Comparison of the Intensity of Biofilm Production by Oral Microflora and Its Adhesion on the Surface of Zirconia Produced in Additive and Subtractive Technology: An In Vitro Study
Abstract
:1. Introduction
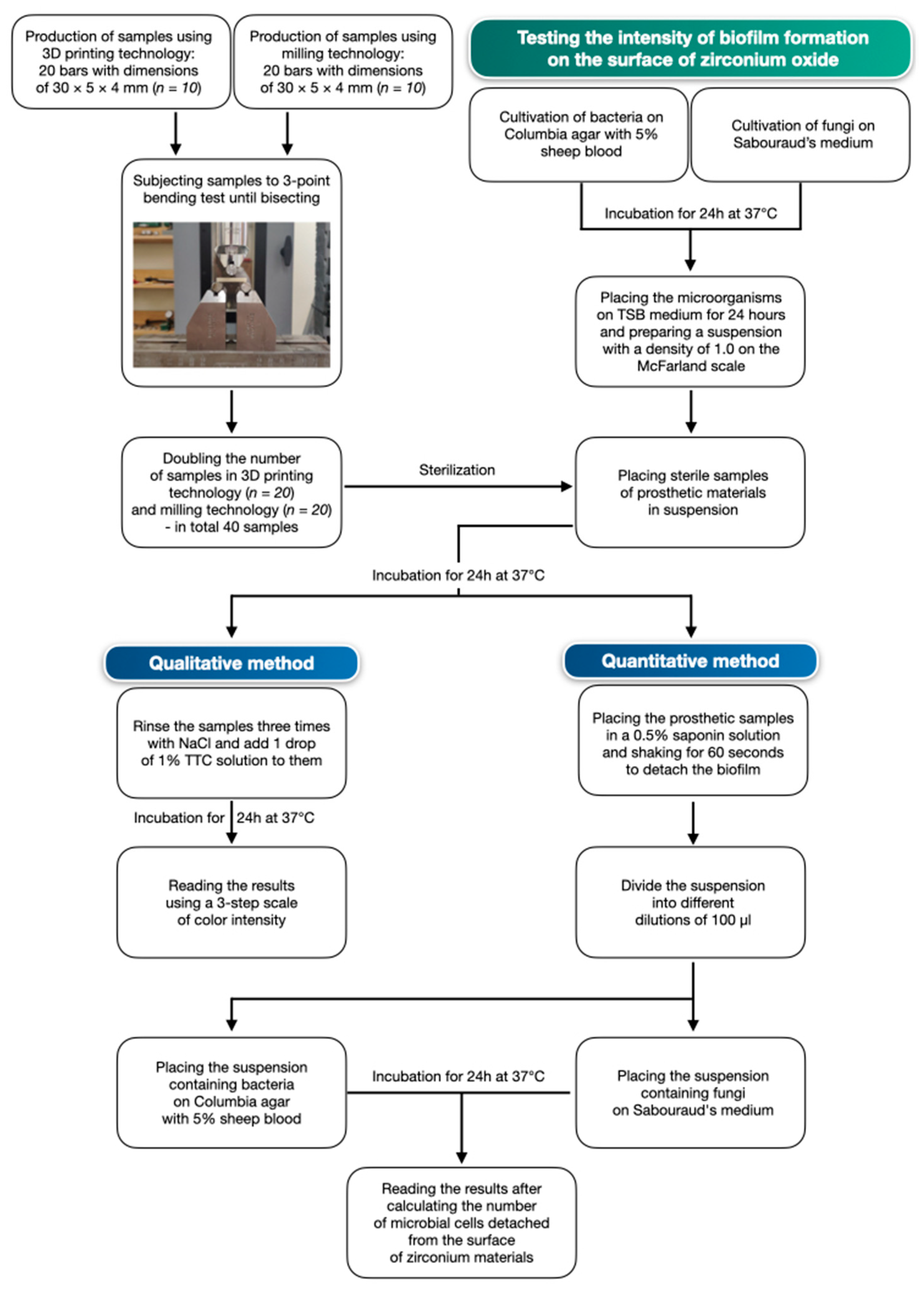
2. Materials and Methods
2.1. Zirconium Oxide Samples
2.2. Bacterial and Fungal Strains
2.3. Study of Biofilm Formation on the Surface of Ceramic Materials
2.3.1. Qualitative Method
2.3.2. Quantitative Method
2.4. Statistical Analysis
3. Results
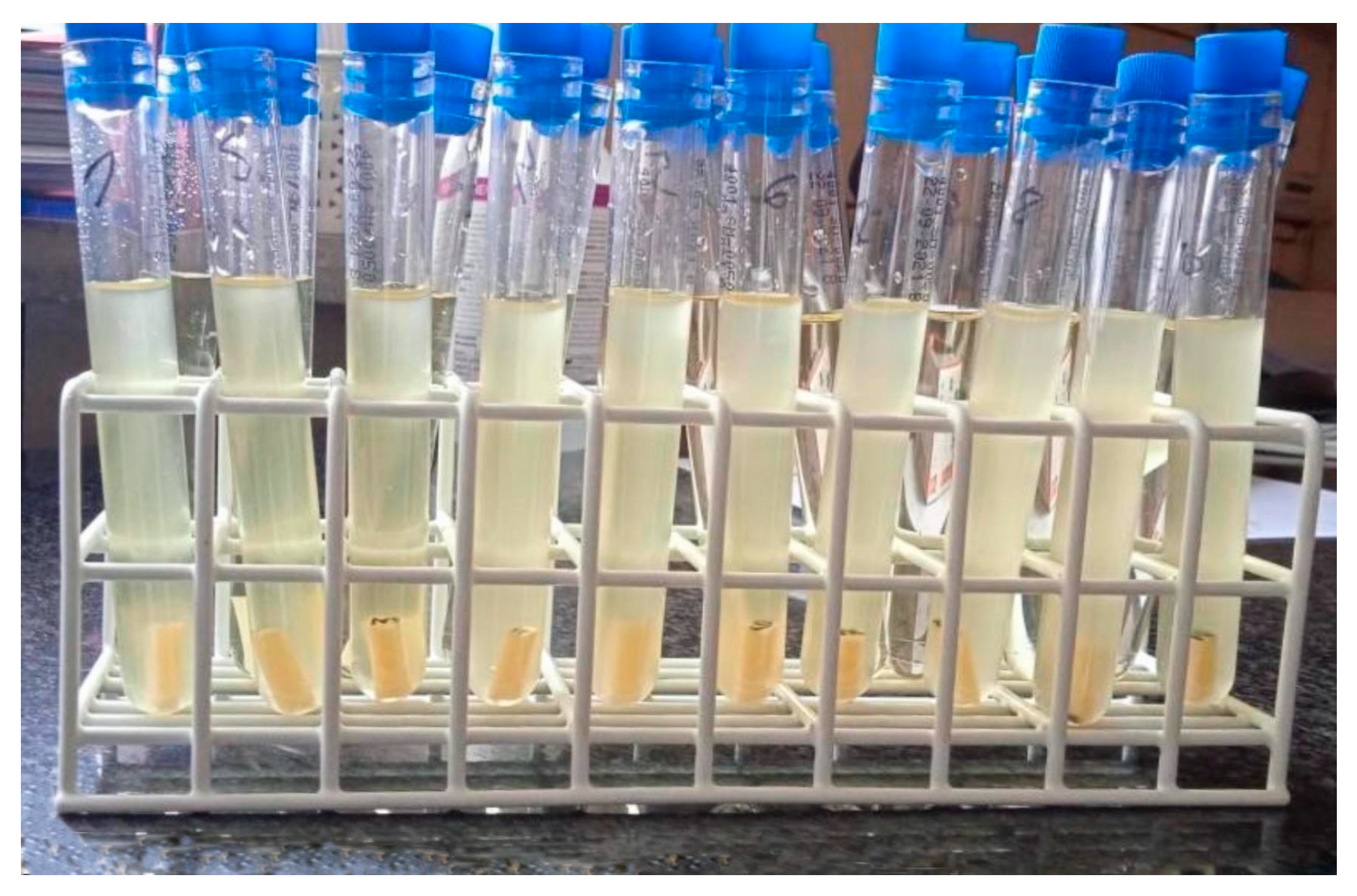
3.1. Qualitative Method
- (-)—absence of cells (non-biofilm forming strain);
- (+)—103–104 CFU/mL (strain with poor biofilm formation);
- (++)—105–106 CFU/mL (strain with strong biofilm formation);
- (+++)—107–108 CFU/mL (strain with very strong biofilm formation).
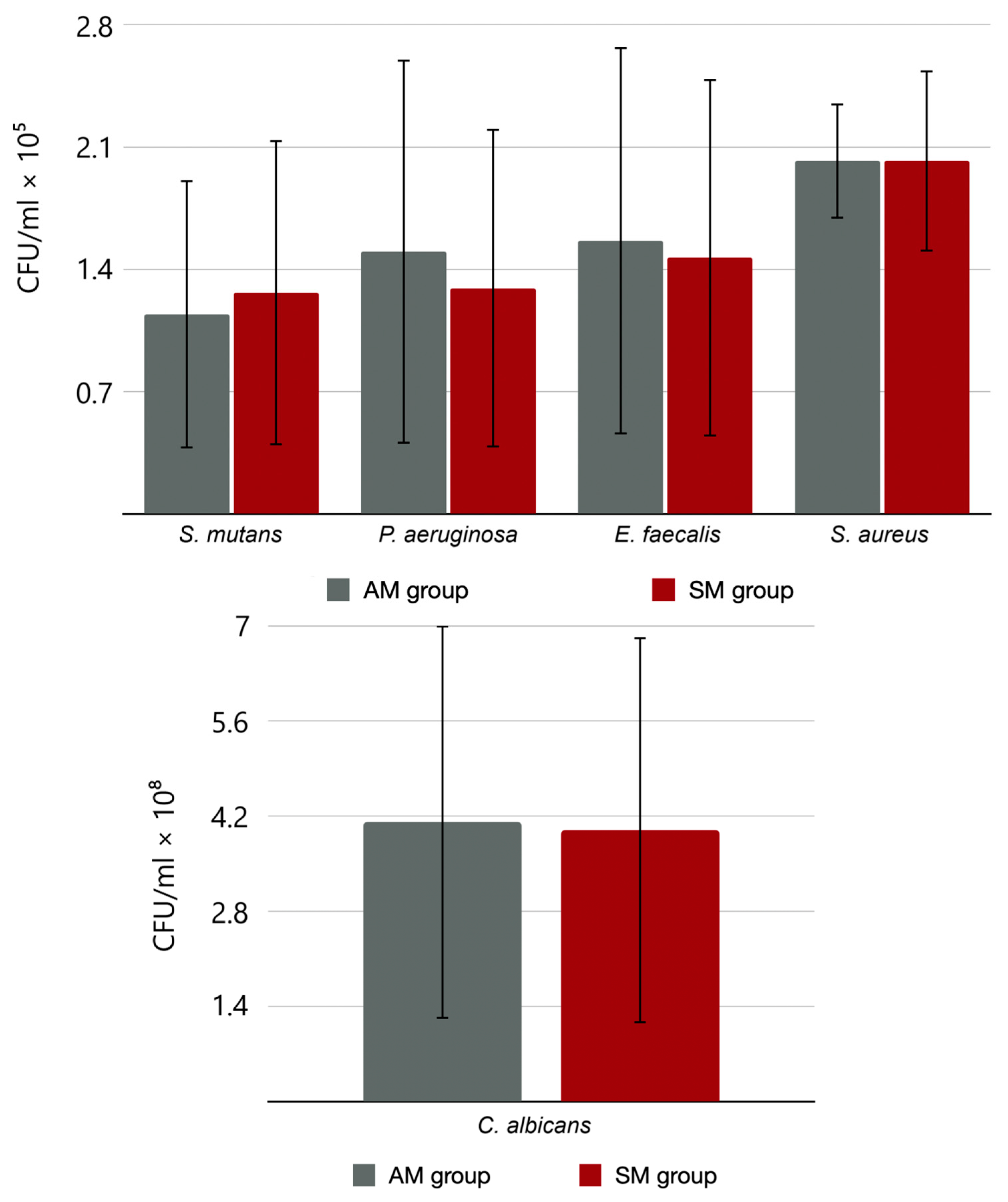
3.2. Quantitative Method
3.3. Statistical Analysis
4. Discussion
Limitations of the Study
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hardan, L.; Mancino, D.; Bourgi, R.; Cuevas-Suárez, C.E.; Lukomska-Szymanska, M.; Zarow, M.; Jakubowicz, N.; Zamarripa-Calderón, J.E.; Kafa, L.; Etienne, O.; et al. Treatment of Tooth Wear Using Direct or Indirect Restorations: A Systematic Review of Clinical Studies. Bioengineering 2022, 9, 346. [Google Scholar] [CrossRef] [PubMed]
- Zimmerli, B.; Strub, M.; Jeger, F.; Stadler, O.; Lussi, A. Composite materials: Composition, properties and clinical applications. A literature review. Schweiz. Monatsschrift Zahnmed. 2010, 120, 972–986. [Google Scholar]
- Von Stein-Lausnitz, M.; Mehnert, A.; Bruhnke, M.; Sterzenbach, G.; Rosentritt, M.; Spies, B.C.; Bitter, K.; Naumann, M. Direct or Indirect Restoration of Endodontically Treated Maxillary Central Incisors with Class III Defects? Composite vs Veneer or Crown Restoration. J. Adhes. Dent. 2018, 20, 519–526. [Google Scholar] [CrossRef]
- Hanawa, T. Zirconia versus titanium in dentistry: A review. Dent. Mater. J. 2020, 39, 24–36. [Google Scholar] [CrossRef] [PubMed]
- Miyazaki, T.; Nakamura, T.; Matsumura, H.; Ban, S.; Kobayashi, T. Current status of zirconia restoration. J. Prosthodont. Res. 2013, 57, 236–261. [Google Scholar] [CrossRef] [PubMed]
- Kaizer, M.R.; Kolakarnprasert, N.; Rodrigues, C.; Chai, H.; Zhang, Y. Probing the interfacial strength of novel multi-layer zirconias. Dent. Mater. 2019, 36, 60–67. [Google Scholar] [CrossRef]
- Schünemann, F.H.; Galárraga-Vinueza, M.E.; Magini, R.; Fredel, M.; Silva, F.; Souza, J.C.; Zhang, Y.; Henriques, B. Zirconia surface modifications for implant dentistry. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 98, 1294–1305. [Google Scholar] [CrossRef] [PubMed]
- Tuominen, H.; Rautava, J. Oral Microbiota and Cancer Development. Pathobiology 2020, 88, 116–126. [Google Scholar] [CrossRef]
- Abranches, J.; Zeng, L.; Kajfasz, J.K.; Palmer, S.R.; Chakraborty, B.; Wen, Z.T.; Richards, V.P.; Brady, L.J.; Lemos, J.A. Biology of Oral Streptococci. Microbiol. Spectr. 2018, 6, 10-1128. [Google Scholar] [CrossRef]
- Antinori, S.; Milazzo, L.; Sollima, S.; Galli, M.; Corbellino, M. Candidemia and invasive candidiasis in adults: A narrative review. Eur. J. Intern. Med. 2016, 34, 21–28. [Google Scholar] [CrossRef]
- Davidowitz, G.; Kotick, P.G. The Use of CAD/CAM in Dentistry. Dent. Clin. North Am. 2011, 55, 559–570. [Google Scholar] [CrossRef]
- Gatto, M.L.; Groppo, R.; Furlani, M.; Giuliani, A.; Mangano, C.; Mangano, F. Lithography-based Ceramic Manufacturing (LCM) versus Milled Zirconia Blocks under uniaxial compressive loading: An in vitro comparative study. J. Dent. 2021, 116, 103886. [Google Scholar] [CrossRef] [PubMed]
- Son, M.; Raju, K.; Lee, J.; Jung, J.; Jeong, S.; Kim, J.-I.; Cho, J. 3D Printing of CNT- and YSZ-Added Dental Resin-Based Composites by Digital Light Processing and Their Mechanical Properties. Materials 2023, 16, 1873. [Google Scholar] [CrossRef] [PubMed]
- Branco, A.; Silva, R.; Santos, T.; Jorge, H.; Rodrigues, A.; Fernandes, R.; Bandarra, S.; Barahona, I.; Matos, A.; Lorenz, K.; et al. Suitability of 3D printed pieces of nanocrystalline zirconia for dental applications. Dent. Mater. 2020, 36, 442–455. [Google Scholar] [CrossRef] [PubMed]
- Revilla-León, M.; Methani, M.M.; Morton, D.; Zandinejad, A. Internal and marginal discrepancies associated with stereolithography (SLA) additively manufactured zirconia crowns. J. Prosthet. Dent. 2020, 124, 730–737. [Google Scholar] [CrossRef] [PubMed]
- Mokhtar, M.; Farahat, D.; Eldars, W.; Osman, M. Physico-mechanical properties and bacterial adhesion of resin composite CAD/CAM blocks: An in-vitro study. J. Clin. Exp. Dent. 2022, 14, e413–e419. [Google Scholar] [CrossRef]
- Vo, D.T.; Arola, D.; Romberg, E.; Driscoll, C.F.; Jabra-Rizk, M.A.; Masri, R. Adherence of Streptococcus mutans on lithium disilicate porcelain specimens. J. Prosthet. Dent. 2015, 114, 696–701. [Google Scholar] [CrossRef]
- Kyung, K.-Y.; Park, J.-M.; Heo, S.-J.; Koak, J.-Y.; Kim, S.-K.; Ahn, J.-S.; Yi, Y. Comparative analysis of flexural strength of 3D printed and milled 4Y-TZP and 3Y-TZP zirconia. J. Prosthet. Dent. 2024, 131, 529.e1–529.e9. Available online: https://www.sciencedirect.com/science/article/pii/S0022391323008399 (accessed on 30 January 2024). [CrossRef]
- Revilla-León, M.; Husain, N.A.-H.; Ceballos, L.; Özcan, M. Flexural strength and Weibull characteristics of stereolithography additive manufactured versus milled zirconia. J. Prosthet. Dent. 2020, 125, 685–690. [Google Scholar] [CrossRef]
- Poole, S.F.; Pitondo-Silva, A.; Oliveira-Silva, M.; Moris, I.C.; Gomes, E.A. Influence of different ceramic materials and surface treatments on the adhesion of Prevotella intermedia. J. Mech. Behav. Biomed. Mater. 2020, 111, 104010. [Google Scholar] [CrossRef]
- Frąckiewicz, W.; Królikowski, M.; Kwiatkowski, K.; Sobolewska, E.; Szymlet, P.; Tomasik, M. Comparison of Dental Zirconium Oxide Ceramics Produced Using Additive and Removal Technology for Prosthodontics and Restorative Dentistry—Strength and Surface Tests: An In Vitro Study. Materials 2023, 17, 168. [Google Scholar] [CrossRef] [PubMed]
- Richards, S.R.; Turner, R.J. A comparative study of techniques for the examination of biofilms by scanning electron microscopy. Water Res. 1984, 18, 767–773. [Google Scholar] [CrossRef]
- Mączyńska, B.; Neumann, K.; Junka, A.; Smutnicka, D.; Secewicz, A.; Bartoszewicz, M.; Wójkowska-Mach, J.; Sękowska, A.; Gospodarek, E.; Burdynowski, K. Analysis of properties related to selection and survival in hospital environment of Klebsiella strains isolated from nosocomial outbreaks. Forum Zakażeń 2013, 4, 77–97. [Google Scholar] [CrossRef]
- ISO 2854; Statistical Interpretation of Data. Techniques of Estimation and Tests Relating to Means and Variances. ISO: Geneva, Switzerland, 1976.
- Zijnge, V.; van Leeuwen, M.B.M.; Degener, J.E.; Abbas, F.; Thurnheer, T.; Gmür, R.; Harmsen, H.J.M. Oral Biofilm Architecture on Natural Teeth. PLoS ONE 2010, 5, e9321. [Google Scholar] [CrossRef] [PubMed]
- Patel, M. Oral Cavity and Candida albicans: Colonisation to the Development of Infection. Pathogens 2022, 11, 335. [Google Scholar] [CrossRef] [PubMed]
- Cepic, L.Z.; Dvorak, G.; Piehslinger, E.; Georgopoulos, A. In vitro adherence of Candida albicans to zirconia surfaces. Oral Dis. 2020, 26, 1072–1080. [Google Scholar] [CrossRef]
- Scotti, R.; Kantorski, K.Z.; Scotti, N.; Monaco, C.; Valandro, L.F.; Bottino, M.A. Early biofilm colonization on polished- and glazed-zirconium ceramic surface. Preliminary results. Minerva Stomatol. 2006, 55, 493–502. [Google Scholar]
- Jaeggi, M.; Gyr, S.; Astasov-Frauenhoffer, M.; Zitzmann, N.U.; Fischer, J.; Rohr, N. Influence of different zirconia surface treatments on biofilm formation in vitro and in situ. Clin. Oral Implant. Res. 2022, 33, 424–432. [Google Scholar] [CrossRef]
- Khattar, A.; Alghafli, J.A.; Muheef, M.A.; Alsalem, A.M.; Al-Dubays, M.A.; AlHussain, H.M.; AlShoalah, H.M.; Khan, S.Q.; AlEraky, D.M.; Gad, M.M. Antibiofilm Activity of 3D-Printed Nanocomposite Resin: Impact of ZrO2 Nanoparticles. Nanomaterials 2023, 13, 591. [Google Scholar] [CrossRef]
- Arweiler, N.B.; Netuschil, L. The Oral Microbiota. Adv. Exp. Med. Biol. 2016, 902, 45–60. [Google Scholar]
- Wiessner, A.; Wassmann, T.; Wiessner, J.M.; Schubert, A.; Wiechens, B.; Hampe, T.; Bürgers, R. In Vivo Biofilm Formation on Novel PEEK, Titanium, and Zirconia Implant Abutment Materials. Int. J. Mol. Sci. 2023, 24, 1779. [Google Scholar] [CrossRef] [PubMed]
- Chiou, L.-L.; Panariello, B.H.D.; Hamada, Y.; Gregory, R.L.; Blanchard, S.; Duarte, S. Comparison of In Vitro Biofilm Formation on Titanium and Zirconia Implants. BioMed Res. Int. 2023, 2023, 8728499. [Google Scholar] [CrossRef] [PubMed]
- Zandinejad, A.; Das, O.; Barmak, A.B.; Kuttolamadom, M.; Revilla-León, M. The Flexural Strength and Flexural Modulus of Stereolithography Additively Manufactured Zirconia with Different Porosities. J. Prosthodont. 2021, 31, 434–440. [Google Scholar] [CrossRef] [PubMed]
- Han, A.; Tsoi, J.K.; Matinlinna, J.P.; Zhang, Y.; Chen, Z. Effects of different sterilization methods on surface characteristics and biofilm formation on zirconia in vitro. Dent. Mater. 2017, 34, 272–281. [Google Scholar] [CrossRef]
- Buj-Corral, I.; Vidal, D.; Tejo-Otero, A.; Padilla, J.A.; Xuriguera, E.; Fenollosa-Artés, F. Characterization of 3D Printed Yttria-Stabilized Zirconia Parts for Use in Prostheses. Nanomaterials 2021, 11, 2942. [Google Scholar] [CrossRef]
- Unkovskiy, A.; Beuer, F.; Metin, D.S.; Bomze, D.; Hey, J.; Schmidt, F. Additive Manufacturing of Lithium Disilicate with the LCM Process for Classic and Non-Prep Veneers: Preliminary Technical and Clinical Case Experience. Materials 2022, 15, 6034. [Google Scholar] [CrossRef]
- ISO 13485; Medical Devices. Quality Management Systems. Requirements for Regulatory Purposes. ISO: Geneva, Switzerland, 2016.

| Strains Used in Biofilm Analysis on 3D Printed Zirconium Oxide Samples | ||||
| S. mutans | P. aeruginosa | E. faecalis | S. aureus | C. albicans |
| + | + | + | + | +++ |
| Strains Used in Biofilm Analysis on Milled Zirconium Oxide Samples | ||||
| S. mutans | P. aeruginosa | E. faecalis | S. aureus | C. albicans |
| + | + | + | + | ++ |
| Trial Number | S. mutans | P. aeruginosa | E. faecalis | S. aureus | C. albicans |
|---|---|---|---|---|---|
| CFU/mL | |||||
| 1 | 1.8 × 105 | 2.1 × 105 | 2.5 × 105 | 1.6 × 105 | 6.1 × 108 |
| 2 | 1.3 × 105 | 1.5 × 104 | 2.5 × 105 | 2.5 × 105 | 6.8 × 108 |
| 3 | 2.1 × 105 | 2.1 × 105 | 2.3 × 104 | 1.9 × 105 | 5.5 × 107 |
| 4 | 2.6 × 104 | 2.6 × 104 | 1.8 × 104 | 2.3 × 105 | 6.5 × 108 |
| 5 | 2.5 × 104 | 2.9 × 105 | 2.4 × 105 | 1.8 × 105 | 6.2 × 107 |
| Trial Number | S. mutans | P. aeruginosa | E. faecalis | S. aureus | C. albicans |
|---|---|---|---|---|---|
| CFU/mL | |||||
| 1 | 2.2 × 105 | 2.1 × 105 | 2.3 × 105 | 1.3 × 105 | 6.7 × 108 |
| 2 | 1.7 × 105 | 1.4 × 104 | 2.2 × 105 | 1.8 × 105 | 5.8 × 108 |
| 3 | 2.0 × 105 | 2.1 × 105 | 2.1 × 104 | 2.9 × 105 | 5.4 × 107 |
| 4 | 1.8 × 104 | 2.2 × 104 | 2.2 × 104 | 2.1 × 105 | 6.4 × 108 |
| 5 | 2.5 × 104 | 1.9 × 105 | 2.4 × 105 | 2.0 × 105 | 5.2 × 107 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frąckiewicz, W.; Pruss, A.; Królikowski, M.; Szymlet, P.; Sobolewska, E. Comparison of the Intensity of Biofilm Production by Oral Microflora and Its Adhesion on the Surface of Zirconia Produced in Additive and Subtractive Technology: An In Vitro Study. Materials 2024, 17, 1231. https://doi.org/10.3390/ma17061231
Frąckiewicz W, Pruss A, Królikowski M, Szymlet P, Sobolewska E. Comparison of the Intensity of Biofilm Production by Oral Microflora and Its Adhesion on the Surface of Zirconia Produced in Additive and Subtractive Technology: An In Vitro Study. Materials. 2024; 17(6):1231. https://doi.org/10.3390/ma17061231
Chicago/Turabian StyleFrąckiewicz, Wojciech, Agata Pruss, Marcin Królikowski, Paweł Szymlet, and Ewa Sobolewska. 2024. "Comparison of the Intensity of Biofilm Production by Oral Microflora and Its Adhesion on the Surface of Zirconia Produced in Additive and Subtractive Technology: An In Vitro Study" Materials 17, no. 6: 1231. https://doi.org/10.3390/ma17061231





