Corrosion Degradation Mechanism of Cr-Coated Zr-4 Alloy under Simulated Nuclear Conditions for Accident-Tolerant Fuel
Abstract
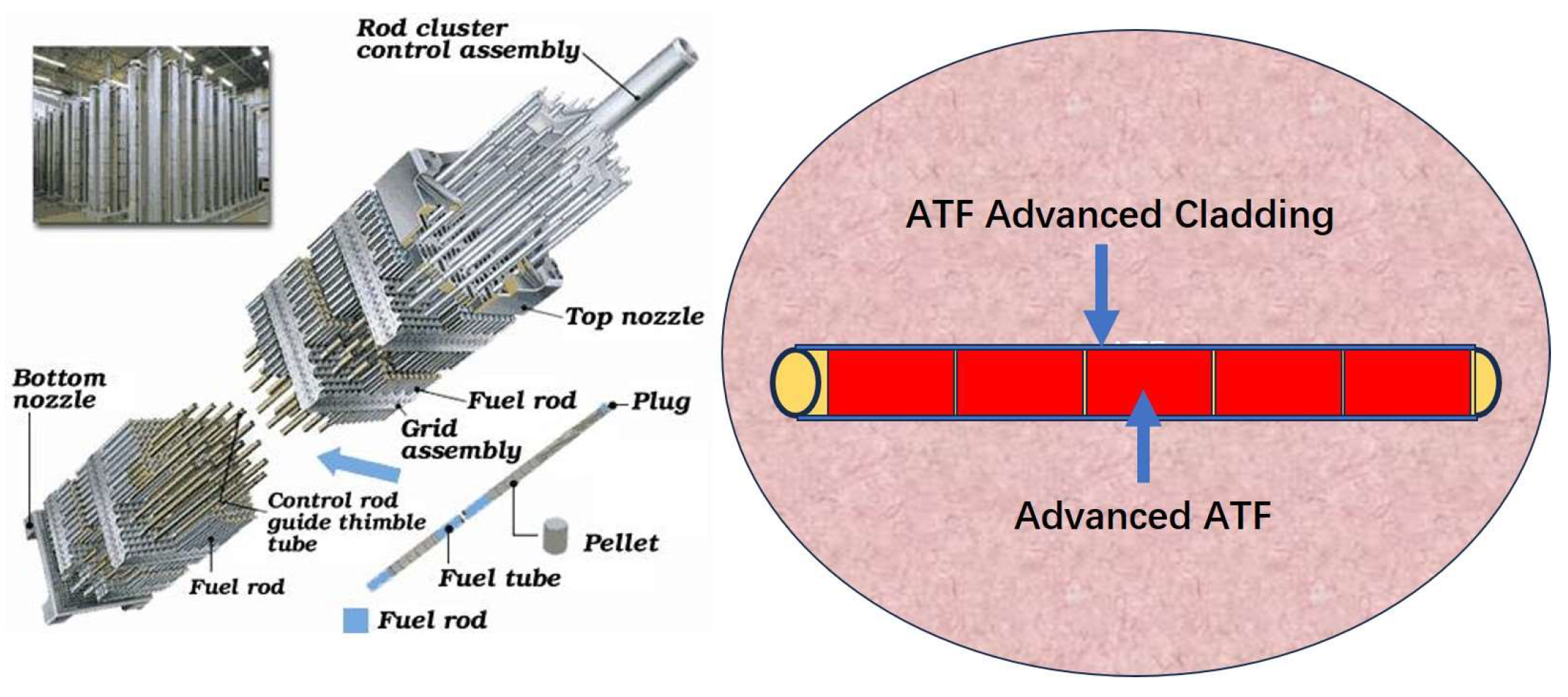
:1. Introduction
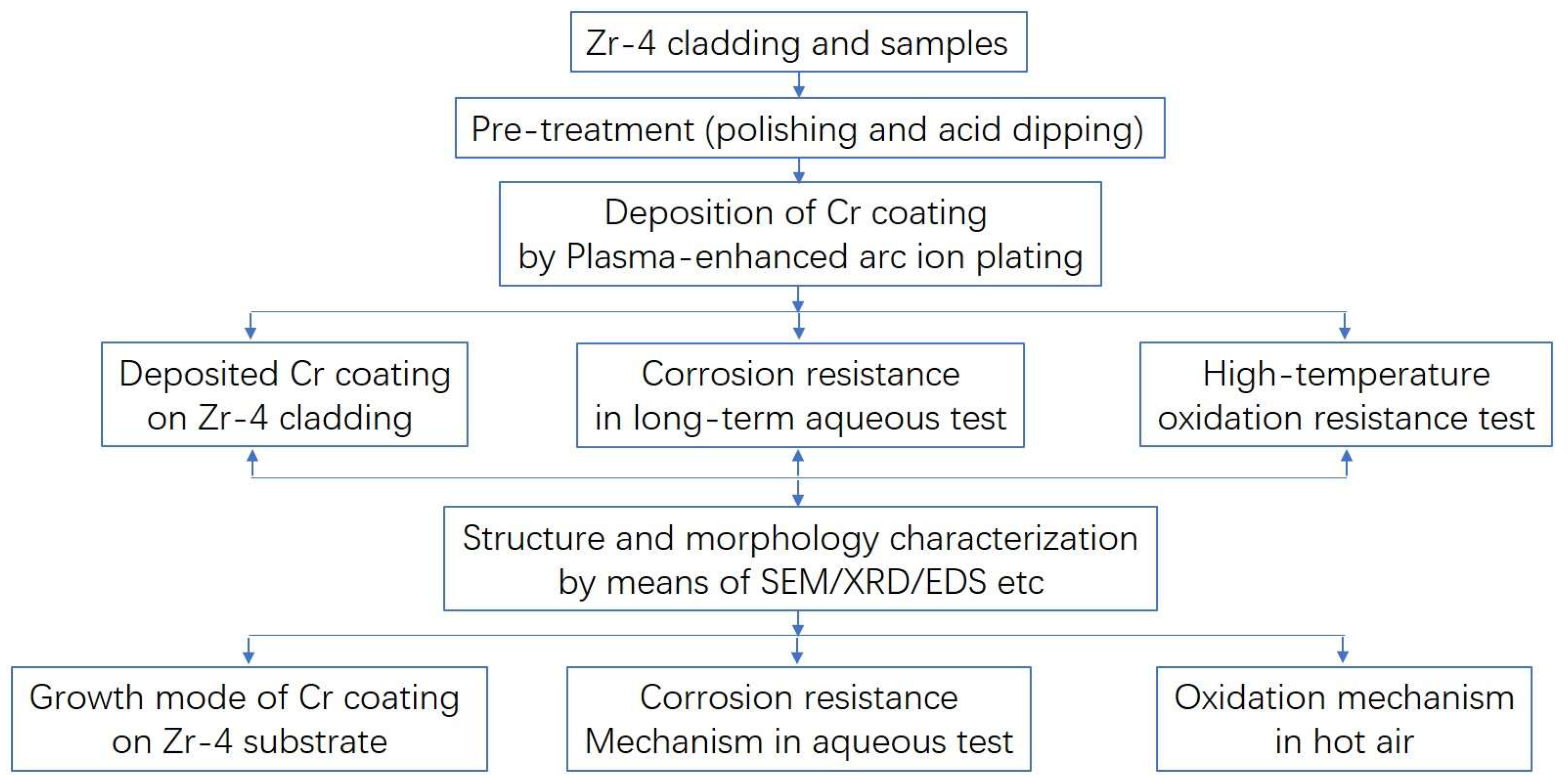
2. Materials and Methods
3. Results
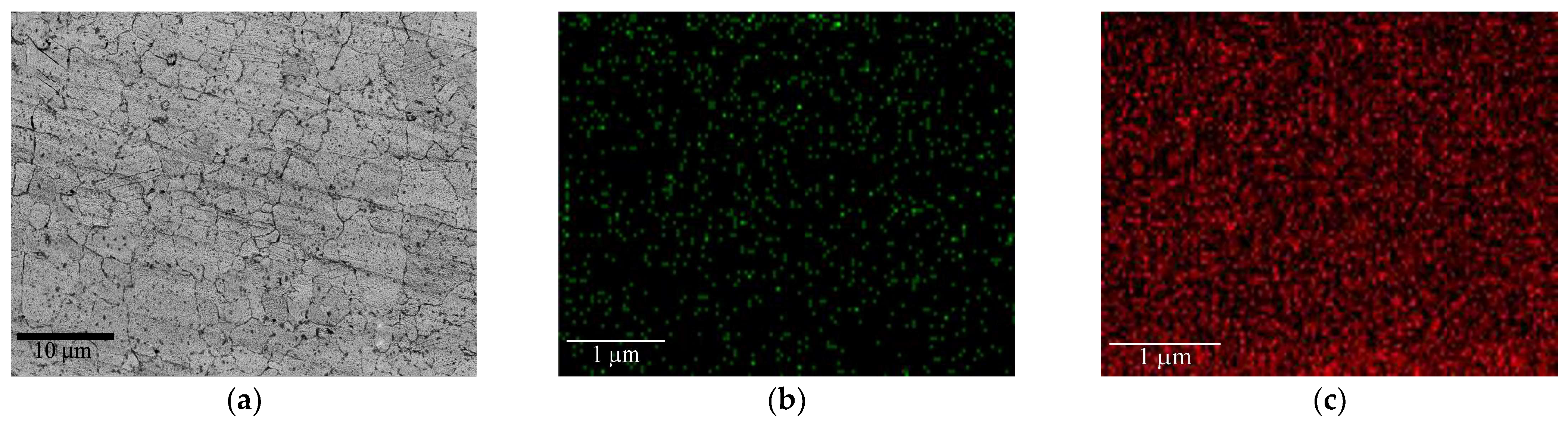
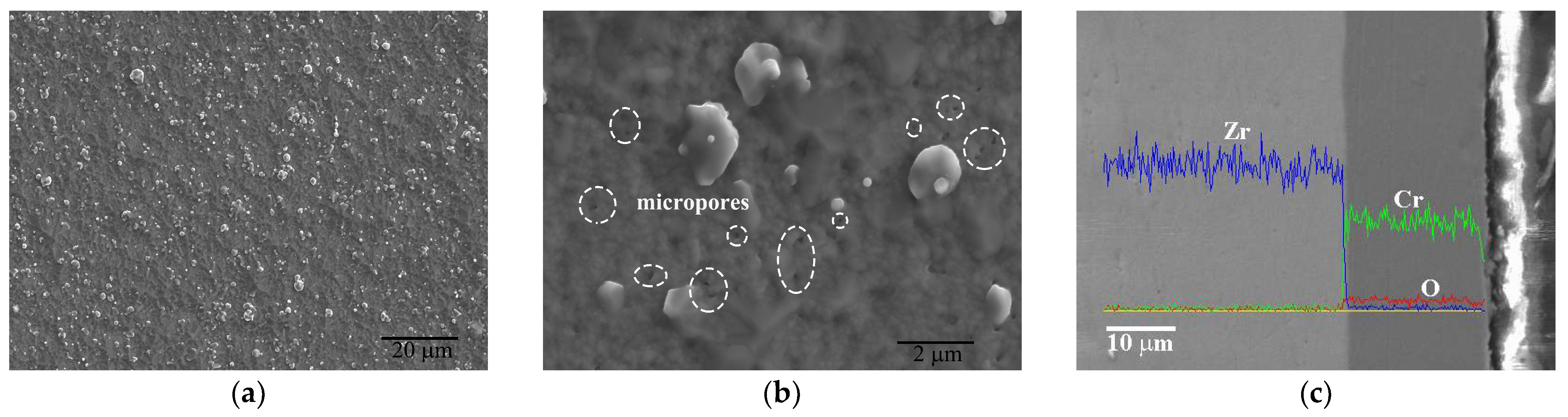
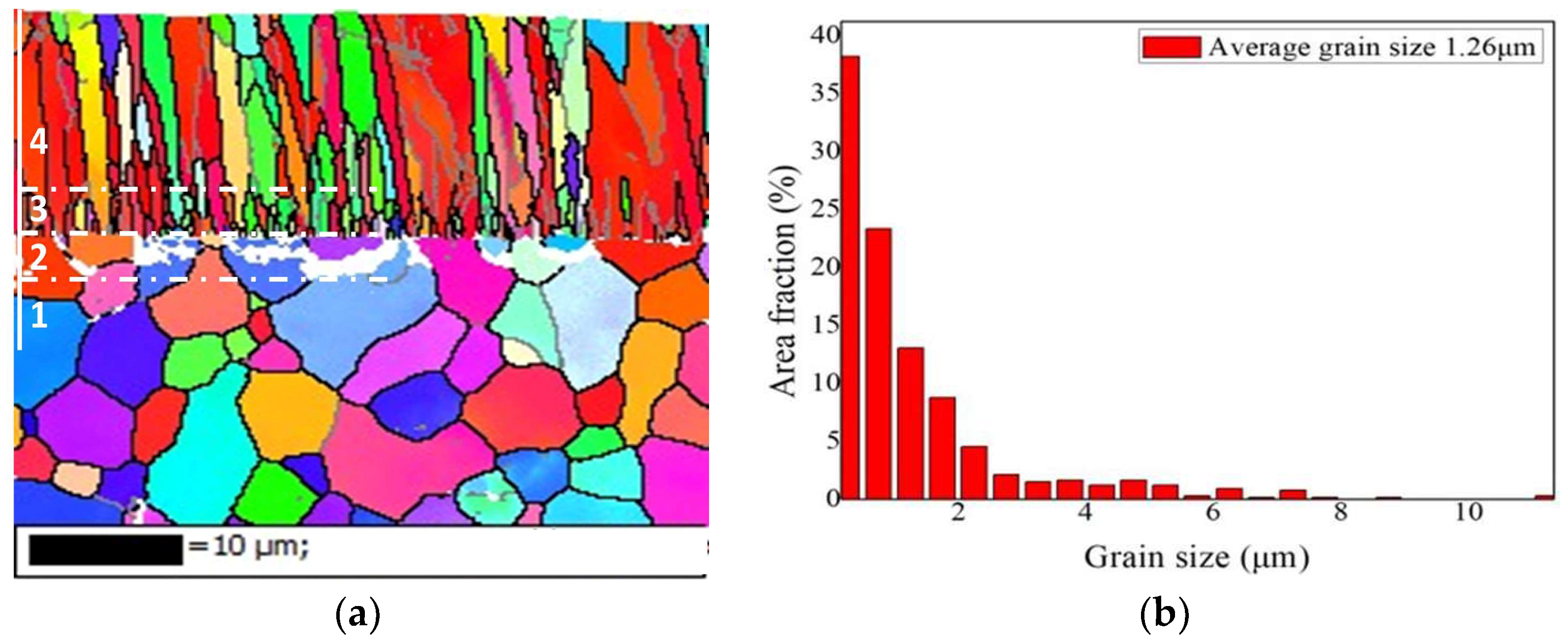
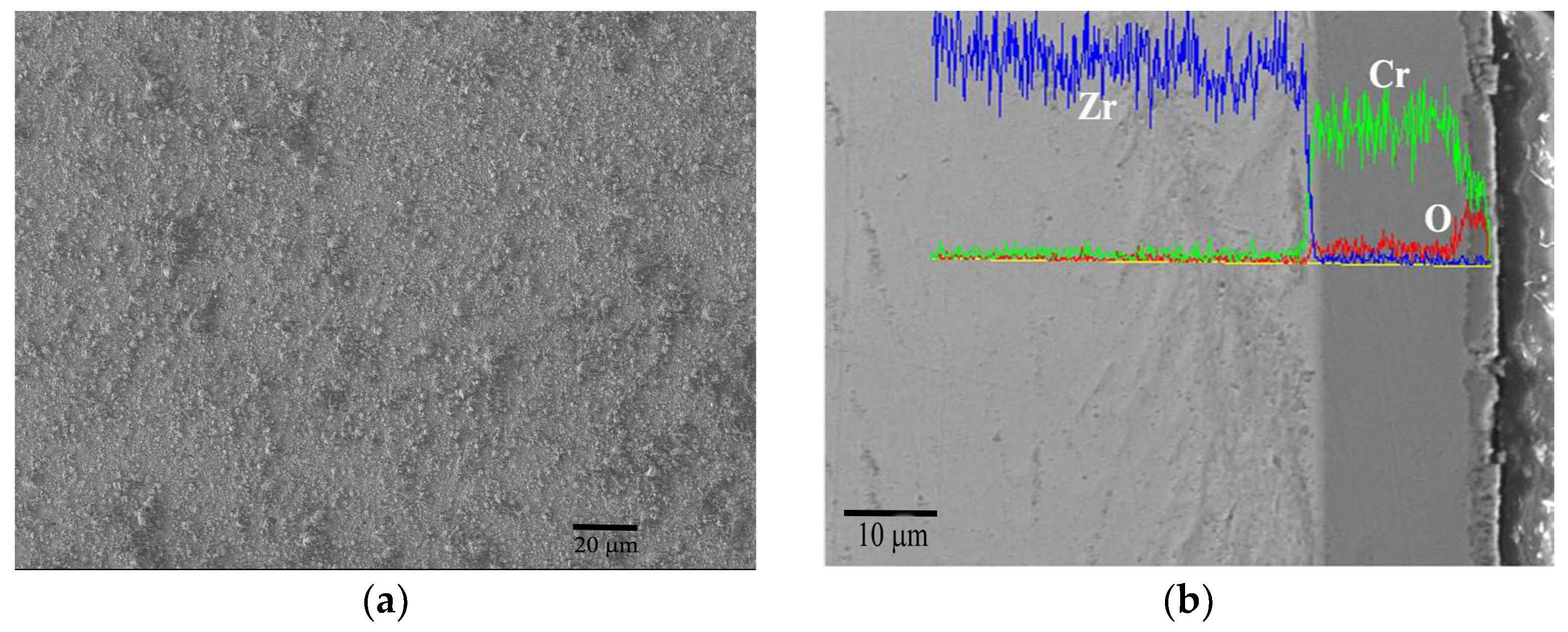
3.1. Microstructure of As-Deposited Cr Coatings
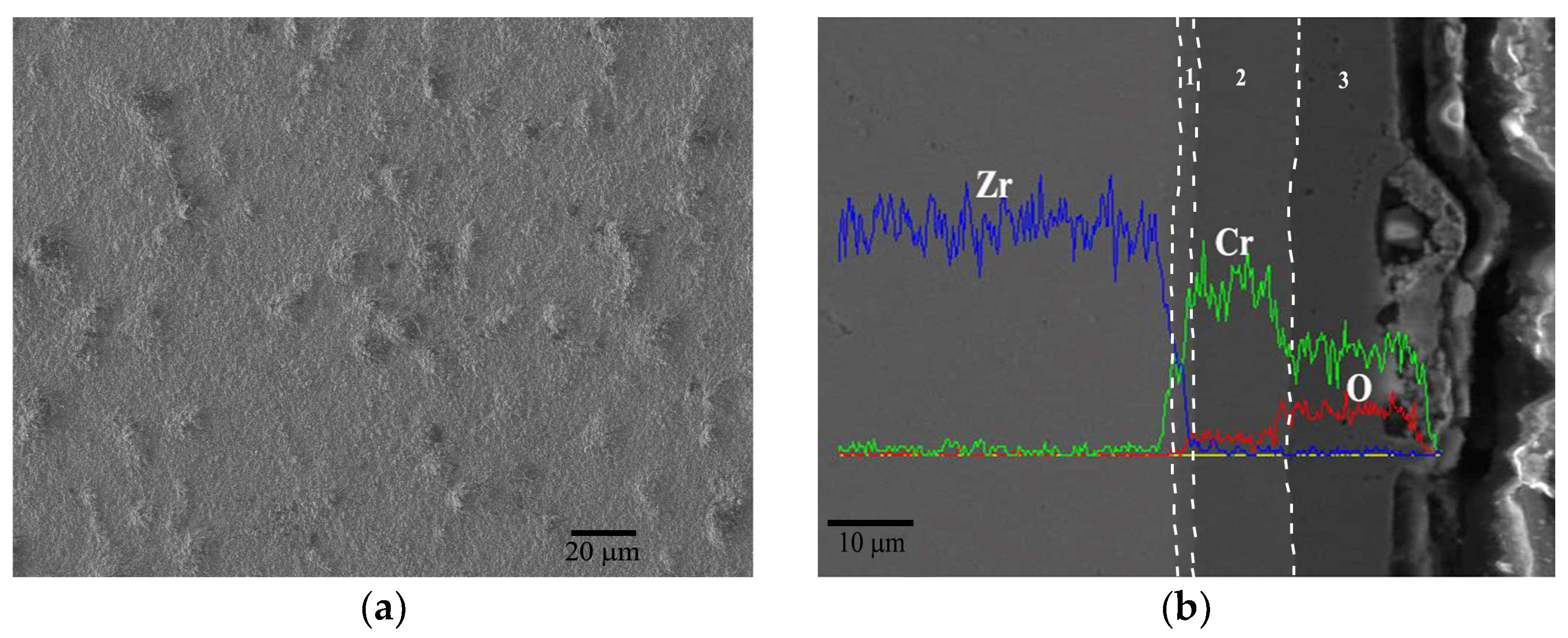
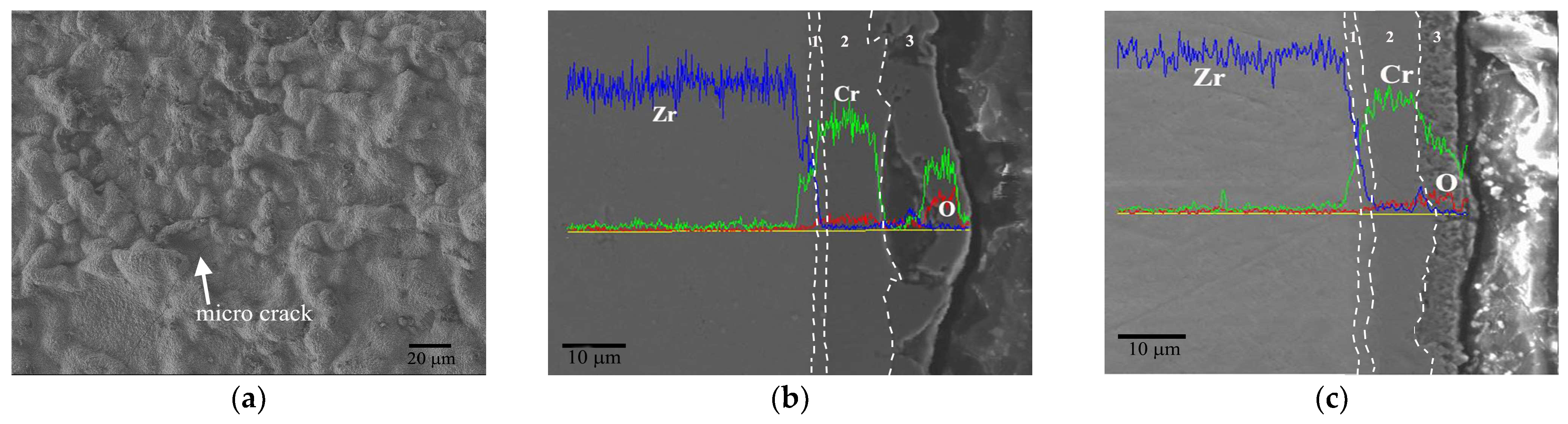
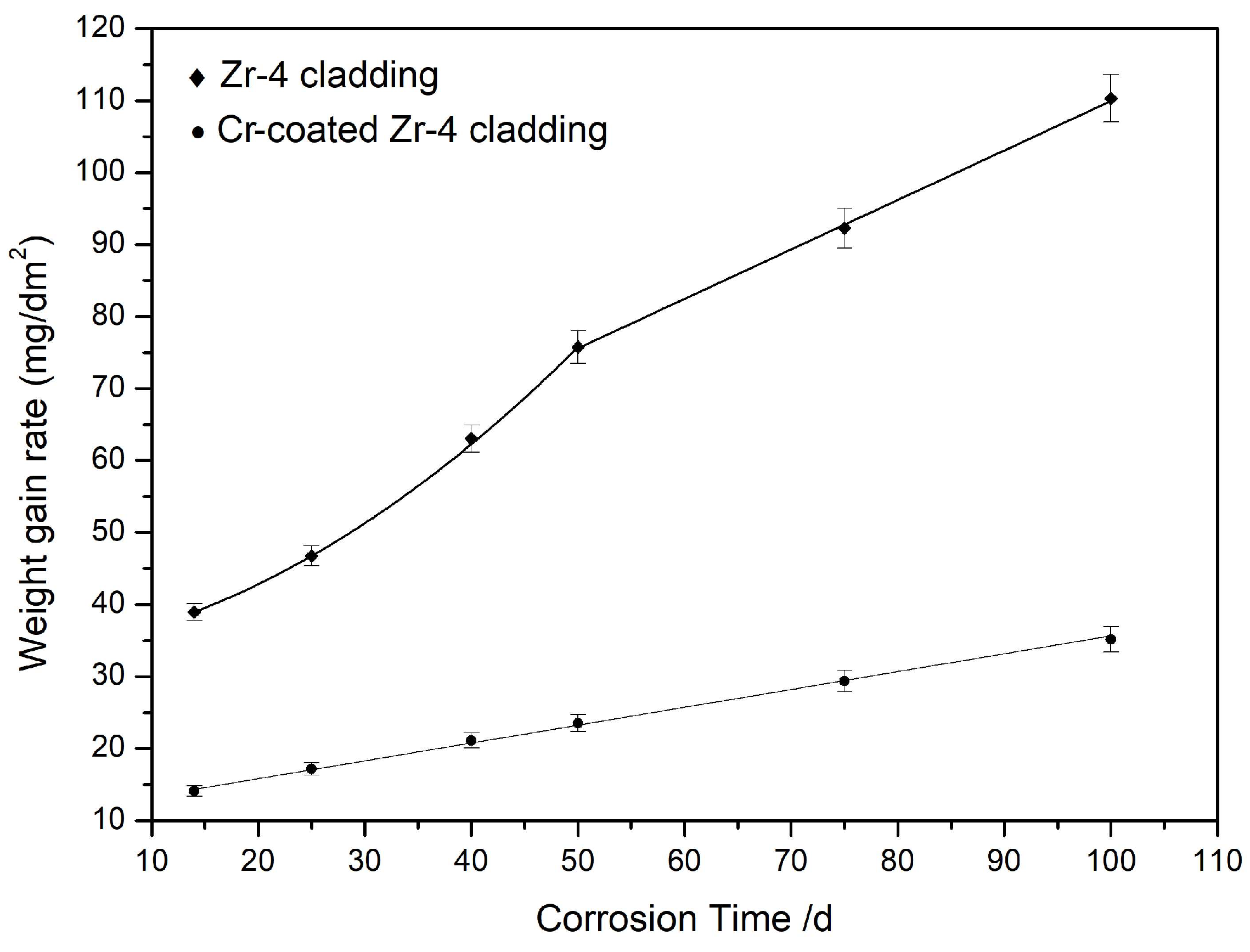
3.2. Durability of Cr Coating under Extreme Conditions
3.3. Durability of the Cr Coating under Extreme Conditions
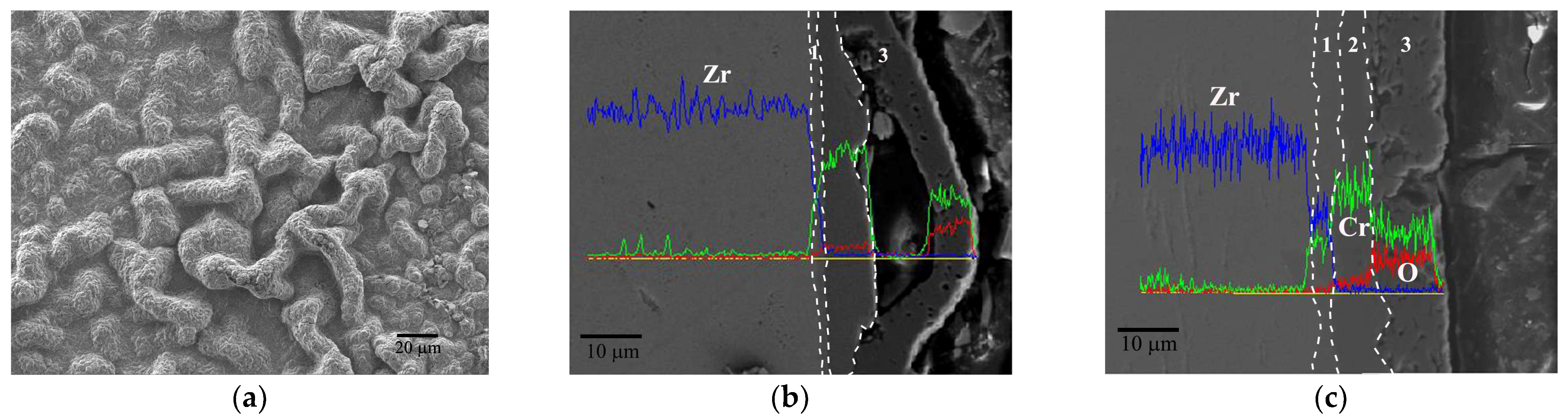
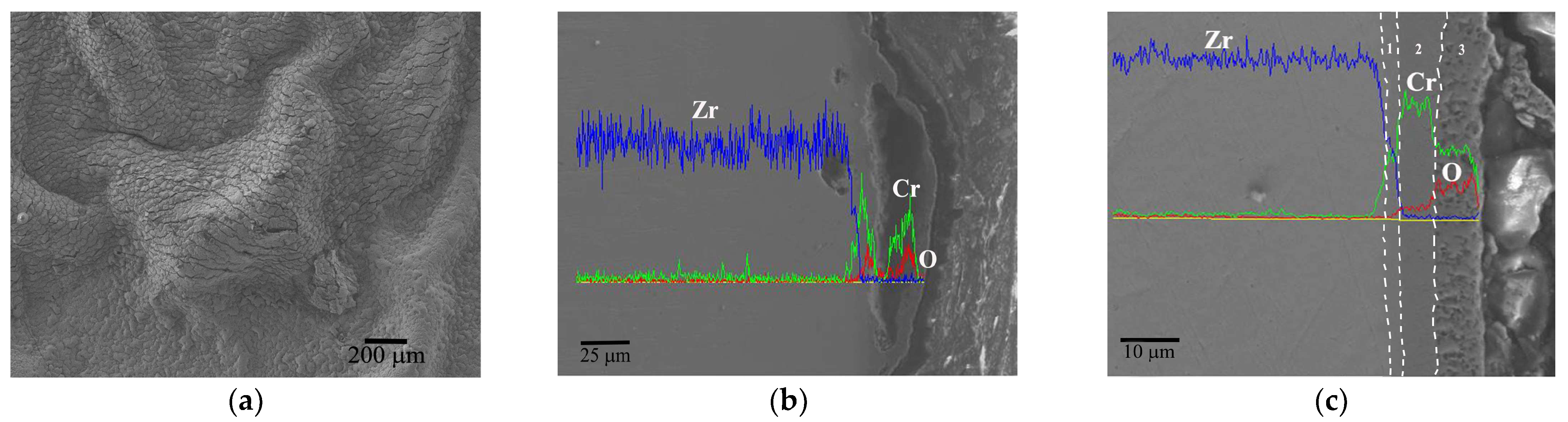
3.3.1. Evolution Behavior of the Cr Coating
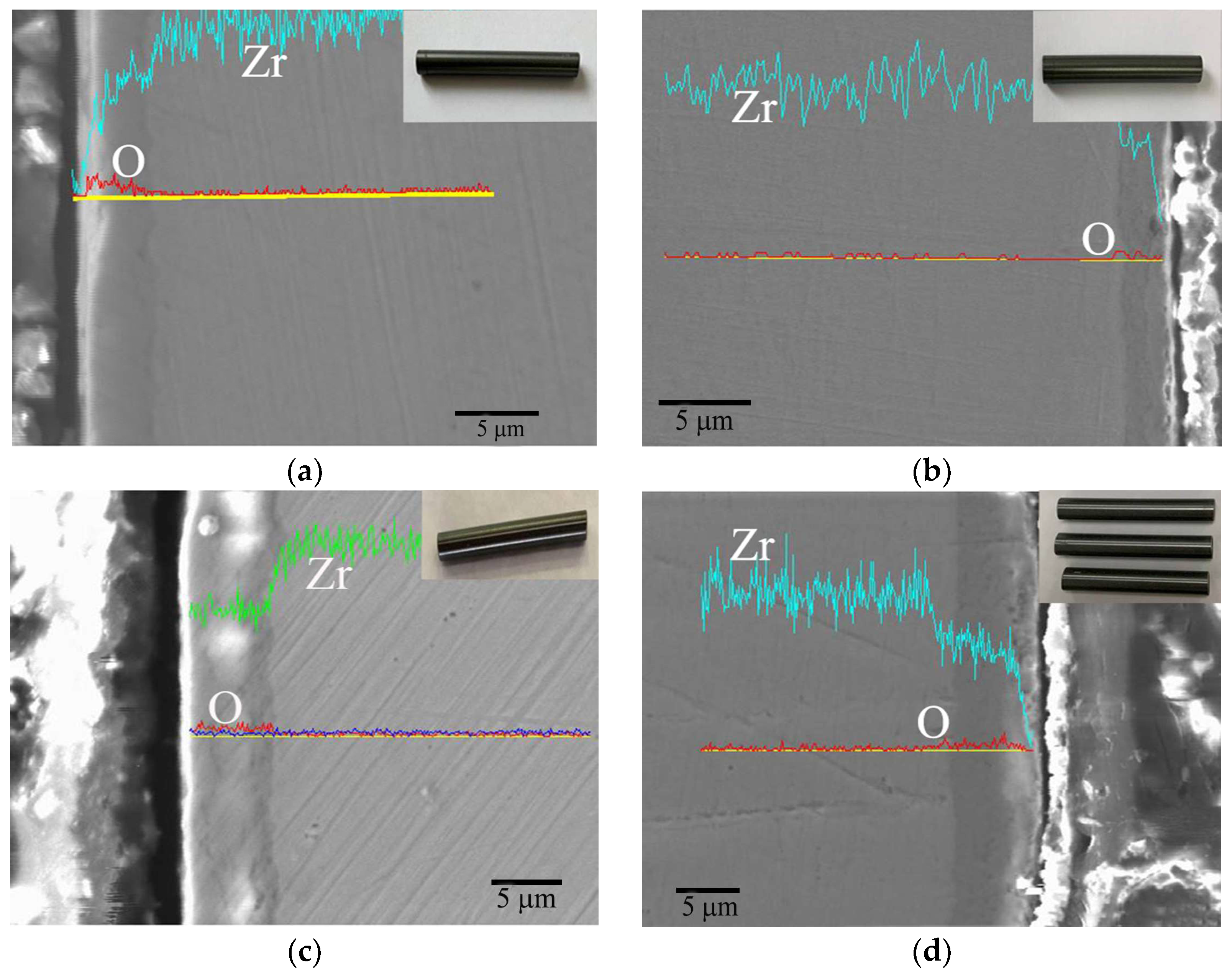
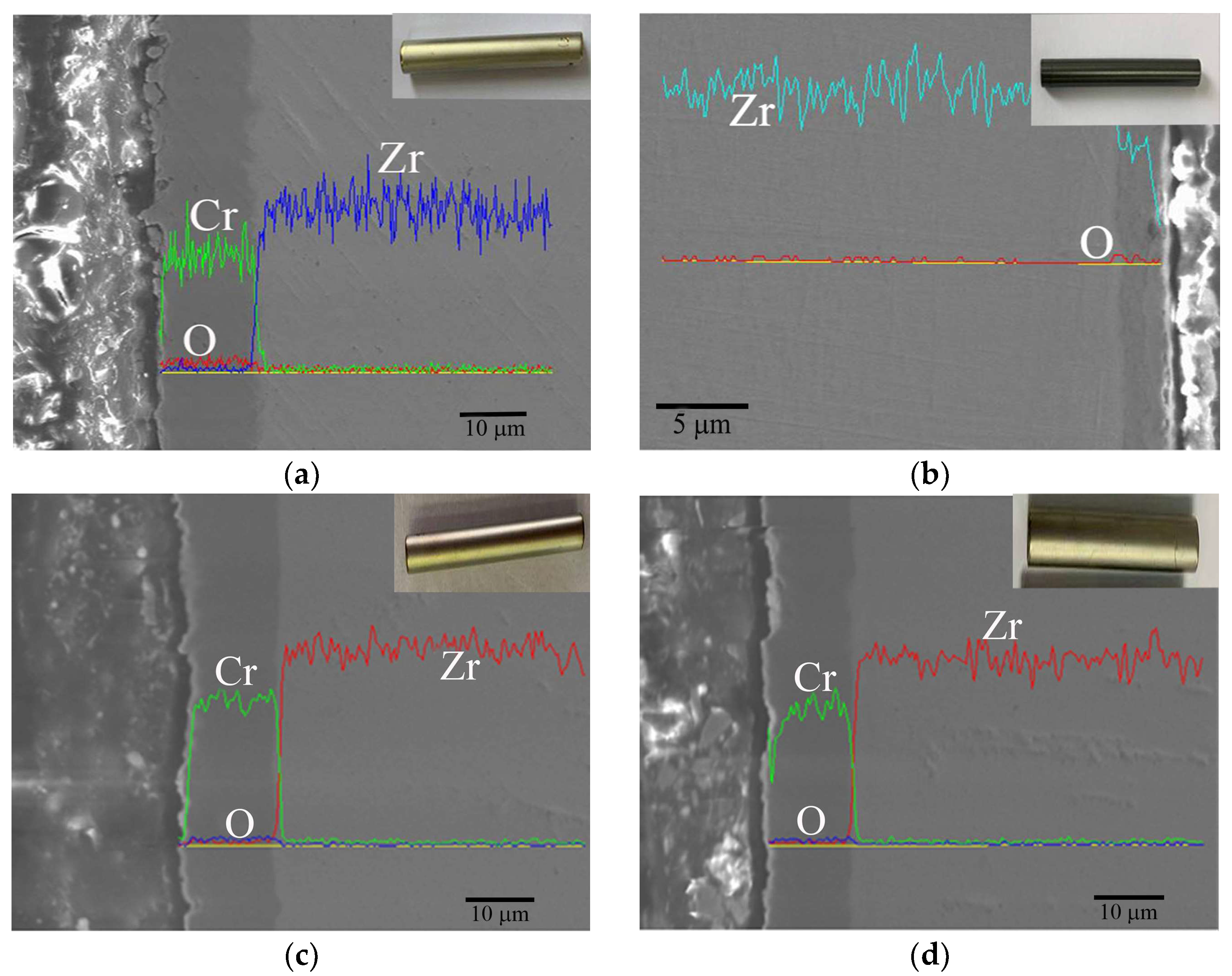
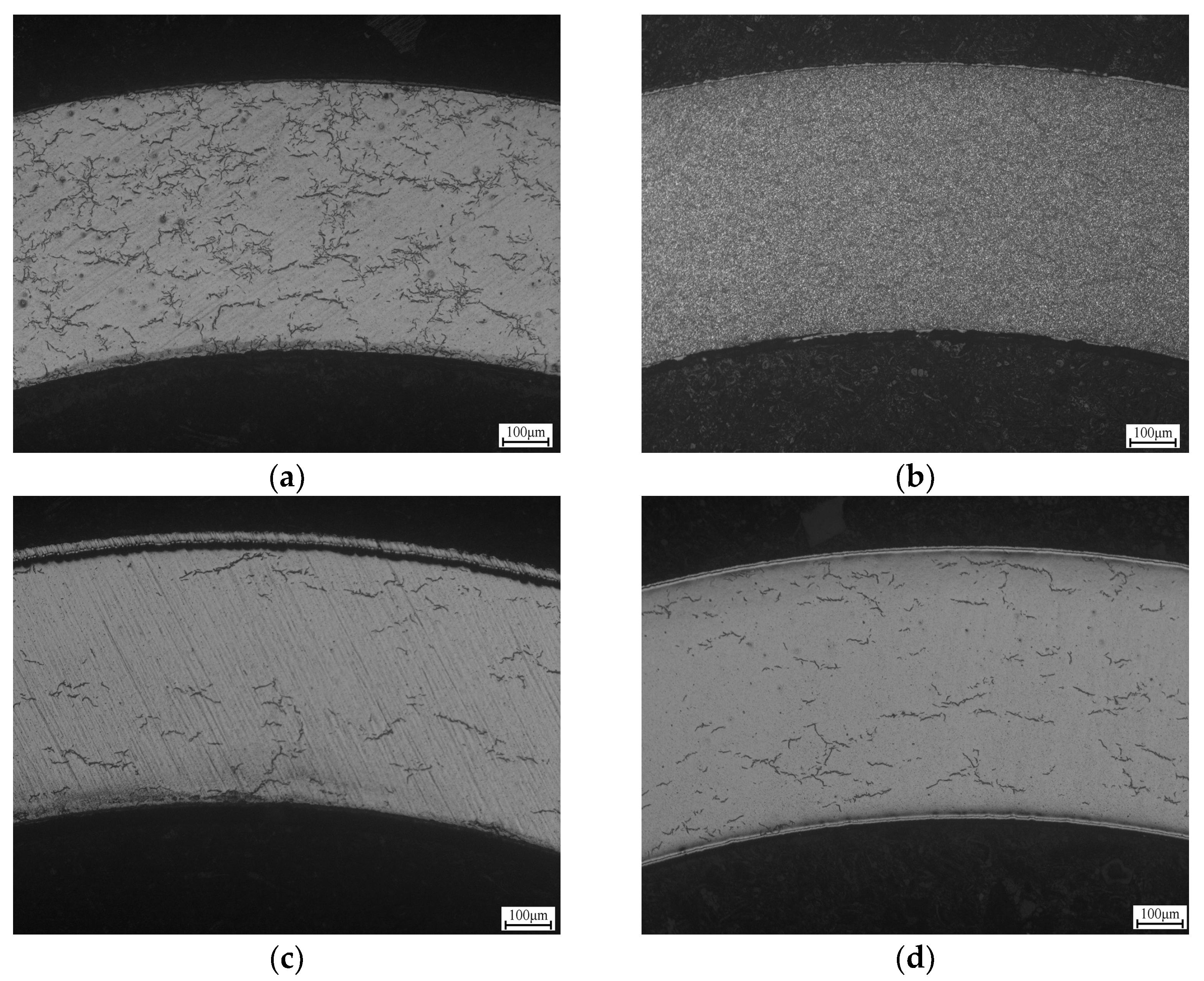
3.3.2. Inhibition of Hydrides by Cr Coating
4. Discussion
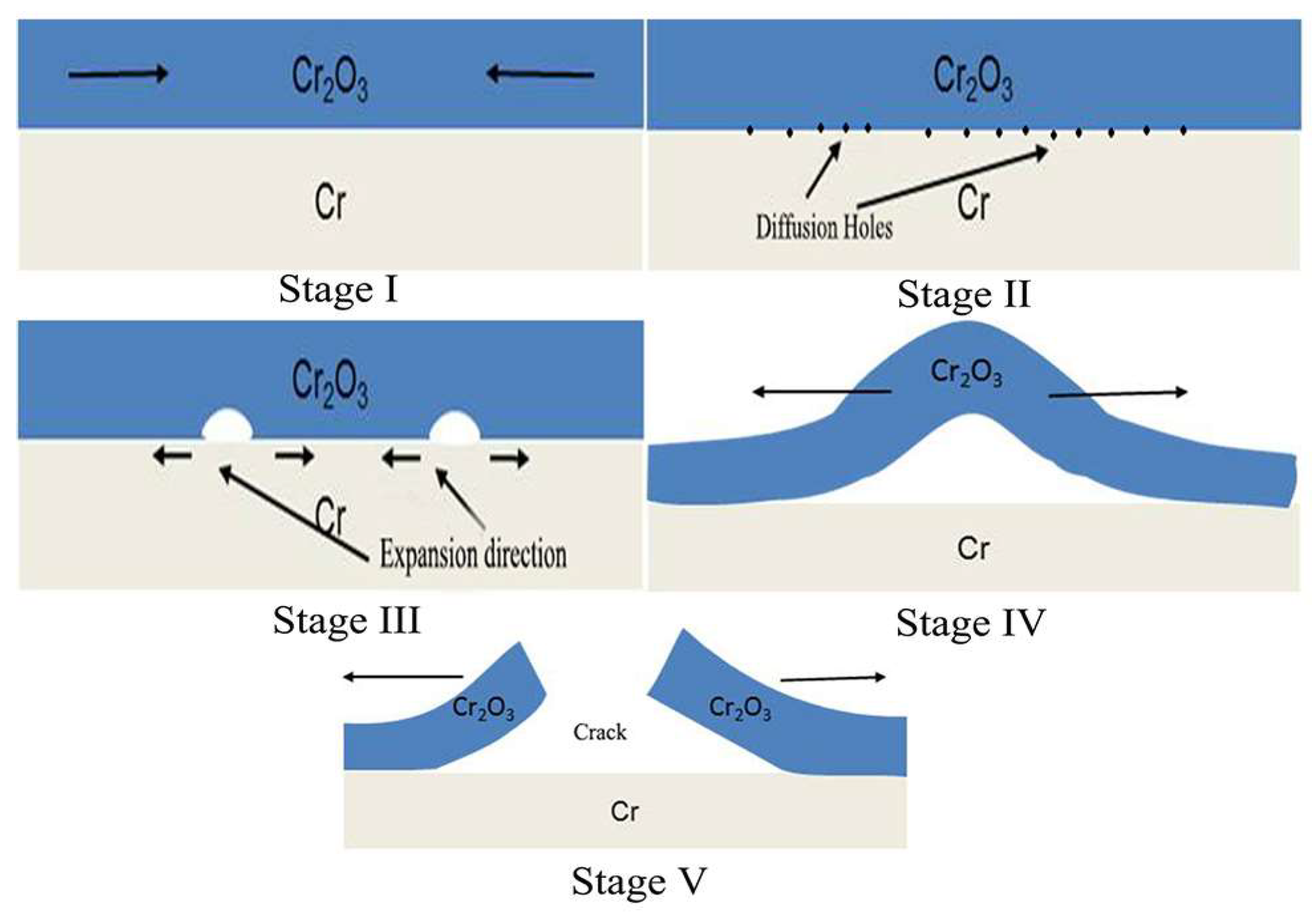
4.1. Oxidation Mechanism of Cr Coating in Hot Air
4.2. Corrosion Mechanism of Cr Coating in Long-Term Aqueous Test
4.3. Perspectives about Cr Coating for ATF
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zinkle, S.; Terrani, K.; Gehin, J.; Ott, L.; Snead, L. Accident tolerant fuels for LWRs: A perspective. J. Nucl. Mater. 2014, 448, 374–379. [Google Scholar] [CrossRef]
- Brachet, J.-C.; Idarraga-Trujillo, I.; Le Flem, M.; Le Saux, M.; Vandenberghe, V.; Urvoy, S.; Rouesne, E.; Guilbert, T.; Toffolon-Masclet, C.; Tupin, M.; et al. Early studies on Cr-coated Zircaloy-4 as enhanced accident tolerant nuclear fuel claddings for light water reactors. J. Nucl. Mater. 2019, 517, 268–285. [Google Scholar] [CrossRef]
- Bragg-Sitton, S. Development of advanced accident-tolerant fuels for commercial LWRs. Nucl. News 2014, 57, 83–86. [Google Scholar]
- Tao, Z.; Wang, P.; Wang, C.; Ma, Z.; Zhang, Y.; Xue, F.; Bai, G.; Yuan, Y.; Lan, R. Design and characterization of AlCrFeCuNbx alloys for accident-tolerant fuel cladding. J. Alloys Compd. 2021, 859, 157805. [Google Scholar] [CrossRef]
- Terrani, K.A. Accident tolerant fuel cladding development: Promise, status, and challenges. J. Nucl. Mater. 2018, 501, 13–30. [Google Scholar] [CrossRef]
- Tang, C.; Stueber, M.; Seifert, H.J.; Steinbrueck, M. Protective coatings on zirconium-based alloys as accident-tolerant fuel (ATF) claddings. Corros. Rev. 2017, 35, 141–165. [Google Scholar] [CrossRef]
- Lee, Y.; Lee, J.I.; No, H.C. Mechanical analysis of surface-coated zircaloy cladding. Nucl. Eng. Technol. 2017, 49, 1031–1043. [Google Scholar] [CrossRef]
- Bischoff, J.; Delafoy, C.; Vauglin, C.; Barberis, P.; Roubeyrie, C.; Perche, D.; Duthoo, D.; Schuster, F.; Brachet, J.-C.; Schweitzer, E.W.; et al. AREVA NP’s enhanced accident-tolerant fuel developments: Focus on Cr-coated M5 cladding. Nucl. Eng. Technol. 2018, 50, 223–228. [Google Scholar] [CrossRef]
- Wang, Y.; Tang, H.; Han, X.; Feng, W.; Zhou, X.; Peng, S.; Zhang, H. Oxidation resistance improvement of Zr-4 alloy in 1000 °C steam environment using ZrO2/FeCrAl bilayer coating. Surf. Coat. Technol. 2018, 349, 807–815. [Google Scholar] [CrossRef]
- Han, X.; Wang, Y.; Peng, S.; Zhang, H. Oxidation behavior of FeCrAl coated Zry-4 under high temperature steam environment. Corros. Sci. 2019, 149, 45–53. [Google Scholar] [CrossRef]
- Kashkarov, E.; Sidelev, D.; Rombaeva, M.; Syrtanov, M.; Bleykher, G.A. Chromium coatings deposited by cooled and hot target magnetron sputtering for accident tolerant nuclear fuel claddings. Surf. Coat. Technol. 2020, 389, 125618. [Google Scholar] [CrossRef]
- Umretiya, R.V.; Elward, B.; Lee, D.; Anderson, M.; Rebak, R.B.; Rojas, J.V. Mechanical and chemical properties of PVD and cold spray Cr-coatings on Zircaloy-4. J. Nucl. Mater. 2020, 541, 152420. [Google Scholar] [CrossRef]
- Maier, B.R.; Garcia-Diaz, B.L.; Hauch, B.; Olson, L.C.; Sindelar, R.L.; Sridharan, K. Cold spray deposition of Ti2AlC coatings for improved nuclear fuel cladding. J. Nucl. Mater. 2015, 466, 712–717. [Google Scholar] [CrossRef]
- Ang, C.; Zinkle, S.; Shih, C.; Silva, C.; Cetiner, N.; Katoh, Y. Phase stability, swelling, microstructure and strength of Ti3SiC2-TiC ceramics after low dose neutron irradiation. J. Nucl. Mater. 2017, 483, 44–53. [Google Scholar] [CrossRef]
- Kam, D.H.; Lee, J.H.; Lee, T.; Jeong, Y.H. Critical heat flux for SiC- and Cr-coated plates under atmospheric condition. Ann. Nucl. Energy 2015, 76, 335–342. [Google Scholar] [CrossRef]
- Al-Olayyan, Y.; Fuchs, G.; Baney, R.; Tulenko, J. The effect of Zircaloy-4 substrate surface condition on the adhesion strength and corrosion of SiC coatings. J. Nucl. Mater. 2005, 346, 109–119. [Google Scholar] [CrossRef]
- Zhong, W.; Mouche, P.A.; Han, X.; Heuser, B.J.; Mandapaka, K.K.; Was, G.S. Performance of iron-chromium-aluminum alloy surface coatings on Zircaloy 2 under high-temperature steam and normal BWR operating conditions. J. Nucl. Mater. 2016, 470, 327–338. [Google Scholar] [CrossRef]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Multilayer (TiN, TiAlN) ceramic coatings for nuclear fuel cladding. J. Nucl. Mater. 2016, 478, 236–244. [Google Scholar] [CrossRef]
- Carr, J.; Vasudevamurthy, G.; Snead, L.; Hinderliter, B.; Massey, C. Investigations of Aluminum-Doped Self-Healing Zircaloy Surfaces in Context of Accident-Tolerant Fuel Cladding Research. J. Mater. Eng. Perform. 2016, 25, 2347–2355. [Google Scholar] [CrossRef]
- Peter, J.; Doyle Stephen, S.; Raiman, R.R.; Kurt, A.T. Characterization of the hydrothermal corrosion behavior of ceramics for accident tolerant fuel cladding. In Proceedings of the 18th International Conference on Environmental Degradation of Materials in Nuclear Power Systems–Water Reactors, Boston, MA, USA, 18–22 August 2019; Springer: Cham, Switzerland, 2018; pp. 269–280. [Google Scholar]
- Alat, E.; Motta, A.T.; Comstock, R.J.; Partezana, J.M.; Wolfe, D.E. Ceramic coating for corrosion (c3) resistance of nuclear fuel cladding. Surf. Coat. Technol. 2015, 281, 133–143. [Google Scholar] [CrossRef]
- Park, J.-Y.; Kim, I.-H.; Jung, Y.-I.; Kim, H.-G.; Park, D.-J.; Kim, W.-J. Long-term corrosion behavior of CVD SiC in 360 °C water and 400 °C steam. J. Nucl. Mater. 2013, 443, 603–607. [Google Scholar]
- Brachet, J.-C.; Rouesne, E.; Ribis, J.; Guilbert, T.; Urvoy, S.; Nony, G.; Toffolon-Masclet, C.; Le Saux, M.; Chaabane, N.; Palancher, H.; et al. High temperature steam oxidation of chromium-coated zirconium-based alloys: Kinetics and process. Corros. Sci. 2020, 167, 108537. [Google Scholar]
- Chen, Q.; Liu, C.; Zhang, R.; Yang, H.; Wei, T.; Wang, Y.; Li, Z.; He, L.; Wang, J.; Wang, L.; et al. Microstructure and high-temperature steam oxidation properties of thick Cr coatings prepared by magnetron sputtering for accident tolerant fuel claddings: The role of bias in the deposition process. Corros. Sci. 2020, 162, 108378. [Google Scholar] [CrossRef]
- Kashkarov, E.B.; Sidelev, D.V.; Syrtanov, M.S.; Tang, C.; Steinbrück, M. Oxidation kinetics of Cr-coated zirconium alloy: Effect of coating thickness and microstructure. Corros. Sci. 2020, 175, 108883. [Google Scholar]
- Park, J.-H.; Kim, H.-G.; Park, J.-y.; Jung, Y.-I.; Park, D.-J.; Koo, Y.-H. High temperature steam-oxidation behavior of arc ion plated Cr coatings for accident tolerant fuel claddings. Surf. Coat. Technol. 2015, 280, 256–259. [Google Scholar] [CrossRef]
- Kim, H.-G.; Kim, I.-H.; Jung, Y.-I.; Park, D.-J.; Park, J.-Y.; Koo, Y.-H. Adhesion property and high-temperature oxidation behavior of Cr-coated Zircaloy-4 cladding tube prepared by 3D laser coating. J. Nucl. Mater. 2015, 465, 531–539. [Google Scholar] [CrossRef]
- Ševeček, M.; Gurgen, A.; Seshadri, A.; Che, Y.; Wagih, M.; Phillips, B.; Champagne, V.; Shirvan, K. Development of Cr cold spray-coated fuel cladding with enhanced accident tolerance. Nucl. Eng. Technol. 2018, 50, 229–236. [Google Scholar] [CrossRef]
- Yeom, H.; Maier, B.; Johnson, G.; Dabney, T.; Lenling, M.; Sridharan, K. High temperature oxidation and microstructural evolution of cold spray chromium coatings on Zircaloy-4 in steam environments. J. Nucl. Mater. 2019, 526, 151737. [Google Scholar] [CrossRef]
- Wang, Y.; Zhou, W.; Wen, Q.; Ruan, X.; Luo, F.; Bai, G.; Qing, Y.; Zhu, D.; Huang, Z.; Zhang, Y.; et al. Behavior of plasma sprayed Cr coatings and FeCrAl coatings on Zr fuel cladding under loss-of-coolant accident conditions. Surf. Coat. Technol. 2018, 344, 41–148. [Google Scholar] [CrossRef]
- Fazi, A.; Aboulfadl, H.; Iyer, A.H.; Sattari, M.; Stiller, K.M.; Lokhande, P.; Thuvander, M.; Andren, H.-O. Characterization of as-deposited cold sprayed Cr-coating on Optimized ZIRLOTM claddings. J. Nucl. Mater. 2021, 549, 152892. [Google Scholar] [CrossRef]
- D’Avico, L.; Beltrami, R.; Lecis, N.; Trasatti, S.P. Corrosion behavior and surface properties of PVD coatings for mold technology applications. Coatings 2019, 9, 7. [Google Scholar] [CrossRef]
- Han, X.; Chen, C.; Tan, Y.; Feng, W.; Peng, S.; Zhang, H. A systematic study of the oxidation behavior of Cr coatings on Zry4 substrates in high temperature steam environment. Corros. Sci. 2020, 174, 108826. [Google Scholar] [CrossRef]
- Ni, N.; Lozano-Perez, S.; Sykes, J.; Smith, G.; Grovenor, C. Focused ion beam sectioning for the 3D characterization of cracking in oxide scales formed on commercial ZIRLO™ alloys during corrosion in high temperature pressurized water. Corros. Sci. 2011, 53, 4073–4083. [Google Scholar] [CrossRef]
- Cox, B. Some thoughts on the mechanisms of in-reactor corrosion of zirconium alloys. J. Nucl. Mater. 2005, 336, 331–368. [Google Scholar] [CrossRef]
- Lyons, J.L.; Partezana, J.; Byers, W.A.; Wang, G.; Parsi, A.; Walters, J.; Romero, J.; Mueller, A.J.; Shah, H.; Oelrich, R., Jr. Westinghouse chromium-coated zirconium alloy cladding development and testing. In Proceedings of the Top Fuel 2019, Seattle, WA, USA, 22–27 September 2019. [Google Scholar]
- Evans, A.; He, M.; Hutchinson, J. Mechanics based scaling laws for the durability of thermal barrier coatings. Prog. Mater. Sci. 2001, 46, 249–271. [Google Scholar] [CrossRef]
- Young, D.J.; Pint, B.A. Chromium volatilization rates from Cr2O3 scales into flowing gases containing water vapor. Oxid. Met. 2006, 66, 137–153. [Google Scholar] [CrossRef]
- Guillou, S.; Cabet, C.; Desgranges, C.; Marchetti, L.; Wouters, Y. Influence of hydrogen and water vapour on the kinetics of chromium oxide growth at high temperature. Oxid. Met. 2011, 76, 193–214. [Google Scholar] [CrossRef]
- Arifin, S.K.; Hamid, M.; Berahim, A.N.; Ani, M.H. Effects of water vapor on protectiveness of Cr2O3 scale at 1073 K. IOP Conf. Ser. Mater. Sci. Eng. 2017, 290, 012085. [Google Scholar] [CrossRef]
- Opila, E.J.; Jacobson, N.S.; Myers, D.L.; Copland, E.H. Predicting oxide stability in high temperature water vapor. JOM 2006, 58, 22–28. [Google Scholar] [CrossRef]
- Isotalo, A.E.; Aarnio, P.A. Comparison of depletion algorithms for large systems of nuclides. Ann. Nucl. Energy 2011, 38, 261–268. [Google Scholar] [CrossRef]
- Stanisz, P.; Oettingen, M.; Cetnar, J. Development of a Trajectory Period Folding Method for Burnup Calculations. Energies 2022, 15, 2245. [Google Scholar] [CrossRef]


| Zr | Sn | Fe | Cr | Ni | Si | Hf |
|---|---|---|---|---|---|---|
| In balance | ~1.5 | ~0.2 | ~0.1 | ~0.12 | ~0.9 | ~0.04 |
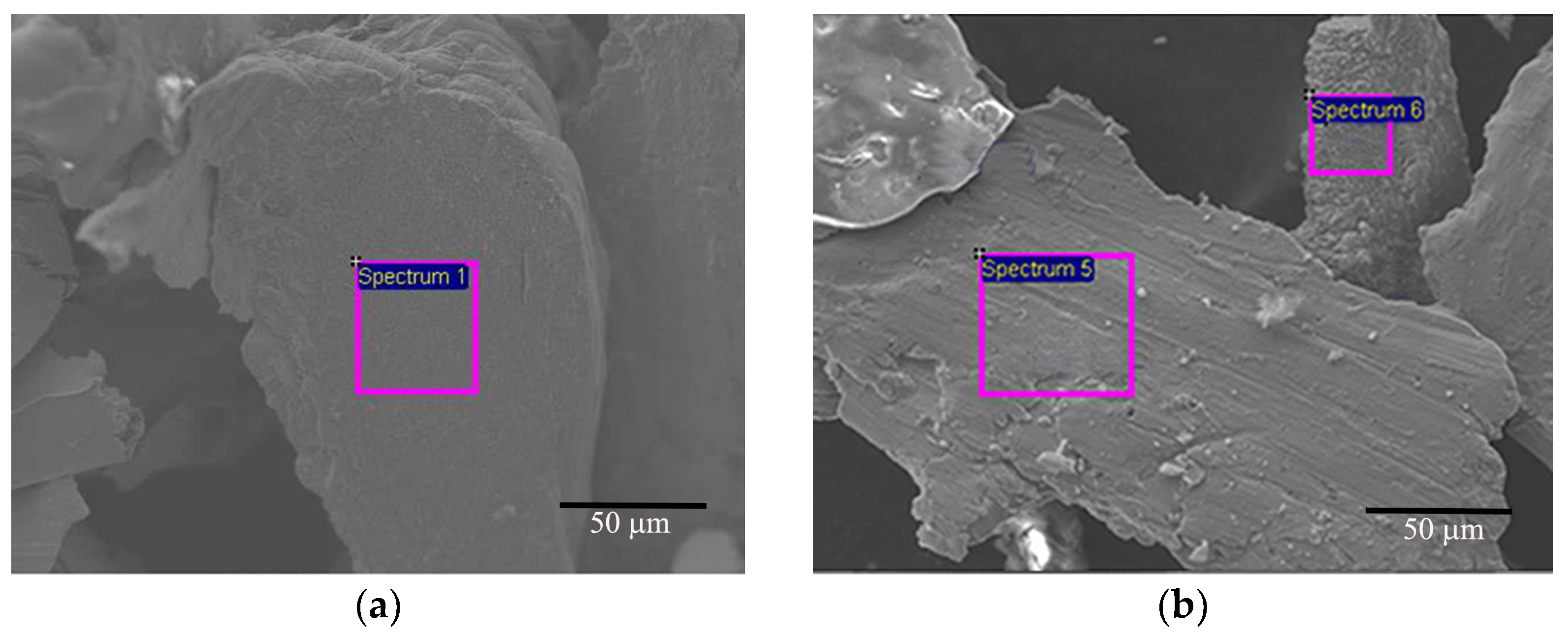
| C | O | Cr | Fe | |
|---|---|---|---|---|
| Spectrum 1 | / | 3.85 | 95.23 | 0.92 |
| Spectrum 5 | 1.03 | 48.80 | 50.17 | / |
| Spectrum 6 | / | 47.36 | 51.32 | 1.32 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Geng, J.; Wang, Y.; Wang, S.; Zhang, C. Corrosion Degradation Mechanism of Cr-Coated Zr-4 Alloy under Simulated Nuclear Conditions for Accident-Tolerant Fuel. Materials 2024, 17, 1240. https://doi.org/10.3390/ma17061240
Wang Y, Geng J, Wang Y, Wang S, Zhang C. Corrosion Degradation Mechanism of Cr-Coated Zr-4 Alloy under Simulated Nuclear Conditions for Accident-Tolerant Fuel. Materials. 2024; 17(6):1240. https://doi.org/10.3390/ma17061240
Chicago/Turabian StyleWang, Yanfeng, Juanjuan Geng, Yun Wang, Shaopeng Wang, and Changwei Zhang. 2024. "Corrosion Degradation Mechanism of Cr-Coated Zr-4 Alloy under Simulated Nuclear Conditions for Accident-Tolerant Fuel" Materials 17, no. 6: 1240. https://doi.org/10.3390/ma17061240





