In-Depth Insight into the Effects of Steel Slag and Calcium Hydroxide on the Properties of a Fly Ash–Red Mud Geopolymer
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Test Methods
3. Results and Discussion
3.1. Raw Material Analysis
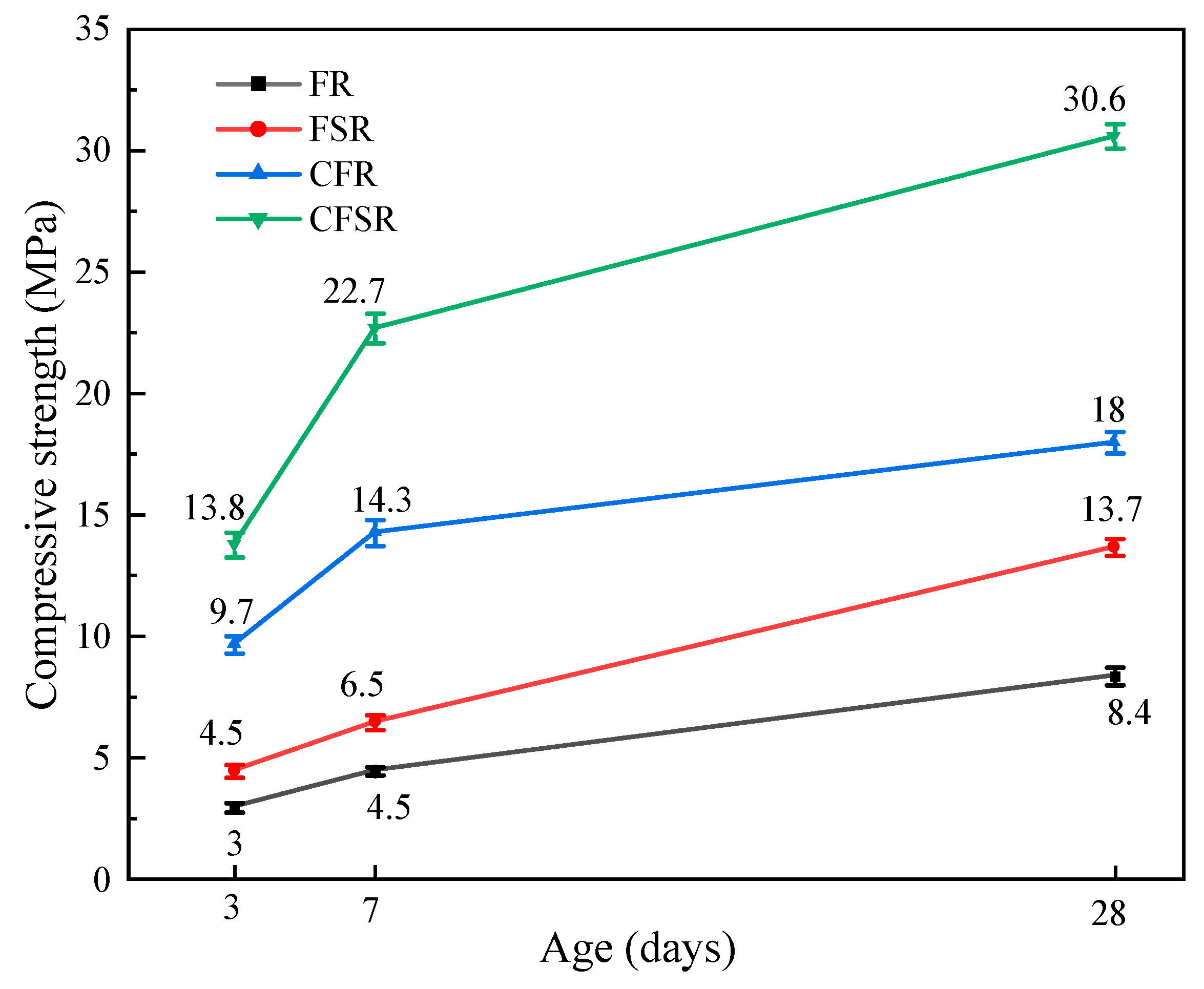
3.2. Mechanical Property Analysis
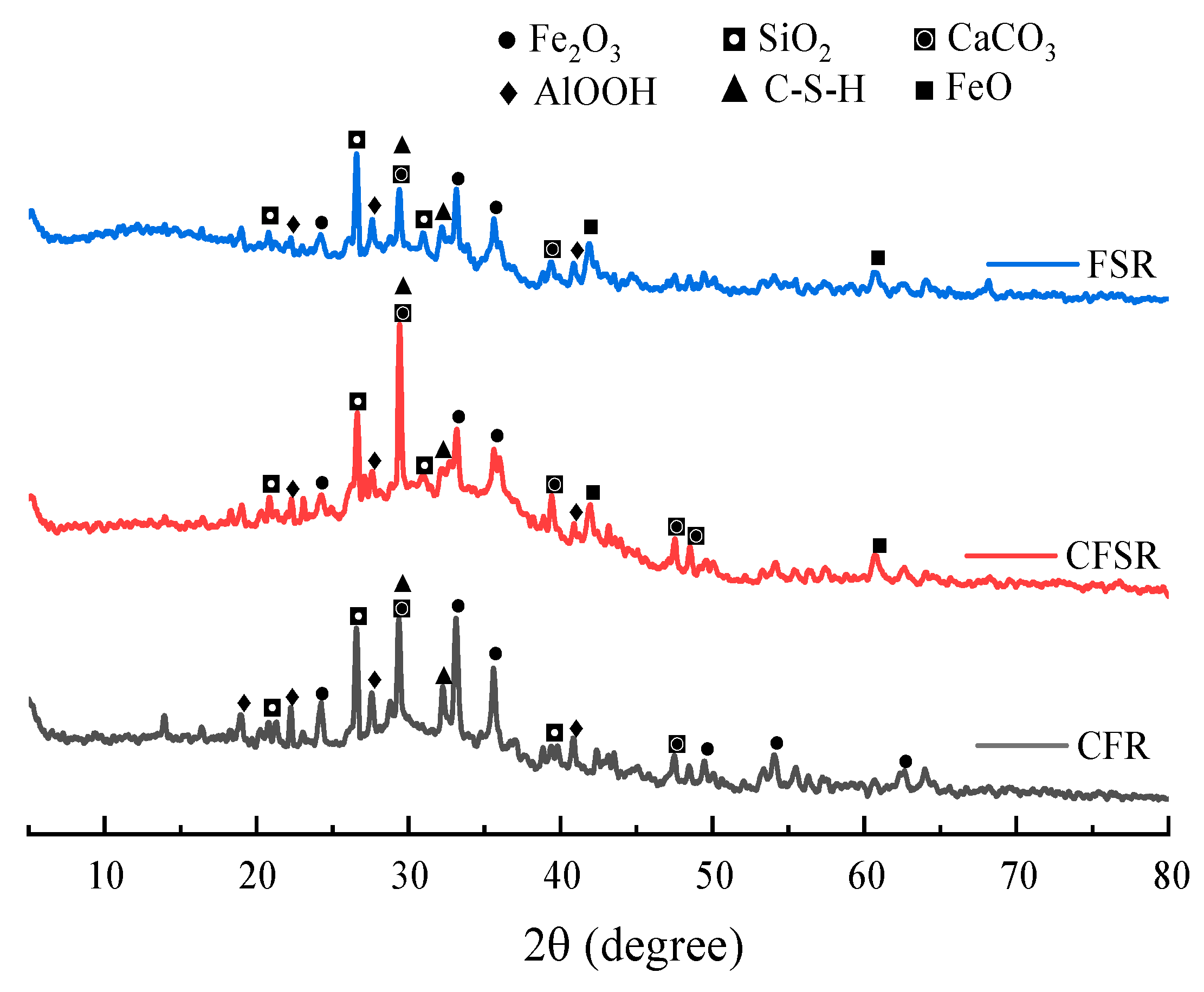
3.3. XRD Analysis
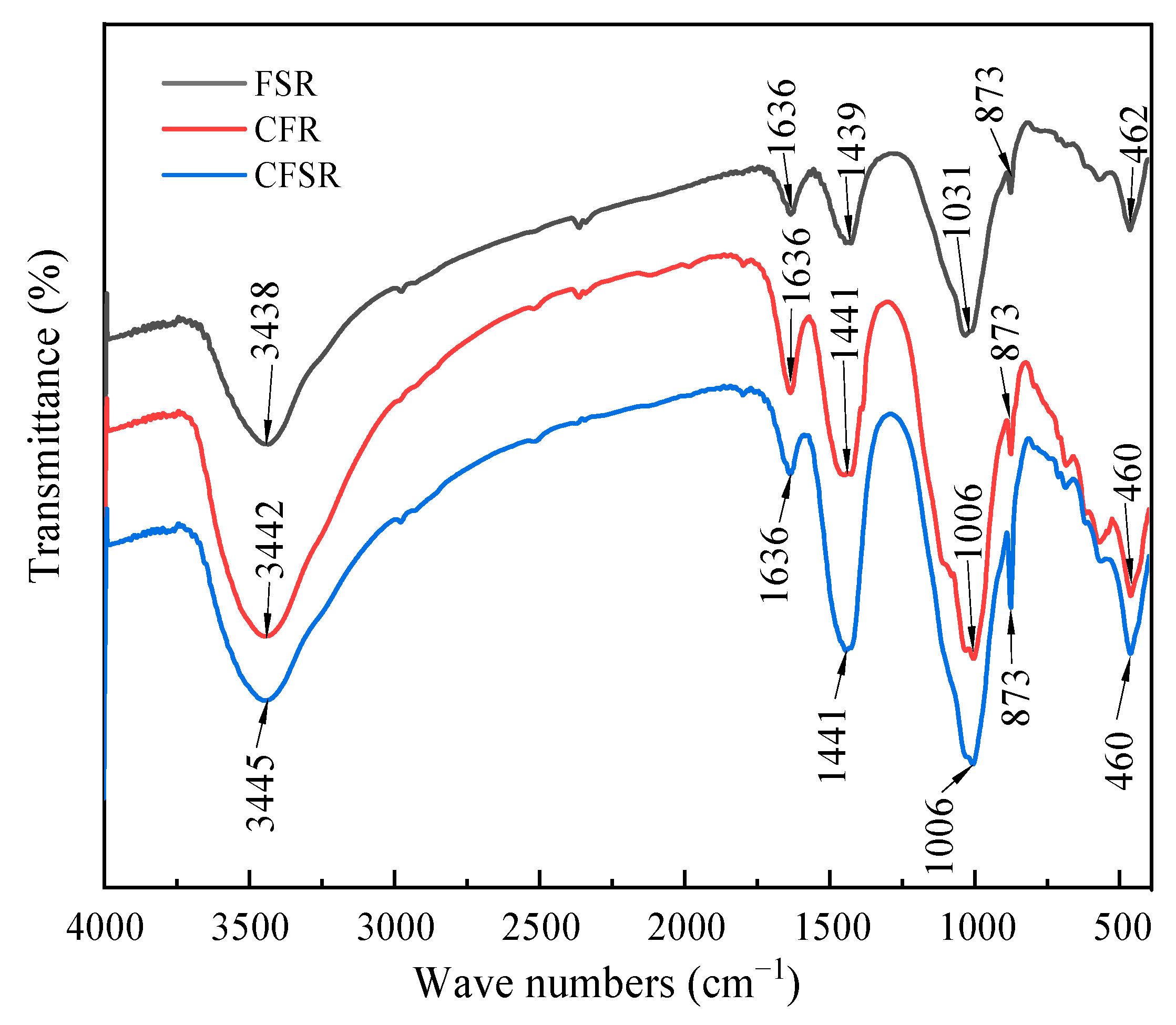
3.4. FTIR Analysis
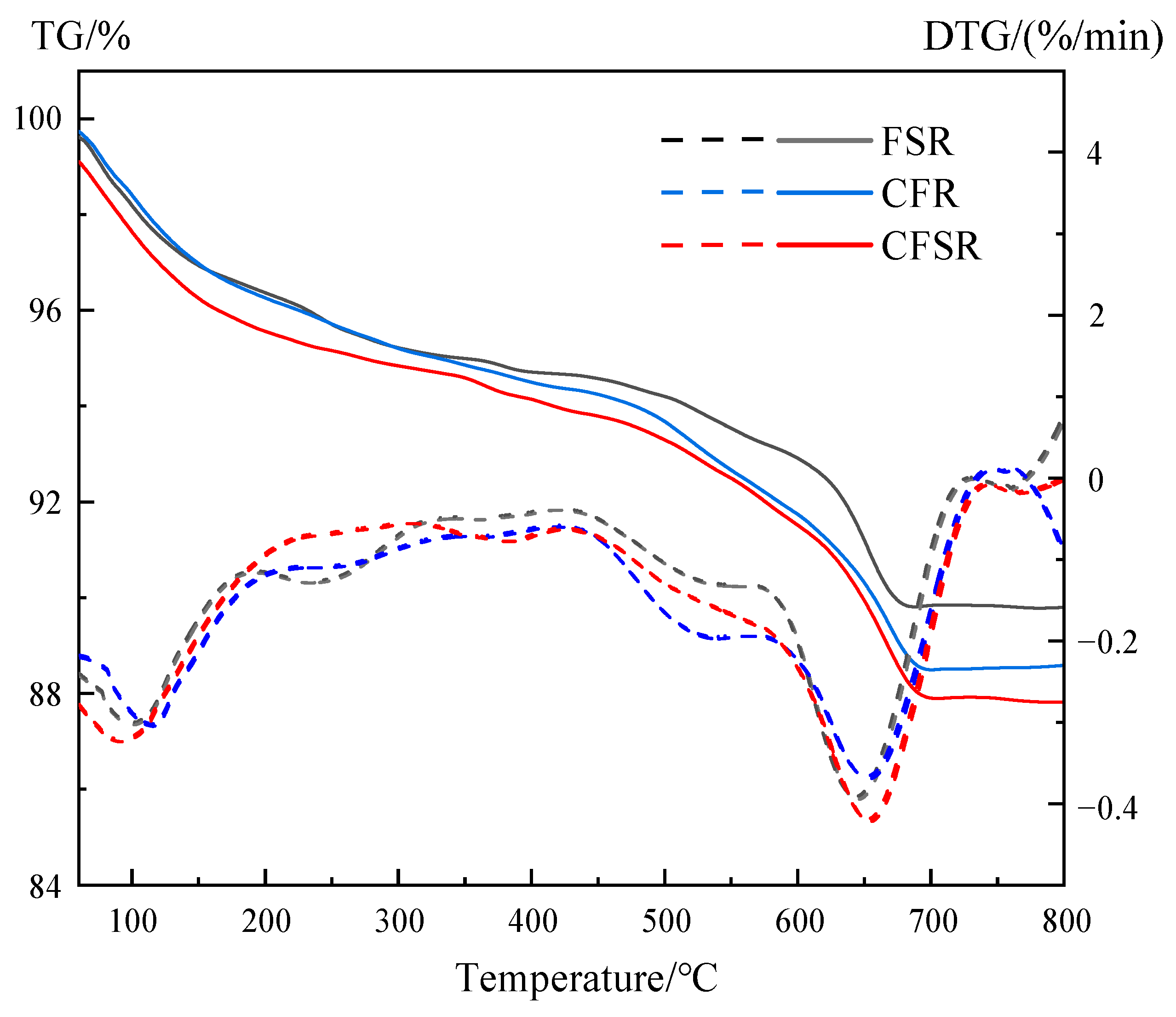
3.5. TG-DTG Analysis
3.6. Mercury Intrusion Porosimetry (MIP) Analysis
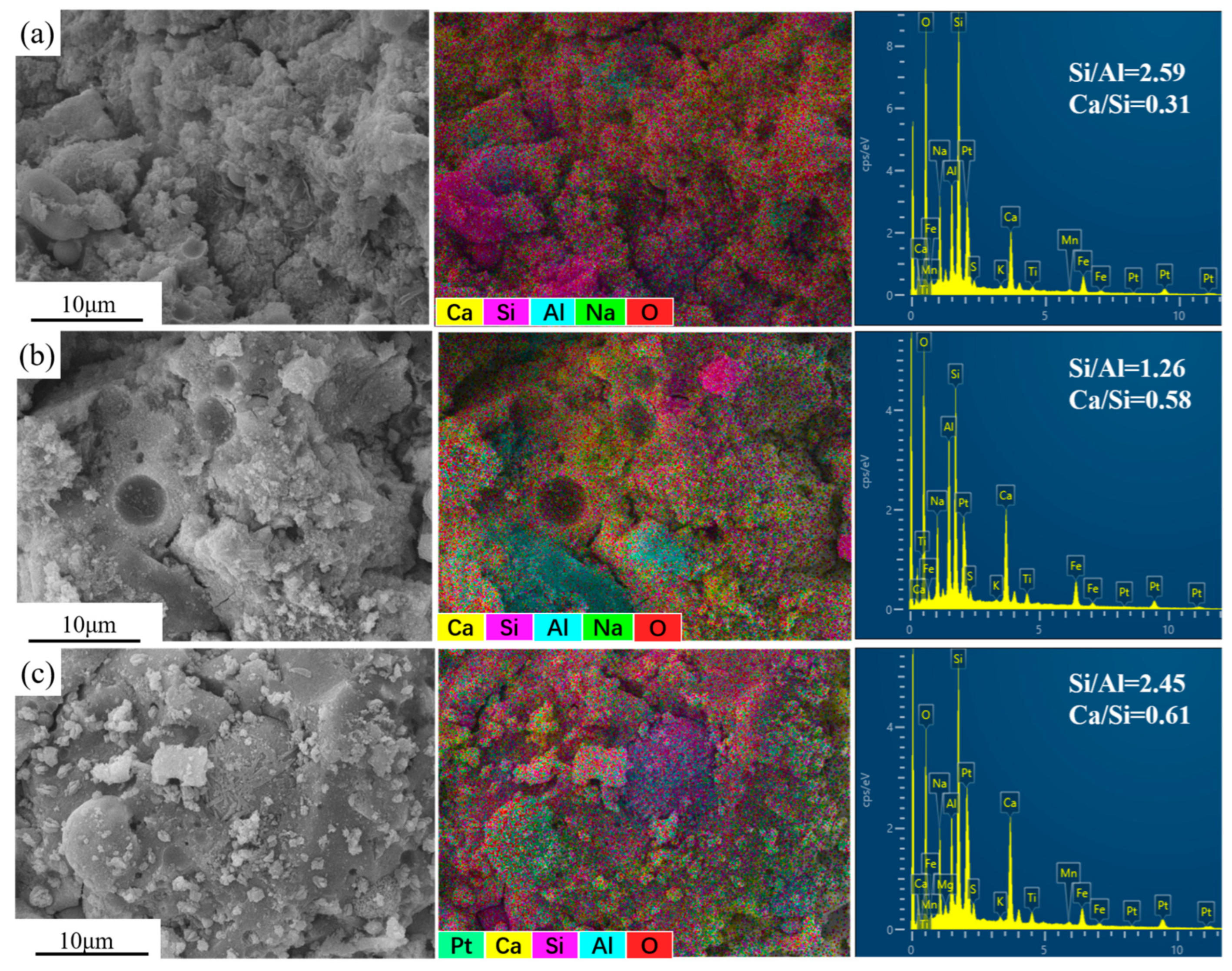
3.7. SEM-EDS Analysis
4. Conclusions
- Both SS substitution of partial RM and Ca(OH)2 incorporation increased the compressive strength of the matrix at all ages. When one of the factors is considered separately, the incorporation of Ca(OH)2 is more obvious than that of SS to replace part of the RM to improve the mechanical properties of the FA-RM geopolymer. When SS replaces 50% RM and 4% Ca(OH)2 is added, the 28 d compressive strength of the prepared geopolymer material reaches 30.6 MPa.
- The results of XRD, FTIR, TG-DTG, and SEM-EDS analysis demonstrated that both SS replacing part of the RM and Ca(OH)2 incorporation could promote the formation of more C(N)-A-S-H and C-S-H gels in the FA-based polymer matrix, as well as optimize the pore structure and reduce the porosity, which could significantly increase the compressive strength of the matrix.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gu, J.; Liu, X.; Zhang, Z. Road base materials prepared by multi-industrial solid wastes in China: A review. Constr. Build. Mater. 2023, 373, 130860. [Google Scholar] [CrossRef]
- Khairul, M.A.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Zhang, M.; El-Korchi, T.; Zhang, G.; Liang, J.; Tao, M. Synthesis factors affecting mechanical properties, microstructure, and chemical composition of red mud–fly ash-based geopolymers. Fuel 2014, 134, 315–325. [Google Scholar] [CrossRef]
- Ziada, M.; Tanyildizi, H.; Uysal, M. The influence of carbon nanotube on underwater geopolymer paste based on metakaolin and slag. Constr. Build. Mater. 2024, 414, 135047. [Google Scholar] [CrossRef]
- Huo, W.; Zhu, Z.; Zhang, J.; Kang, Z.; Pu, S.; Wan, Y. Utilization of OPC and FA to enhance reclaimed lime-fly ash macadam based geopolymers cured at ambient temperature. Constr. Build. Mater. 2021, 303, 124378. [Google Scholar] [CrossRef]
- He, J.; Jie, Y.; Zhang, J.; Yu, Y.; Zhang, G. Synthesis and characterization of red mud and rice husk ash-based geopolymer composites. Cem. Concr. Compos. 2013, 37, 108–118. [Google Scholar] [CrossRef]
- Liu, J.; Doh, J.H.; Ong, D.E.; Wang, S.; Yang, Y.; Dinh, H.L.; Zi, G. Correlation between dissolubilities of Si, Al, and Fe from aluminosilicate precursor and strength of fly ash-based geopolymer. Constr. Build. Mater. 2023, 393, 132107. [Google Scholar] [CrossRef]
- Power, G.; Gräfe, M.; Klauber, C. Bauxite residue issues: I. Current management, disposal and storage practices. Hydrometallurgy 2011, 108, 33–45. [Google Scholar] [CrossRef]
- Kumar, A.; Kumar, S. Development of paving blocks from synergistic use of red mud and fly ash using geopolymerization. Constr. Build. Mater. 2013, 38, 865–871. [Google Scholar] [CrossRef]
- Gräfe, M.; Klauber, C. Bauxite residue issues: IV. Old obstacles and new pathways for in situ residue bioremediation. Hydrometallurgy 2011, 108, 46–59. [Google Scholar] [CrossRef]
- Mudgal, M.; Singh, A.; Chouhan, R.K.; Acharya, A.; Srivastava, A.K. Fly ash red mud geopolymer with improved mechanical strength. Clean. Eng. Technol. 2021, 4, 100215. [Google Scholar] [CrossRef]
- Xie, T.; Ozbakkaloglu, T. Behavior of low-calcium fly and bottom ash-based geopolymer concrete cured at ambient temperature. Ceram. Int. 2015, 41, 5945–5958. [Google Scholar] [CrossRef]
- Soutsos, M.; Boyle, A.P.; Vinai, R.; Hadjierakleous, A.; Barnett, S.J. Factors influencing the compressive strength of fly ash based geopolymers. Constr. Build. Mater. 2016, 110, 355–368. [Google Scholar] [CrossRef]
- Hu, W.; Nie, Q.; Huang, B.; Shu, X.; He, Q. Mechanical and microstructural characterization of geopolymers derived from red mud and fly ashes. J. Clean. Prod. 2018, 186, 799–806. [Google Scholar] [CrossRef]
- Diaz, E.I.; Allouche, E.N.; Eklund, S. Factors affecting the suitability of fly ash as source material for geopolymers. Fuel 2010, 89, 992–996. [Google Scholar] [CrossRef]
- Alexander, A.E.; Shashikala, A.P. Studies on the microstructure and durability characteristics of ambient cured FA-GGBS based geopolymer mortar. Constr. Build. Mater. 2022, 347, 128538. [Google Scholar] [CrossRef]
- Khan, M.S.H.; Castel, A.; Akbarnezhad, A.; Foster, S.J.; Smith, M. Utilisation of steel furnace slag coarse aggregate in a low calcium fly ash geopolymer concrete. Cem. Concr. Res. 2016, 89, 220–229. [Google Scholar] [CrossRef]
- Jiang, Y.; Jia, L.J.; Weng, M.Y. Preparation of Alkali-activated Fly Ash/Steel Slag Cementitious Materials. Bull. Chin. Ceram. Soc. 2019, 38, 2152–2156; 2161. [Google Scholar]
- Niklioć, I.; Marković, S.; Janković–Častvan, I.; Radmilović, V.V.; Karanović, L.; Babić, B.; Radmilović, V.R. Modification of mechanical and thermal properties of fly ash-based geopolymer by the incorporation of steel slag. Mater. Lett. 2016, 176, 301–305. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Properties of low-calcium fly ash based geopolymer concrete incorporating OPC as partial replacement of fly ash. Constr. Build. Mater. 2017, 150, 792–807. [Google Scholar] [CrossRef]
- GB/T 17671-2021; Cement Mortar Strength Test Method (ISO Method). General Administration for National Market Supervision: Beijing, China, 2021.
- Vitor APaulo, N.; Borges, H.R. Recent advances in the reuse of steel slags and future perspectives as binder and aggregate for alkali-activated materials. Constr. Build. Mater. 2021, 281, 122605. [Google Scholar]
- Sun, J.; Zhang, Z.; Zhuang, S.; He, W. Hydration properties and microstructure characteristics of alkali–activated steel slag. Constr. Build. Mater. 2020, 241, 118141. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, D.W.; Li, L.I.; Wang, J.X.; Shao, N.N.; Wang, D.M. Microstructure and phase evolution of alkali-activated steel slag during early age. Constr. Build. Mater. 2019, 204, 158–165. [Google Scholar] [CrossRef]
- Temuujin, J.; van Riessen, A.; Williams, R. Influence of calcium compounds on the mechanical properties of fly ash geopolymer pastes. J. Hazard. Mater. 2009, 167, 82–88. [Google Scholar] [CrossRef]
- Wang, S.; Yu, L.; Huang, L.; Wu, K.; Yang, Z. Incorporating steel slag in the production of high heat resistant FA based geopolymer paste via pressure molding. J. Clean. Prod. 2021, 325, 129265. [Google Scholar] [CrossRef]
- Hao, X.; Liu, X.; Zhang, Z.; Zhang, W.; Lu, Y.; Wang, Y.; Yang, T. In-depth insight into the cementitious synergistic effect of steel slag and red mud on the properties of composite cementitious materials. J. Build. Eng. 2022, 52, 104449. [Google Scholar] [CrossRef]
- Lu, B.; Shi, C.; Zhang, J.; Wang, J. Effects of carbonated hardened cement paste powder on hydration and microstructure of Portland cement. Constr. Build. Mater. 2018, 186, 699–708. [Google Scholar] [CrossRef]
- Li, Q.; Yang, Y.; Yang, K.; Chao, Z.; Tang, D.; Tian, Y.; Wu, F.; Basheer, M.; Yang, C. The role of calcium stearate on regulating activation to form stable, uniform and flawless reaction products in alkali-activated slag cement. Cem. Concr. Compos. 2019, 103, 242–251. [Google Scholar] [CrossRef]
- Yang, J.; Zhang, D.; Hou, J.; He, B.; Xiao, B. Preparation of glass-ceramics from red mud in the aluminium industries. Ceram. Int. 2008, 34, 125–130. [Google Scholar] [CrossRef]
- Ylmén, R.; Jäglid, U.; Steenari, B.M.; Panas, I. Early hydration and setting of Portland cement monitored by IR, SEM and Vicat techniques. Cem. Concr. Res. 2009, 39, 433–439. [Google Scholar] [CrossRef]
- de Vargas, A.S.; Dal Molin, D.C.; Masuero, Â.B.; Vilela, A.C.; Castro-Gomes, J.; de Gutierrez, R.M. Strength development of alkali-activated fly ash produced with combined NaOH and Ca(OH)2 activators. Cem. Concr. Compos. 2014, 53, 341–349. [Google Scholar] [CrossRef]
- Heah, C.Y.; Kamarudin, H.; Al Bakri, A.M.; Bnhussain, M.; Luqman, M.; Nizar, I.K.; Ruzaidi, C.M.; Liew, Y.M. Study on solids-to-liquid and alkaline activator ratios on kaolin-based geopolymers. Constr. Build. Mater. 2012, 35, 912–922. [Google Scholar] [CrossRef]
- Bernal, S.A.; Provis, J.L.; Walkley, B.; San Nicolas, R.; Gehman, J.D.; Brice, D.G.; Kilcullen, A.R.; Duxson, P.; van Deventer, J.S. Gel nanostructure in alkali-activated binders based on slag and fly ash, and effects of accelerated carbonation. Cem. Concr. Res. 2013, 53, 127–144. [Google Scholar] [CrossRef]
- Burciaga-Díaz, O.; Escalante-García, J.I. Comparative performance of alkali activated slag/metakaolin cement pastes exposed to high temperatures. Cem. Concr. Compos. 2017, 84, 157–166. [Google Scholar] [CrossRef]
- Zhou, J.; Ye, G.; van Breugel, K. Characterization of pore structure in cement-based materials using pressurization–depressurization cycling mercury intrusion porosimetry (PDC-MIP). Cem. Concr. Res. 2010, 40, 1120–1128. [Google Scholar] [CrossRef]
- Diamond, S. The microstructure of cement paste and concrete––A visual primer. Cem. Concr. Compos. 2004, 26, 919–933. [Google Scholar] [CrossRef]
- Lodeiro, I.G.; Macphee, D.E.; Palomo, A.; Fernández-Jiménez, A. Effect of alkalis on fresh C–S–H gels. FTIR analysis. Cem. Concr. Res. 2009, 39, 147–153. [Google Scholar] [CrossRef]
- Liu, Y.; Ceng, X.; Wang, B.W. Preparation and Strength Mechanism of Alkali-Activated Fly Ash-Slag-Carbide Slag Based Geopolymer. Bull. Chin. Ceram. Soc. 2023, 42, 1353–1362. [Google Scholar]
- Huisheng, S.; Ming, X.; Xiaolu, G. Research Development on Mechanism of Fly Ash-based Geopolymer and Effect of Each Component. J. Chin. Ceram. Soc. 2013, 41, 972–980. [Google Scholar]
- Song, W.L.; Zhu, Z.D.; Pu, S.Y.; Song, S.; Peng, Y.; Gu, X.; Wei, Y. Mechanical Performance and Micro-mechanism of Alkali-activated Binary/Ternary Composite Industrial Waste Residues Cementitious Materials. Mater. Rep. 2020, 34, 22070–22077. [Google Scholar]



| Samples | SS | RM | FA | NaOH Pellet | Sodium Silicate | Ca(OH)2 | Sand | Water |
|---|---|---|---|---|---|---|---|---|
| FR | 0 | 225 | 225 | 18.83 | 141.49 | 0 | 1350 | 79.91 |
| FSR | 112.5 | 112.5 | 225 | 18.83 | 141.49 | 0 | 1350 | 79.91 |
| CFR | 0 | 225 | 225 | 18.83 | 141.49 | 18 | 1350 | 79.91 |
| CFSR | 112.5 | 112.5 | 225 | 18.83 | 141.49 | 18 | 1350 | 79.91 |
| Binder | SiO2 | Al2O3 | Fe2O3 | MgO | CaO | Na2O | K2O | SO3 | TiO2 | MnO | Other |
|---|---|---|---|---|---|---|---|---|---|---|---|
| FA | 48.47 | 21.11 | 8.40 | 2.11 | 9.15 | 0.74 | 0.98 | \ | 0.81 | 0.09 | 8.14 |
| RM | 13.49 | 20.39 | 30.58 | 0.32 | 15.82 | 9.15 | 0.19 | 0.66 | 7.80 | \ | 1.6 |
| SS | 26.95 | 5.66 | 24.15 | 5.33 | 29.67 | 0.09 | 0.08 | \ | 0.57 | 5.39 | 2.11 |
| Samples | Total Pore Volume (mL/g) | Average Pore Diameter (nm) | Porosity (%) | Pore Size Distribution (%) | ||
|---|---|---|---|---|---|---|
| <20 nm | 20–50 nm | >50 nm | ||||
| FSR | 0.31 | 37.23 | 42.82 | 10.63 | 48.79 | 40.58 |
| CFR | 0.29 | 19.07 | 41.98 | 39.72 | 29.99 | 30.29 |
| CFSR | 0.25 | 14.99 | 39.52 | 48.67 | 38.18 | 13.15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, P.; Chen, P.; Ming, Y.; Li, Q.; Dong, X. In-Depth Insight into the Effects of Steel Slag and Calcium Hydroxide on the Properties of a Fly Ash–Red Mud Geopolymer. Materials 2024, 17, 1249. https://doi.org/10.3390/ma17061249
Wang P, Chen P, Ming Y, Li Q, Dong X. In-Depth Insight into the Effects of Steel Slag and Calcium Hydroxide on the Properties of a Fly Ash–Red Mud Geopolymer. Materials. 2024; 17(6):1249. https://doi.org/10.3390/ma17061249
Chicago/Turabian StyleWang, Penghuai, Ping Chen, Yang Ming, Qing Li, and Xuanxuan Dong. 2024. "In-Depth Insight into the Effects of Steel Slag and Calcium Hydroxide on the Properties of a Fly Ash–Red Mud Geopolymer" Materials 17, no. 6: 1249. https://doi.org/10.3390/ma17061249




