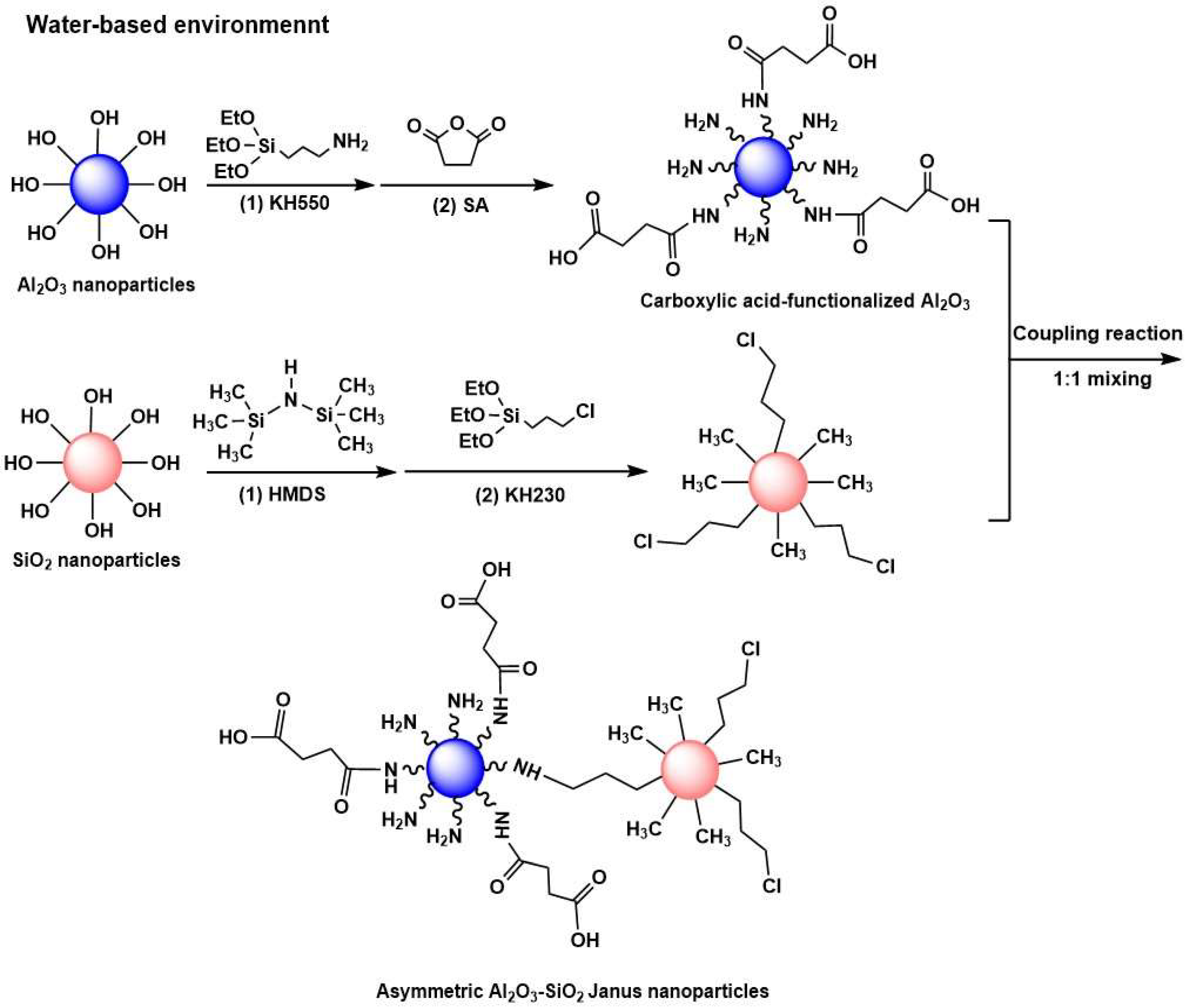
3.2. Carboxylic Acid-Functionalized Al2O3 Nanoparticles
The solid samples for FTIR analysis were obtained by drying the modified Al
2O
3 hydrosol at 100 °C and washing it thoroughly with ethanol and water to remove unreacted SA.
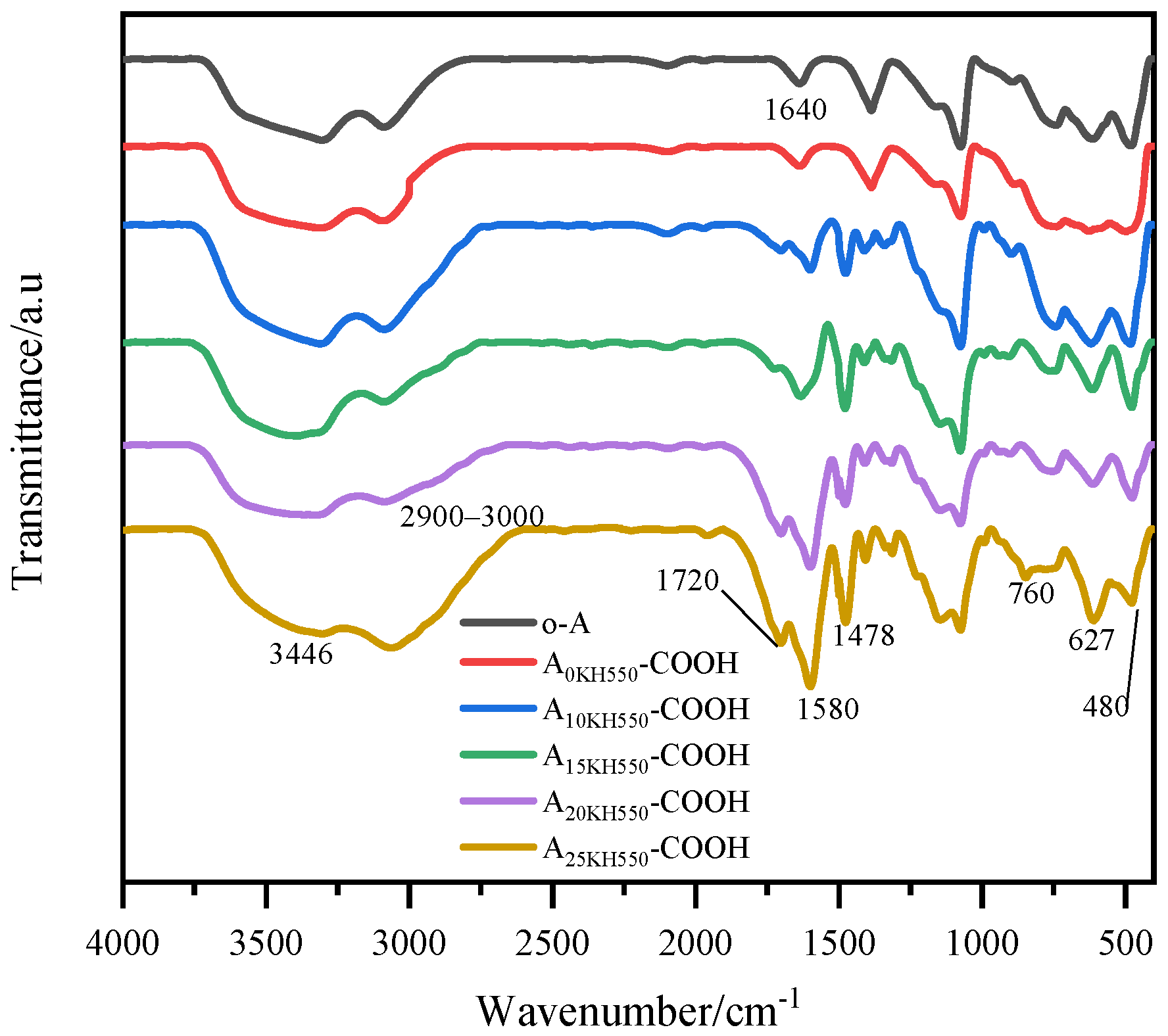
Figure 2 exhibits the FTIR spectra of carboxylic acid-functionalized Al
2O
3 nanoparticles in the range 400–4000 cm
−1. The peaks at 3446 and 1640 cm
−1 were attributed to the stretching and bending vibrations of the hydroxyl group, respectively, while the peaks at 760, 627, and 480 cm
−1 were caused by the vibration of the Al-O bonds of Al
2O
3 nanoparticles. After modification, the peak at about 2900–3000 cm
−1 can be ascribed to the absorption of −CH
2−, which corresponds to the −CH
2− groups of KH550 and SA. A new absorption peak of carboxylic acid-functionalized Al
2O
3 nanoparticles appeared at 1720 cm
−1 corresponding to O=C-OH [
31,
32]. Furthermore, the peak at 1580 cm
−1 was attributed to the vibration of the amide bond, suggesting that the conjugation of amino-functionalized Al
2O
3 nanoparticles with SA was achieved through a ring opening linker elongation reaction of the amine functions with SA. In addition, a control experiment was conducted under the same conditions by mixing the Al
2O
3 hydrosol with SA (A
0KH550-COOH), but without KH550. As shown in
Figure 2, the absorption peaks of carboxyl and amide were not observed in the FTIR of A
0KH550-COOH, indicating that the reaction between SA and Al
2O
3 nanoparticles did not occur, and the unreacted SA can be effectively washed off.
The −COOH groups on the surface of Al
2O
3 nanoparticles were quantitatively titrated using the simplest acid–base titration method and the detailed titration process is provided in the
Supplementary Materials Text S1. The grafting ratio of SA on the surface of modified Al
2O
3 nanoparticles was calculated using Equation (1), and the results are presented in
Table 1. In the control experiment, the grafting ratio of SA in A
0KH550-COOH was negligible, suggesting the absence of −NH
2 groups on the surface of Al
2O
3. The grafting ratios of SA in carboxylic acid-functionalized Al
2O
3 nanoparticles increased from 17% to 44% with the simultaneous increase in KH550 and SA. The amount of KH550 used was in excess compared to SA in the reaction system to ensure that there were remaining −NH
2 groups on the surface of Al
2O
3 nanoparticles for subsequent coupling reactions. The conversion rate (CR) of SA in different samples was calculated according to Equation (S1), ranging from 76 to 81%, indicating a relatively complete reaction between SA and amino-functionalized Al
2O
3 nanoparticles.
where
in 1 g of sample is explicitly given by Equation (S2) and the
represents the grafting ratio of SA calculated by acid–base titration, namely the proportion of the mass of SA involved in the reaction to the mass of sample.
is the molar mass of SA,
is the molecular weight of SA and
is the mass of the sample.
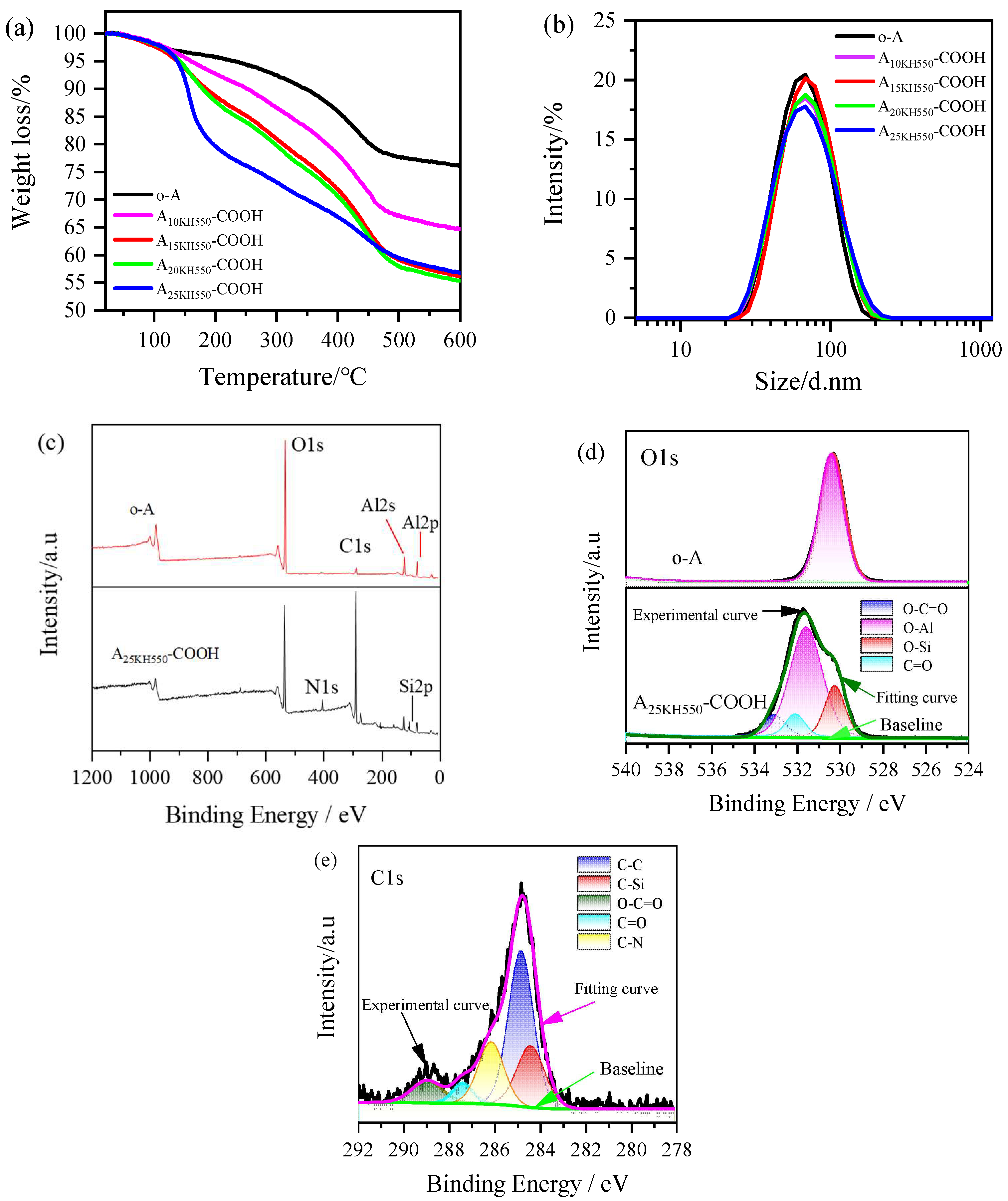
Figure 3a shows the TGA curves of Al
2O
3 nanoparticles before and after modification. The weight loss at temperatures lower than 140 °C is attributed to the evaporation of physis orbed water from the surface of o-A. The weight loss of o-A in the range of 140–600 °C is around 20%, which corresponds to the slow condensation of Al-OH. According to Equation (2) [
33], the −OH group content can be calculated as 22.22 mmol per gram of Al
2O
3 at T
0. After modification, the weight loss curves of four samples at temperatures lower than 140 °C are similar. However, these curves in the range of 140–600 °C are always below the curve of O-A, mainly due to the decomposition of organic components grafted on the surface of Al
2O
3 nanoparticles and the condensation of residual −OH groups at higher temperatures. As the samples were washed with ethanol and deionized water beforehand, the weight loss of the physical adsorption SA could be neglected. For inorganic oxide nanoparticles, surface modification was achieved through the reaction between the modifier and the −OH groups on the surface of the nanoparticles. Therefore, the grafting ratio of the modifier can be defined as the ratio of the mass of the modifier grafted to the nanoparticle surface to the total mass of the −OH groups of the nanoparticle [
34]. According to Equation (3), the total grafting ratios of KH550 and SA on the surface of the four modified Al
2O
3 nanoparticles were calculated to be approximately 50%, 58%, 69%, and 77%, respectively.
where
is the weight loss (wt.%) in the temperature region from
T0 to
,
is the molecular weight of H
2O, the
represents the grafting ratio of SA determined by TGA analysis,
is the mass of modifiers grated on the surface of nanoparticles and
is the mass of the −OH group in nanoparticles.
Based on the TGA curve of KH550-modified Al
2O
3 nanoparticles shown in
Figure S1, the grafting ratios of KH550 in A
10KH550, A
15KH550, A
20KH550, and A
25KH550, calculated using Equation (3), were determined to be 37%, 40%, 48%, and 53%, respectively. Consequently, the grafting ratios of SA on the surface of Al
2O
3 nanoparticles were calculated as 13%, 18%, 21%, and 24%, respectively. Note that the grafting ratio of SA increased with the increase in the amount of modifier using both methods, but the grafting ratio of SA calculated by the thermogravimetric method was found to be lower than that calculated by the titration method. This difference can be attributed to the fact that the −OH groups measured by thermogravimetric analysis include both surface and internal −OH groups. Consequently, the measured content of −OH groups is higher, resulting in a lower grafting ratio.
The particle size distributions of the original Al
2O
3 nanoparticles (o-A) and carboxylic-functionalized Al
2O
3 nanoparticles were shown in
Figure 3b. The average size of o-A was 61 ± 1 nm and the polydispersity index (PDI) value was below 0.13, indicating good dispersity. The average particle sizes of the carboxy-functionalized products (A
10KH550-COOH, A
15KH550-COOH, A
20KH550-COOH, and A
25KH550-COOH) were 62 ± 1, 64 ± 1, 61 ± 1, and 62 ± 1 nm, respectively. It can be observed that the average particle size of the resulting products did not change significantly compared to o-A. The PDI values for the carboxy-functionalized products ranged from 0.15 to 0.17, which indicates that Al
2O
3 nanoparticles before and after surface modification also have good dispersity in hydrosol. Furthermore, the carboxylic acid-functionalized Al
2O
3 nanoparticle hydrosols were found to be stable for more than two months.
XPS analysis of Al
2O
3 nanoparticles before and after modification was performed to confirm the change in chemical bond on the surface of nanoparticles, as shown in
Figure 3c–e. The electron core level XPS spectra of o-A, namely Al2p, Al2s, and O1s, indicate the presence of aluminum and oxygen components in the sample. The weak carbon signal peak in the XPS full survey spectra is attributed to the carbon pollution of the instrument itself. For the carboxylic acid-functionalized Al
2O
3 nanoparticles (A
25KH550-COOH), in addition to Al and O elements, N and Si signal peaks also appeared, and the signal peak of C was significantly enhanced, indicating that KH550 was grafted on the surface of Al
2O
3 nanoparticles (
Figure 3c). The O1s spectra signal peak in o-A is about 531.1 eV, while the O1s spectra of A
25KH550-COOH can be fitted into four peaks at 531.8 eV, 530 eV, 532 eV and 533.1 eV, corresponding to O-Al, O-Si, C=O and O-C=O, respectively (
Figure 3d). The shift of O-Al binding energy in A
25KH550-COOH towards the left may stem from the reduction in electron density on the surface after the chemical reaction between the −OH groups on the surface of Al
2O
3 and the modifier. The C1s spectra of A
25KH550-COOH can be deconstructed into five individual peaks, each representing a separate carbon bond, namely C-C (284.8 eV), C-Si (284.2 eV), C-N (286.6 eV), C=O (287.4 eV) and O-C=O (289.0 eV) [
35] (
Figure 3e), further indicating the successful preparation of carboxylic acid-functionalized Al
2O
3 nanoparticles.
3.3. Hydrophobic Modification of SiO2 Nanoparticles by HMDS
The SiO
2 powders obtained by SiO
2 hydrosol were washed with ethanol and deionized water using a similar protocol as that of the Al
2O
3 nanoparticles prior to their FTIR and TGA analysis.
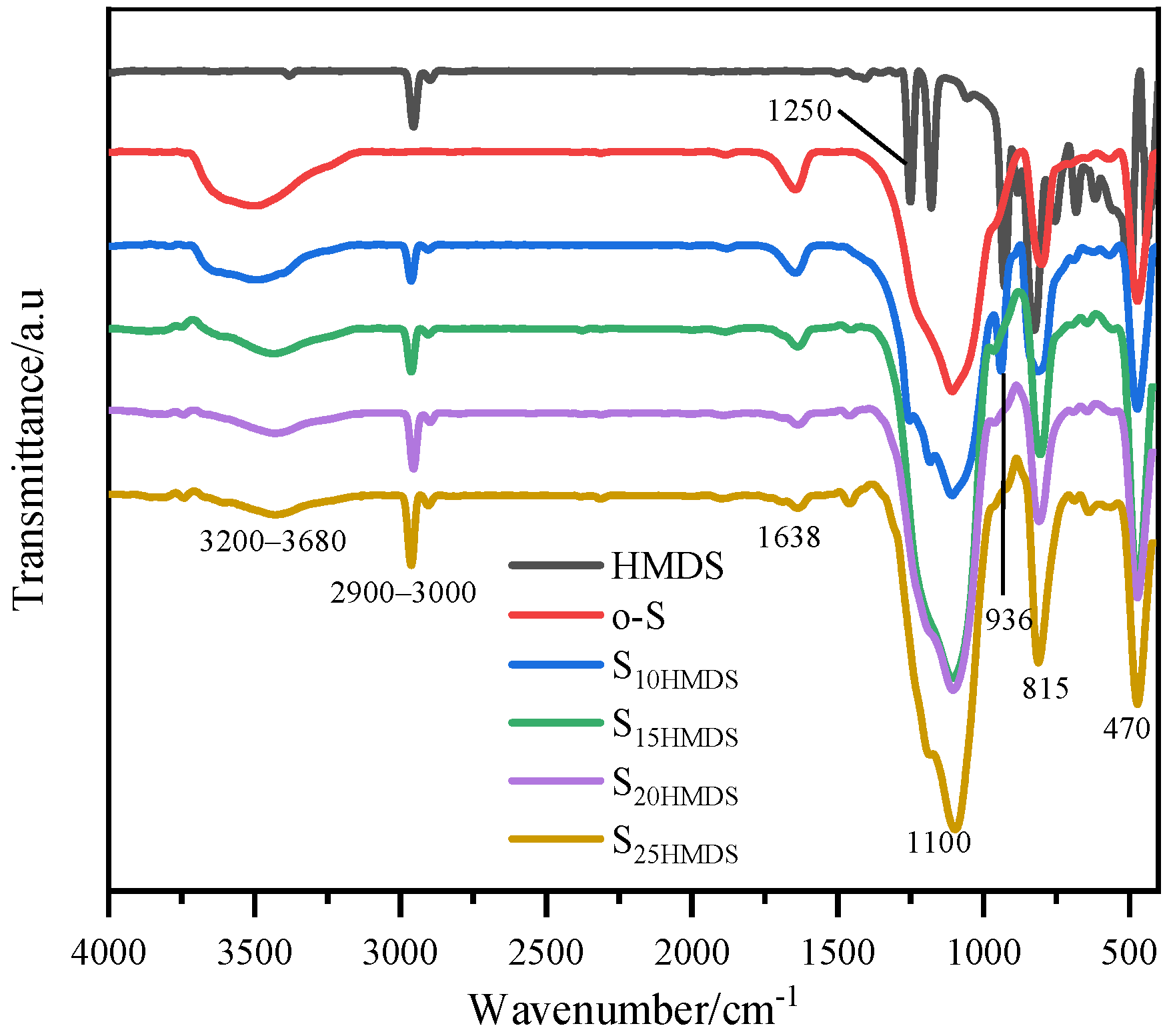
Figure 4 shows the FTIR spectra of SiO
2 nanoparticles before and after modification with HMDS. For the original SiO
2 nanoparticles (o-S), the broadband at 3200–3680 cm
−1 is associated with the asymmetric stretching vibration of O-H bonds in −OH groups bonded on the surface of SiO
2, while the peak at 1638 cm
−1 corresponds to the bending vibration of O-H bonds. The characteristic peaks of 1100, 815, and 470 cm
−1 correspond to asymmetric stretching vibration, symmetric stretching vibration, and bending vibration of Si-O-Si bonds of SiO
2 nanoparticles. After modification with HMDS, several new peaks appeared in the SiO
2 nanoparticles. The asymmetric and symmetric stretching vibrations of C-H bonds in −CH
3 groups united on the surface of SiO
2 nanoparticles were observed at 2900–3000 cm
−1. Additionally, the peaks at 1250 and 936 cm
−1 correspond to the stretching vibration and bending vibration of −CH
3 groups directly connected with Si. As the amount of HMDS added increases, the characteristic absorption of −OH groups at 3200–3680 cm
−1 and 1638 cm
−1 gradually weakened, indicating that HMDS has been grafted onto the silica surface in the form of new Si-O-Si bonds, and the polar −OH groups on silica surface are progressively replaced by nonpolar −CH
3 groups.
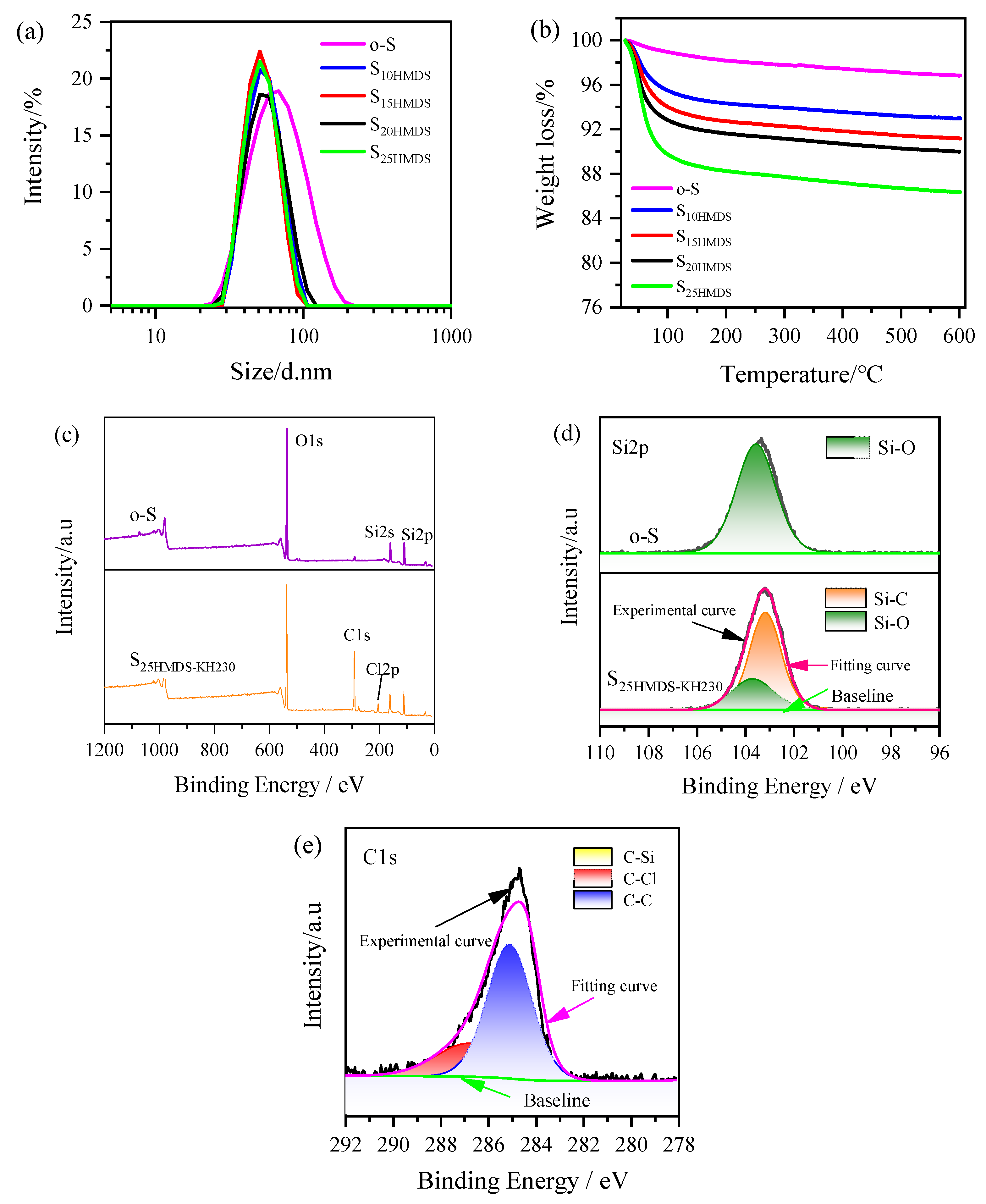
As presented in
Figure 5a, the average particle size of o-S is 60 ± 1 nm with a PDI value of 0.19. After modification with HMDS, the average particle sizes of S
10HMDS, S
15HMDS, S
20HMDS, and S
25HMDS were measured to be 52 ± 2, 50 ± 1, 53 ± 2, and 51 ± 1 nm, respectively. Note that the modification process caused a change in the particle size and distribution, resulting in a reduction in the average particle size from 60 nm to 50 nm, which suggests that the agglomeration of SiO
2 nanoparticles was reduced due to the grafting of the −CH
3 groups onto the surface of SiO
2 nanoparticles during modification. With an increase in the amount of HMDS added, the PDI of the resulting products decreased, with values of 0.04, 0.01, 0.07, and 0.05, respectively. This indicates that surface-modified SiO
2 nanoparticles with HMDS exhibited good monodispersity in the aqueous phase, as confirmed by the narrow distribution of modified SiO
2 nanoparticles shown in
Figure 5a compared with o-S. Furthermore, the modified SiO
2 hydrosol was quite stable during the aging process, lasting for up to three months.
The TGA curve of SiO
2 nanoparticles before and after modification with HMDS is illustrated in
Figure 5b. The weight loss below 100 °C for o-S is mainly due to the physically adsorbed water on the silica surface. Between 100 and 600 °C, o-S showed a weight loss of about 2%, which can be assigned to the condensation of silanols. The −OH group content of the SiO
2 nanoparticle was calculated as 2.22 mmol using Equation (2) in
Section 3.2, which is lower than that of Al
2O
3 nanoparticles. The weight loss of S
10HMDS, S
15HMDS, S
20HMDS, and S
25HMDS can be ascribed to the thermal decomposition of HMDS chemically bonded to the surface of SiO
2 and the condensation of residual hydroxyl groups at higher temperatures. The physical adsorption of HMDS can also be disregarded since the samples were pre-washed with ethanol and deionized water. According to Equation (3), the grafting ratio of S
10HMDS, S
15HMDS, S
20HMDS and S
25HMDS can be calculated as 26%, 32%, 34%, and 39%, respectively.
In order to react with carboxyl acid-functionalized Al
2O
3 nanoparticles, further modification of HMDS-modified SiO
2 was carried out using KH230 to graft −CH
2Cl groups onto its surface.
Figure 5c shows the XPS full survey spectra of o-S and S
25HMDS-KH230 and the Si2p signal at 103.5 eV reveals the existence of pure SiO
2. The O1s spectra signal peak absorbed at 532.3 eV clearly indicates the higher contribution of oxygen with silica. A small hump near 284 eV indicates the presence of residual carbon in the sample, potentially originating from carbon contamination within the instrument itself, similar to Al
2O
3. Apart from the above-mentioned elements, a Cl2p signal peak was detected at 199.8 eV in the sample S
25HMDS-KH230, and there was a notable increase in the C1s signal due to the modification of the silica surface by the modifier. The single peak at 103.6 eV in the Si2p spectra of o-S was assigned to the Si-O bonds in SiO
2 nanoparticles (
Figure 5d). After modification, two peaks centered at 103.6 and 103 eV were identified in the Si2p spectra of S
25HMDS-KH230, attributed to the Si-O and Si-C bonds, respectively. The deconvolution of C1s spectra of S
25HMDS-KH230 shown in
Figure 5e suggested the presence of C-C, C-Si and C-Cl bonds, with peaks at 285.1, 284.2 and 287 eV, respectively. These results suggest that KH230 is grafted onto the surface of HMDS-modified SiO
2 nanoparticles to introduce −CH
2Cl functional groups.
3.4. Coupling Reaction of Asymmetric Al2O3-SiO2 Janus Nanoparticles
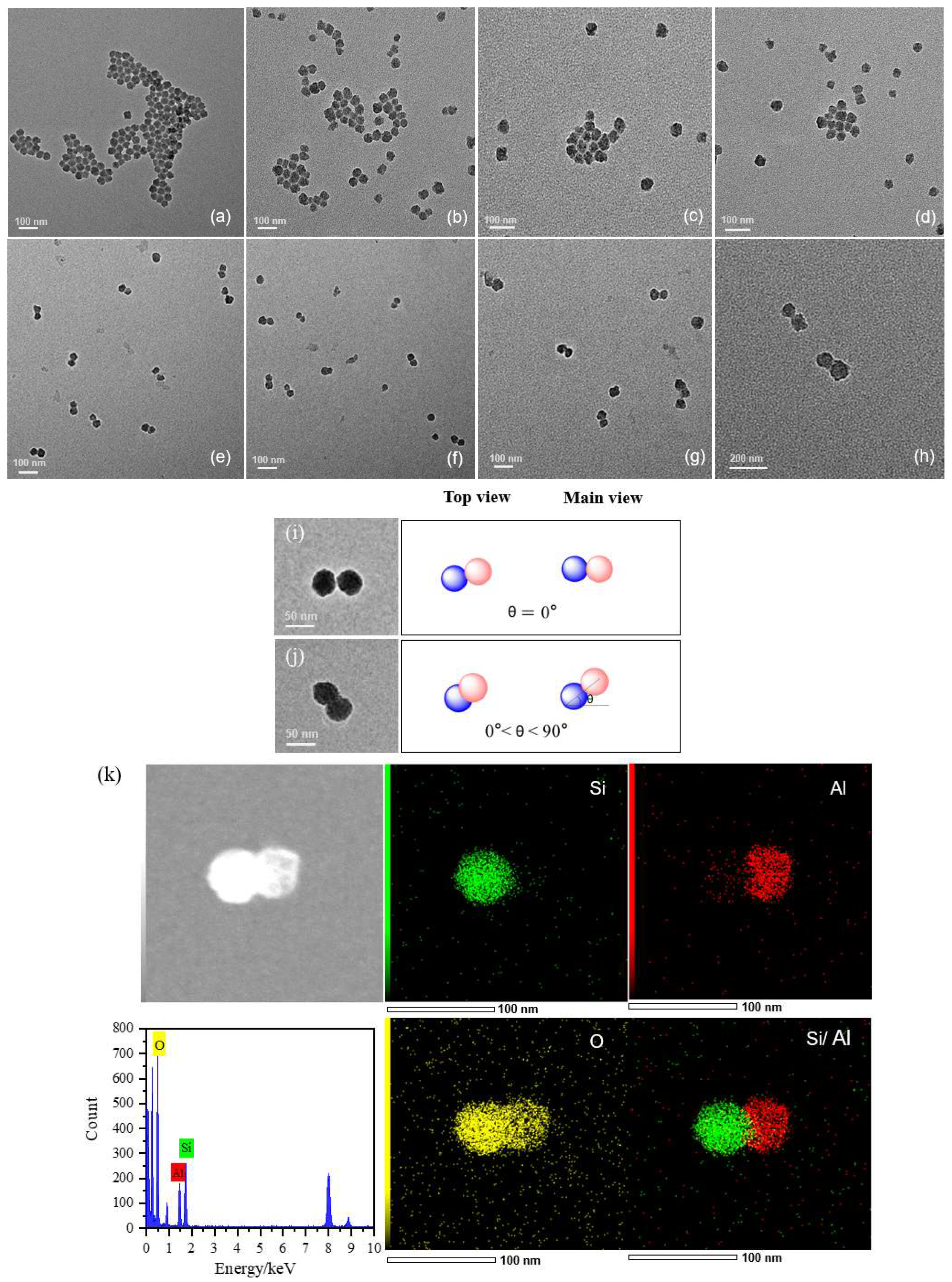
The morphology of Al
2O
3 and SiO
2 nanoparticles before and after coupling was investigated by TEM, as shown in
Figure 6. The o-A and o-S are all uniformly spherical and well-shaped nanoparticles, but there was some obvious coalescence between them (
Figure 6a,b). This can be attributed to the high specific surface area of Al
2O
3 and SiO
2 nanoparticles and the large number of −OH groups distributed on their surfaces, making them prone to adhesion to each other. However, when the two types of nanoparticles were covalently connected in a one-to-one manner in the aqueous phase, the Al
2O
3-SiO
2 nanoparticles with a dumbbell-like structure (A
25KH550-COOH@S
25HMDS-KH230) were clearly observed in different positions on the background (
Figure 6e–h), which has different postures in the TEM images since they were randomly deposited on the background. Usually, the shooting angle of TEM is the top view. After the sample is dropped on the copper mesh and dried using an infrared lamp, the dispersant evaporates rapidly, causing the random deposition of dumbbell-like Al
2O
3-SiO
2 nanoparticles onto the copper mesh background. Consequently, the nanoparticles display diverse orientations on the copper mesh surface, as depicted in
Figure 6i,j. Moreover, the elements of Si and Al are independently distributed in the SiO
2 and Al
2O
3 ends of the dumbbell-like Janus nanoparticles (as illustrated in
Figure 6k), while O elements are dispersed throughout the entire structure of the dumbbell-like Al
2O
3-SiO
2 nanoparticles, suggesting that these Janus nanoparticles are composed of spheroidal Al
2O
3 and SiO
2 nanoparticles. In the comparative experiments, hydrophilic nanoparticles A
25KH550-COOH were mixed with o-S (A
25KH550-COOH @ o-S,
Figure 6c). Severe agglomeration of the two nanoparticles was observed and no dumbbell-like structure was formed due to the absence of one of the reactive groups in the mixture. The same phenomenon was observed in another controlled experiment (
Figure 6d), where hydrophilic nanoparticles A
25KH550-COOH were mixed with hydrophobic nanoparticles S
25HMDS without prior modification with KH230. Furthermore, no multiple structures were observed in
Figure 6e–h, which is closely related to the steric hindrance effect in the dumbbell structure.
From the above analysis on the morphology of Al
2O
3 and SiO
2 nanoparticles before and after coupling, the two nanoparticles were covalently connected on a one-to-one basis through chemical bonds among surface groups to form dominant dumbbell-like nanoparticles and further reaction with other nanoparticles can be prevented by the strong steric hindrance around the dominant nanoparticles. The steric hindrance on the surface of nanoparticles is primarily imparted by the modifier, which condenses with −OH groups on the nanoparticle surface after modification, weakening the hydrogen bonding between particles and increasing the steric hindrance among them. Consequently, the dumbbell-like Al
2O
3-SiO
2 Janus nanoparticles reached a thermodynamically stable state after the initial coupling reaction. Thus, the predominant entities within this reaction system are the dumbbell-like Al
2O
3-SiO
2 Janus nanoparticles, aligning with our previous research findings [
29,
30]. Other studies have also demonstrated the critical role of steric hindrance in preventing the formation of multiple structures, such as the synthesis of dissymmetrical snowman and dumbbell-like silica/polymer colloidal particles through emulsion polymerization [
16], and the assembly of DNA-linked colloidal gold NP building blocks for programmable matter [
36], which further indicates the importance of steric hindrance in the formation of the asymmetric Al
2O
3-SiO
2 Janus nanoparticles with a dumbbell-like structure.
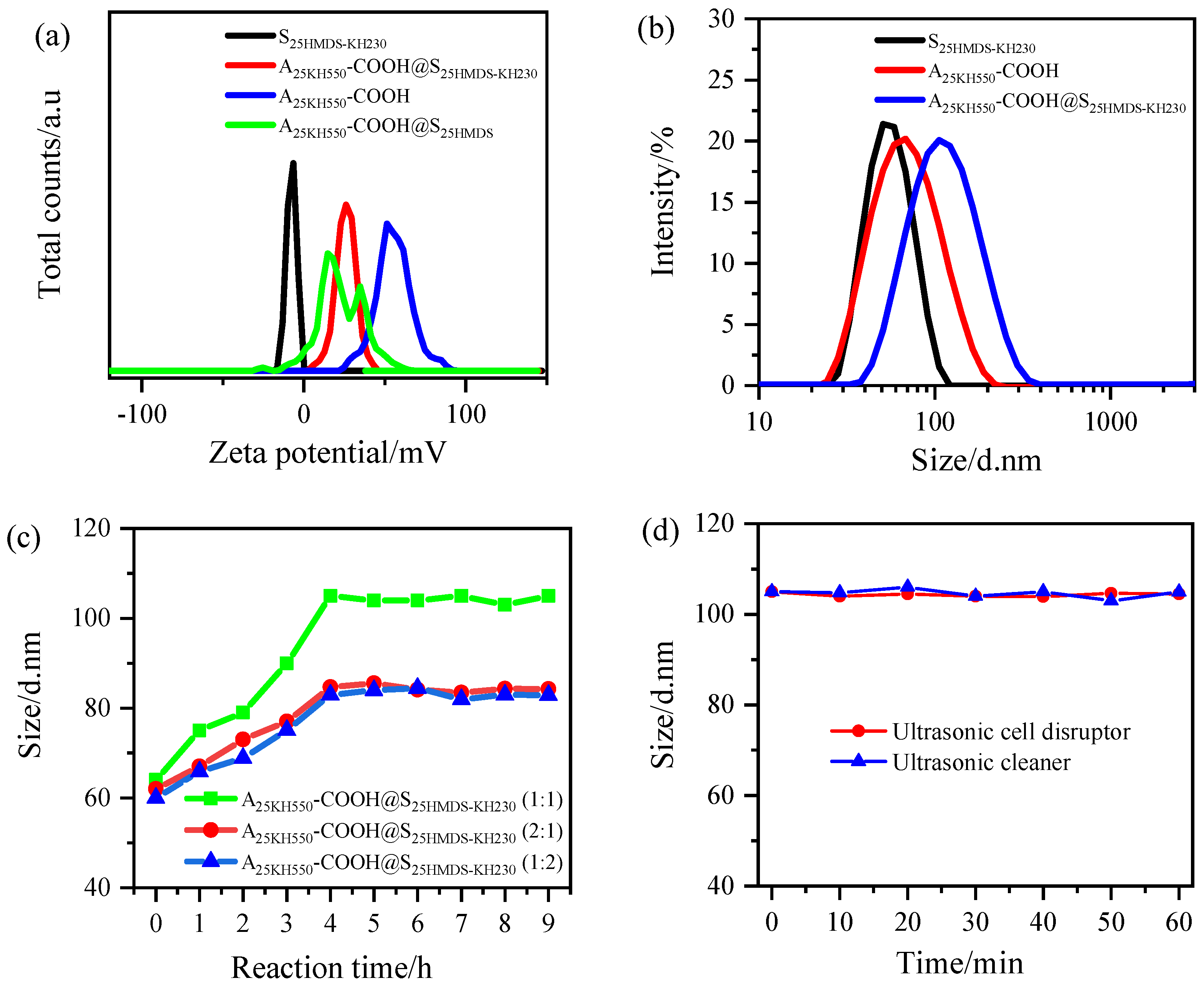
The formation of asymmetric Al
2O
3-SiO
2 nanoparticles can be indirectly proved by the change in zeta potential (ZP) of Al
2O
3 and SiO
2 nanoparticles before and after coupling. As shown in
Figure S2, the ZP of o-A and o-S before modification was approximately 42 mV and −30 mV, respectively, indicating a stable system. After modification, the ZP of A
25KH550 increased to about 54 mV, while the ZP of S
25HMDS changed from −30 mV to −9 mV. The results showed that −NH
2 groups and −CH
3 groups were successfully grafted on the surface of Al
2O
3 and SiO
2 nanoparticles, respectively. The ZP changes after further functionalization of Al
2O
3 and SiO
2 nanoparticles are shown in
Figure 7a. The ZP values of S
25HMDS-KH230 and A
25KH550-COOH were −8 and 43 mV, respectively. A 1:1 mixture of the two nanoparticles produced dumbbell-like nanoparticles with a ZP value of 26 mV. Compared with the carboxylic acid-functionalized Al
2O
3 nanoparticles, the ZP value of the dumbbell-like Al
2O
3-SiO
2 nanoparticles decreased significantly, which can be attributed to the reaction of the positively charged −NH
2 groups on the surface of carboxylic-functionalized Al
2O
3 nanoparticles with the −CH
2Cl groups on the hydrophobic SiO
2 nanoparticles. The positively charged −NH
2 groups are deprotonated due to the nucleophilic substitution with the −CH
2Cl groups, so the ZP value of the A
25KH550-COOH@S
25HMDS−KH230 decreased. However, the zeta potential distribution of A
25KH550-COOH@S
25HMDS exhibited a multi-peak distribution in the controlled experiment, indicating that the system was a mixed system without a coupling reaction. It can be concluded that the asymmetric Al
2O
3-SiO
2 Janus nanoparticles with the dumbbell-like structure were successfully prepared via the nucleophilic substitution reaction of −NH
2 and −CH
2Cl groups.
Figure 7b illustrates the particle size distribution of samples obtained by mixing equal amounts of two nanoparticle hydrosols. The average particle size of A
25KH550-COOH@S
25HMDS-KH230 increased from 60 ± 1 nm to 105 ± 1 nm, while the PDI value remained below 0.17, indicating good monodispersity of the coupled nanoparticles in the aqueous phase, which is beneficial for study of particle size changes before and after coupling. The particle size evolution of A
25KH550-COOH@S
25HMDS-KH230 prepared under different mass ratios within 9 h was also investigated and is shown in
Figure 7c. The particle size of all samples increased immediately with the increase in reaction time, and reached a stable value after 4 h, indicating that the coupling reaction was almost complete. Interestingly, even when one of the modified nanoparticles was present in excess, the measured particle sizes of the 2:1 and 1:2 ratios (about 84 nm) were smaller than the value of the 1:1 ratio (about 105 nm), suggesting that excess nanoparticles did not participate in the reaction but instead decreased the average particle size of the system along with the dumbbell-like Al
2O
3-SiO
2 nanoparticles formed. These findings imply that the coupling reaction occurred between modified Al
2O
3 and SiO
2 nanoparticles in a one-to-one manner through a chemical bond, and no higher nanoparticle structures were formed. To distinguish the chemically bonded Al
2O
3-SiO
2 nanoparticles from physically attached ones, two kinds of sonication instruments, an ultrasonic cell disruptor (TL92-IID, Tianling Co., Ltd., Yancheng, China) and ultrasonic cleaner (SCIENTC, SB25-12DT, Ningbo, China), were applied to treat sample 6. As depicted in
Figure 7d, the particle size of A
25KH550-COOH@S
25HMDS-KH230 (1:1) remained stable throughout the 60 min of sonication treatment. This indicates that there is a chemical bond between the Al
2O
3 and SiO
2 nanoparticles and hard agglomeration cannot be disrupted by simple mechanical forces [
37].
The results of the particle size distribution of different proportions of modified Al
2O
3 and SiO
2 nanoparticles mixed in specific mass ratios, along with three control experiments, are summarized in
Table 2. The average particle size of samples 1 to 6 increased gradually from 86 ± 1 nm to 105 ± 1 nm, which is related to the increase in reactive groups on the surface of the two nanoparticles. Compared to sample 6, the average particle sizes of samples 7 and 8 were about 86 and 84 nm, respectively, which are similar to those of samples 1 and 2, indicating that the system with few reactive groups behaves similarly to the system with an excess of one of the modified nanoparticles, resulting in an incomplete coupling reaction and a reduction in the overall average particle size. In the control sample 9 and sample 10, the average particle size measured was approximately 66 nm. In another controlled experiment (sample 11), A
25KH550-COOH was mixed with S
25HMDS without KH230 modification under the same reaction conditions and the average particle size was measured to be 68 ± 1 nm. These results suggest that no coupling reaction occurred between A
25KH550-COOH and o-S or o-A and S
25HMDS or A
25KH550-COOH and S
25HMDS in the water system. It is worth noting that KH230 plays a critical bridging role in forming the asymmetric Al
2O
3-SiO
2 Janus nanoparticles.
Based on our previous work [
29], since the particle size distribution was analyzed by dynamic light scattering (DLS), the measurement particle size of anisotropic nanoparticles is expressed in terms of equivalent diameter. Therefore, the equivalent rod-like model proposed by Briard et al. [
38] can be applied to the particle size measurement of asymmetric Al
2O
3-SiO
2 Janus nanoparticles. The equivalent diameter can be calculated using Equation (4).
where
L and
p represent the length and aspect ratio of the rod-like nanoparticles, respectively,
is the equivalent diameter of the rod-like nanoparticles,
f is the translational friction coefficient of the rod-like nanoparticle and
f0 is the translational friction coefficient of a sphere that has the same volume as the rod-like nanoparticle. In this study, it is assumed that the nanoparticle mixture after the coupling reaction contains three different components: unreacted single nanoparticles, coupled dumbbell-like nanoparticles, and triple structure nanoparticles, which are denoted as d
1, d
2, and d
3, respectively. The previous measurement results obtained by DLS have shown that the particle sizes of Al
2O
3 (d
A1) and SiO
2 (ds
1) are approximately equal to 60 nm, so it can be succinctly considered that
dA1 = ds1 =d. Finally,
d1 = d,
d2 = 1.6
d, and
d3 = 2
d were obtained according to the previous study. Then, the peak fittings of the particle size distribution curves of the coupled Al
2O
3-SiO
2 Janus nanoparticles were performed using the method from [
29] and the coupling reaction between the two types of nanoparticles was quantitatively analyzed by combining Equations (5) and (6) inspired by Rayleigh Approximation Theory [
39].
where size
d1,
d2, and
d3 represent the diameter of single nanoparticles, dumbbell-like nanoparticles, and triple structure nanoparticles.
N1,
N2, and
N3 are particle numbers with sizes d
1, d
2, and d
3. In addition, %I
1 and %N
1 present intensity-weighted distribution and number-weighted distribution for small particles with size
d1, based on the relative amount of intensity of particles and the number of particles with size d
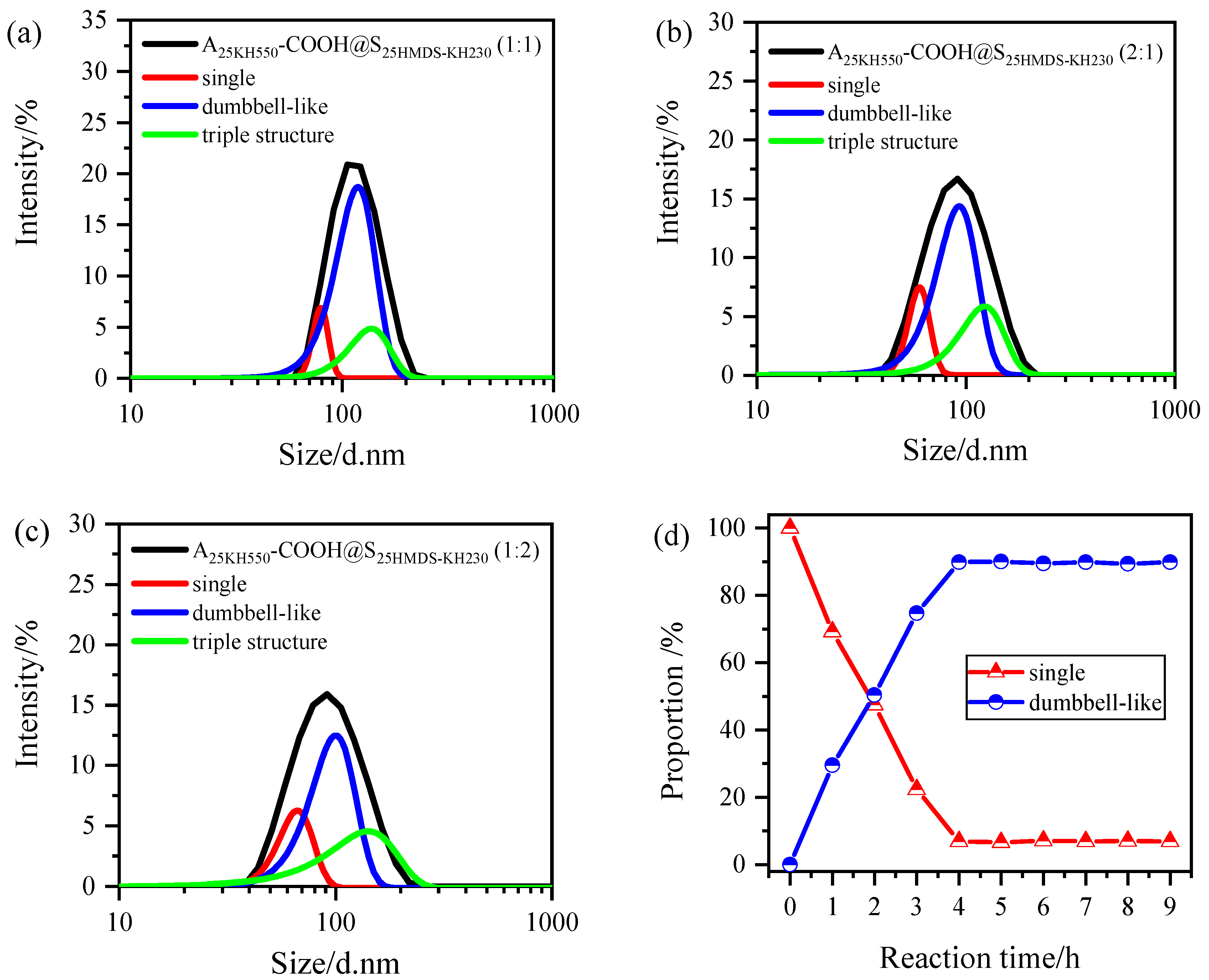
1, respectively. When A
25KH550-COOH was mixed with S
25HMDS-KH230 in a 1:1 ratio, the obtained intensity ratios of single nanoparticles, coupled dumbbell-like nanoparticles, and triple structure nanoparticles were plotted in
Figure 8a, which were 0.7% (%I
1), 89.2% (%I
2), and 10.1% (%I
3), respectively. Next, by combing Equations (5) and (6) with the values of the intensity ratios of the different nanoparticles, the ratios
N1:
N2 = 0.15:1, and
N3:
N2 = 0.03:1 were obtained. The asymmetric Al
2O
3-SiO
2 Janus nanoparticles with a dumbbell-like structure accounted for about 90% of the total number of nanoparticles in system after the coupling reaction, whereas the triple structure accounted for only 3.2% (results are shown in
Table 3). When the two nanoparticles were mixed in a 2:1 or 1:2 ratio, the particle size distribution of the samples was also analyzed by the same method, as shown in
Figure 8b,c and the proportions of coupled Al
2O
3-SiO
2 Janus nanoparticles with a dumbbell-like structure were 61.4% and 67.5%, respectively, while that of triple structure nanoparticles were 5.9% and 5.6%, respectively. This indicated that a relatively complete transformation from single nanoparticles to dumbbell-like nanoparticles occurred in a 1:1 hybrid water system compared with 2:1 or 1:2, in which the higher structures, such as triple structure nanoparticles, are negligibly small.
In addition, the effect of the amounts of modifiers on the coupling reaction in a 1:1 hybrid water system was also investigated in
Figure S3a–e, and the obtained results are listed in
Table 3. The proportion of Al
2O
3-SiO
2 nanoparticles with a dumbbell-like structure in the total number of nanoparticles in the system after the coupling reaction increased with the increase in modifier dosage, ranging from 56% to 82%, indicating that the amount of modifier is related to the reactive groups on the surface of the nanoparticles. Specifically, a lower amount of modifier resulted in fewer single nanoparticles participating in the coupling reaction, while a higher amount of modifier led to a higher transformation rate from single nanoparticles to dumbbell-like nanoparticles. It can be concluded that the dominant structure in the coupling reaction is the dumbbell-like structure, while the proportion of triple structure nanoparticles is very small. Therefore, the presence of higher structures, such as quadruple structure nanoparticles, can be disregarded.
Furthermore, the peak fitting process was conducted on the particle size distribution curves of different reaction times using sample 6 (A
25KH550-COOH@S
25MDS-KH230) as an example to investigate the changes in the proportion of single nanoparticles and dumbbell-like structures as the reaction time increased. The results are depicted in
Figure 8d. It was observed that the proportion of single nanoparticles decreased significantly within 4 h, accompanied by a noticeable increase in the proportion of dumbbell-like structures. After 4 h, the reaction was essentially completed, with approximately 90% of the single nanoparticles transformed into dumbbell-like nanoparticles.
3.5. Application of Asymmetric Al2O3-SiO2 Janus Nanoparticles in Surfactant
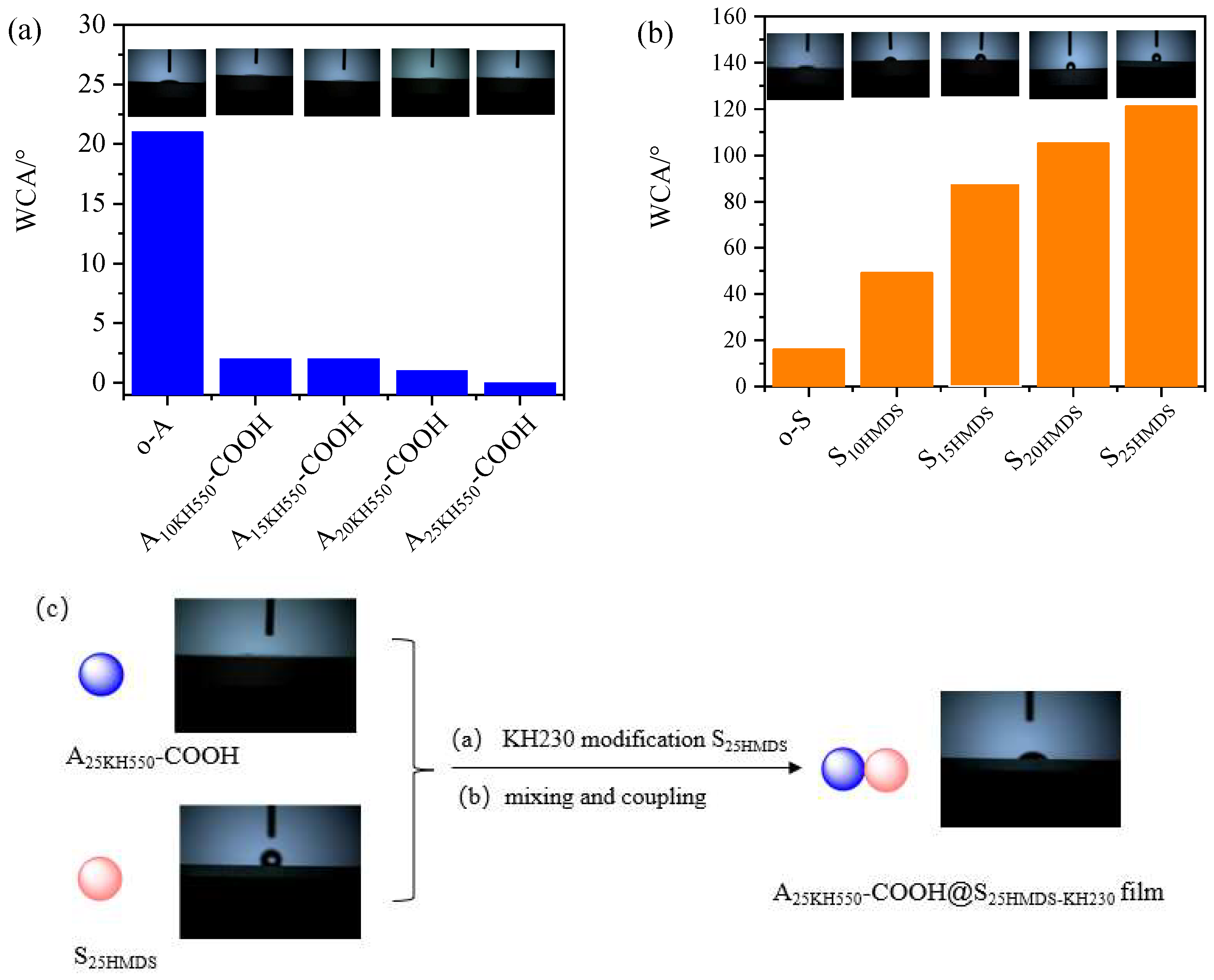
The hydrophilicity of nanoparticles was characterized by water contact angle (WCA) measurement, as presented in
Figure 9. Before measuring the WCA, Al
2O
3 and SiO
2 nano hydrosol must be deposited onto the silicon wafer using a simple sol–gel dip-coating process. Detailed experimental procedures are provided in
Text S2. The WCA values of o-A, A
10KH550-COOH, A
15KH550-COOH, A
20KH550-COOH, and A
25KH550-COOH were 21°, 2°, 2°, 1°, and 0° (
Figure 9a), indicating a superhydrophilic surface, while that of o-S, S
10HMDS, S
15HMDS, S
20HMDS, and S
25HMDS were 16°, 49°, 88°, 105°, and 121°, respectively (
Figure 9b). The WCA values exhibited significant changes with an increasing HMDS content. When the HMDS content reached 25% of the mass of SiO
2 in hydrosol, the WCA reached 121°, indicating a better hydrophobic modification of SiO
2 under these conditions. Therefore, two types of nanoparticles with significant differences in hydrophilicity were obtained after modification. The superhydrophilic A
25KH550-COOH and hydrophobic S
25HMDS were then coupled to obtain asymmetric Al
2O
3-SiO
2 Janus nanoparticles (A
25KH550-COOH@S
25HMDS-KH230) with a WCA of 44° (
Figure 9c). Evidently, A
25KH550-COOH@S
25HMDS-KH230 possess an amphiphilic property in addition to chemical asymmetry. As a result, these nanoparticles can be regarded as amphiphilic Janus nanoparticles to emulsify the oil–water system.
The amphiphilicity of asymmetric Al
2O
3-SiO
2 Janus nanoparticles was tested using A
25KH550-COOH@S
25HMDS-KH230 to stabilize the oil–water system. Different model compounds such as cyclohexane–water, toluene–water, silicone oil–water, and vegetable oil–water were used for this purpose. Digital photographs of samples suspended in a dual-phase mixture of various oils and water are presented in
Figure S4 and o-A, A
25KH550-COOH, and o-S dispersed only in water as a result of their hydrophilic hydroxyl groups or carboxyl groups, while S
25HMDS was mainly dispersed in oil due to the introduction of hydrophobic alkyl groups. In a control experiment, the oil–water emulsions were not stabilized by the simple physical mixture (A
25KH550-COOH@S
25HMDS), which was separated into two layers within 5 min. However, A
25KH550-COOH@S
25HMDS-KH230 could effectively stabilize emulsions of different oil and water mixtures, forming a stable emulsion layer with a thickness of 3.2cm. The effect of amphiphilic asymmetric Al
2O
3-SiO
2 Janus nanoparticles on the stability of four oil–water emulsions was then further evaluated by Turbiscan based on the backscattering technology [
40,
41]. Given that one end of Al
2O
3-SiO
2 Janus nanoparticles exhibits anion properties, a common anionic surfactant, namely sodium dodecyl sulfate, was selected for comparison with A
25KH550-COOH@S
25HMDS-KH230 and A
25KH550-COOH@S
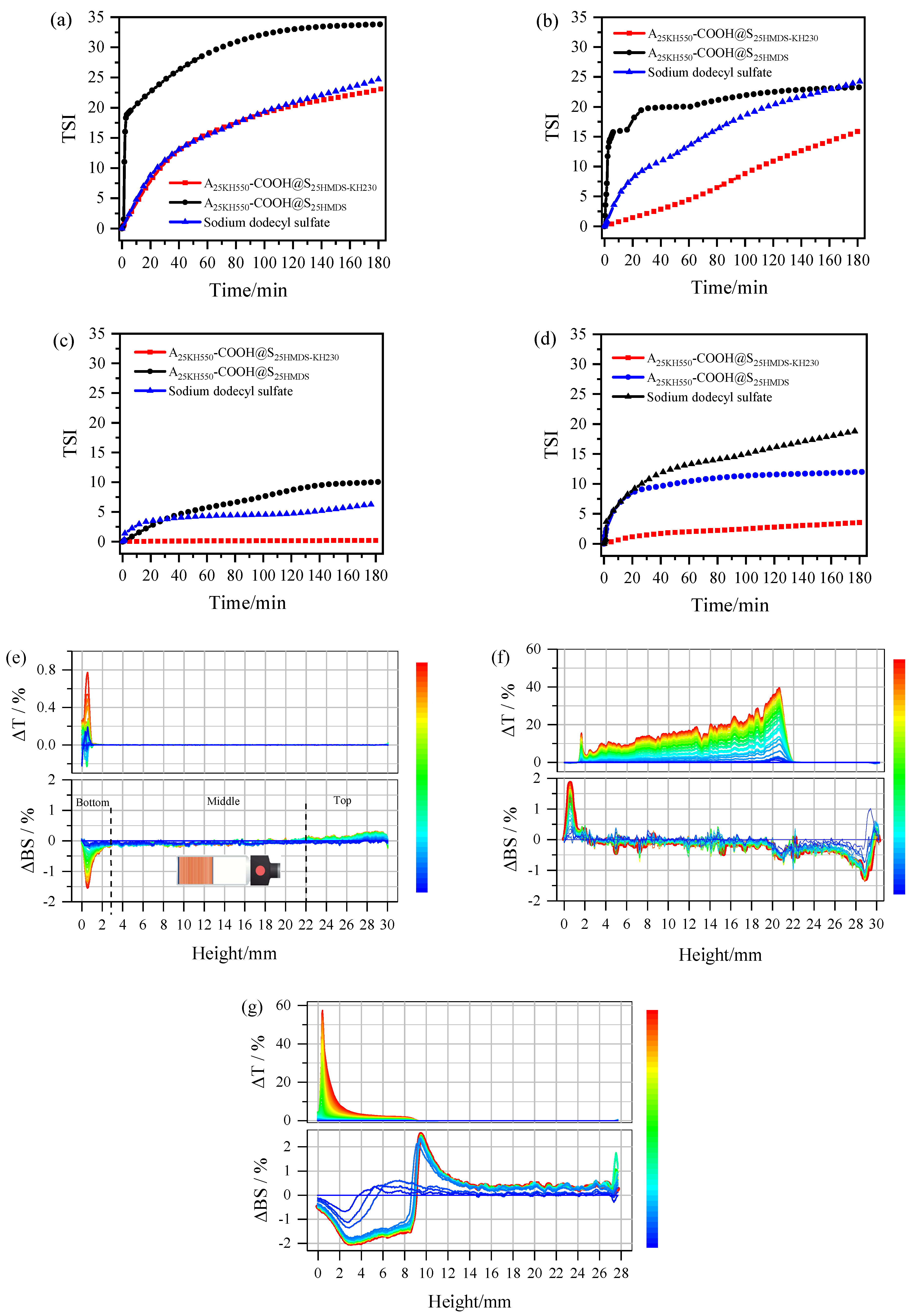
25HMDS. The Turbiscan Stability Index (TSI) curves for different emulsions are depicted in
Figure 10. The changes in the TSI values of emulsions indicated physical stability during storage. The higher the TSI value, the lower the stability of the system [
42]. The TSI curves of various oil–water systems emulsified by A
25KH550-COOH@S
25HMDS-KH230 consistently exhibit lower values compared to those of sodium dodecyl sulfate and A
25KH550-COOH@S
25HMDS, except for the similarity in emulsification capabilities between A
25KH550-COOH@S
25HMDS-KH230 and sodium dodecyl sulfate within the initial 2 h, as observed in
Figure 10a.
Table 4 presents the TSI values of different oil–water systems at 3 h and the stability provided by A
25KH550-COOH@S
25HMDS and sodium dodecyl sulfate for the four oil–water systems is inferior to that of A
25KH550-COOH@S
25HMDS-KH230. These results indicated that the dumbbell-like Al
2O
3-SiO
2 Janus nanoparticles with amphiphilic properties have a good affinity for both water and oil and exhibit significantly higher interfacial activity than their homogeneous nanoparticles, effectively lowering the interfacial tension between oil and water.
To further characterize the physical stability of emulsions, Turbiscan delta transmission (ΔT) and delta backscattering (ΔBS) data were plotted against sample height over time (
Figure 10e–g, using silicone oil–water emulsions as an example). For the silicone oil–water systems emulsified by A
25KH550-COOH@S
25HMDS-KH230 (
Figure 10e), the change in ΔT signal increased between 0 and 1 mm over the entire height of the sample during the scan (3 h), indicating that this zone was starting to become clear. However, the T signal remained close to zero in the middle and top of the samples, indicating a high degree of emulsion stability with no apparent demulsification. Conversely, between 0 and 3 mm heights at the bottom of the sample vials, an apparent decrease in the BS signal was observed, suggesting slight flocculation or coalescence-induced sedimentation in silicone oil–water emulsions during storage [
43]. No obvious changes in backscattered light were observed in the middle of the sample, indicating no droplet size change phenomenon. The BS signal at the top of sample vials increased with time due to the formation of the upper emulsion layer. In the case of A
25KH550-COOH@S
25HMDS (
Figure 10f), an obvious increase in the T signal was observed in the 0–22 mm region from the bottom to the middle with the formation of a thicker water-clearing layer in that area, indicating the significant stratification and instability of the system. The increase in the BS signal in the bottom indicated a higher droplet concentration in the emulsion layer, whereas the decrease in the BS signal at the top regions indicated a lower droplet concentration. Similarly, in the silicone oil–water systems emulsified by sodium dodecyl sulfate (
Figure 10g), a layer thickness of around 9 mm was observed at the top in the ΔT spectrum. Correspondingly, the BS signal decreased in the bottom regions (0–9 mm), while it increased in the middle region (9–14 mm) and top region (14–28 mm), indicating the formation of a water layer at the bottom. Furthermore, the T and BS changes in
Figure 10e,f are basically identical, so the emulsions obtained are of the same type, that is, O/W emulsion. The ΔT and ΔBS profiles of the other oil–water systems emulsified by A
25KH550-COOH@S
25HMDS-KH230, A
25KH550-COOH@S
25HMDS and sodium dodecyl sulfate are shown in
Figures S5–S7 and the analytical methods of T and BS spectra are the same as above. Collectively, the clarification at the bottom of the sample is associated with the increase in the T signal, while the droplet concentration (emulsification) at the top of the sample was linked with an increased BS signal. These changes in TSI, T data, and BS data of the emulsions suggested that the asymmetric Al
2O
3-SiO
2 Janus nanoparticles with dumbbell-like structures exhibit significantly enhanced interfacial activity. This enhancement effectively reduces the interfacial tension between oil and water, highlighting their potential as promising surfactants for emulsifying certain oil–water emulsions.