Synergistic Activation of Electric Furnace Ferronickel Slag by Mechanical Grinding and Chemical Activators to Prepare Cementitious Composites
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Ferronickel Slag
2.1.2. Other Raw Materials and Additives
2.2. Exprerimental Methods
2.2.1. Preparation of Materials
2.2.2. Property Analysis of Materials
2.2.3. Characteristics Analysis
3. Results and Discussion
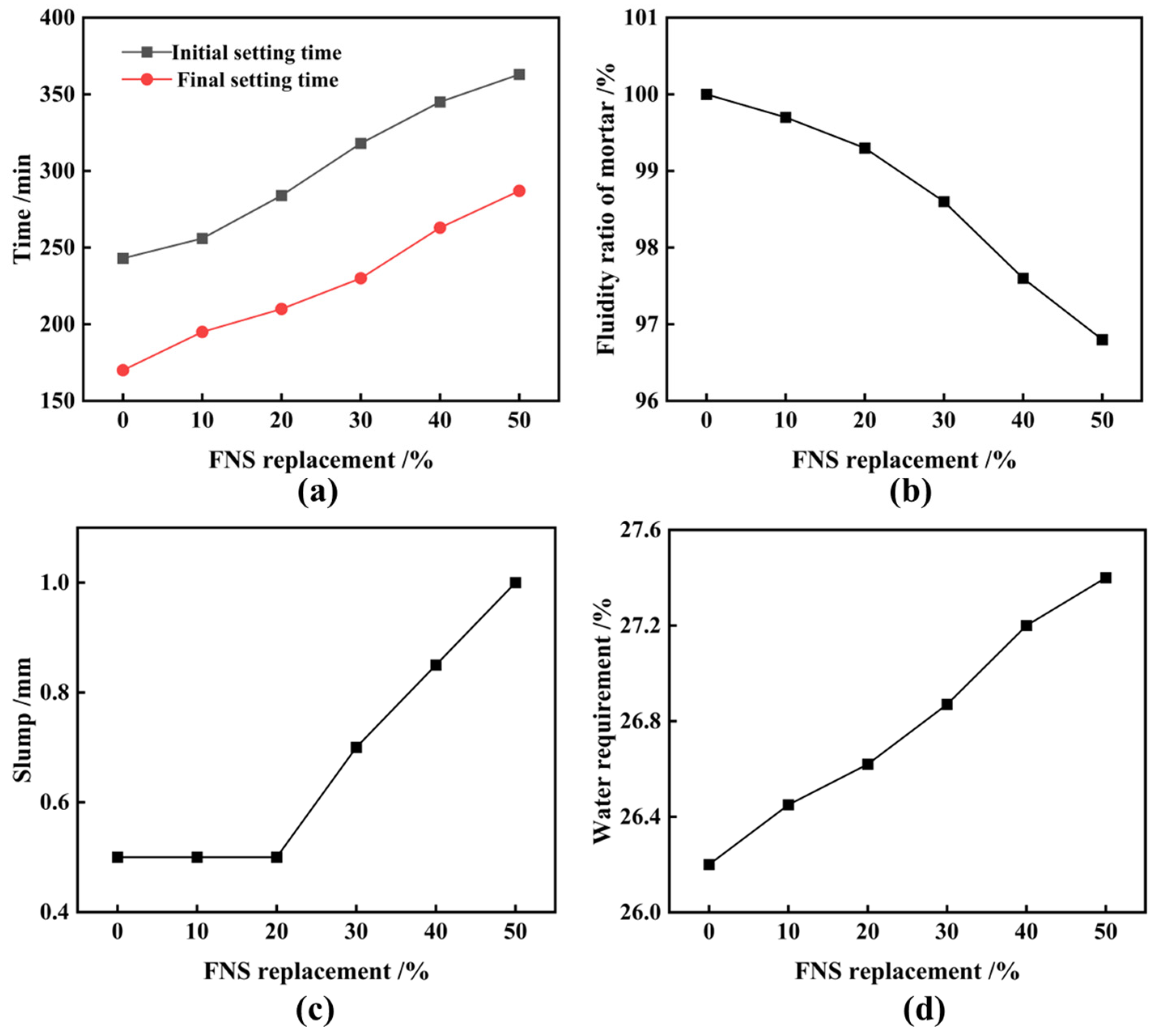
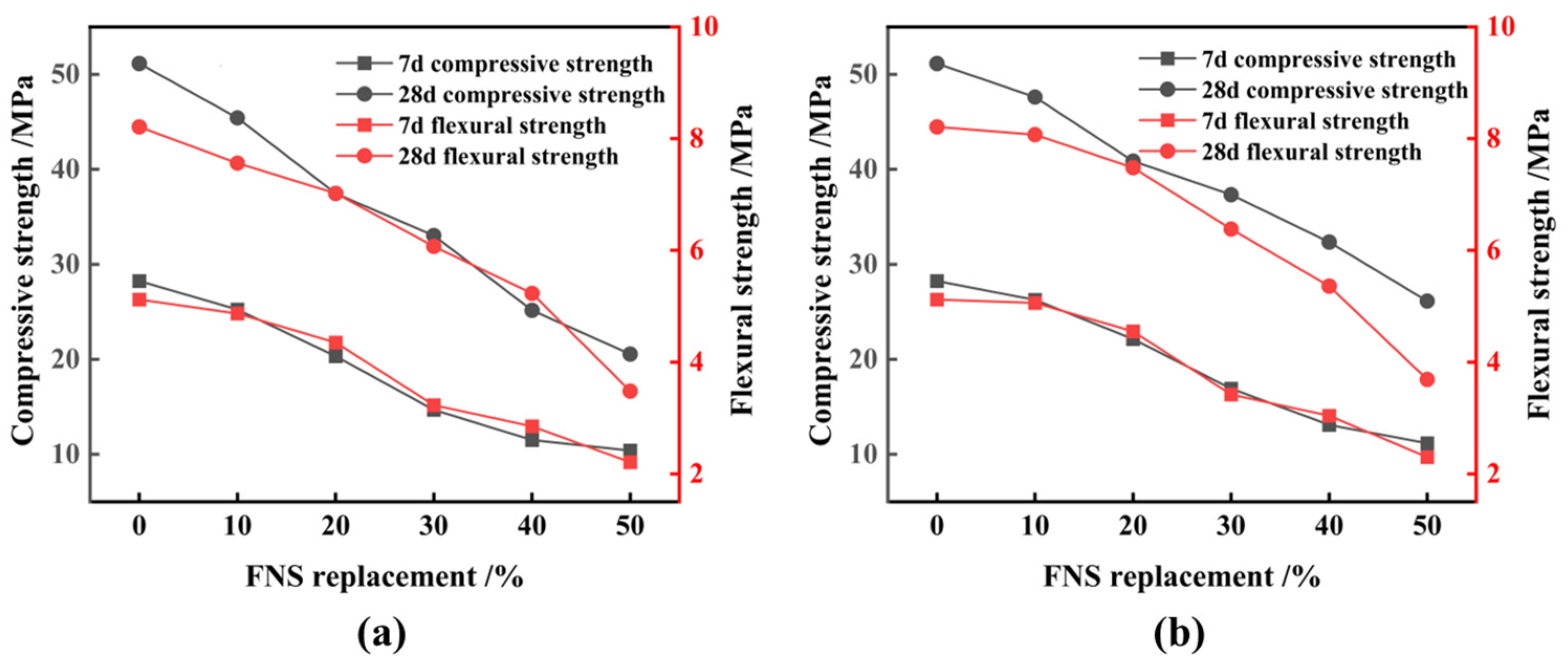
3.1. Ferronickel Slag Used as Cement Admixture
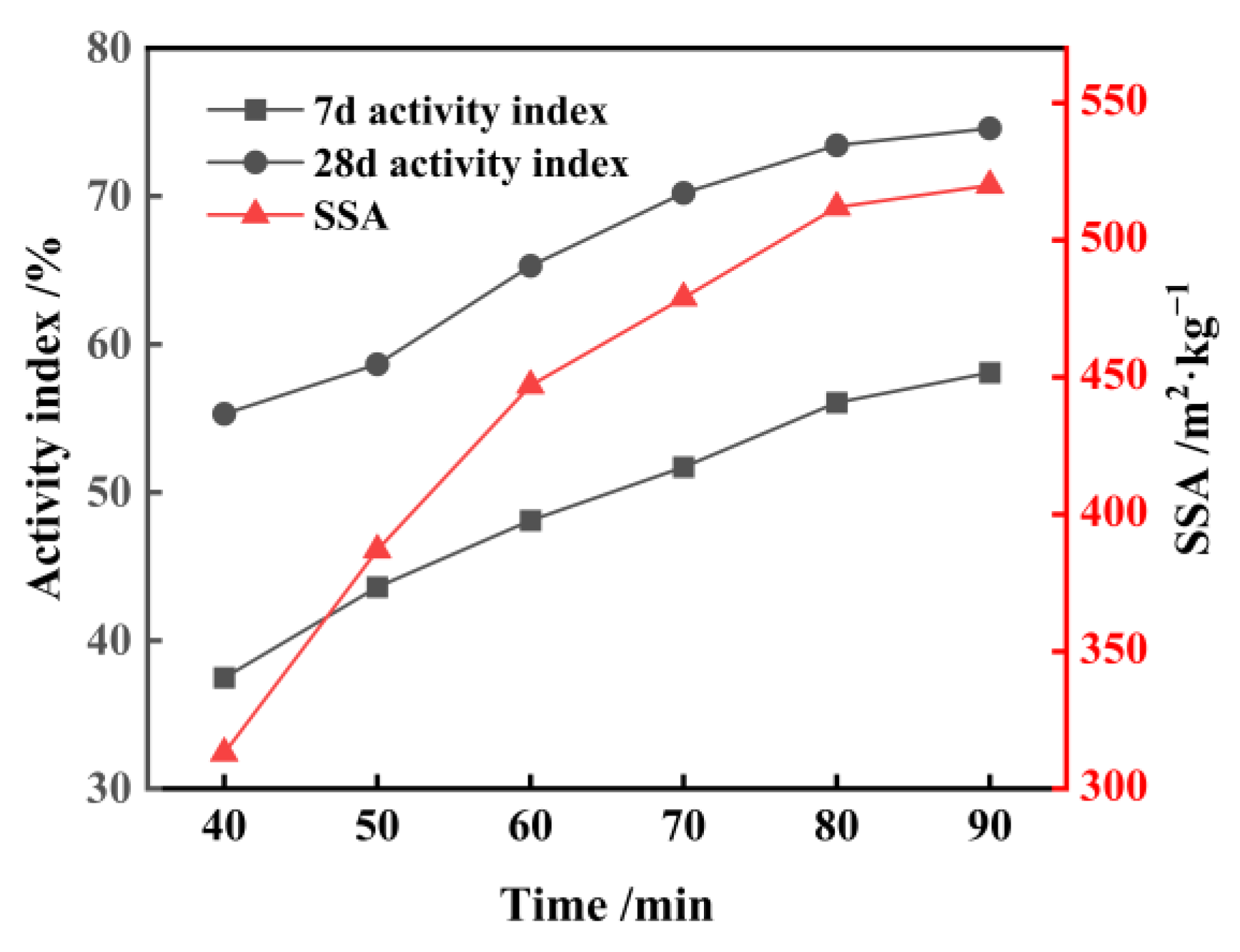
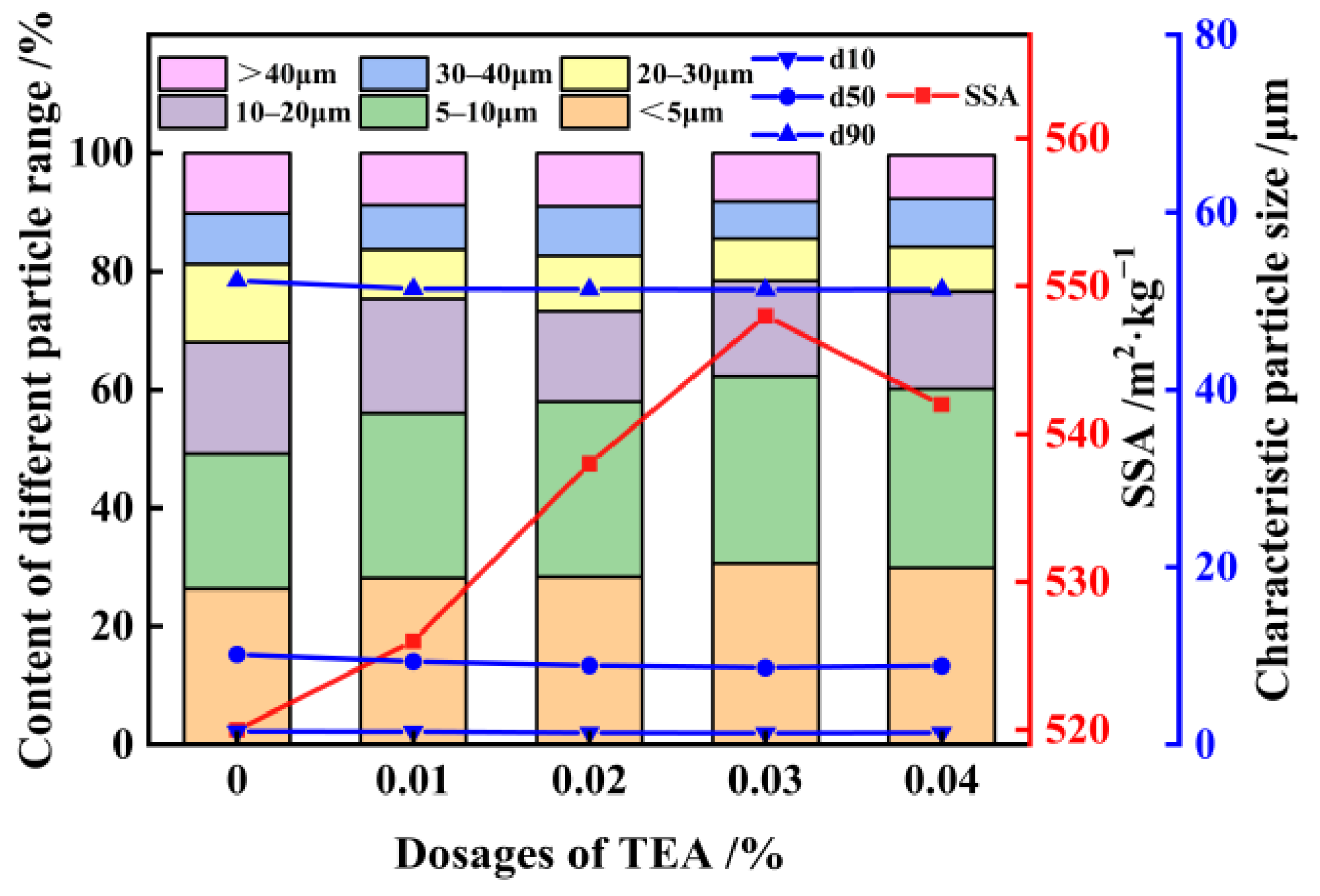
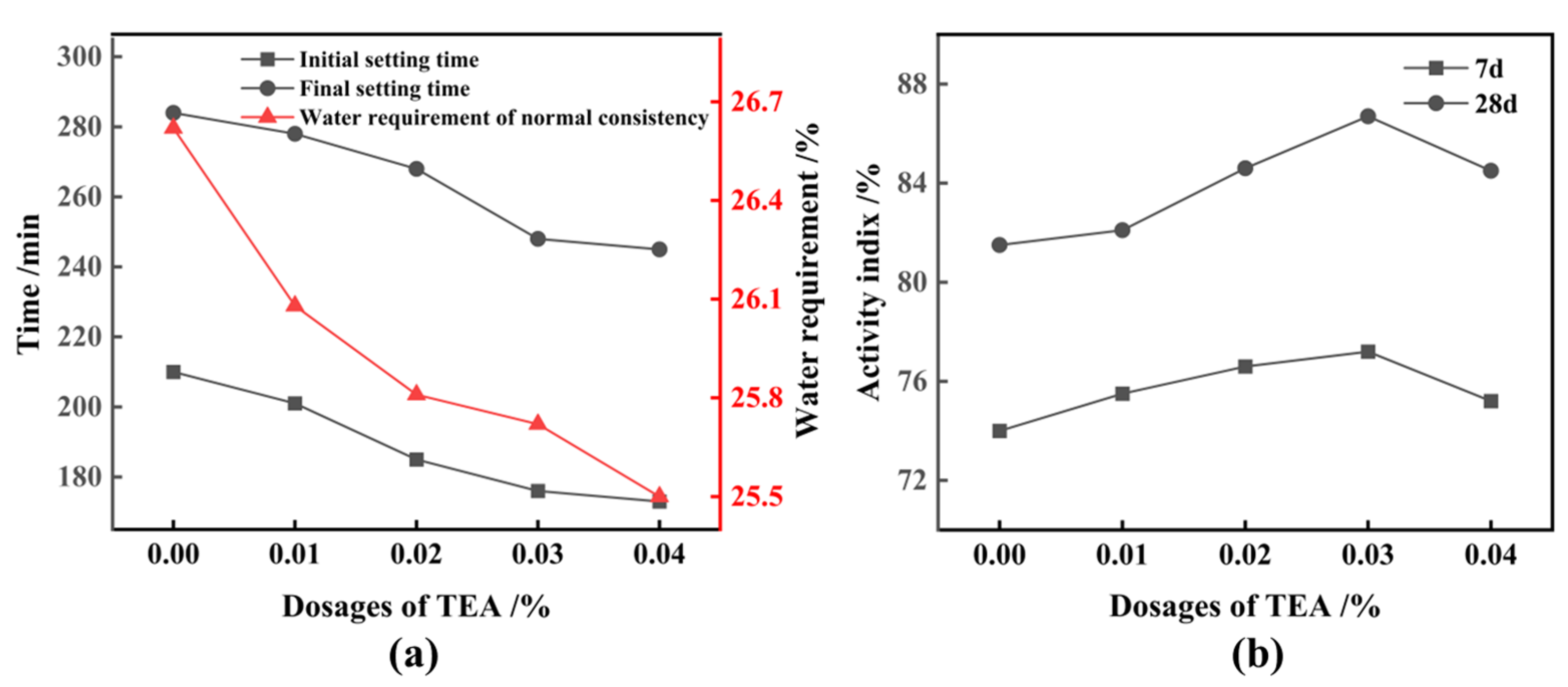
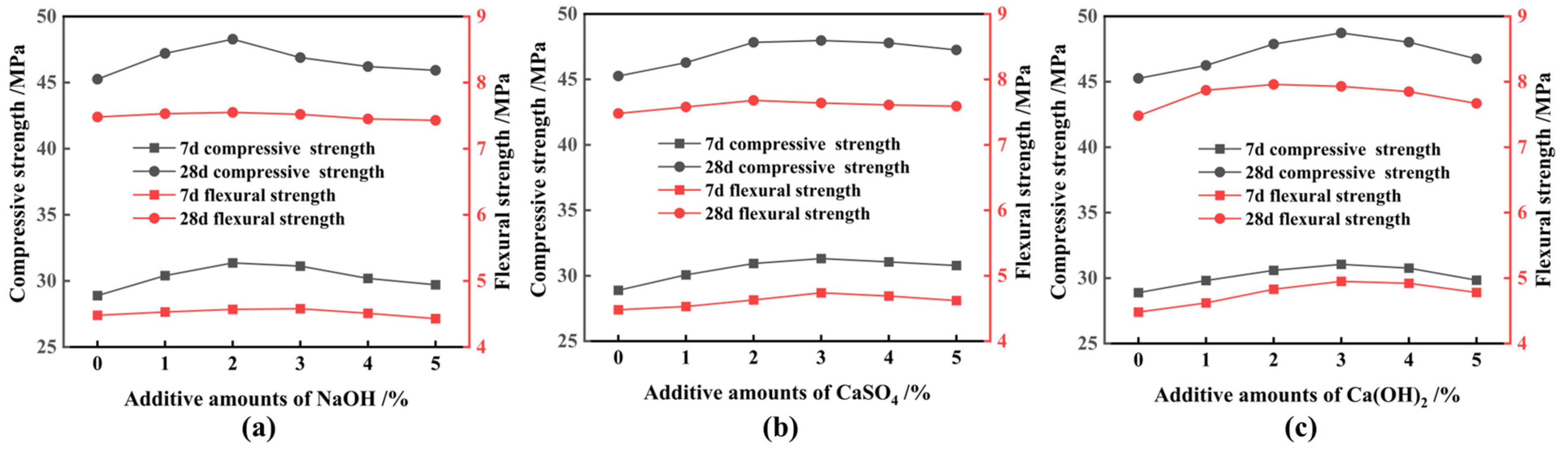
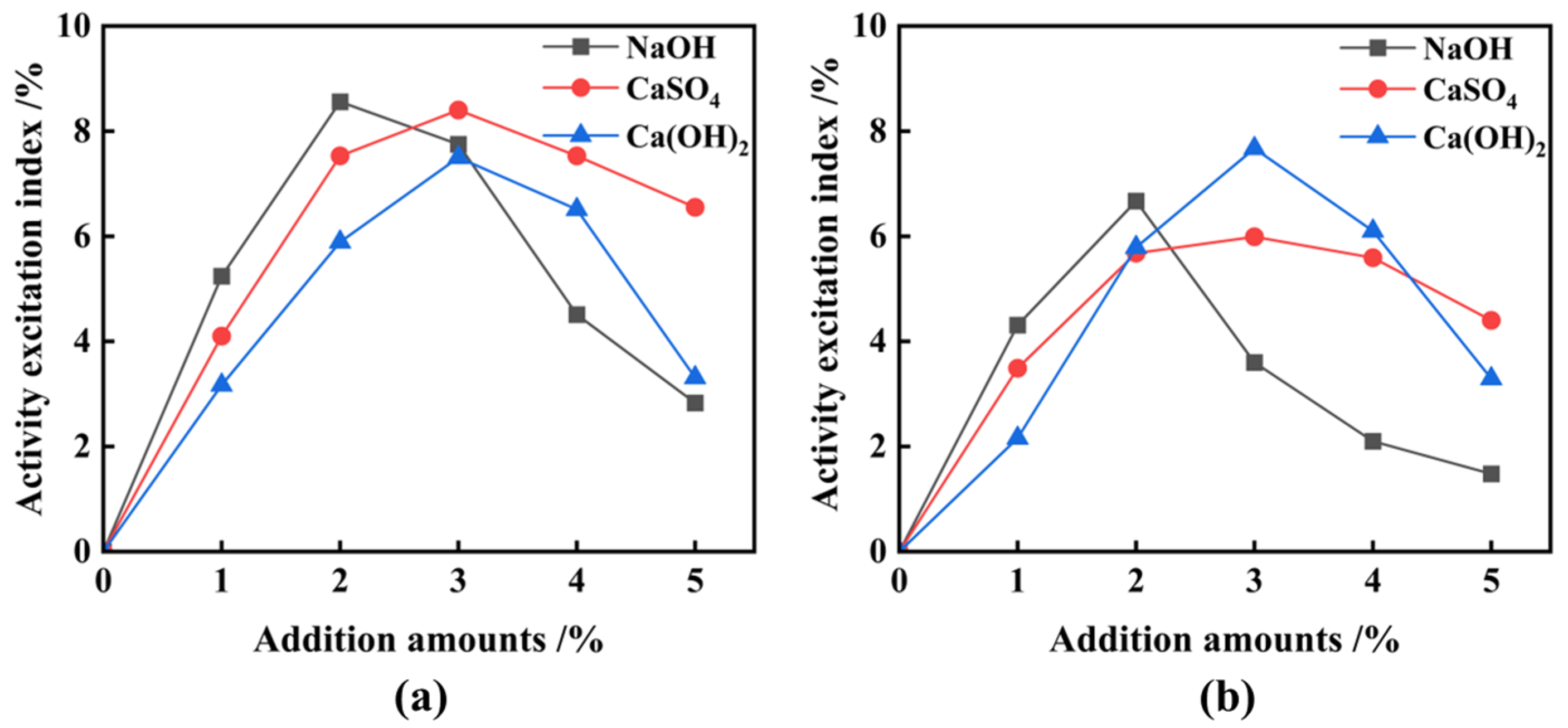
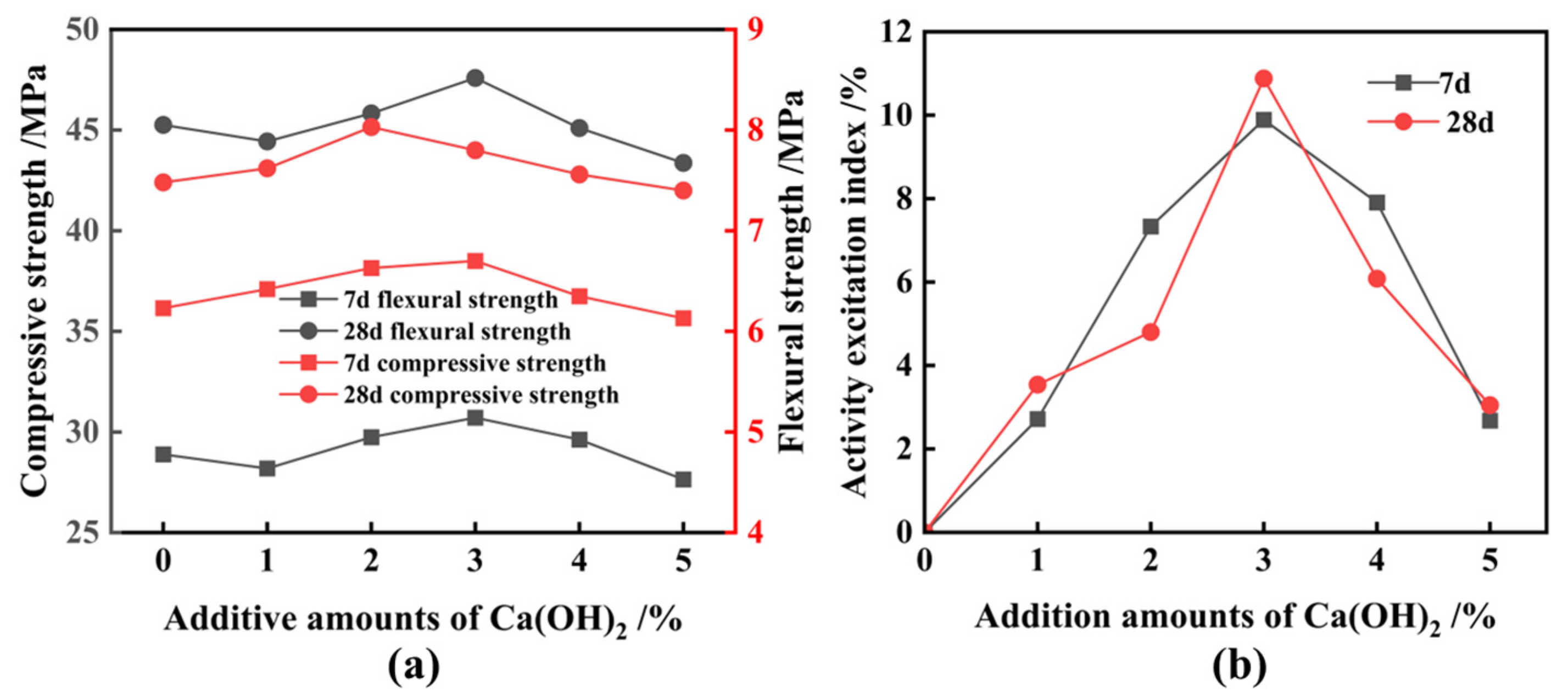
3.2. Activation of FNS
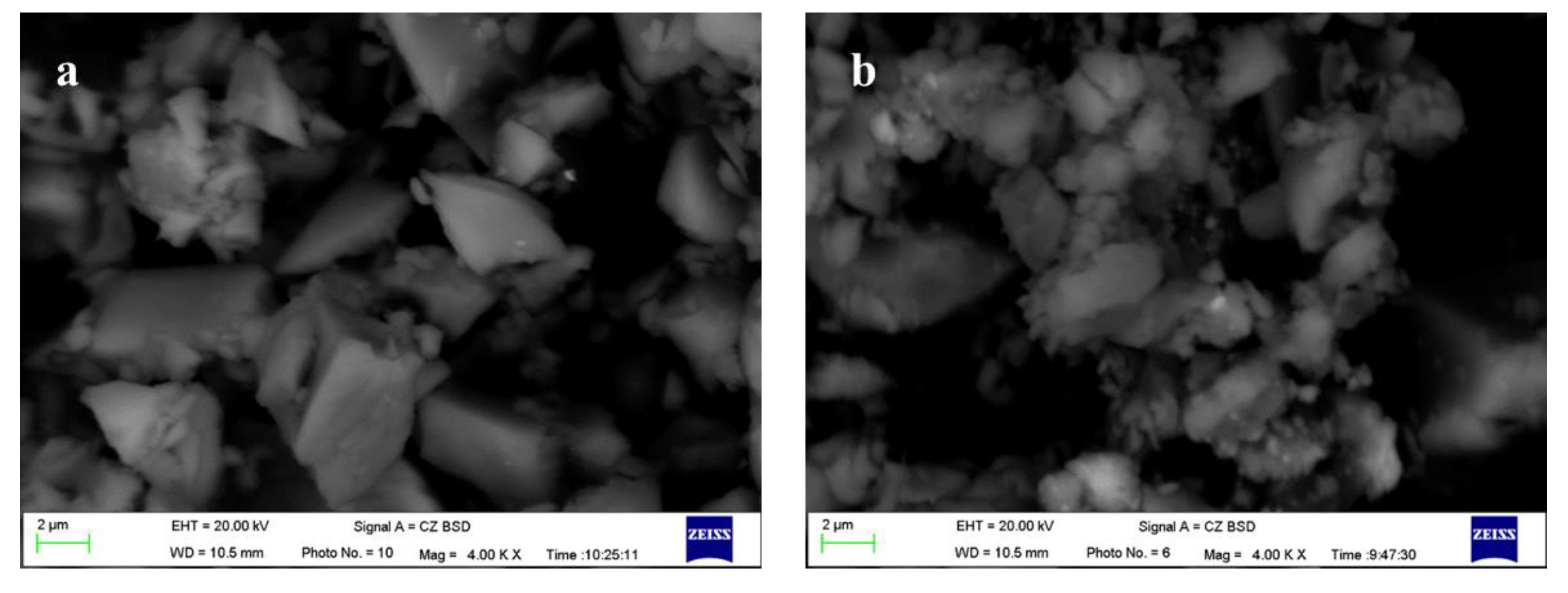
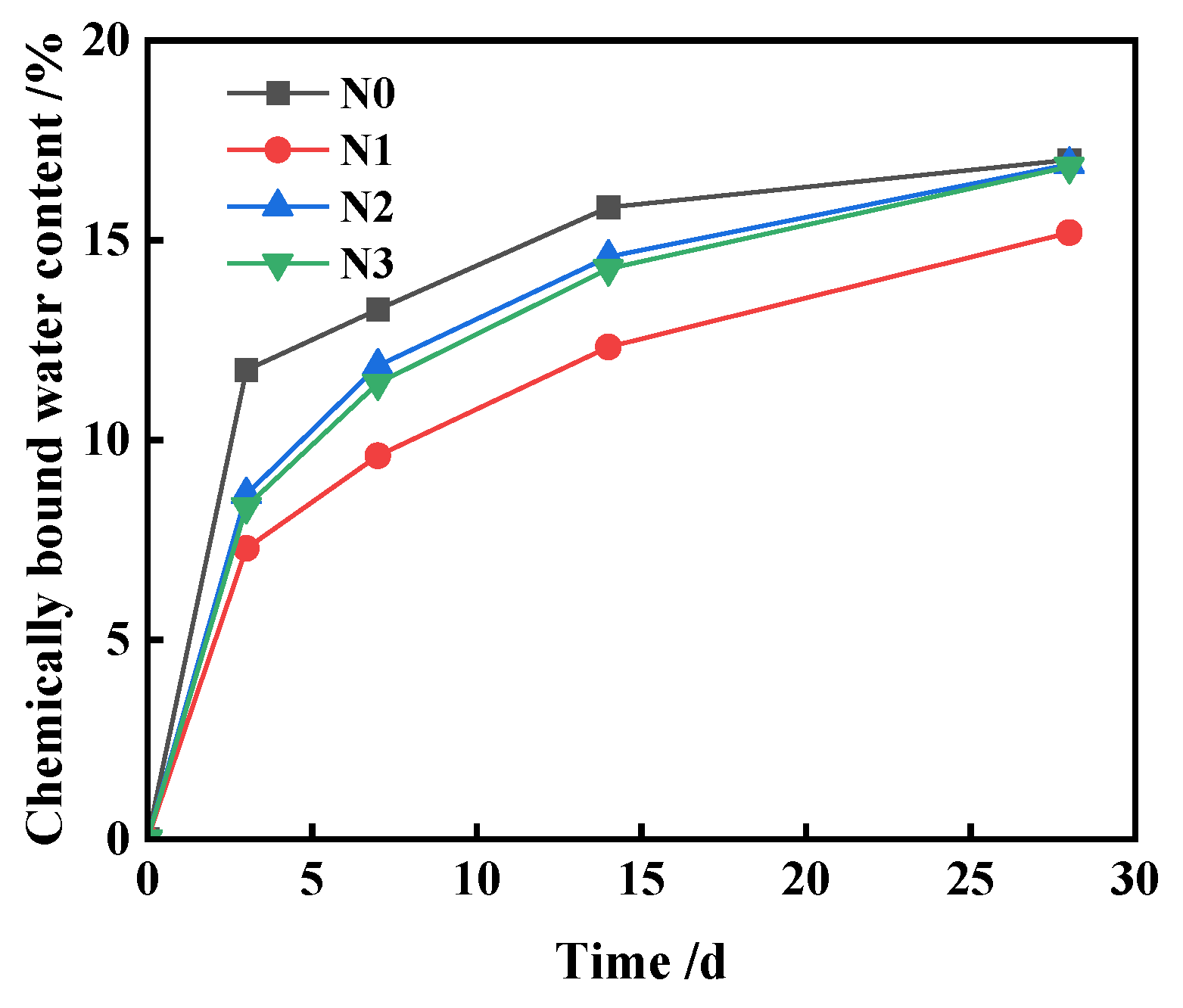
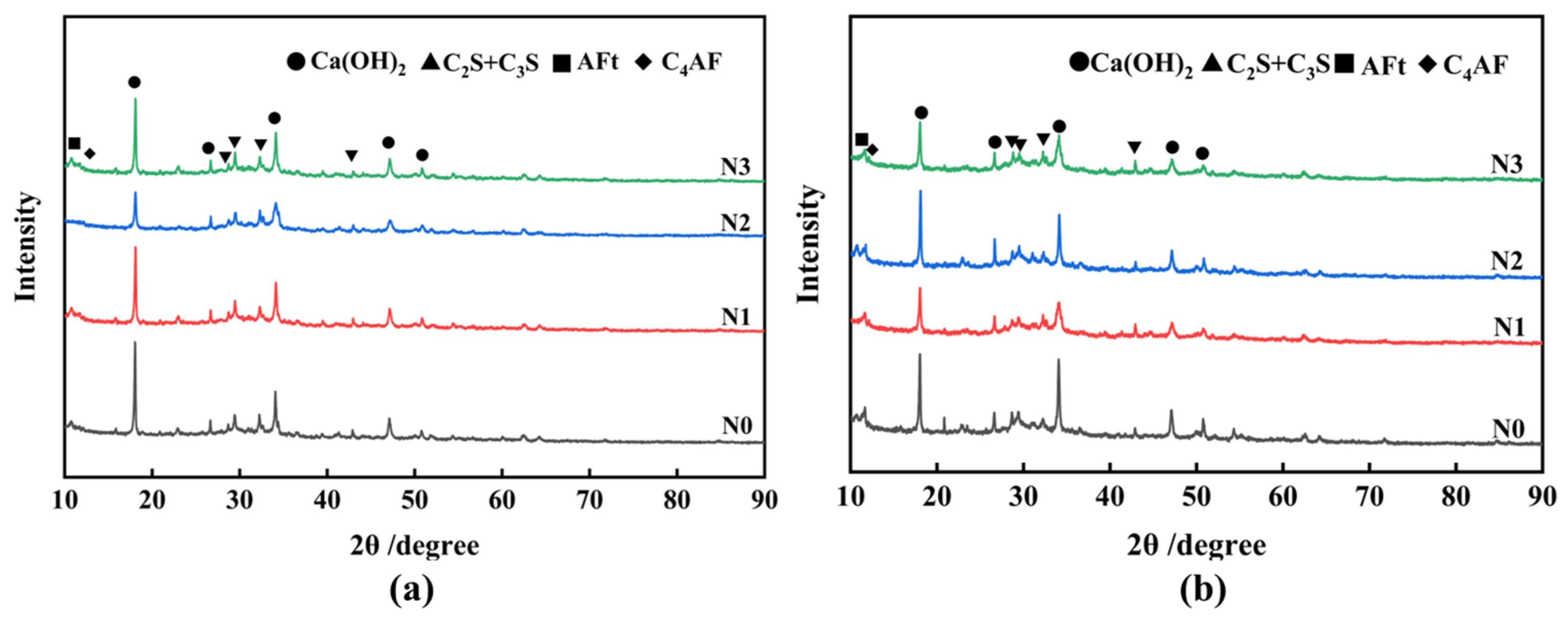
3.3. Analysis of Synergistic Hydration Mechanism
4. Conclusions
- Finely ground FNS powder with an SSA of 447 m2/kg or more can fulfill the activity requirements of cementitious composites. However, its replacement of cement should be limited to less than 10%. FNS does not compromise stability or exceed leaching toxicity standards. Nevertheless, it does increase the water requirement of normal consistency and the setting time of the cementitious composites while decreasing the material’s fluidity;
- Ca(OH)2 is the best chemical excitation elagent compared to NaOH and CaSO4; this is mainly due to it providing a high alkaline environment and amounts of calcium ions during the secondary hydration reaction, and the above-mentioned function is not provided simultaneously by NaOH or CaSO4;
- When FNS is subjected to mixed grinding with 3% Ca(OH)2 and 0.03% TEA additives for 81 min, resulting in a micropowder with an SSA of 522 m2/kg, which exhibits favorable properties when replacing 20% of the cement. The 7-day and 28-day activities of the FNS micropowder reach 81.0% and 95.1%, respectively, which is comparable to the activity of S95 slag powder. The FNS and 0.03% TEA additives were firstly ground for 82 min to obtain a micropowder with an SSA of 522 m2/kg, followed by activating with the addition of 3% Ca(OH)2; this FNS micropowder, however, cannot meet the standard of S95 slag powder. The synergistic activation effect of combining Ca(OH)2 and TEA additives during griding is found to be significant;
- The investigation into the hydration mechanism indicates that the increase in FNS activity is due to the improved alkalinity of the hydration environment caused by Ca(OH)2, the positive influence of TEA additives on the particle size and interfacial characteristics of FNS powder, and the associated synergistic effects of these two, which accelerate the depolymerization of the FNS vitreous body, promote the release of more calcium ions for secondary hydration reactions, and lead to the generation of additional hydration products, resulting in a denser slurry structure and higher strength.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cho, W.J.; Kim, M.J.; Kim, J.S. Study on the Pore Structure Characteristics of Ferronickel-Slag-Mixed Ternary-Blended Cement. Materials 2020, 13, 4863. [Google Scholar] [CrossRef] [PubMed]
- Guan, Q.F.; Xia, J.L.; Wang, J.; Leng, F.G.; Zhou, Y.X.; Cao, C.W. Recycling Blast Furnace Ferronickel Slag as a Replacement for Paste in Mortar: Formation of Carboaluminate, Reduction of White Portland Cement, and Increase in Strength. Materials 2021, 16, 2687. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Bartzas, G. Effect of sulphate and nitrate anions on heavy metal immobilisation in ferronickel slag geopolymers. Appl. Clay Sci. 2013, 73, 103–109. [Google Scholar] [CrossRef]
- Rahman, M.A.; Sarker, P.K.; Shaikh, F.U.A.; Saha, A.K. Soundness and compressive strength of Portland cement blended with ground granulated ferronickel slag. Constr. Build. Mater. 2017, 140, 194–202. [Google Scholar] [CrossRef]
- Chandra, K.J.; Kumar, S.P.; Ahmed, S.F.U. Sulphuric acid resistance of ground ferronickel slag blended fly ash geopolymer mortar. Constr. Build. Mater. 2021, 313, 125505. [Google Scholar]
- Kang, S.S.; Park, K.; Kim, D. Potential Soil Contamination in Areas Where Ferronickel Slag Is Used for Reclamation Work. Materials 2014, 7, 7157–7172. [Google Scholar] [CrossRef]
- Tang, H.M.; Peng, Z.W.; Gu, F.Q.; Ye, L.; Hwang, J.; Rao, M.J.; Li, G.H.; Jiang, T. Alumina-enhanced valorization of ferronickel slag into refractory materials under microwave irradiation. Ceram. Int. 2020, 46, 6828–6837. [Google Scholar] [CrossRef]
- Komnitsas, K.; Zaharaki, D.; Perdikatsis, V. Geopolymerisation of low calcium ferronickel slags. J. Mater. Sci. 2007, 42, 3073–3082. [Google Scholar] [CrossRef]
- Saha, A.K.; Sarker, P.K. Sustainable use of ferronickel slag fine aggregate and fly ash in structural concrete: Mechanical properties and leaching study. J. Clean. Prod. 2017, 162, 438–448. [Google Scholar] [CrossRef]
- Choi, Y.C.; Choi, S. Alkali-silica reactivity of cementitious materials using ferro-nickel slag fine aggregates produced in different cooling conditions. Constr. Build. Mater. 2015, 99, 279–287. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, M.; Sohn, J.; Park, H. Applicability of gold tailings, waste limestone, red mud, and ferronickel slag for producing glass fibers. J. Clean. Prod. 2018, 203, 957–965. [Google Scholar] [CrossRef]
- Dana, A.; Iluti, V.; Claudiu, A. Metallurgical Wastes as Resources for Sustainability of the Steel industry. Materials 2022, 14, 5488. [Google Scholar]
- Nuruzzaman, M.; Casimiro, J.O.C.; Sarker, P.K. Fresh and hardened properties of high strength self-compacting concrete using by-product ferronickel slag fine aggregate. J. Build. Eng. 2020, 32, 101686. [Google Scholar] [CrossRef]
- Duan, X.Q.; Lu, S.Y.; Jiang, X.C.; Liu, T.; Yang, H.F. Preparation of Silicon Carbide Powder from Amorphous Silica and Investigation of Synthesis Mechanism. Minerals 2024, 14, 189. [Google Scholar] [CrossRef]
- Saha, A.K.; Khan, M.N.N.; Sarker, P.K. Value added utilization of by-product electric furnace ferronickel slag as construction materials: A review. Resour. Conserv. Recycl. 2018, 134, 10–24. [Google Scholar] [CrossRef]
- Zulhan, Z.; Agustina, N. A novel utilization of ferronickel slag as a source of magnesium metal and ferroalloy production. J. Clean. Prod. 2021, 292, 125307. [Google Scholar] [CrossRef]
- Kim, H.; Lee, C.H.; Ann, K.Y. Feasibility of ferronickel slag powder for cementitious binder in concrete mix. Constr. Build. Mater. 2019, 207, 693–705. [Google Scholar] [CrossRef]
- Han, F.H.; Zhang, H.B.; Li, Y.C.; Zhang, Z.Q. The Laterite Nickel Ore Smelting Slag Used as Cement Admixture. J. Clean. Prod. 2023, 414, 137633. [Google Scholar] [CrossRef]
- Song, L.Q.; Wang, F.; Cai, X.T.; Nie, W.H.; Wang, J.H. Research on the Performance of Nickel-iron Slag as Cement Mixture. Cem. Technol. 2016, 4, 34–37. [Google Scholar]
- Tian, Q.; Qu, M.J.; Yao, T.S.; Qi, S.; Wang, C.; Ruan, M.Y. Coupling Effect of Chemical Excitation and Heat Treatment on Active Excitation of Recycled Powder. Bull. Chin. Ceram. Soc. 2023, 42, 1400–1408. [Google Scholar]
- Maragkos, I.; Giannopoulou, I.P.; Panias, D. Synthesis of ferronickel slag-based geopolymers. Miner Eng. 2009, 22, 196–203. [Google Scholar] [CrossRef]
- Chen, F.; Tong, S.H.; Lai, W.T. Environment Impermeability of Cement Soil Mixed with Ferronickel Slag Powder in Seawater. J. Build. Mater. 2022, 25, 1190–1194. [Google Scholar]
- Komnitsas, K.; Bartzas, G.; Karmali, V.; Petrakis, E.; Kurylak, W.; Pietek, G.; Kanasiewicz, J. Assessment of Alkali Activation Potential of a Polish Ferronickel Slag. Sustainability 2019, 11, 1863. [Google Scholar] [CrossRef]
- Rosales, J.; Agrela, F.; Diaz-López, J.L.; Cabrera, M. Alkali-Activated Stainless Steel Slag as a Cementitious Material in the Manufacture of Self-Compacting Concrete. Materials 2021, 14, 3945. [Google Scholar] [CrossRef]
- Lu, N.; Ran, X.; Pan, Z.; Korayem, A.H. Use of Municipal Solid Waste Incineration Fly Ash in Geopolymer Masonry Mortar Manufacturing. Materials 2022, 15, 8689. [Google Scholar] [CrossRef] [PubMed]
- GB/T18046-2017; Ground Granulated Blast Furnace Slag Used for Cement, Mortar and Concrete. Standards Press of China: Beijing, China, 2017.
- GB/T17671-2021; Test Method of Cement Mortar Strength (ISO Method). Standards Press of China: Beijing, China, 2021.
- GB-175; Common Portland Cement. Standards Press of China: Beijing, China, 2007.
- GB/T12957-2005; Test Method for Activity of Industrial Waste Slag Used as Addition to Cement. China Architecture and Building Press: Beijing, China, 2005.
- GB/T2847-2022; Pozzolanic Materials Used for Cement Production. China Architecture and Building Press: Beijing, China, 2022.
- GB/T1346-2011; Test Methods for Water Requirement of Normal Consistency, Setting Time and Soundness of the Portland Cement. China Architecture and Building Press: Beijing, China, 2011.
- GB/T2419-2005; Test Method for Fluidity of Cement Mortar. China Architecture and Building Press: Beijing, China, 2005.
- GB/T30810-2014; Method for Determination of Leachable Heavy Metals in Cement Mortar. China Architecture and Building Press: Beijing, China, 2014.
- ISO 679:2009; Cement-Test Methods-Determination of Strength. The International Organization for Standardization: Geneva, Switzerland, 2009.
- Park, B.; Choi, Y.C. Effects of fineness and chemical activators on the hydration and physical properties of high-volume fly-ash cement pastes. J. Build. Eng. 2022, 51, 104274. [Google Scholar] [CrossRef]
- Bulejko, P.; Šuleková, N.; Vlasák, J.; Tuunila, R.; Kinnarinen, T.; Svěrák, T.; Häkkinen, A. Ultrafine wet grinding of corundum in the presence of triethanolamine. Powder Technol. 2022, 395, 556–561. [Google Scholar] [CrossRef]
- Xie, H.; Liu, X.; Zheng, Y.S.; Chi, B.C.; Guo, J.; Dai, X.Q.; Zhang, Z.W.; Sun, M.Q.; Duan, L.Q.; Wang, Z.M.; et al. Effect of complexation of alkanolamine in accelerators on the initial stage of cement hydration. Constr. Build. Mater. 2023, 393, 132105. [Google Scholar] [CrossRef]
- Kirchberger, I.; Goetz-Neunhoeffer, F.; Neubauer, J. Enhancing the aluminate reaction during OPC hydration by combining increased sulfate content, triethanolamine and tartaric acid. Cem. Concr. Res. 2023, 170, 107188. [Google Scholar] [CrossRef]
- Seongwoo, G.; Cheol, C.Y.; Myoungsu, S. Effect of plant cellulose microfibers on hydration of cement composites. Constr. Build. Mater. 2021, 267, 121734. [Google Scholar]
- Kapeluszna, E.; Kotwica, L. The Effect of Various Grinding Aids on the Properties of Cement and Its Compatibility with Acrylate-Based Superplasticizer. Materials 2022, 15, 614. [Google Scholar] [CrossRef] [PubMed]
- Yaphary, Y.L.; Yu, Z.C.; Lam, R.H.W.; Lau, D. Effect of triethanolamine on cement hydration toward initial setting time. Constr. Build. Mater. 2023, 16, 7041. [Google Scholar] [CrossRef]
- Lee, T.; Lee, J.; Choi, H.; Lee, D.E. The Effects of Fineness and TEA-Based Chemical Admixture on Early Strength Development of Concrete in Construction Site Applications. Materials 2020, 13, 2027. [Google Scholar] [CrossRef]
- Jiang, J.; Ye, Z.M.; Wu, J.M.; Yang, Q.C.; Li, Q.L.; Kong, X.M. Impact of triethanolamine on the hydration of Portland cement in the presence of high pozzolanic activity supplementary cementitious materials. Cem. Concr. Compos. 2024, 147, 105435. [Google Scholar] [CrossRef]
- Schade, T.; Bellmann, F.; Middendorf, B. Quantitative analysis of C-(K)-A-S-H-amount and hydrotalcite phase content in finely ground highly alkali-activated slag/silica fume blended cementitious material. Cem. Concr. Res. 2022, 153, 106706. [Google Scholar] [CrossRef]
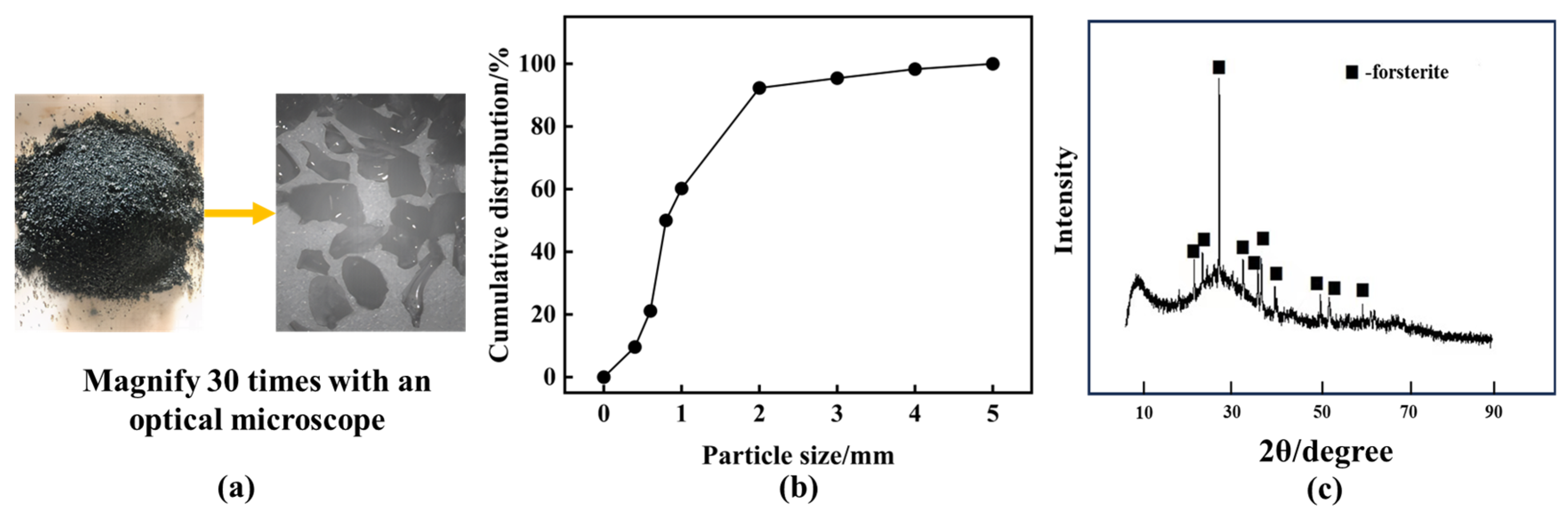


| Oxide | SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 |
|---|---|---|---|---|---|---|
| Content | 48.17 | 4.10 | 6.89 | 1.10 | 37.43 | 0.07 |
| Oxide | K2O | TiO2 | MnO | Cr2O3 | NiO | Na2O |
| Content | 0.05 | 0.05 | 1.04 | 1.25 | 0.03 | 0.06 |
| Sample Number | Cement Content/% | FNS Replacement/% | SSA/m2·kg−1 | Dosages of TEA/% | Additions of Ca(OH)2/% | Grinding Time/min | Adding Way of Ca(OH)2 |
|---|---|---|---|---|---|---|---|
| N0 | 100 | 0 | - | - | - | - | - |
| N1 | 80 | 20 | 520 | 0 | 0 | 90 | - |
| N2 | 80 | 20 | 548 | 3 | 3 | 90 | During grinding |
| N3 | 80 | 20 | 522 | 3 | 3 | 81 | After grinding |
| Hydration Age | 7 d | 28 d | ||||||
|---|---|---|---|---|---|---|---|---|
| Test block | N0 | N1 | N2 | N3 | N0 | N1 | N2 | N3 |
| Test result | 2.12 | 1.83 | 1.85 | 1.89 | 2.38 | 1.63 | 1.61 | 1.53 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, Y.; Duan, X.; Li, B.; Lu, S.; Liu, T.; Li, Y. Synergistic Activation of Electric Furnace Ferronickel Slag by Mechanical Grinding and Chemical Activators to Prepare Cementitious Composites. Materials 2024, 17, 1247. https://doi.org/10.3390/ma17061247
Jiang Y, Duan X, Li B, Lu S, Liu T, Li Y. Synergistic Activation of Electric Furnace Ferronickel Slag by Mechanical Grinding and Chemical Activators to Prepare Cementitious Composites. Materials. 2024; 17(6):1247. https://doi.org/10.3390/ma17061247
Chicago/Turabian StyleJiang, Yanjun, Xuqin Duan, Bohua Li, Shuaiyu Lu, Tong Liu, and Yunyun Li. 2024. "Synergistic Activation of Electric Furnace Ferronickel Slag by Mechanical Grinding and Chemical Activators to Prepare Cementitious Composites" Materials 17, no. 6: 1247. https://doi.org/10.3390/ma17061247





