and Feldspar to Create One-Part Geopolymer Cement. Constr. Build. Mater. 2018, 187, 231–237. [Google Scholar] [CrossRef]
- Ahmari, S.; Zhang, L. Utilization of Cement Kiln Dust (Ckd) to Enhance Mine Tailings-Based Geopolymer Bricks. Constr. Build. Mater. 2013, 40, 1002–1011. [Google Scholar] [CrossRef]
- Yaseri, S.; Verki, V.M.; Mahdikhani, M. Utilization of High Volume Cement Kiln Dust and Rice Husk Ash in the Production of Sustainable Geopolymer. J. Clean. Prod. 2019, 230, 592–602. [Google Scholar] [CrossRef]
- Provis, J.L.; Palomo, A.; Shi, C. Advances in Understanding Alkali-Activated Materials. Cem. Concr. Res. 2015, 78, 110–125. [Google Scholar] [CrossRef]
- Li, Z.; Liang, X.; Liu, C.; Liang, M.; van Breugel, K.; Ye, G. Thermal Deformation and Stress of Alkali-Activated Slag Concrete under Semi-Adiabatic Condition: Experiments and Simulations. Cem. Concr. Res. 2022, 159, 106887. [Google Scholar] [CrossRef]
- Walkley, B.; Ke, X.; Provis, J.L.; Bernal, S.A. Activator Anion Influences the Nanostructure of Alkali-Activated Slag Cements. J. Phys. Chem. C 2021, 125, 20727–20739. [Google Scholar] [CrossRef]
- Yi, Y.; Li, C.; Liu, S. Alkali-Activated Ground-Granulated Blast Furnace Slag for Stabilization of Marine Soft Clay. J. Mater. Civ. Eng. 2015, 27, 04014146. [Google Scholar] [CrossRef]
- Rafeet, A.; Vinai, R.; Soutsos, M.; Sha, W. Guidelines for Mix Proportioning of Fly Ash/Ggbs Based Alkali Activated Concretes. Constr. Build. Mater. 2017, 147, 130–142. [Google Scholar] [CrossRef]
- Gökçe, H.; Tuyan, M.; Nehdi, M. Alkali-Activated and Geopolymer Materials Developed Using Innovative Manufacturing Techniques: A Critical Review. Constr. Build. Mater. 2021, 303, 124483. [Google Scholar] [CrossRef]
- Cai, G.H.; Zhou, Y.F.; Li, J.S.; Han, L.J.; Poon, C.S. Deep Insight into Mechanical Behavior and Microstructure Mechanism of Quicklime-Activated Ground Granulated Blast-Furnace Slag Pastes. Cem. Concr. Compos. 2022, 134, 104767. [Google Scholar] [CrossRef]
- Chaunsali, P.; Peethamparan, S. Evolution of Strength, Microstructure and Mineralogical Composition of a Ckd–Ggbfs Binder. Cem. Concr. Res. 2011, 41, 197–208. [Google Scholar] [CrossRef]
- Chaunsali, P.; Peethamparan, S. Novel Cementitious Binder Incorporating Cement Kiln Dust: Strength and Durability. ACI Mater. J. 2013, 110, 297–304. [Google Scholar] [CrossRef]
- Abdel-Gawwad, H.A.; Sanad, S.A.; Mohammed, M.S. A clean approach through sustainable utilization of cement kiln dust, hazardous lead-bearing, and sewage sludges in the production of lightweight bricks. J. Clean. Prod. 2020, 273, 123129. [Google Scholar] [CrossRef]
- Ibrahim, M.; Rahman, M.K.; Najamuddin, S.K.; Alhelal, Z.S.; Acero, C.E. A Review on Utilization of Industrial by-Products in the Production of Controlled Low Strength Materials and Factors Influencing the Properties. Constr. Build. Mater. 2022, 325, 126704. [Google Scholar] [CrossRef]
- Wang, J.; Lyu, X.; Wang, L.; Cao, X.; Liu, Q.; Zang, H. Influence of the Combination of Calcium Oxide and Sodium Carbonate on the Hydration Reactivity of Alkali-Activated Slag Binders. J. Clean. Prod. 2018, 171, 622–629. [Google Scholar] [CrossRef]
- Lachemi, M.; Şahmaran, M.; Hossain, K.M.A.; Lotfy, A.; Shehata, M. Properties of Controlled Low-Strength Materials Incorporating Cement Kiln Dust and Slag. Cem. Concr. Compos. 2010, 32, 623–629. [Google Scholar] [CrossRef]
- GB/T8077-2012; Methods for Testing Uniformity of Concrete Admixture. China Standards Press: Beijing, China, 2012.
- GB/T 1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. China Standards Press: Beijing, China, 2011.
- ASTM D4219-08; Standard Test Method for Unconfined Compressive Strength Index of Chemical-Grouted Soils. ASTM International: West Conshohocken, PA, USA, 2008.
- Liu, X.; Gao, P.; Han, Y. Resource Utilization of Slag from Desulphurization and Slag Skimming: A Comprehensive Recycling Process of All Components. Int. J. Min. Sci. Technol. 2022, 32, 585–593. [Google Scholar] [CrossRef]
- Hojati, M.; Radlińska, A. Shrinkage and Strength Development of Alkali-Activated Fly Ash-Slag Binary Cements. Constr. Build. Mater. 2017, 150, 808–816. [Google Scholar] [CrossRef]
- Yusuf, M.O.; Johari, M.A.M.; Ahmad, Z.A.; Maslehuddin, M. Effects of Addition of Al(OH)3 on the Strength of Alkaline Activated Ground Blast Furnace Slag-Ultrafine Palm Oil Fuel Ash (Aagu) Based Binder. Constr. Build. Mater. 2014, 50, 361–367. [Google Scholar] [CrossRef]
- Heikal, M.; Zaki, M.; Alshammari, A. Preparation and Characterization of an Eco-Friendly Binder from Alkali-Activated Aluminosilicate Solid Industrial Wastes Containing Ckd and Ggbs. J. Mater. Civ. Eng. 2018, 30, 04018093. [Google Scholar] [CrossRef]
- Qin, L.; Gao, X.; Li, Q. Upcycling Carbon Dioxide to Improve Mechanical Strength of Portland Cement. J. Clean. Prod. 2018, 196, 726–738. [Google Scholar] [CrossRef]
- Zhou, Y.F.; Li, J.S.; Lu, J.X.; Cheeseman, C.; Poon, C.S. Sewage Sludge Ash: A Comparative Evaluation with Fly Ash for Potential Use as Lime-Pozzolan Binders. Constr. Build. Mater. 2020, 242, 118160. [Google Scholar] [CrossRef]
- Shen, X.; Feng, P.; Zhang, Q.; Lu, J.; Liu, X.; Ma, Y.; Jin, P.; Wang, W.; Ran, Q.; Hong, J. Toward the Formation Mechanism of Synthetic Calcium Silicate Hydrate (C-S-H)-Ph and Kinetic Considerations. Cem. Concr. Res. 2023, 172, 107248. [Google Scholar] [CrossRef]
- Nguyen, H.A.; Chang, T.P.; Shih, J.Y.; Chen, C.T. Influence of Low Calcium Fly Ash on Compressive Strength and Hydration Product of Low Energy Super Sulfated Cement Paste. Cem. Concr. Compos. 2019, 99, 40–48. [Google Scholar] [CrossRef]
- Chen, Z.; Li, J.S.; Zhan, B.J.; Sharma, U.; Poon, C.S. Compressive Strength and Microstructural Properties of Dry-Mixed Geopolymer Pastes Synthesized from Ggbs and Sewage Sludge Ash. Constr. Build. Mater. 2018, 182, 597–607. [Google Scholar] [CrossRef]
- Zhao, X.; Liu, C.; Zuo, L.; Wang, L.; Zhu, Q.; Wang, M. Investigation into the Effect of Calcium on the Existence Form of Geopolymerized Gel Product of Fly Ash Based Geopolymers. Cem. Concr. Compos. 2019, 103, 279–292. [Google Scholar] [CrossRef]
- Yu, J.; Qian, J.; Wang, F.; Li, Z.; Jia, X. Preparation and Properties of a Magnesium Phosphate Cement with Dolomite. Cem. Concr. Res. 2020, 138, 106235. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Zhang, Z. Effect of Na2O Concentration and Water/Binder Ratio on Carbonation of Alkali-Activated Slag/Fly Ash Cements. Constr. Build. Mater. 2021, 269, 121258. [Google Scholar] [CrossRef]
- Su, L.; Fu, G.; Liang, B.; Sun, Q.; Zhang, X. Mechanical Properties and Microstructure Evaluation of Fly Ash-Slag Geopolymer Foaming Materials. Ceram. Int. 2022, 48, 18224–18237. [Google Scholar] [CrossRef]
- Akatov, A.A.; Nikonov, B.S.; Omel’yanenko, B.I.; Stefanovsky, S.V.; Marra, J.C. Structure of Borosilicate Glassy Materials with High Concentrations of Sodium, Iron, and Aluminum Oxides. Glass Phys. Chem. 2009, 35, 245–259. [Google Scholar] [CrossRef]
- Li, H.; Zhang, S.; Wang, K.; Zhang, X.; Jiang, Y. Effect of Co2 Capture on the Performance of Cao-Activated Slag Pastes and Their Acid Resistance. Constr. Build. Mater. 2023, 365, 130039. [Google Scholar] [CrossRef]
- Bernard, E.; Yan, Y.; Lothenbach, B. Effective Cation Exchange Capacity of Calcium Silicate Hydrates (C-S-H). Cem. Concr. Res. 2021, 143, 106393. [Google Scholar] [CrossRef]
- Cuesta, A.; Santacruz, I.; Angeles, G.; Dapiaggi, M.; Zea-Garcia, J.D.; Aranda, M.A. Local Structure and Ca/Si Ratio in Csh Gels from Hydration of Blends of Tricalcium Silicate and Silica Fume. Cem. Concr. Res. 2021, 143, 106405. [Google Scholar] [CrossRef]
- Manzano, H.; Dolado, J.; Guerrero, A.; Ayuela, A. Mechanical Properties of Crystalline Calcium-Silicate-Hydrates: Comparison with Cementitious C-S-H Gels. Phys. Status Solidi (A) 2007, 204, 1775–1780. [Google Scholar] [CrossRef]
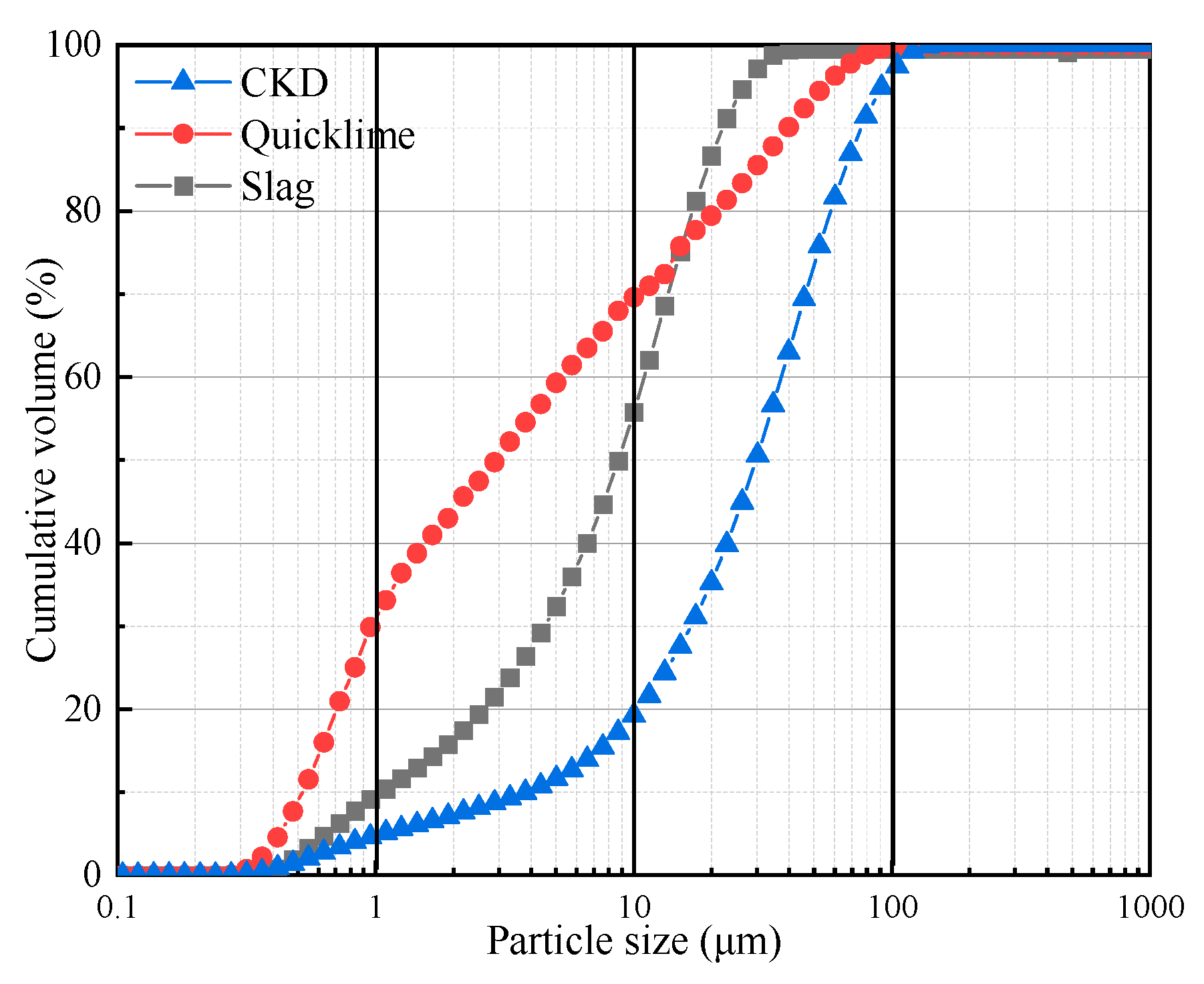
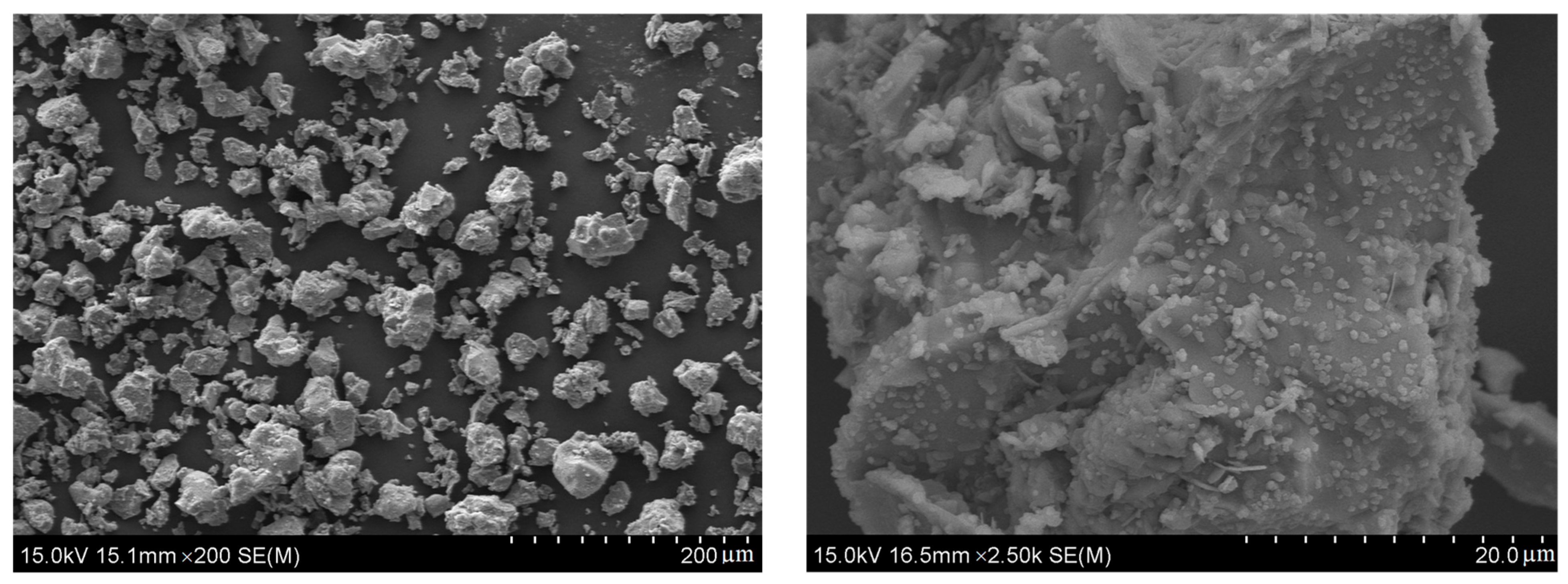
| Element | CKD | Slag | Quicklime |
|---|---|---|---|
| Dv(10) 1 | 4.37 | 1.14 | 0.60 |
| Dv(50) | 34.20 | 9.89 | 1.98 |
| Dv(90) | 87.11 | 24.89 | 45.40 |
| D(3,2) 2 | 7.34 | 3.46 | 1.48 |
| D(4,3) 3 | 40.98 | 11.68 | 13.08 |
| Chemical Composition | CaO | SiO2 | Al2O3 | MgO | Fe2O3 | K2O | Na2O | SO3 | LOI |
|---|---|---|---|---|---|---|---|---|---|
| CKD | 83.20 | 6.21 | 0.61 | 0.13 | 6.36 | 1.20 | 0.14 | 0.95 | 23.65 |
| Slag | 68.22 | 12.61 | 5.95 | 3.85 | 2.05 | 0.62 | 0.21 | 0.80 | 1.32 |
| Quicklime | 98.21 | 0.18 | 0.10 | 1.03 | 0.40 | - | - | 0.08 | 8.37 |
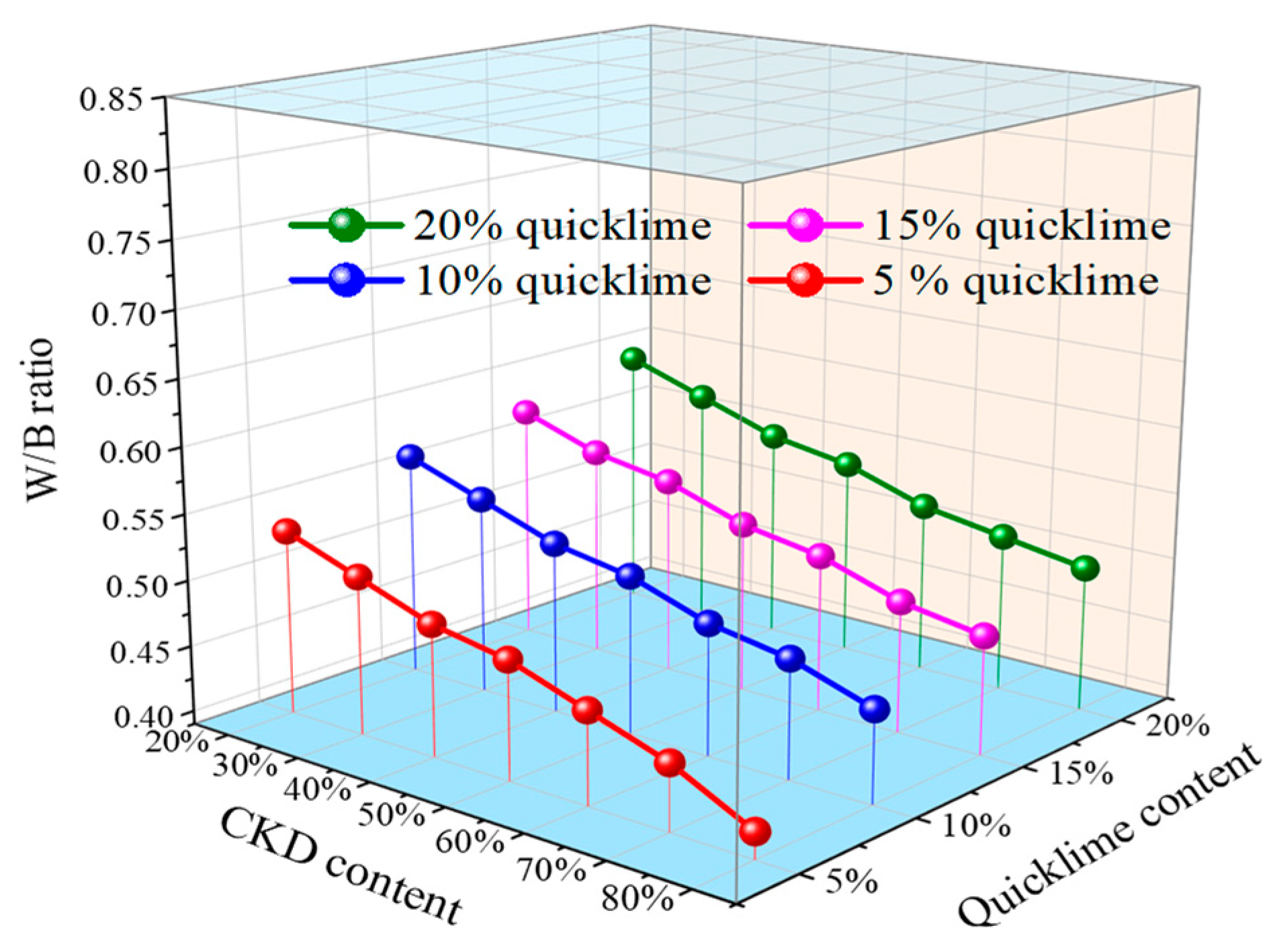
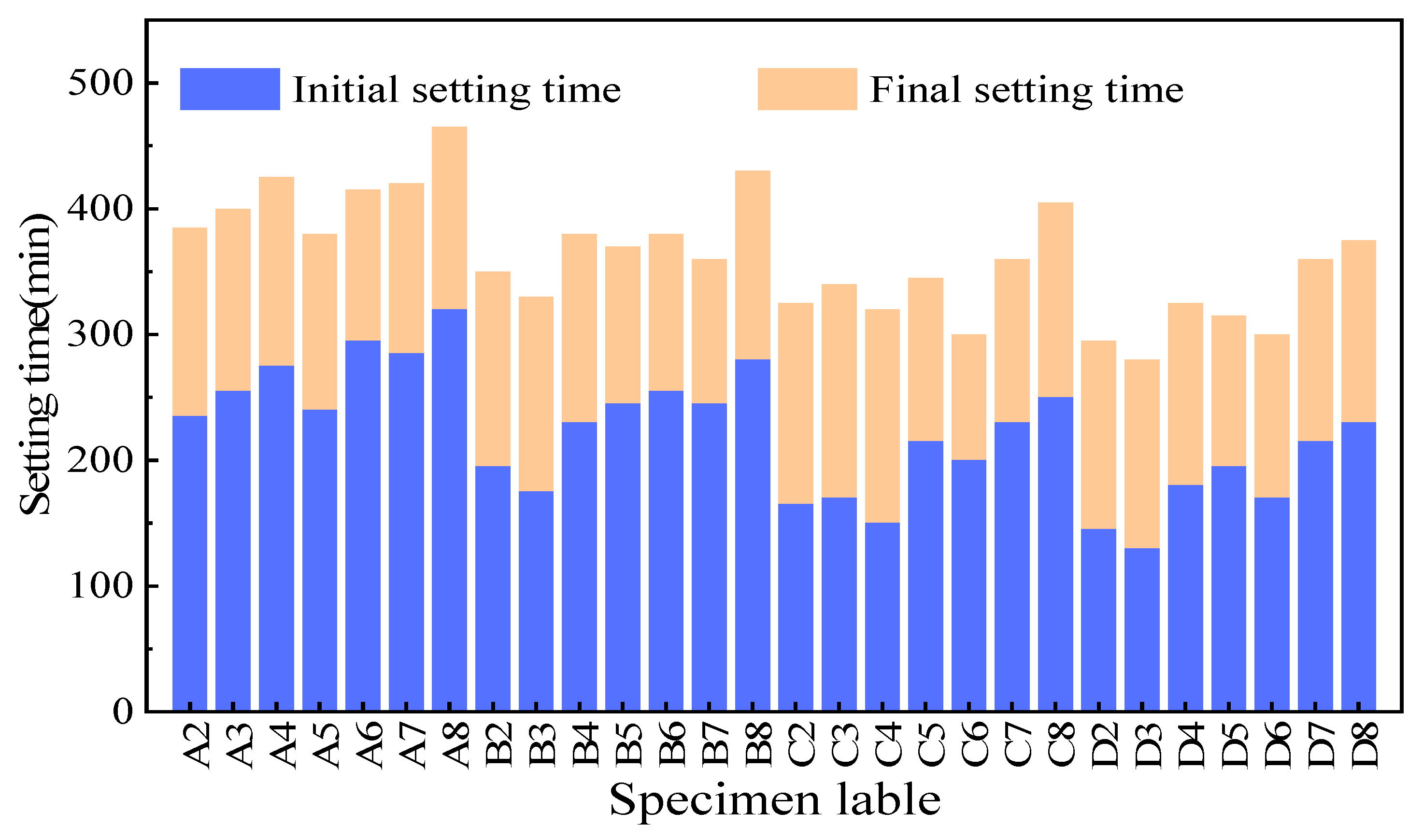
| ID | Quicklime (%) | CKD (%) | Slag (%) | W/B Ratio | Flow (mm) | Initial Setting Time (min) | Final Setting Time (min) |
|---|---|---|---|---|---|---|---|
| A2 | 5 | 19 | 76 | 0.53 | 203 | 235 | 385 |
| A3 | 5 | 29 | 66 | 0.51 | 200 | 255 | 400 |
| A4 | 5 | 38 | 57 | 0.49 | 198 | 275 | 425 |
| A5 | 5 | 48 | 47 | 0.48 | 201 | 240 | 380 |
| A6 | 5 | 57 | 38 | 0.46 | 206 | 295 | 415 |
| A7 | 5 | 67 | 28 | 0.44 | 200 | 285 | 420 |
| A8 | 5 | 76 | 19 | 0.41 | 197 | 320 | 465 |
| B2 | 10 | 18 | 72 | 0.56 | 201 | 195 | 350 |
| B3 | 10 | 27 | 63 | 0.54 | 203 | 175 | 330 |
| B4 | 10 | 36 | 54 | 0.52 | 198 | 230 | 380 |
| B5 | 10 | 45 | 45 | 0.51 | 200 | 245 | 370 |
| B6 | 10 | 54 | 36 | 0.49 | 202 | 255 | 380 |
| B7 | 10 | 63 | 27 | 0.48 | 199 | 245 | 360 |
| B8 | 10 | 72 | 18 | 0.46 | 203 | 280 | 430 |
| C2 | 15 | 17 | 68 | 0.57 | 204 | 165 | 325 |
| C3 | 15 | 26 | 59 | 0.55 | 202 | 170 | 340 |
| C4 | 15 | 34 | 51 | 0.54 | 200 | 150 | 320 |
| C5 | 15 | 43 | 42 | 0.52 | 198 | 215 | 345 |
| C6 | 15 | 51 | 34 | 0.51 | 201 | 200 | 300 |
| C7 | 15 | 60 | 25 | 0.49 | 203 | 230 | 360 |
| C8 | 15 | 68 | 17 | 0.48 | 200 | 250 | 405 |
| D2 | 20 | 16 | 64 | 0.59 | 200 | 145 | 295 |
| D3 | 20 | 24 | 56 | 0.57 | 202 | 130 | 280 |
| D4 | 20 | 32 | 48 | 0.55 | 199 | 180 | 325 |
| D5 | 20 | 40 | 40 | 0.54 | 201 | 195 | 315 |
| D6 | 20 | 48 | 32 | 0.52 | 203 | 170 | 300 |
| D7 | 20 | 56 | 24 | 0.51 | 201 | 215 | 360 |
| D8 | 20 | 64 | 16 | 0.50 | 202 | 230 | 375 |
| Wavenumber (cm−1) | Forms | Characteristic |
|---|---|---|
| 840–900 | Q0Si | Four non-bridge oxygen in [SiO4] |
| 900–950 | Q1Si | Three non-bridge oxygen in [SiO4] |
| 950–1030 | Q2Si | Two non-bridge oxygen in [SiO4] |
| 1030–1120 | Q3Si | One non bridge oxygen in [SiO4] |
| 1120–1300 | Q4Si | Completely polymerized [SiO4] |
| ID | Region | Elements (Atomic (%)) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Ca | Si | Al | Na | S | Ca/Si | Ca/Al | Si/Al | ||
| A4 | Spot 1 | 35.00 | 4.40 | 1.76 | 0.00 | 0.00 | 7.95 | 19.89 | 2.50 |
| Spot 2 | 31.26 | 13.7 | 4.14 | 1.24 | 0.00 | 2.28 | 7.55 | 3.31 | |
| B2 | Spot 1 | 17.23 | 10.15 | 4.59 | 1.69 | 1.76 | 1.70 | 3.75 | 2.21 |
| Spot 2 | 16.04 | 9.67 | 6.05 | 1.9 | 1.16 | 1.66 | 2.65 | 1.60 | |
| B6 | Spot 1 | 19.84 | 14.33 | 3.98 | 0.94 | 1.01 | 1.38 | 4.98 | 3.60 |
| Spot 2 | 16.14 | 10.21 | 4.95 | 0.96 | 0.98 | 1.58 | 3.26 | 2.06 | |
| B8 | Spot 1 | 32.65 | 13.11 | 0.65 | 0.00 | 0.88 | 2.49 | 50.23 | 20.17 |
| Spot 2 | 18.37 | 9.06 | 1.47 | 0.89 | 1.53 | 2.03 | 12.50 | 6.16 | |
| D6 | Spot 1 | 34.74 | 2.15 | 0.00 | 0.00 | 0.74 | 16.16 | 0.00 | 0.00 |
| Spot 2 | 40.57 | 2.72 | 1.94 | 0.00 | 0.65 | 14.92 | 20.91 | 1.40 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, M.; Dong, T.; Cui, Z.; Li, Z. Mechanical Behavior and Microstructure Evaluation of Quicklime-Activated Cement Kiln Dust-Slag Binder Pastes. Materials 2024, 17, 1253. https://doi.org/10.3390/ma17061253
Hu M, Dong T, Cui Z, Li Z. Mechanical Behavior and Microstructure Evaluation of Quicklime-Activated Cement Kiln Dust-Slag Binder Pastes. Materials. 2024; 17(6):1253. https://doi.org/10.3390/ma17061253
Chicago/Turabian StyleHu, Minhui, Tianwen Dong, Zhenglong Cui, and Zhuo Li. 2024. "Mechanical Behavior and Microstructure Evaluation of Quicklime-Activated Cement Kiln Dust-Slag Binder Pastes" Materials 17, no. 6: 1253. https://doi.org/10.3390/ma17061253





