Finite Element Analysis and Fatigue Test of INTEGRA Dental Implant System
Abstract
1. Introduction
2. Materials and Methods
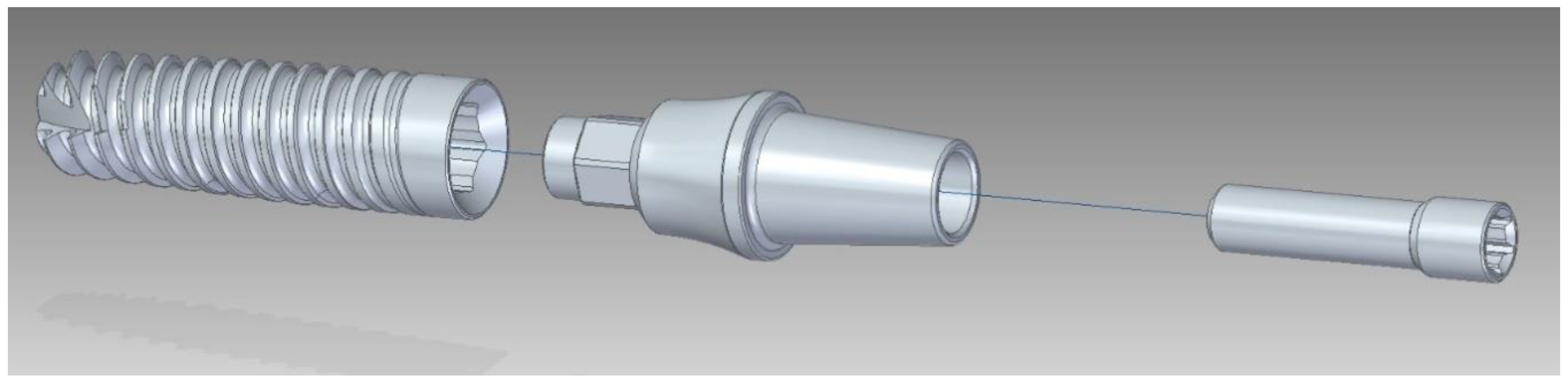
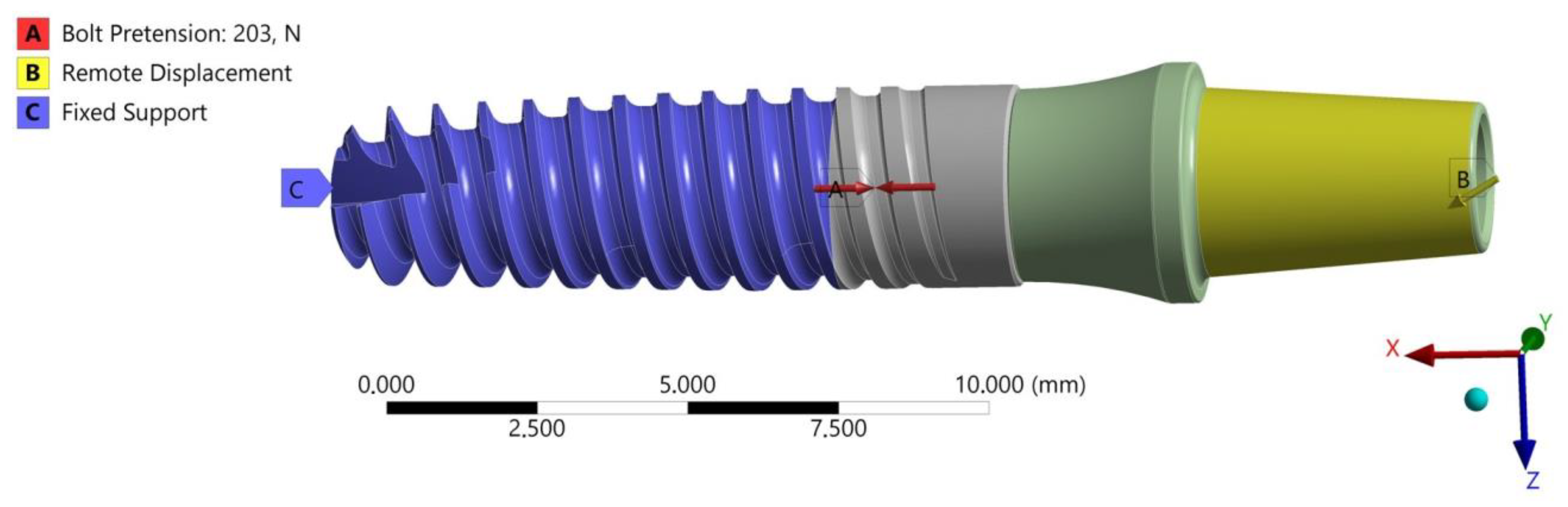
2.1. Description of the Set
2.2. FEA of the INTEGRA OPTIMA 3.35
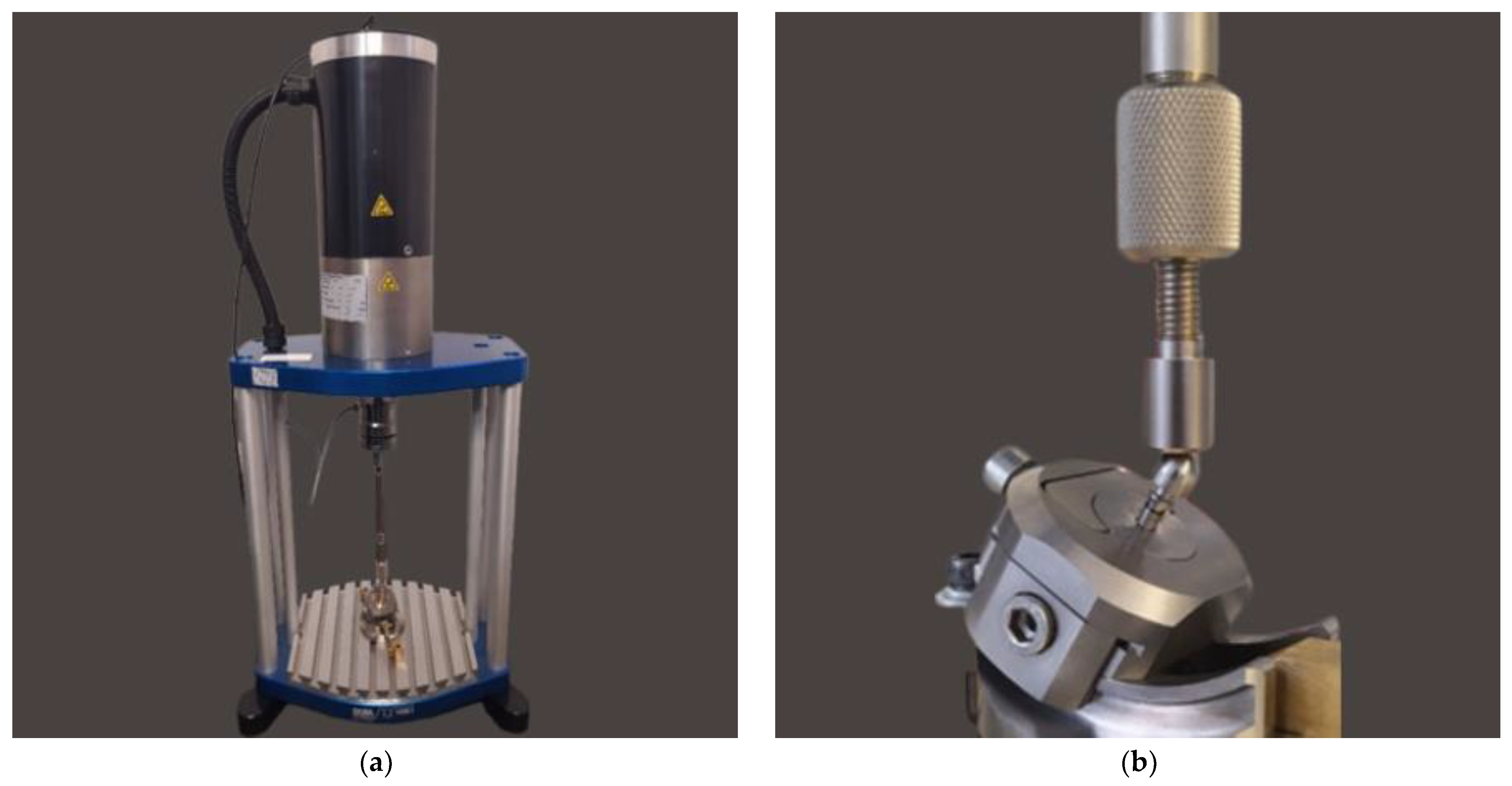
2.3. Fatigue Strength Testing
2.3.1. Description of Wöhler Curve
2.3.2. Fatigue Test of the INTEGRA OPTIMA 3.35
2.4. Hypothesis Zero
3. Results
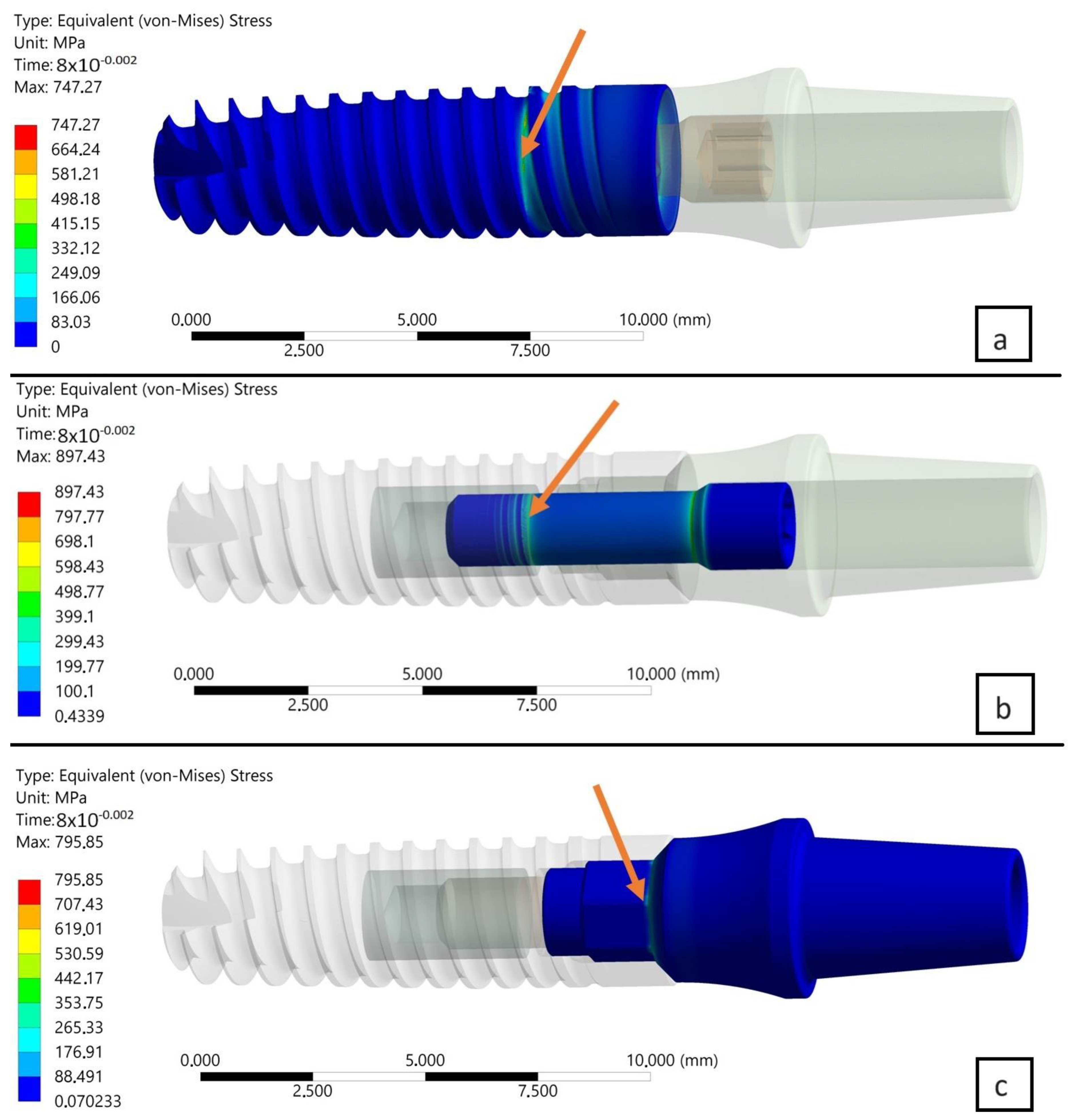
3.1. Results of FEA of INTEGRA OPTIMA 3.35
- The area of the external thread of the endosteal implant at its “exit” from the fixed support reflecting the assembly’s anchoring in the substrate (jawbone or maxilla).
- The area of the screw thread corresponding to the position where the connector screw exits the implant.
- The area of the upper part of the hexagonal-cylindrical peg of the prosthetic connector.
- Comparing the two planes of load with each other, i.e., X-Z and X-Y, in subsequent time steps, a decrease in stress was observed in the area of the implant by, respectively, 6% (0.08 s) and 5.3% (0.1 s).
- Comparing the two planes of load with each other, i.e., X-Z and X-Y, in subsequent time steps, an increase in stress was observed in the area of the connecting screw by, respectively, 1.3% (0.08 s) and 1.9% (0.1 s).
- Comparing the two planes of load with each other, i.e., X-Z and X-Y, in subsequent time steps, an increase in stress was observed in the area of the connector by, respectively, 4.4% (0.08 s) and 5.5% (0.1 s).
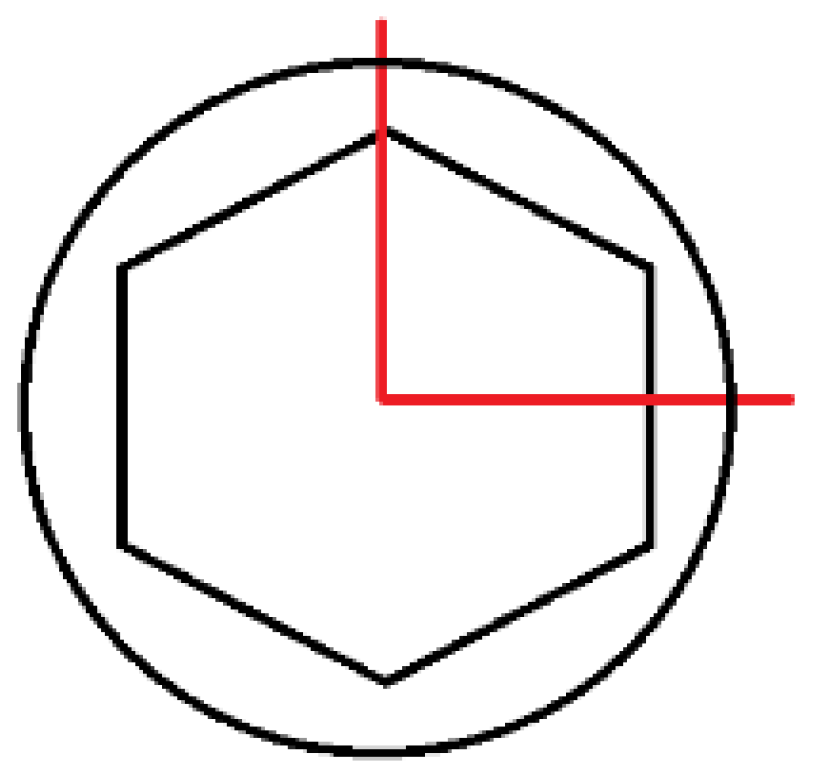
- The change in the loaded cross-sectional area resulting from a different position of the hexagonal-cylindrical peg of the connector relative to the action of the loading plane (Figure 7).
- The stiffness of the support fixing the implant, the greater it is, the greater the increase in stress concentration in the area where the implant exits the fixation area [holder, bone] (Figure 9).
3.2. Results of Fatigue Tests
4. Discussion
4.1. Fatigue Test
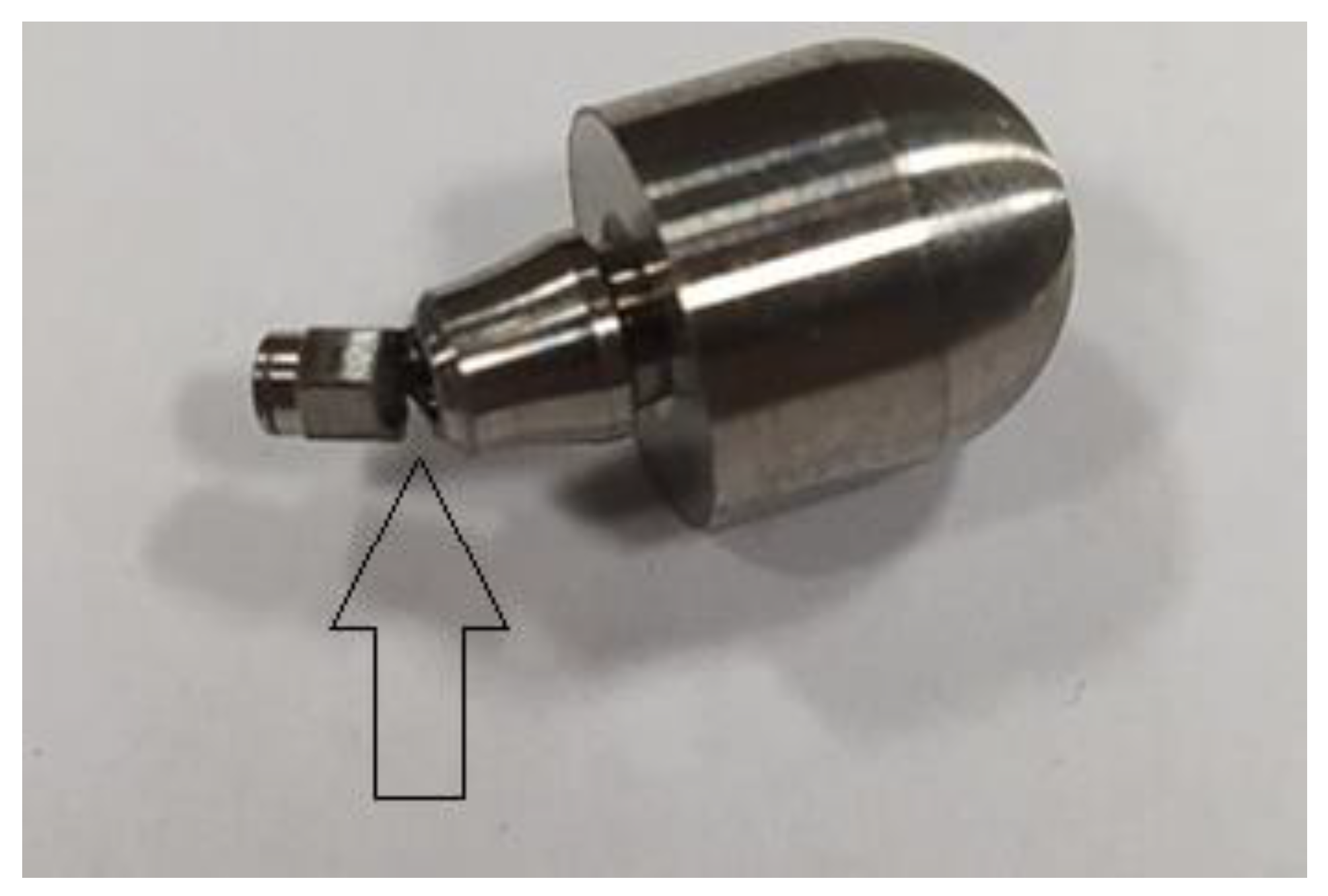
- The area of the upper part of the hexagonal-cylindrical peg of the prosthetic connector.
- The area of the thread of the screw connecting the prosthetic connector to the endosteal implant.
4.2. Clinical Application of Narrow Implants—Survival, Success, and Marginal Bone Level under Functional Loading
5. Conclusions
- The comparison of results presented on the Wöhler curve prepared for selected systems suggests that the unlimited strength for the Optima 3.35 system is indicated at a load force level of F = 200–210 N.
- Comparing the results of the fatigue test with the qualitative FEA analysis, it can be concluded that the value of unlimited fatigue strength at a load force level of F = 200–210 N is consistent with the qualitative results of numerical calculations where the load force was estimated at F = 180 N. The differences that were revealed probably result from the change in the pre-tension of the connecting screw during the assembly of the system as well as the method of mounting the implant with the base.
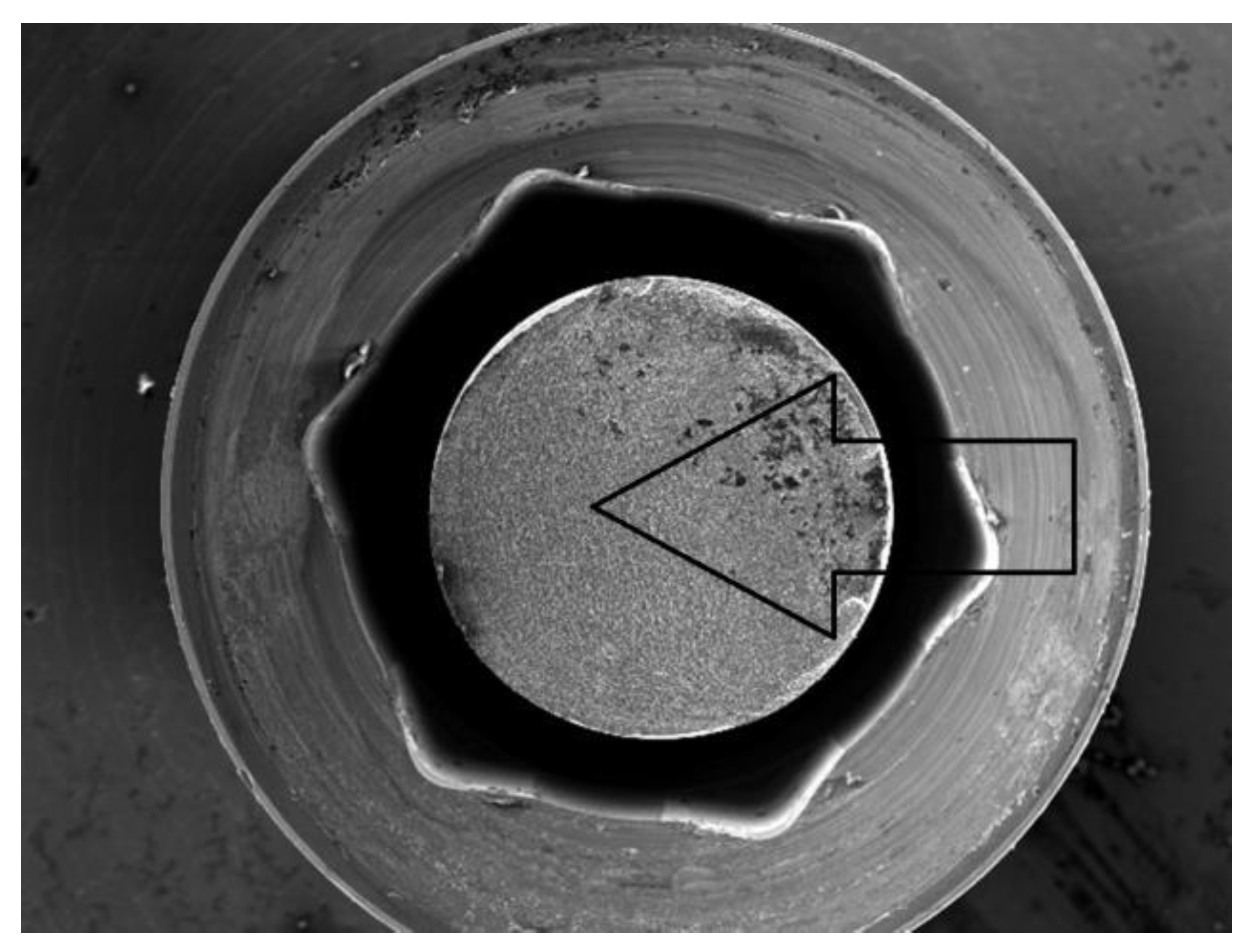
- Observation of the prosthetic connector in the OPTIMA 3.35 system, cyclically loaded with a force of F ≈ 300 N in the area of the upper hexagonal peg, revealed a fatigue fracture consisting of a perifocal zone, primary displacements, and fatigue lines. Fatigue foci are located on both opposite sides (corners) of the hexagonal peg.
- From the comparison of the numerical analysis with the SEM image of the fatigue fracture in the area of the upper part of the hexagonal peg of the prosthetic connector for the OPTIMA 3.35 system, satisfactory convergence of results was obtained. The areas indicated in the numerical analysis confirm that they are potential sites for the development of cracks and propagation of fatigue foci in the analyzed system.
- The observed change in stress peak in the dental connector for two different force application surfaces shows that the positioning of the dental system (setting of the socket in relation to the force action plane) is significant in estimating the limited fatigue strength. This difference can be seen in the Wöhler curve characteristics by observing the load level for a force close to F ≈ 300 N, where significant differences in the life cycle count of the tested structure were obtained.
- The method of modeling, correlated with fatigue testing, not only explains the impact of the analyzed geometry of the system on the test results but also explains phenomena occurring during loading, which have a significant impact on the estimation of the unlimited fatigue strength of the system.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pjetursson, B.E.; Asgeirsson, A.G.; Zwahlen, M.; Sailer, I. Improvements in implant dentistry over the last decade: Comparison of survival and complication rates in older and newer publications. Int. J. Oral Maxillofac. Implant. 2014, 29, 308–324. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.L.; Lin, H.; Zhang, Y.D.; Han, J.M. Comparison of technical, biological, and esthetic parameters of ceramic and metal-ceramic implant-supported fixed dental prostheses: A systematic review and meta-analysis. J. Prosthet. Dent. 2020, 124, 26–35. [Google Scholar] [CrossRef] [PubMed]
- Gaddale, R.; Mishra, S.K.; Chowdhary, R. Complications of screw- and cement-retained implant-supported full-arch restorations: A systematic review and meta-analysis. Int. J. Oral Implantol. 2020, 13, 11–40. [Google Scholar]
- Sghaireen, M.G.; Shrivastava, D.; Alnusayri, M.O.; Alahmari, A.D.; Aldajani, A.M.; Srivastava, K.C.; Alam, M.K. Bone Grafts in Dental Implant Management: A Narrative Review. Curr. Pediatr. Rev. 2022, 19, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Farias, D.; Caceres, F.; Sanz, A.; Olate, S. Horizontal Bone Augmentation in the Posterior Atrophic Mandible and Dental Implant Stability Using the Tenting Screw Technique. Int. J. Periodontics Restor. Dent. 2021, 41, e147–e155. [Google Scholar] [CrossRef] [PubMed]
- Kalsi, A.S.; Kalsi, J.S.; Bassi, S. Alveolar ridge preservation: Why, when and how. Br. Dent. J. 2019, 227, 264–274. [Google Scholar] [CrossRef]
- Frost, H.M. Bone “mass” and the “mechanostat”: A proposal. Anat. Rec. 1987, 219, 1–9. [Google Scholar] [CrossRef]
- Frost, H.M. A 2003 update of bone physiology and Wolff’s law for clinicians. Angle Orthod. 2004, 74, 3–15. [Google Scholar]
- Chang, Y.; Tambe, A.A.; Maeda, Y.; Wada, M.; Gonda, T. Finite element analysis of dental implants with validation: To what extent can we expect the model to predict biological phenomena? A literature review and proposal for classification of a validation process. Int. J. Implant. Dent. 2018, 4, 7. [Google Scholar] [CrossRef]
- Pesqueira, A.A.; Goiato, M.C.; Filho, H.G.; Monteiro, D.R.; Santos, D.M.D.; Haddad, M.F.; Pellizzer, E.P. Use of stress analysis methods to evaluate the biomechanics of oral rehabilitation with implants. J. Oral Implantol. 2014, 40, 217–228. [Google Scholar] [CrossRef]
- Ey-Chmielewska, H.; Chruściel-Nogalska, M.; Frączak, B. Photogrammetry and Its Potential Application in Medical Science on the Basis of Selected Literature. Adv. Clin. Exp. Med. 2015, 24, 737–741. [Google Scholar] [CrossRef] [PubMed]
- Bonfante, E.A.; Coelho, P.G. A Critical Perspective on Mechanical Testing of Implants and Prostheses. Adv. Dent. Res. 2016, 28, 18–27. [Google Scholar] [CrossRef]
- Kelly, J.R.; Cesar, P.F.; Scherrer, S.S.; Della Bona, A.; van Noort, R.; Tholey, M.; Vichi, A.; Lohbauer, U. ADM guidance-ceramics: Fatigue principles and testing. Dent. Mater. 2017, 33, 1192–1204. [Google Scholar] [CrossRef]
- PN-EN 14801; Dentistry—Implants—Dynamic Fatigue Test for Endosseous Dental Implants. ISO: Geneve, Switzerland, 2016.
- ISO 5832-2:2018; Implants for Surgery—Metallic Materials—Part 2: Unalloyed Titanium. ISO: Geneve, Switzerland, 2018.
- Wiskott, H.W.; Nicholls, J.I.; Belser, U.C. Fatigue resistance of soldered joints: A methodological study. Dent. Mater. 1994, 10, 215–220. [Google Scholar] [CrossRef] [PubMed]
- Balfour, A.; O’Brien, G.R. Comparative study of antirotational single tooth abutments. J. Prosthet. Dent. 1995, 73, 36–43. [Google Scholar] [CrossRef]
- Huang, H.M.; Tsai, C.M.; Chang, C.C.; Lin, C.T.; Lee, S.Y. Evaluation of loading conditions on fatigue-failed implants by fracture surface analysis. Int. J. Oral Maxillofac. Implant. 2005, 20, 854–859. [Google Scholar]
- Gehrke, P.; Dhom, G.; Brunner, J.; Wolf, D.; Degidi, M.; Piattelli, A. Zirconium implant abutments: Fracture strength and influence of cyclic loading on retaining-screw loosening. Quintessence Int. 2006, 37, 19–26. [Google Scholar]
- Seetoh, Y.L.; Tan, K.B.; Chua, E.K.; Quek, H.C.; Nicholls, J.I. Load fatigue performance of conical implant-abutment connections. Int. J. Oral Maxillofac. Implant. 2011, 26, 797–806. [Google Scholar]
- Marchetti, E.; Ratta, S.; Mummolo, S.; Tecco, S.; Pecci, R.; Bedini, R.; Marzo, G. Mechanical Reliability Evaluation of an Oral Implant-Abutment System According to UNI EN ISO 14801 Fatigue Test Protocol. Implant. Dent. 2016, 25, 613–618. [Google Scholar] [CrossRef]
- Canullo, L.; Coelho, P.G.; Bonfante, E.A. Mechanical testing of thin-walled zirconia abutments. J. Appl. Oral Sci. 2013, 21, 20–24. [Google Scholar] [CrossRef][Green Version]
- Marchetti, E.; Ratta, S.; Mummolo, S.; Tecco, S.; Pecci, R.; Bedini, R.; Marzo, G. Evaluation of an endosseous oral implant system according to UNI EN ISO 14801 Fatigue Test Protocol. Implant. Dent. 2014, 23, 665–671. [Google Scholar] [CrossRef]
- Park, J.M.; Baek, C.H.; Heo, S.J.; Kim, S.K.; Koak, J.Y.; Kim, S.K.; Belser, U.C. An in vitro evaluation ofthe loosening of different interchangeable abutments in internal-connection-type implants. Int. J. Oral Maxillofac. Implant. 2017, 32, 350–355. [Google Scholar] [CrossRef]
- Spies, B.C.; Nold, J.; Vach, K.; Kohal, R.J. Two-piece zirconia oral implants withstand masticatory loads: An investigation in the artificial mouth. J. Mech. Behav. Biomed. Mater. 2016, 53, 1–10. [Google Scholar] [CrossRef]
- Joda, T.; Voumard, B.; Zysset, P.K.; Brägger, U.; Ferrari, M. Ultimate force and stiffness of 2-piece zirconium dioxide implants with screw-retained monolithic lithium disilicate reconstructions. J. Prosthodont. Res. 2018, 62, 258–263. [Google Scholar] [CrossRef]
- Choi, J.W.; Choi, K.H.; Chae, H.J.; Chae, S.K.; Bae, E.B.; Lee, J.J.; Lee, S.H.; Jeong, C.M.; Huh, J.B. Load-Bearing Capacity and Retention of Newly Developed Micro-Locking Implant Prosthetic System: An In Vitro Pilot Study. Materials 2018, 11, 564. [Google Scholar] [CrossRef]
- Renouard, F.; Nisand, D. Impact of implant length and diameter on survival rates. Clin. Oral Implant. Res. 2006, 17 (Suppl. S2), 35–51. [Google Scholar] [CrossRef] [PubMed]
- Mijiritsky, E.; Mazor, Z.; Lorean, A.; Levin, L. Implant diameter and length influence on survival: Interim results during the first 2 years of function of implants by a single manufacturer. Implant. Dent. 2013, 22, 394–398. [Google Scholar] [CrossRef] [PubMed]
- Jackson, B.J. Small diameter implants: Specific indications and considerations for the posterior mandible: A case report. J. Oral Implantol. 2011, 37, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Sohn, D.S.; Bae, M.S.; Heo, J.U.; Park, J.S.; Yea, S.H.; Romanos, G.E. Retrospective multicenter analysis of immediate provisionalization using one-piece narrow-diameter (3.0-mm) implants. Int. J. Oral Maxillofac. Implant. 2011, 26, 163–168. [Google Scholar]
- Galindo-Moreno, P.; Nilsson, P.; King, P.; Becktor, J.; Speroni, S.; Schramm, A.; Maiorana, C. Clinical and radiographic evaluation of early loaded narrow diameter implants—1-year follow-up. Clin. Oral Implant. Res. 2012, 23, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Mazor, Z.; Lorean, A.; Mijiritsky, E.; Levin, L. Replacement of a molar with 2 narrow diameter dental implants. Implant. Dent. 2012, 21, 36–38. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, J.B.; Alam, M.N.; Salman, A.; Chandrasekaran, S.C. Narrow diameter implant in posterior region. J. Indian. Soc. Periodontol. 2012, 16, 610–613. [Google Scholar] [PubMed]
- Sallam, H.; Kheiralla, L.S.; Aldawakly, A. Microstrains around standard and mini implants supporting different bridge designs. J. Oral Implantol. 2012, 38, 221–229. [Google Scholar] [CrossRef] [PubMed]
- Olate, S.; Lyrio, M.C.; de Moraes, M.; Mazzonetto, R.; Moreira, R.W.F. Influence of diameter and length of implant on early dental implant failure. J. Oral Maxillofac. Surg. 2010, 68, 414–419. [Google Scholar] [CrossRef]
- Yi, Y.S.; Emanuel, K.M.; Chuang, S.K. Short (5.0 × 5.0 mm) implant placements and restoration with integrated abutment crowns. Implant. Dent. 2011, 20, 125–130. [Google Scholar] [CrossRef]
- Klein, M.O.; Schiegnitz, E.; Al-Nawas, B. Systematic Review on Success of Narrow-Diameter Dental Implants. Int. J. Oral Maxillofac. Implant. 2014, 29, 43–54. [Google Scholar] [CrossRef]
- Slagter, K.W.; Meijer, H.J.A.; Hentenaar, D.F.M.; Vissink, A.; Raghoebar, G.M. Immediate single-tooth implant placement with simultaneous bone augmentation versus delayed implant placement after alveolar ridge preservation in bony defect sites in the esthetic region: A 5-year randomized controlled trial. J. Periodontol. 2021, 92, 1738–1748. [Google Scholar] [CrossRef]





| Density | 465 | kg/m3 |
| Young’s Modulus | 110.000 | MPa |
| Poisson’s Ratio | 0.34 | - |
| Yield Strength | 848 | MPa |
| Tangent Modulus | 30.000 | MPa |
| Area of force application | X-Z | X-Y | X-Z | X-Y |
| Temporal point of analysis | 0.08 [s] | 0.08 [s] | 0.1 [s] | 0.1 [s] |
| Value of the peak of reduced von Mises stresses in the endosteal implant | 747.27 [MPa] | 704.84 [MPa] | 854.54 [MPa] | 810.97 [MPa] |
| 6% | 5.3% | |||
| Value of the peak of reduced von Mises stresses in the connector screw | 897.43 [MPa] | 909.45 [MPa] | 916.37 [MPa] | 934.18 [MPa] |
| 1.3% | 1.9% | |||
| Value of the peak of reduced von Mises stresses in the abutment | 795.85 [MPa] | 831.07 [MPa] | 795.68 [MPa] | 839.50 [MPa] |
| 4.4% | 5.5% | |||
| Force F [N] | Standard Deviation ± [N] | Number of Cycles [n] |
|---|---|---|
| 195,100 | 5525 | 5,000,000 |
| 198,300 | 5625 | 5,000,000 |
| 198,400 | 5183 | 5,000,000 |
| 206,600 | 6769 | 5,000,000 |
| 218,000 | 4853 | 146,546 |
| 228,200 | 4846 | 93,242 |
| 228,200 | 4336 | 66,917 |
| 248,100 | 4169 | 58,217 |
| 297,000 | 5835 | 8010 |
| 297,700 | 4146 | 21,362 |
| 297,500 | 4711 | 14,276 |
| 297,700 | 4313 | 20,461 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zieliński, R.; Lipa, S.; Piechaczek, M.; Sowiński, J.; Kołkowska, A.; Simka, W. Finite Element Analysis and Fatigue Test of INTEGRA Dental Implant System. Materials 2024, 17, 1213. https://doi.org/10.3390/ma17051213
Zieliński R, Lipa S, Piechaczek M, Sowiński J, Kołkowska A, Simka W. Finite Element Analysis and Fatigue Test of INTEGRA Dental Implant System. Materials. 2024; 17(5):1213. https://doi.org/10.3390/ma17051213
Chicago/Turabian StyleZieliński, Rafał, Sebastian Lipa, Martyna Piechaczek, Jerzy Sowiński, Agata Kołkowska, and Wojciech Simka. 2024. "Finite Element Analysis and Fatigue Test of INTEGRA Dental Implant System" Materials 17, no. 5: 1213. https://doi.org/10.3390/ma17051213
APA StyleZieliński, R., Lipa, S., Piechaczek, M., Sowiński, J., Kołkowska, A., & Simka, W. (2024). Finite Element Analysis and Fatigue Test of INTEGRA Dental Implant System. Materials, 17(5), 1213. https://doi.org/10.3390/ma17051213







