Effect of K+ Diffusion on Hydration of Magnesium Potassium Phosphate Cement with Different Mg/P Ratios: Experiments and Molecular Dynamics Simulation Calculations
Abstract
1. Introduction
2. Methodology
2.1. Experimental Method
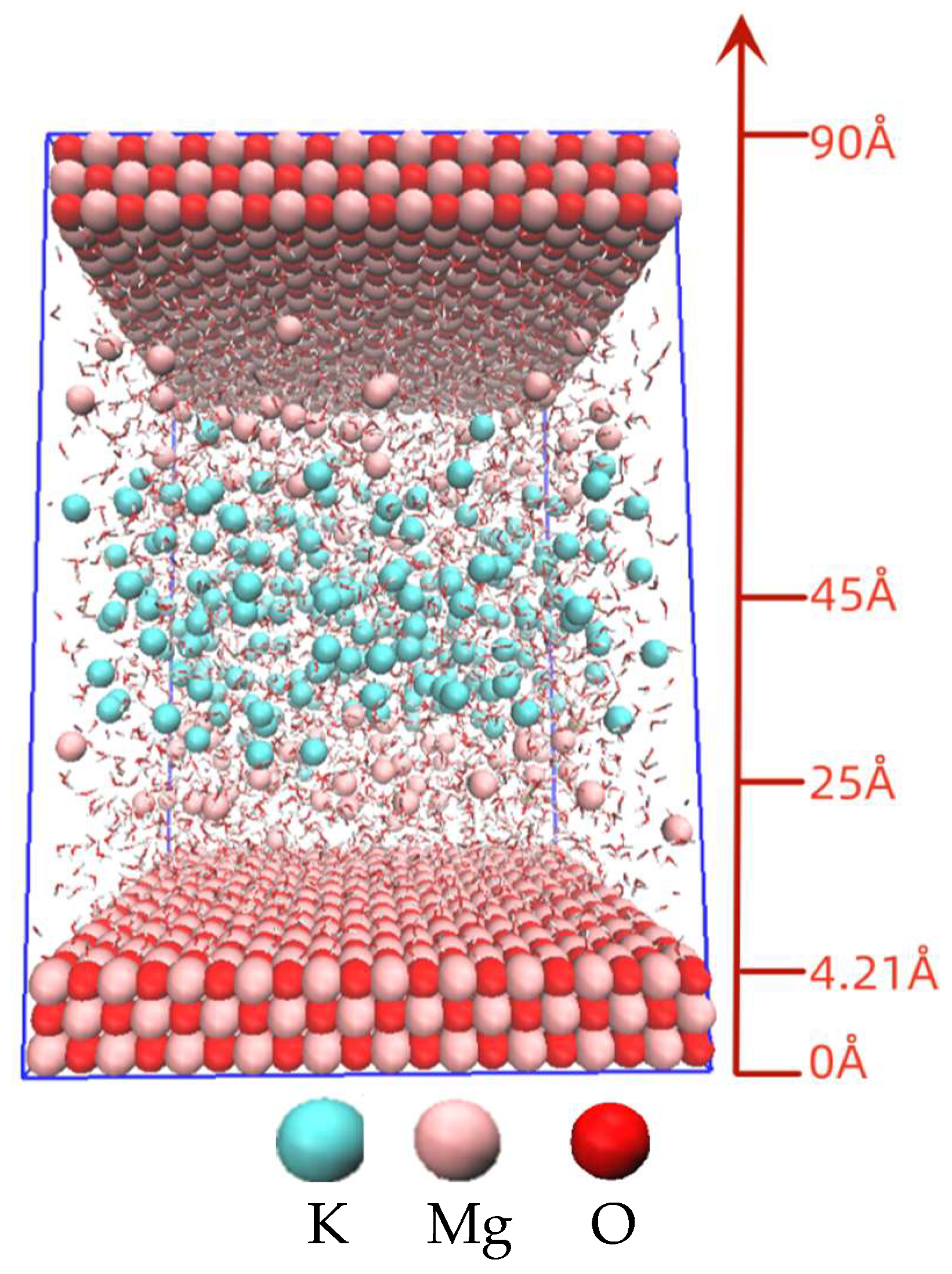
2.2. Computational Method
3. Results and Discussion
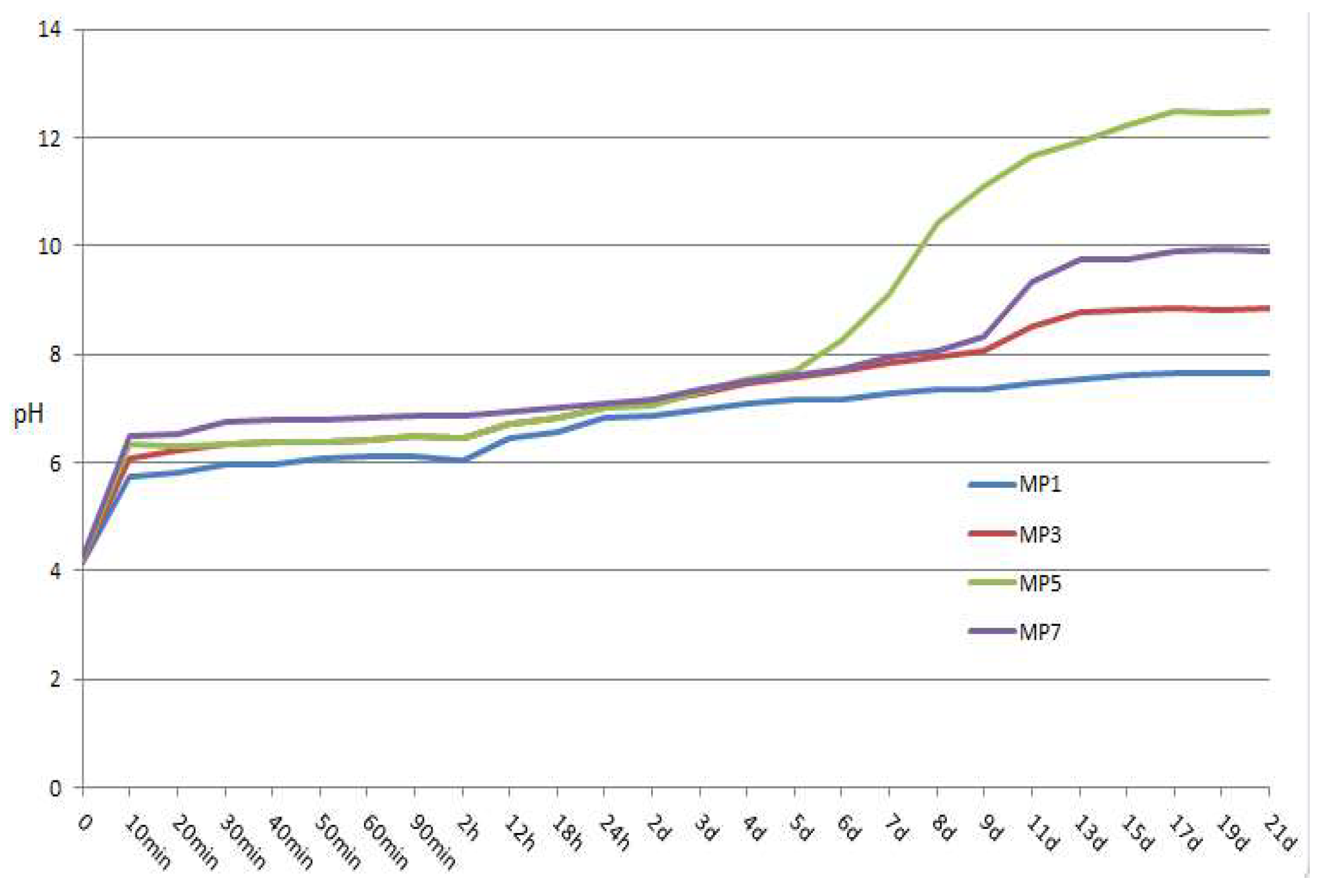
3.1. pH Value
3.2. Concentrations of Phosphorus and Potassium in Leachate
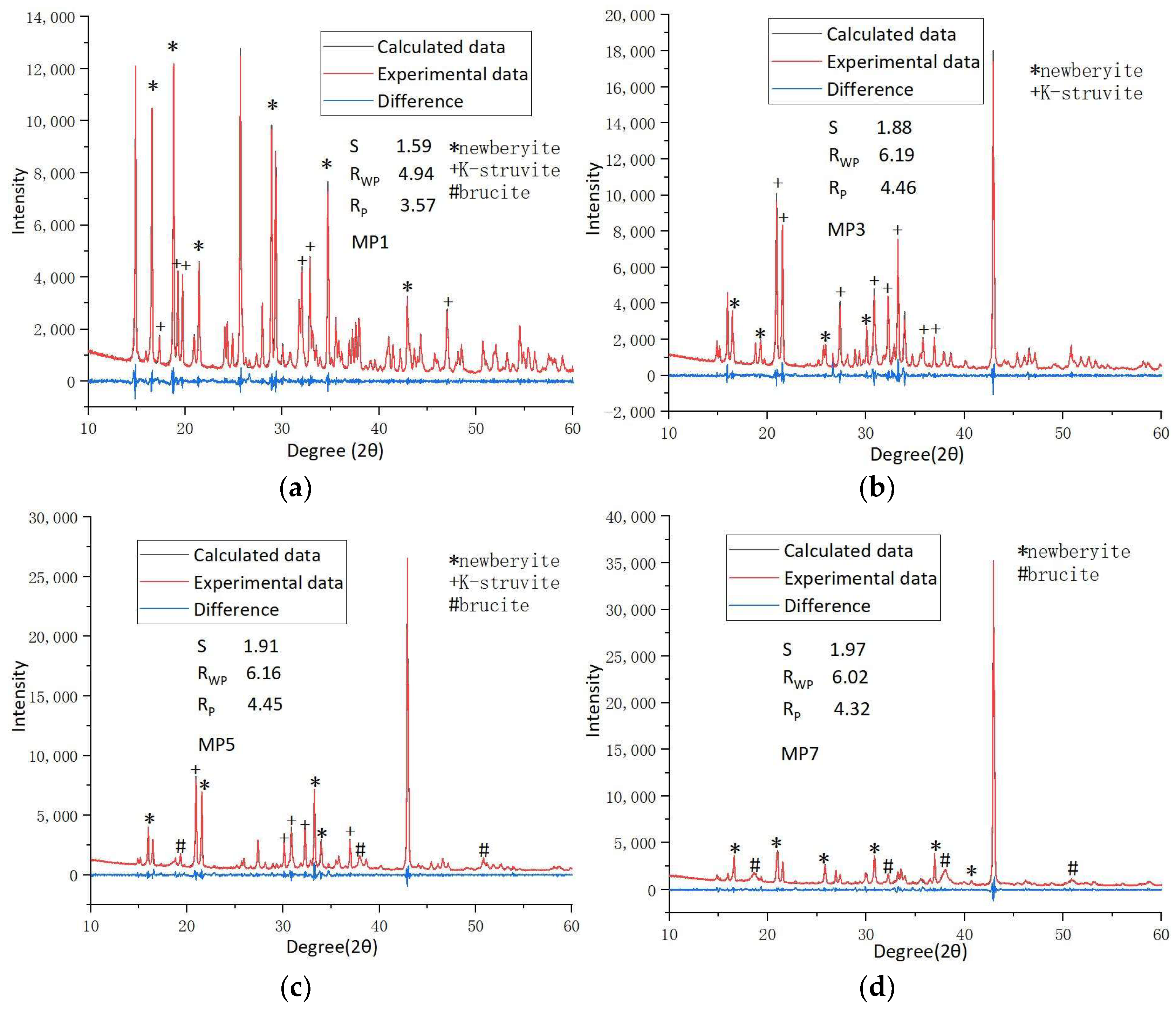
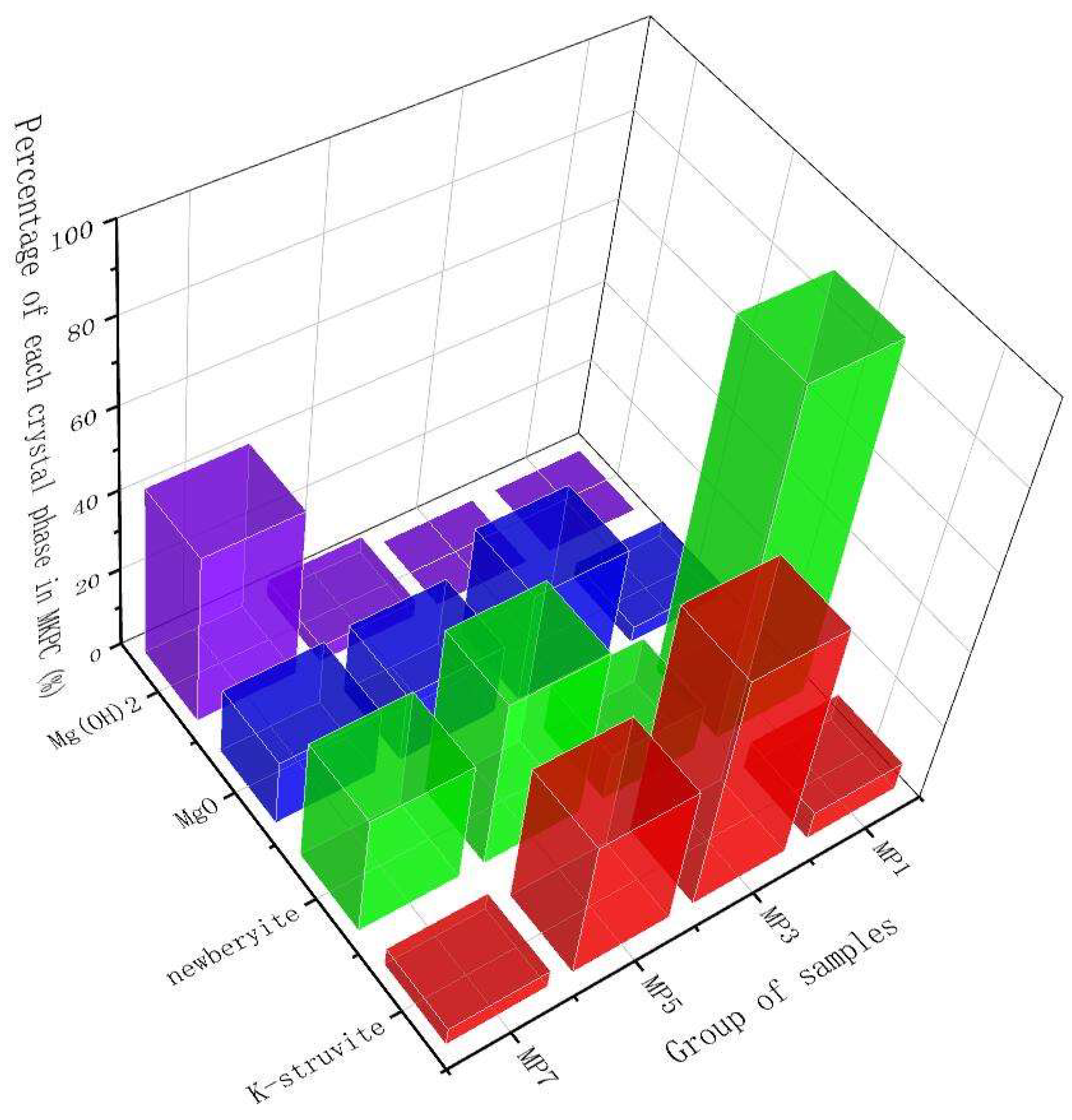
3.3. Rietveld XRD Refinement
3.4. SEM and EDS Analysis
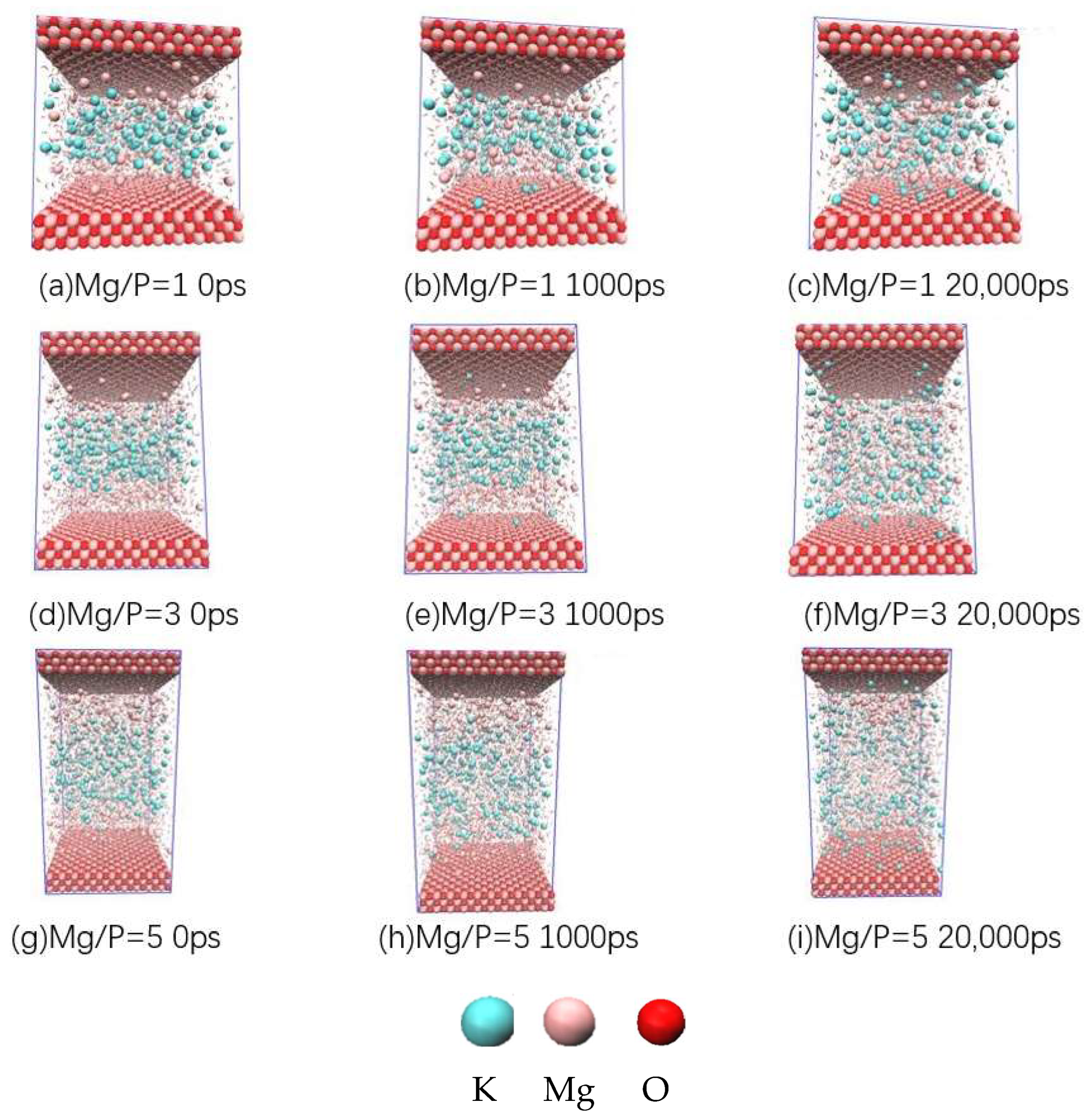
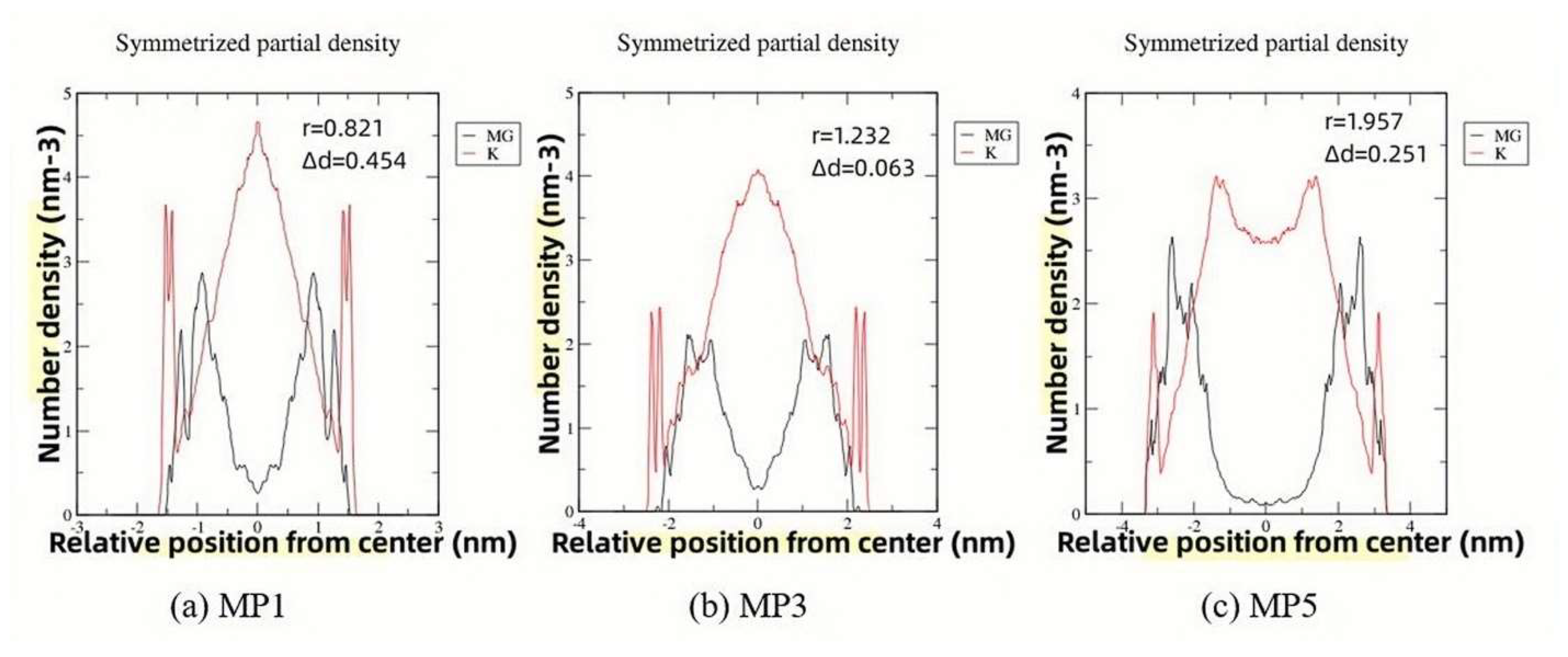
3.5. Analysis of Molecular Dynamics Simulation Calculations
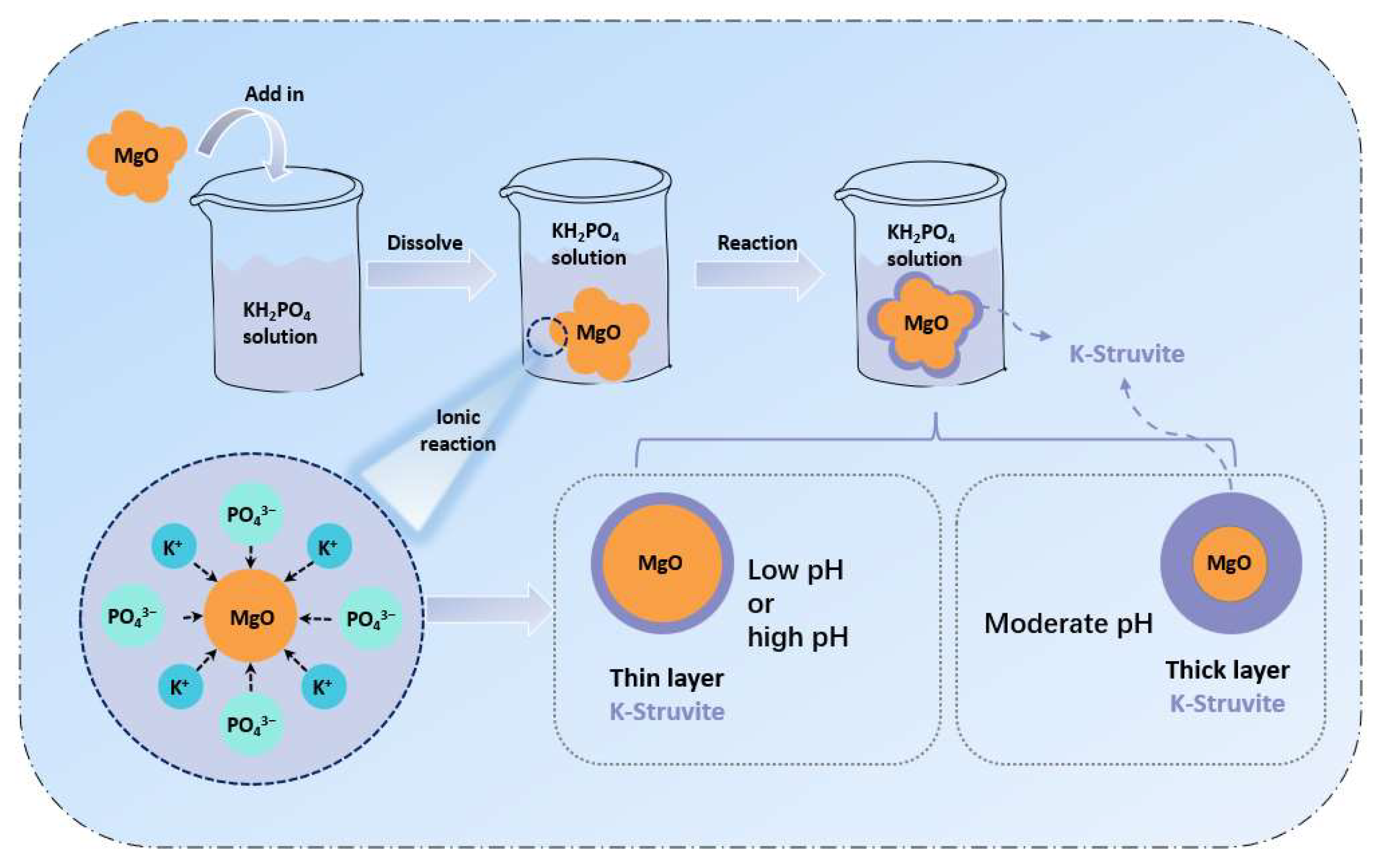
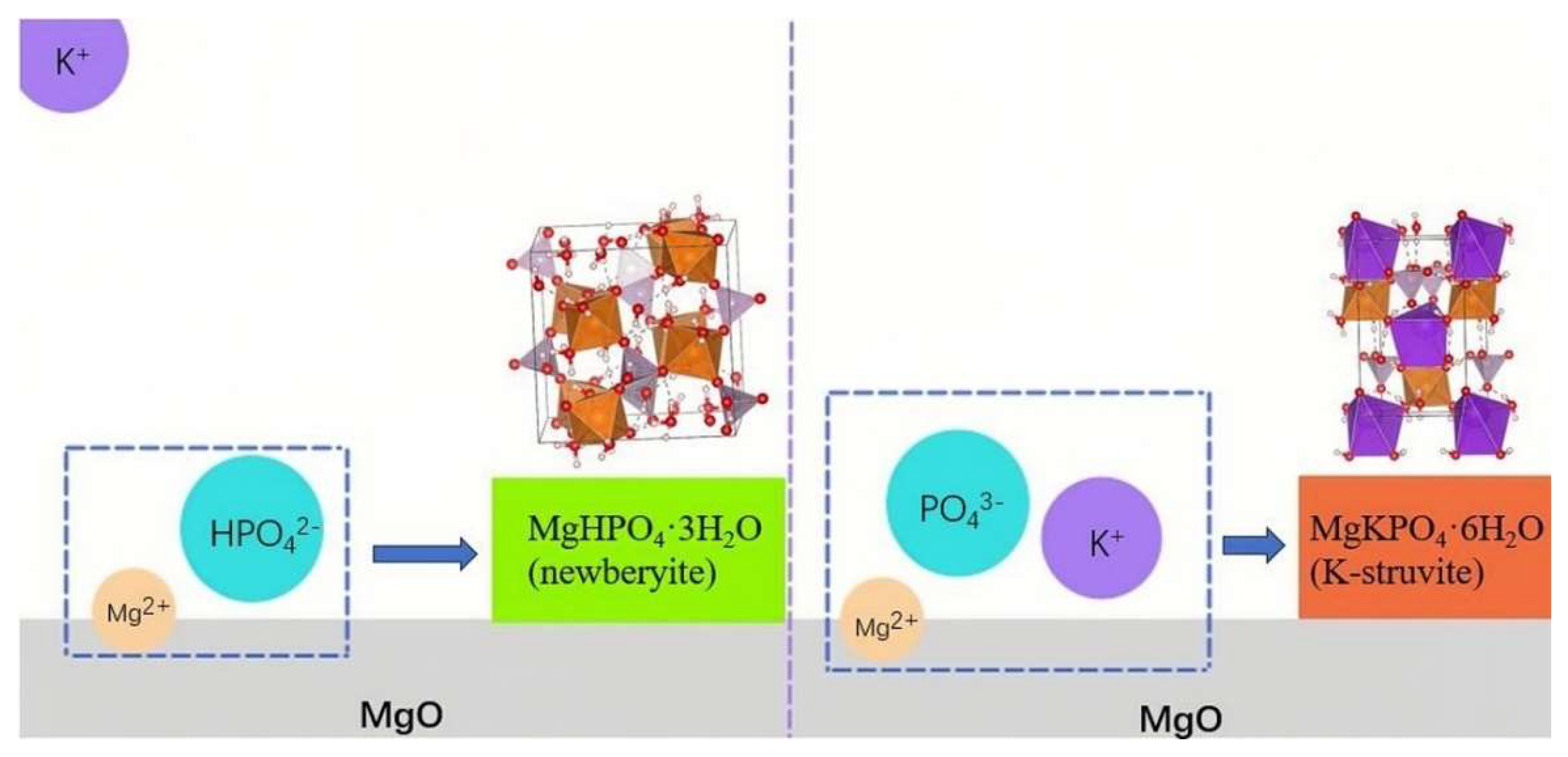
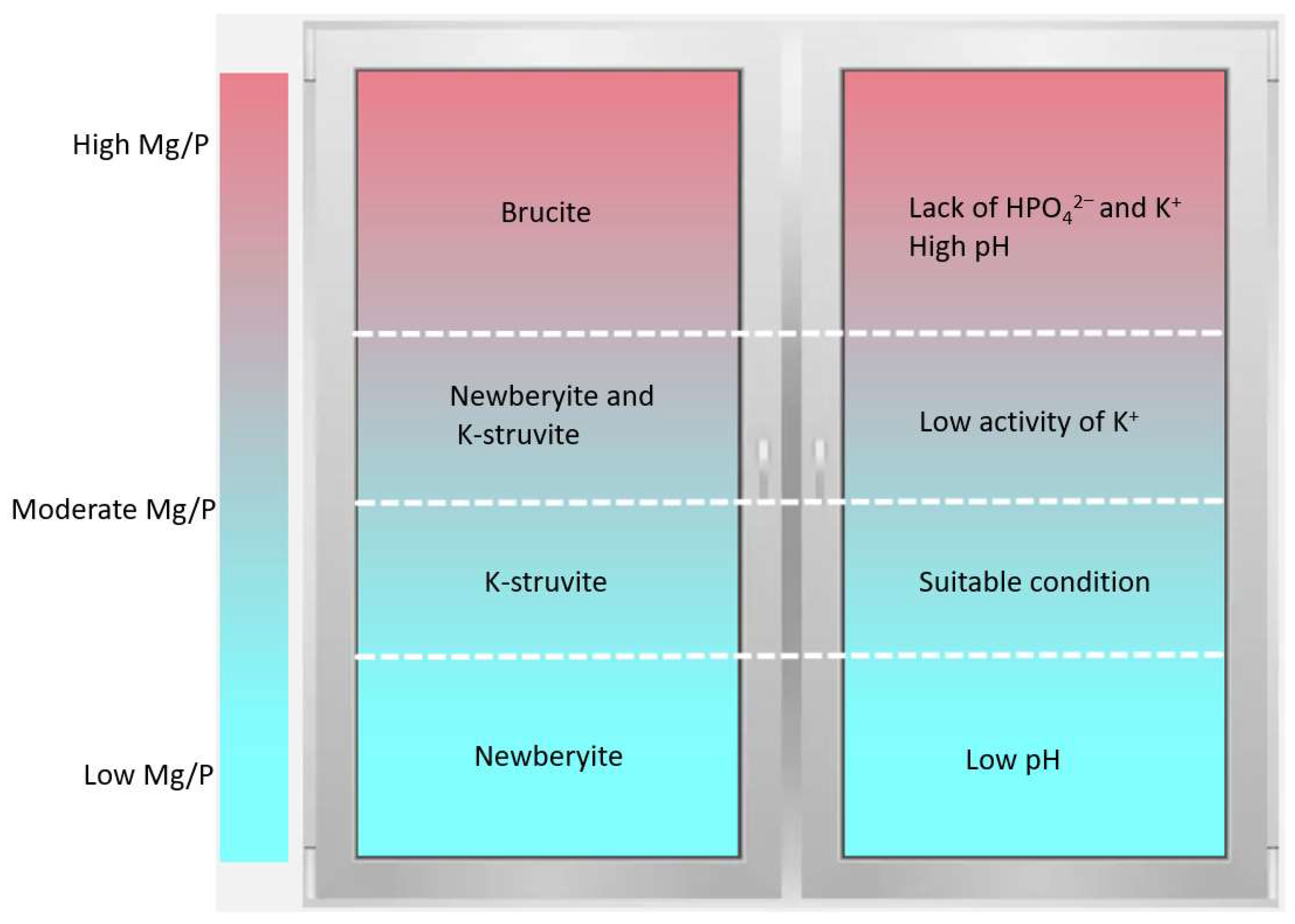
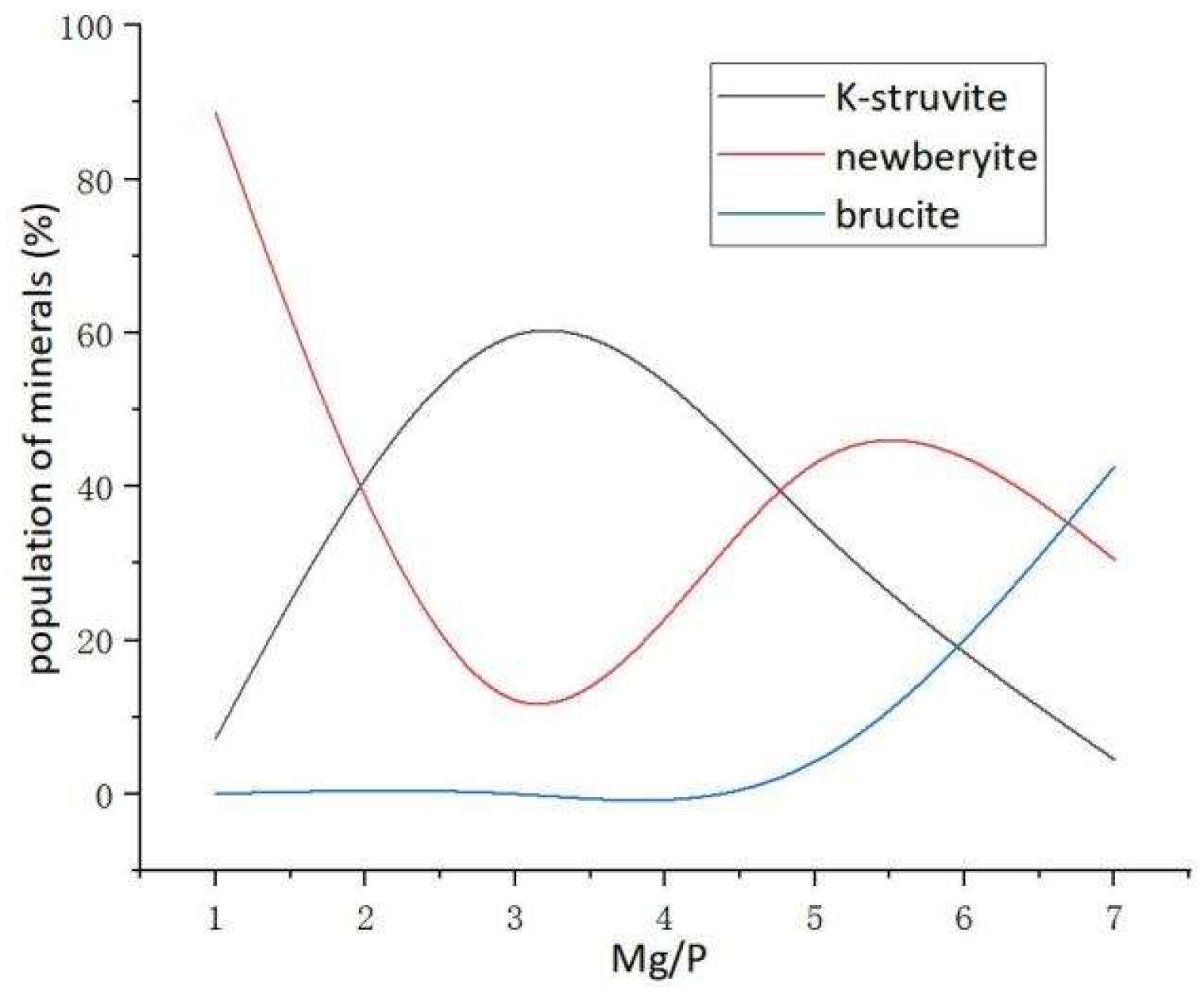
3.6. Effects of K+ Diffusion with Different Mg/P on the Conversion Path of MgO
4. Conclusions
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wilson, A.D. The chemistry of dental cements. Chem. Soc. Rev. 1978, 7, 265–296. [Google Scholar] [CrossRef]
- Kingery, W.D. Fundamental Study of Phosphate Bonding in Refractories: I, Literature Review. J. Am. Ceram. Soc. 1950, 33, 239–241. [Google Scholar] [CrossRef]
- Kaiser, F.; Schröter, L.; Stein, S.; Krüger, B.; Weichhold, J.; Stahlhut, P.; Ignatius, A.; Gbureck, U. Accelerated bone regeneration through rational design of magnesium phosphate cements. Acta Biomater. 2022, 145, 358–371. [Google Scholar] [CrossRef]
- Gardner, L.J.; Walling, S.A.; Corkhill, C.L.; Bernal, S.A.; Lejeune, V.; Stennett, M.C.; Provis, J.L.; Hyatt, N.C. Temperature transformation of blended magnesium potassium phosphate cement binders. Cem. Concr. Res. 2021, 141, 106332. [Google Scholar] [CrossRef]
- Wagh, A.S.; Jeong, S.Y. Chemically Bonded Phosphate Ceramics: I, A Dissolution Model of Formation. J. Am. Ceram. Soc. 2003, 86, 1838–1844. [Google Scholar] [CrossRef]
- Singh, D.; Wagh, A.S.; Tlustochowicz, M. Phosphate ceramic process for macroencapsulation and stabilization of low-level debris wastes. Waste Manag. 1998, 18, 135–143. [Google Scholar] [CrossRef]
- Yang, Q.; Zhu, B.; Wu, X. Characteristics and durability test of magnesium phosphate cement-based material for rapid repair of concrete. Mater. Struct. 2000, 33, 229–234. [Google Scholar] [CrossRef]
- Buj, I.; Torras, J.; Rovira, M.; de Pablo, J. Leaching behaviour of magnesium phosphate cements containing high quantities of heavy metals. J. Hazard. Mater. 2010, 175, 789–794. [Google Scholar] [CrossRef] [PubMed]
- Leng, D.; Li, X.; Lv, Y.; Tan, H.; Li, N.; Liu, Z.; Jiang, W.; Jiang, D. Cesium immobilization by K-struvite crystal in aqueous solution: Ab initio calculations and experiments. J. Hazard. Mater. 2020, 387, 121872. [Google Scholar] [CrossRef] [PubMed]
- Leng, D.; Li, X.; Lv, Y.; Jian, S.; Li, N.; Dan, J.; Jiang, W.; Jiang, D.; He, C. Comprehending the stability of Sr2+ immobilization in chemically bonded phosphate ceramic system: A mechanism study. Ceram. Int. 2022, 48, 10209–10219. [Google Scholar] [CrossRef]
- Walling, S.A.; Provis, J.L. Magnesia-Based Cements: A Journey of 150 Years, and Cements for the Future? Chem. Rev. 2016, 116, 4170–4204. [Google Scholar] [CrossRef]
- Opara, E.U.; Karthäuser, J.; Köhler, R.; Kowald, T.; Koddenberg, T.; Mai, C. Low-carbon magnesium potassium phosphate cement (MKPC) binder comprising caustic calcined magnesia and potassium hydroxide activated biochar from softwood technical lignin. Constr. Build. Mater. 2023, 398, 132475. [Google Scholar] [CrossRef]
- De Campos, M.; Davy, C.A.; Djelal, N.; Rivenet, M.; Garcia, J. Development of a stoichiometric magnesium potassium phosphate cement (MKPC) for the immobilization of powdered minerals. Cem. Concr. Res. 2021, 142, 106346. [Google Scholar] [CrossRef]
- Gardner, L.J.; Corkhill, C.L.; Walling, S.A.; Vigor, J.E.; Murray, C.A.; Tang, C.C.; Provis, J.L.; Hyatt, N.C. Early age hydration and application of blended magnesium potassium phosphate cements for reduced corrosion of reactive metals. Cem. Concr. Res. 2021, 143, 106375. [Google Scholar] [CrossRef]
- Yesigat, A.; Worku, A.; Mekonnen, A.; Bae, W.; Feyisa, G.L.; Gatew, S.; Han, J.-L.; Liu, W.; Wang, A.; Guadie, A. Phosphorus recovery as K-struvite from a waste stream: A review of influencing factors, advantages, disadvantages and challenges. Environ. Res. 2022, 214, 114086. [Google Scholar] [CrossRef]
- Li, Y.; Li, Y.; Li, H. Multi-scale investigation of creep properties of potassium magnesium phosphate cement. J. Build. Eng. 2022, 48, 103949. [Google Scholar] [CrossRef]
- Xu, B.; Winnefeld, F.; Ma, B.; Rentsch, D.; Lothenbach, B. Influence of aluminum sulfate on properties and hydration of magnesium potassium phosphate cements. Cem. Concr. Res. 2022, 156, 106788. [Google Scholar] [CrossRef]
- Cau Dit Coumes, C.; Rousselet, A.; Xu, B.; Mercier, C.A.; Gauffinet, S. Investigation of aluminum nitrate as a set retarder of magnesium potassium phosphate cement: Mechanisms involved in diluted suspension. Cem. Concr. Res. 2021, 150, 106608. [Google Scholar] [CrossRef]
- Shi, J.; Zhao, J.; Chen, H.; Hou, P.; Kawashima, S.; Qin, J.; Zhou, X.; Qian, J.; Cheng, X. Sulfuric acid-resistance performances of magnesium phosphate cements: Macro-properties, mineralogy and microstructure evolutions. Cem. Concr. Res. 2022, 157, 106830. [Google Scholar] [CrossRef]
- Peng, L.; Chen, B. Mechanical behavior, durability, thermal performances and microstructure of GGBFS—Modified MPC solidified dredged sludge. Constr. Build. Mater. 2021, 303, 124557. [Google Scholar] [CrossRef]
- Yu, J.; Qian, J.; Wang, F.; Li, Z.; Jia, X. Preparation and properties of a magnesium phosphate cement with dolomite. Cem. Concr. Res. 2020, 138, 106235. [Google Scholar] [CrossRef]
- Xu, B.; Lothenbach, B.; Winnefeld, F. Influence of wollastonite on hydration and properties of magnesium potassium phosphate cements. Cem. Concr. Res. 2020, 131, 106012. [Google Scholar] [CrossRef]
- Pang, B.; Liu, J.; Wang, B.; Liu, R.; Yang, Y. Effects of K-struvite on hydration behavior of magnesium potassium phosphate cement. Constr. Build. Mater. 2021, 275, 121741. [Google Scholar] [CrossRef]
- Luo, Z.; Ma, Y.; Mu, W.; Liu, J.; He, J.; Zhou, X. Magnesium phosphate cement prepared with electric furnace ferronickel slag: Properties and its hydration mechanism. Constr. Build. Mater. 2021, 300, 123991. [Google Scholar] [CrossRef]
- Ruan, W.; Li, F.; Liao, J.; Gu, X.; Mo, J.; Shen, Y.; Zhu, Y.; Ma, X. Effects of water purifying material waste on properties and hydration mechanism of magnesium phosphate cement-based grouting materials. Constr. Build. Mater. 2022, 349, 128676. [Google Scholar] [CrossRef]
- Liu, Z.; Lai, Z.; Luo, X.; Xiao, R.; Liu, M.; Deng, Q.; Chen, J.; Lu, Z.; Lv, S. Effect of titanium slag on the properties of magnesium phosphate cement. Constr. Build. Mater. 2022, 343, 128132. [Google Scholar] [CrossRef]
- Haque, M.A.; Chen, B.; Liu, Y. The role of bauxite and fly-ash on the water stability and microstructural densification of magnesium phosphate cement composites. Constr. Build. Mater. 2020, 260, 119953. [Google Scholar] [CrossRef]
- Ruan, W.; Liao, J.; Li, F.; Gu, X.; Zhou, A.; Xie, Y. Effects of water purifying material waste and red mud on performance of magnesium phosphate cement. Constr. Build. Mater. 2021, 303, 124563. [Google Scholar] [CrossRef]
- Xiao, R.; Lai, Z.; Luo, X.; Liu, Z.; Liu, M.; Deng, Q.; Chen, J.; Lu, Z.; Lv, S. Effects of sodium dihydrogen phosphate on properties of magnesium phosphate cement. J. Build. Eng. 2022, 60, 105216. [Google Scholar] [CrossRef]
- Soudée, E.; Péra, J. Mechanism of setting reaction in magnesia-phosphate cements. Cem. Concr. Res. 2000, 30, 315–321. [Google Scholar] [CrossRef]
- Wagh, A.S. Chemically Bonded Phosphate Ceramics: Twenty-First Century Materials with Diverse Applications; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Le Rouzic, M.; Chaussadent, T.; Platret, G.; Stefan, L. Mechanisms of k-struvite formation in magnesium phosphate cements. Cem. Concr. Res. 2017, 91, 117–122. [Google Scholar] [CrossRef]
- Lahalle, H.; Coumes, C.C.D.; Mesbah, A.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Investigation of magnesium phosphate cement hydration in diluted suspension and its retardation by boric acid. Cem. Concr. Res. 2016, 87, 77–86. [Google Scholar] [CrossRef]
- Xu, B.; Winnefeld, F.; Kaufmann, J.; Lothenbach, B. Influence of magnesium-to-phosphate ratio and water-to-cement ratio on hydration and properties of magnesium potassium phosphate cements. Cem. Concr. Res. 2019, 123, 105781. [Google Scholar] [CrossRef]
- Xu, B.; Lothenbach, B.; Leemann, A.; Winnefeld, F. Reaction mechanism of magnesium potassium phosphate cement with high magnesium-to-phosphate ratio. Cem. Concr. Res. 2018, 108, 140–151. [Google Scholar] [CrossRef]
- Lahalle, H.; Coumes, C.C.D.; Mercier, C.; Lambertin, D.; Cannes, C.; Delpech, S.; Gauffinet, S. Influence of the w/c ratio on the hydration process of a magnesium phosphate cement and on its retardation by boric acid. Cem. Concr. Res. 2018, 109, 159–174. [Google Scholar] [CrossRef]
- Josephson, G.B.; Westsik, J.H.; Pires, R.P.; Bickford, J.; Foote, M.W. Engineering-Scale Demonstration of DuraLith and Ceramicrete Waste Forms; PNNL: Richland, WA, USA, 2011. [Google Scholar]
- Haque, M.A.; Chen, B.; Maierdan, Y. Influence of supplementary materials on the early age hydration reactions and microstructural progress of magnesium phosphate cement matrices. J. Clean. Prod. 2022, 333, 130086. [Google Scholar] [CrossRef]
- Páll, S.; Abraham, M.J.; Kutzner, C.; Hess, B.; Lindahl, E. Tackling Exascale Software Challenges in Molecular Dynamics Simulations with GROMACS. In Exascale Applications and Software Conference; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Pronk, S.; Páll, S.; Schulz, R.; Larsson, P.; Bjelkmar, P.; Apostolov, R.; Shirts, M.R.; Smith, J.C.; Kasson, P.M.; van der Spoel, D.; et al. GROMACS 4.5: A high-throughput and highly parallel open source molecular simulation toolkit. Bioinformatics 2013, 29, 845–854. [Google Scholar] [CrossRef]
- Van Der Spoel, D.; Lindahl, E.; Hess, B.; Groenhof, G.; Mark, A.E.; Berendsen, H.J. GROMACS: Fast, flexible, and free. J. Comput. Chem. 2005, 26, 1701–1718. [Google Scholar] [CrossRef]
- Berendsen, H.J.; van der Spoel, D.; van Drunen, R. GROMACS: A message-passing parallel molecular dynamics implementation—ScienceDirect. Comput. Phys. Commun. 1995, 91, 43–56. [Google Scholar] [CrossRef]
- Hess, B.; Kutzner, C.; Van Der Spoel, D.; Lindahl, E. GROMACS 4: Algorithms for Highly Efficient, Load-Balanced, and Scalable Molecular Simulation. J. Chem. Theory Comput. 2008, 4, 435–447. [Google Scholar] [CrossRef] [PubMed]
- Abraham, M.J.; Murtola, T.; Schulz, R.; Páll, S.; Smith, J.C.; Hess, B.; Lindahl, E. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX 2015, 1–2, 19–25. [Google Scholar] [CrossRef]
- Martínez, L.; Andrade, R.; Birgin, E.G.; Martínez, J.M. PACKMOL: A package for building initial configurations for molecular dynamics simulations. J. Comput. Chem. 2010, 30, 2157–2164. [Google Scholar] [CrossRef] [PubMed]
- Holt, S.A.; Jones, C.F.; Watson, G.S.; Crossley, A.; Johnston, C.; Sofield, C.J.; Myhra, S. Surface modification of mgo substrates from aqueous exposure—An atomic force microscopy study. Thin Solid Film. Int. J. Sci. Technol. Thin Thick Film. 1997, 292, 96–102. [Google Scholar] [CrossRef]
- Ribeiro, D.V.; Paula, G.R.; Morelli, M.R. Effect of boric acid content on the properties of magnesium phosphate cement. Constr. Build. Mater. 2019, 214, 557–564. [Google Scholar] [CrossRef]
- Shi, C.; Yang, J.; Yang, N.; Chang, Y. Effect of waterglass on water stability of potassium magnesium phosphate cement paste. Cem. Concr. Compos. 2014, 53, 83–87. [Google Scholar] [CrossRef]
- Viani, A.; Mácová, P.; Ševčík, R.; Zárybnická, L. Mechanism of magnesium phosphate cement retardation by citric acid. Ceram. Int. 2023, 49, 11112–11122. [Google Scholar] [CrossRef]
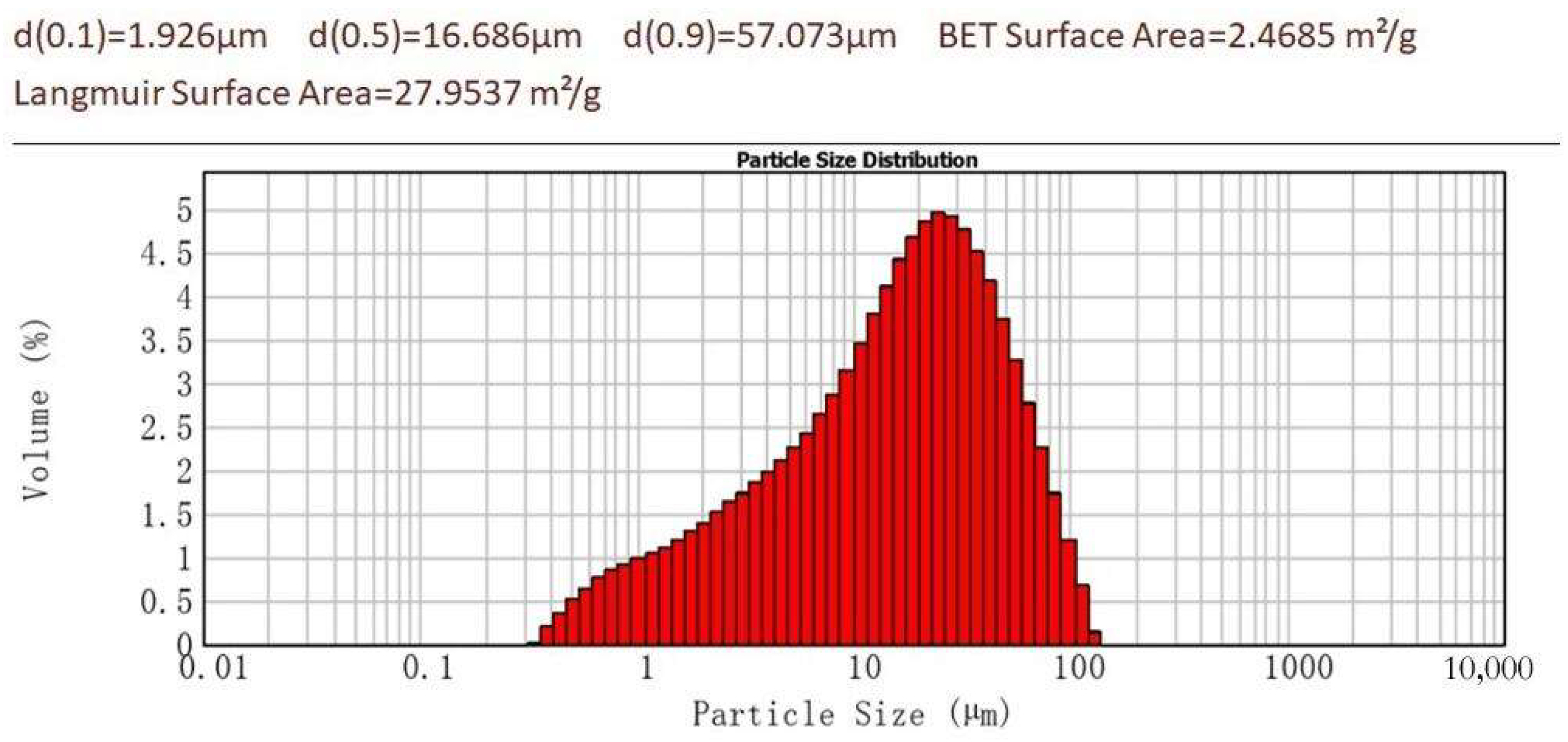


| Oxide | wt% |
|---|---|
| MgO | 85.592 |
| SiO2 | 5.221 |
| CaO | 3.014 |
| Fe2O3 | 1.824 |
| Al2O3 | 1.239 |
| SO3 | 0.251 |
| Na2O | 0.182 |
| P2O5 | 0.138 |
| MnO | 0.113 |
| Cr2O3 | 0.067 |
| K2O | 0.047 |
| TiO2 | 0.037 |
| Cl | 0.017 |
| SrO | 0.007 |
| CO2 | 2.251 |
| MgO | KH2PO4 | w/s | |
|---|---|---|---|
| MP1 | 0.5 | 0.5 | 10 |
| MP3 | 0.75 | 0.25 | |
| MP5 | 0.83 | 0.17 | |
| MP7 | 0.875 | 0.125 |
| Mg/P Ratio | 0–4.21 Å | 5–25 Å | 25–45 Å |
|---|---|---|---|
| 1:1 | Solid matrix MgO containing 1200 Mg atoms and 1200 O atoms | 2227 H2O | |
| 80 Mg2+ | 170 K+ | ||
| 80 HPO42− | 80 HPO42− | ||
| \ | 10 H2PO4− | ||
| 3:1 | Solid matrix MgO containing 1200 Mg atoms and 1200 O atoms | 3245 H2O | |
| 100 Mg2+ | 230 K+ | ||
| 100 HPO42− | 100 HPO42− | ||
| \ | 30 H2PO4− | ||
| 5:1 | Solid matrix MgO containing 1200 Mg atoms and 1200 O atoms | 4200 H2O | |
| 120 Mg2+ | 280 K+ | ||
| 120 HPO42− | 120 HPO42− | ||
| \ | 34 H2PO4− | ||
| cPi | cPf | Pl (%) | Ps (%) | cKi | cKf | Kl (%) | Ks (%) | |
|---|---|---|---|---|---|---|---|---|
| MP1 | 0.567 | 0.218 | 38.48 | 61.52 | 0.567 | 0.495 | 87.28 | 12.72 |
| MP3 | 0.389 | 0.085 | 21.92 | 78.08 | 0.389 | 0.179 | 46 | 54 |
| MP5 | 0.296 | 0.021 | 6.48 | 93.52 | 0.296 | 0.088 | 29.68 | 70.32 |
| MP7 | 0.239 | 0.058 | 24.08 | 75.92 | 0.239 | 0.124 | 52 | 48 |
| nPi | nPf | nKi | nKf | |
|---|---|---|---|---|
| MP1 | 0.125 | 0.0481 | 0.125 | 0.1091 |
| MP3 | 0.125 | 0.0274 | 0.125 | 0.0575 |
| MP5 | 0.125 | 0.0081 | 0.125 | 0.0371 |
| MP7 | 0.125 | 0.0301 | 0.125 | 0.0650 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leng, D.; Fu, Q.; Ge, Y.; He, C.; Lv, Y.; Li, X. Effect of K+ Diffusion on Hydration of Magnesium Potassium Phosphate Cement with Different Mg/P Ratios: Experiments and Molecular Dynamics Simulation Calculations. Materials 2024, 17, 1151. https://doi.org/10.3390/ma17051151
Leng D, Fu Q, Ge Y, He C, Lv Y, Li X. Effect of K+ Diffusion on Hydration of Magnesium Potassium Phosphate Cement with Different Mg/P Ratios: Experiments and Molecular Dynamics Simulation Calculations. Materials. 2024; 17(5):1151. https://doi.org/10.3390/ma17051151
Chicago/Turabian StyleLeng, Difei, Qiuyan Fu, Yunlu Ge, Chenhao He, Yang Lv, and Xiangguo Li. 2024. "Effect of K+ Diffusion on Hydration of Magnesium Potassium Phosphate Cement with Different Mg/P Ratios: Experiments and Molecular Dynamics Simulation Calculations" Materials 17, no. 5: 1151. https://doi.org/10.3390/ma17051151
APA StyleLeng, D., Fu, Q., Ge, Y., He, C., Lv, Y., & Li, X. (2024). Effect of K+ Diffusion on Hydration of Magnesium Potassium Phosphate Cement with Different Mg/P Ratios: Experiments and Molecular Dynamics Simulation Calculations. Materials, 17(5), 1151. https://doi.org/10.3390/ma17051151





