Design of Bismuth Tungstate Bi2WO6 Photocatalyst for Enhanced and Environmentally Friendly Organic Pollutant Degradation
Abstract
1. Introduction
2. Experimental Section
2.1. BWO Synthesis
2.2. Material Characterization
2.3. Photocatalytic Procedure
2.4. Measurement of Zero Charge Point
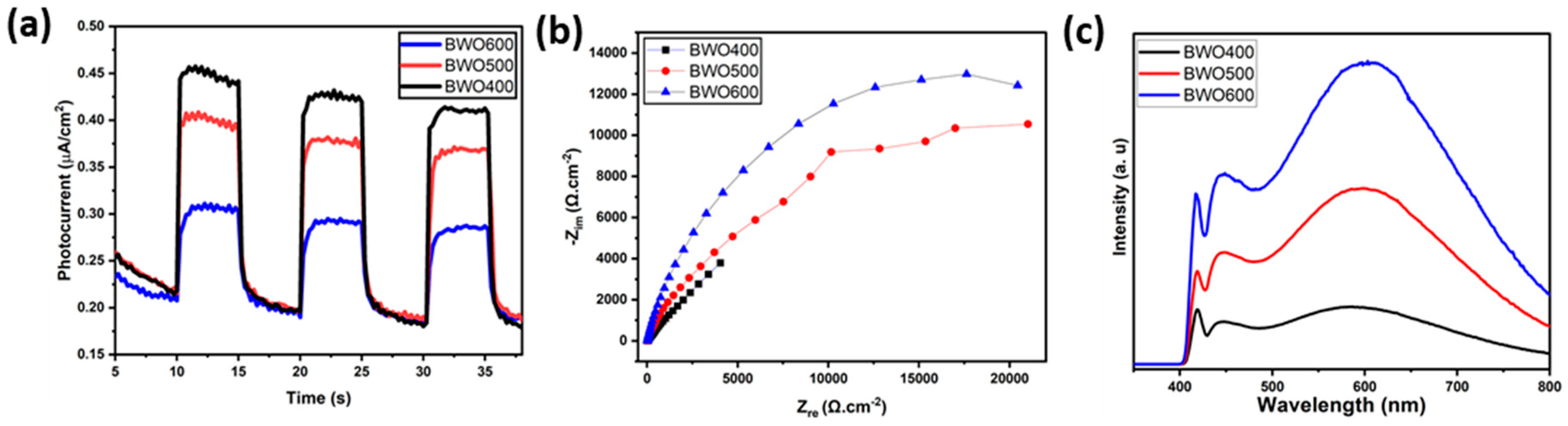
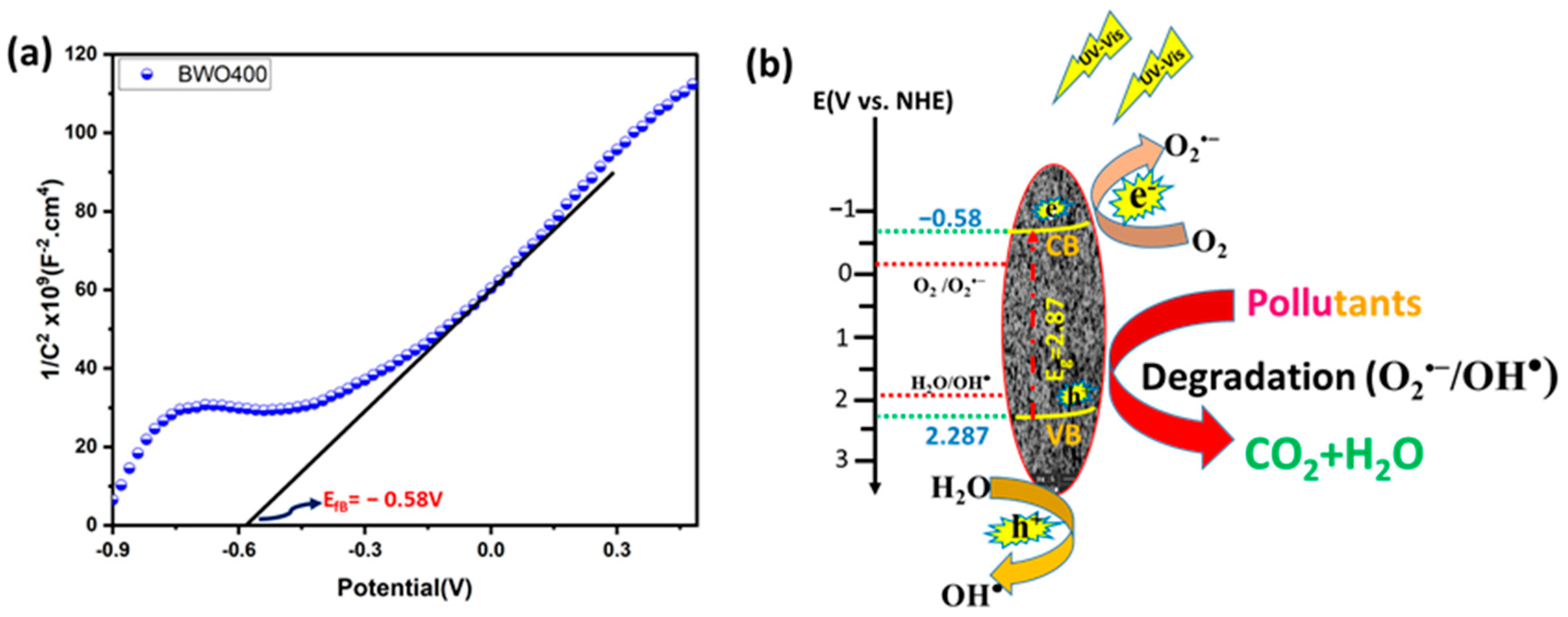
2.5. Photoelectrochemical Measurement
2.6. Chemical Oxygen Demand (COD)
3. Results and Discussion
3.1. Structural Investigation
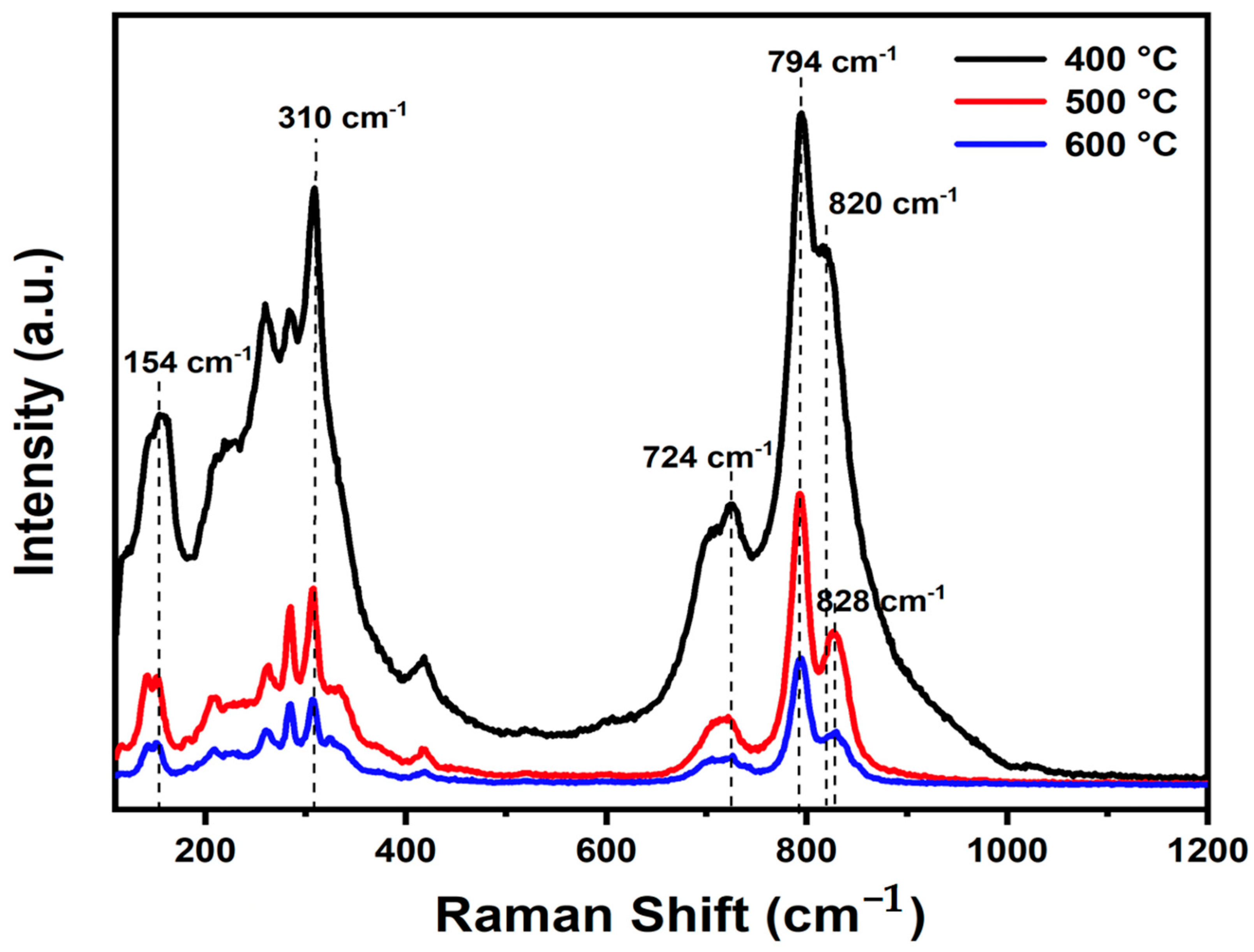
3.2. Vibrational Spectroscopy
3.3. Morphology Analysis
3.4. Optical Properties
3.5. X-ray Photoelectron Spectroscopy (XPS)
4. Assessment of the Photocatalytic Performance of BWO Photocatalyst
4.1. Adsorption and Photolysis Investigation
4.2. Photocatalytic Activity and Kinetic Investigation of BWO
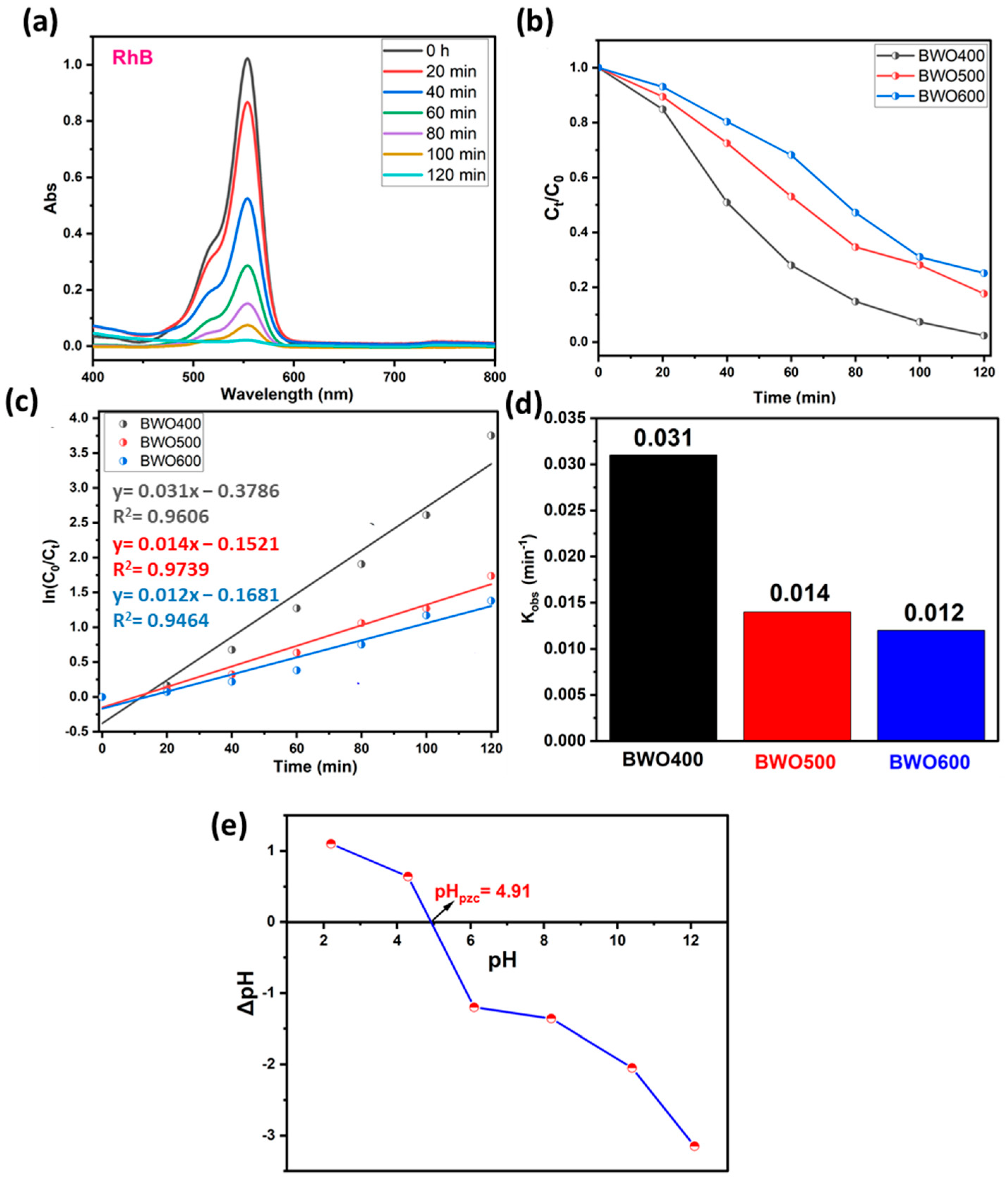
4.2.1. The Case of Rhodamine B
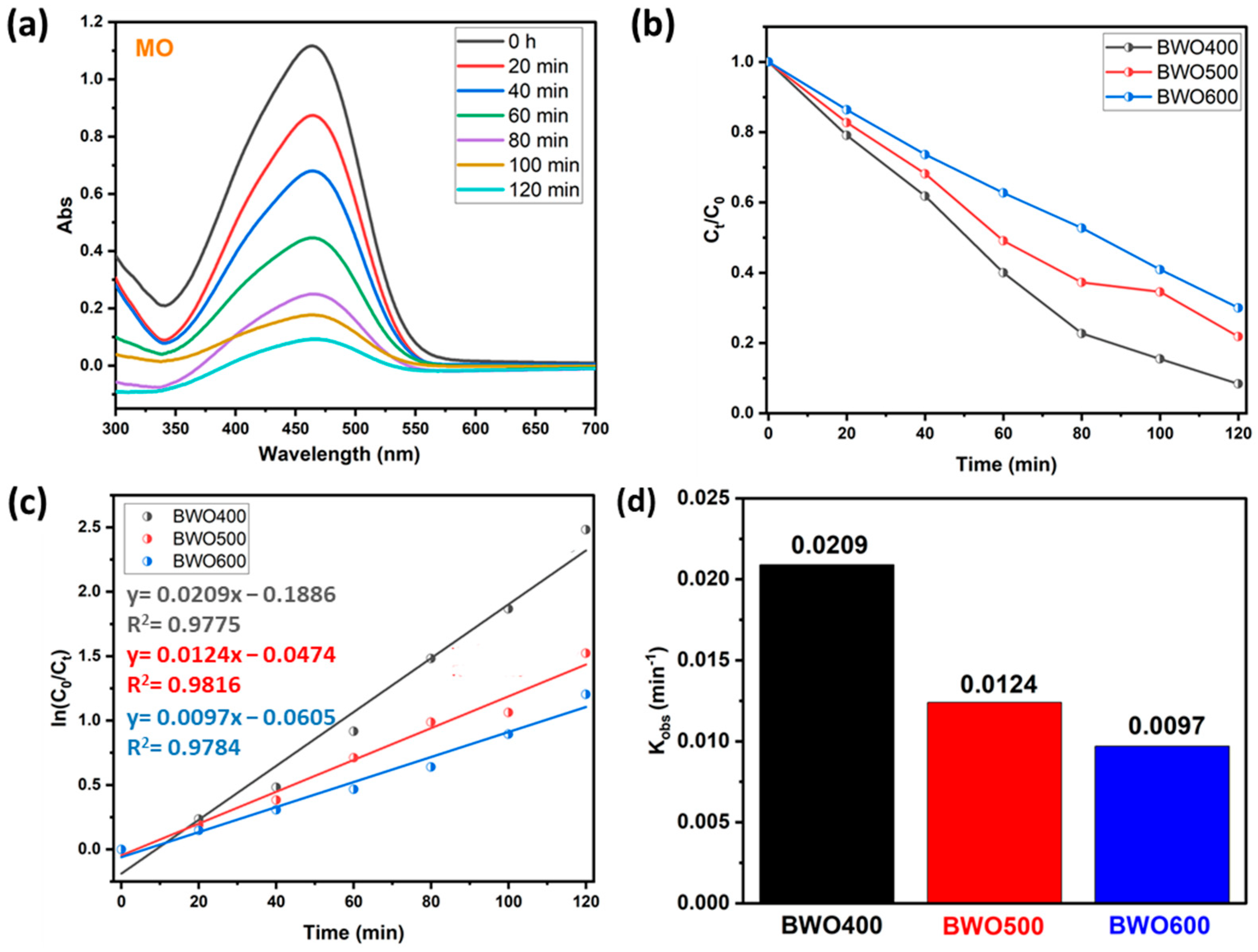
4.2.2. The Case of Methyl Orange
4.3. Investigation of the Photocatalytic Mechanism
4.4. Trapping Test
4.5. Recyclability
4.6. Proposed Mechanism
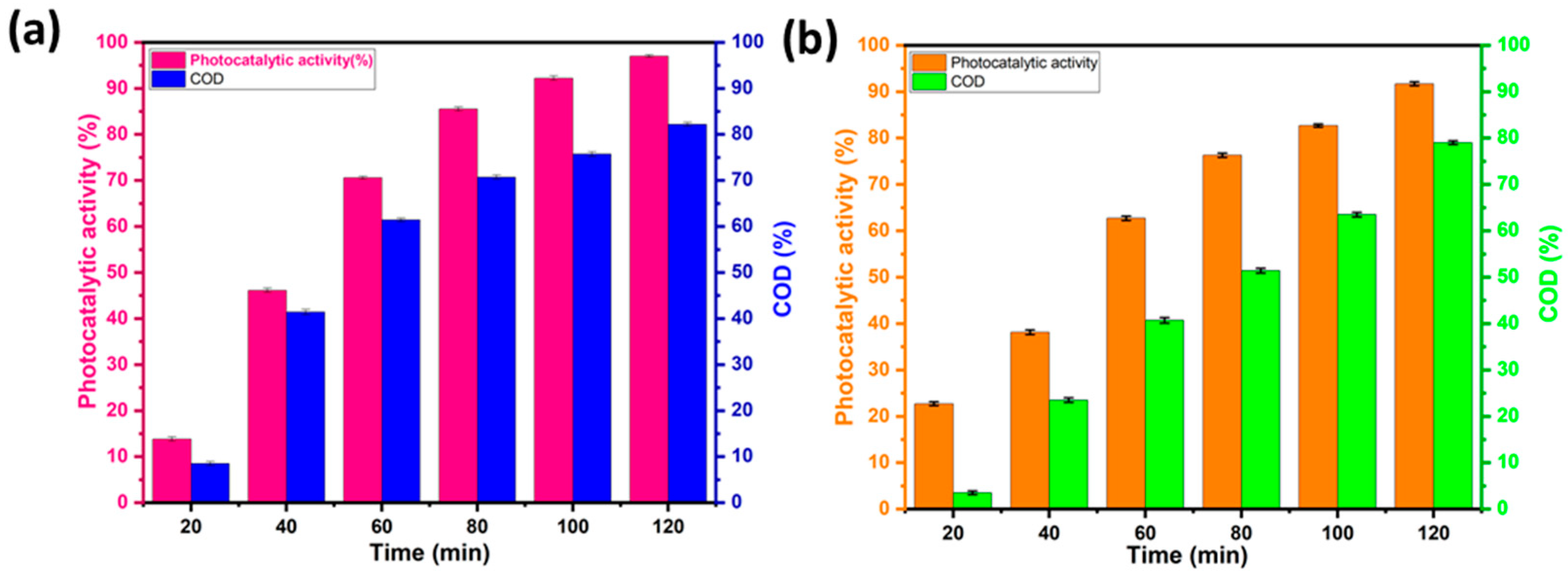
4.7. Estimation of RhB and MO Mineralization by COD Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Channab, B.-E.; Ouardi, M.E.; Layachi, O.A.; Marrane, S.E.; Idrissi, A.E.; BaQais, A.A.; Ahsaine, H.A. Recent Trends on MIL-Fe Metal-Organic Frameworks: Synthesis Approaches, Structural Insights, and Applications in Organic Pollutant Adsorption and Photocatalytic Degradation: A Review. Environ. Sci. Nano 2023, 10, 2957–2988. [Google Scholar] [CrossRef]
- Khanal, V.; Balayeva, N.O.; Günnemann, C.; Mamiyev, Z.; Dillert, R.; Bahnemann, D.W.; Subramanian, V. (Ravi) Photocatalytic NOx Removal Using Tantalum Oxide Nanoparticles: A Benign Pathway. Appl. Catal. B Environ. 2021, 291, 119974. [Google Scholar] [CrossRef]
- Hamou, A.B.; Enneiymy, M.; Farsad, S.; Amjlef, A.; Chaoui, A.; Nouj, N.; Majdoub, A.; Jada, A.; Ez-zahery, M.; Alem, N.E. Novel Chemically Reduced Cobalt-Doped g-C3N4 (CoCN-x) as a Highly Heterogeneous Catalyst for the Super-Degradation of Organic Dyes via Peroxymonosulfate Activation. Mater. Adv. 2024. [Google Scholar] [CrossRef]
- Farsad, S.; Amjlef, A.; Chaoui, A.; Hamou, A.B.; Hamma, C.; Benafqir, M.; Jada, A.; Alem, N.E. Harnessing Carbon-Based Material from Food Waste Digestate for Dye Adsorption: The Role of Hydrogel Beads in Enhancing Stability and Regenerative Capacity. Mater. Adv. 2023, 4, 6599–6611. [Google Scholar] [CrossRef]
- Farsad, S.; Anfar, Z.; Ait El Fakir, A.; Amjlef, A.; El Alem, N.; Ionel, I. Methane Recovery from the Leachate of Municipal Solid Waste Landfill by Using Anaerobic Digestion. Case Study. In Proceedings of the 30th European Biomass Conference and Exhibition Proceedings, Online, 9–12 May 2022; pp. 223–227. [Google Scholar] [CrossRef]
- El Idrissi, A.; Channab, B.; Essamlali, Y.; Zahouily, M. Superabsorbent Hydrogels Based on Natural Polysaccharides: Classification, Synthesis, Physicochemical Properties, and Agronomic Efficacy under Abiotic Stress Conditions: A Review. Int. J. Biol. Macromol. 2024, 258, 128909. [Google Scholar] [CrossRef] [PubMed]
- Channab, B.-E.; El Idrissi, A.; Essamlali, Y.; Zahouily, M. Nanocellulose: Structure, Modification, Biodegradation and Applications in Agriculture as Slow/Controlled Release Fertilizer, Superabsorbent, and Crop Protection: A Review. J. Environ. Manag. 2024, 352, 119928. [Google Scholar] [CrossRef] [PubMed]
- El Ouardi, M.; El aouni, A.; Ahsaine, H.A.; Zbair, M.; BaQais, A.; Saadi, M. ZIF-8 Metal Organic Framework Composites as Hydrogen Evolution Reaction Photocatalyst: A Review of the Current State. Chemosphere 2022, 308, 136483. [Google Scholar] [CrossRef]
- El ouardi, M.; El Idrissi, A.; Arab, M.; Zbair, M.; Haspel, H.; Saadi, M.; Ait Ahsaine, H. Review of Photoelectrochemical Water Splitting: From Quantitative Approaches to Effect of Sacrificial Agents, Oxygen Vacancies, Thermal and Magnetic Field on (Photo)Electrolysis. Int. J. Hydrogen Energy 2023, 51, 1044–1067. [Google Scholar] [CrossRef]
- Chakhtouna, H.; El Allaoui, B.; Zari, N.; Bouhfid, R.; Qaiss, A.e.K. Bio-Inspired Polymers as Organic Electrodes for Batteries. In Organic Electrodes: Fundamental to Advanced Emerging Applications; Gupta, R.K., Ed.; Engineering Materials; Springer International Publishing: Cham, Switzerland, 2022; pp. 189–206. ISBN 978-3-030-98021-4. [Google Scholar]
- El Allaoui, B.; Benzeid, H.; Zari, N.; Qaiss, A.e.K.; Bouhfid, R. Functional Cellulose-Based Beads for Drug Delivery: Preparation, Functionalization, and Applications. J. Drug Deliv. Sci. Technol. 2023, 88, 104899. [Google Scholar] [CrossRef]
- El Allaoui, B.; Chakhtouna, H.; Zari, N.; Benzeid, H.; Qaiss, A.e.K.; Bouhfid, R. Superhydrophobic Alkylsilane Functionalized Cellulose Beads for Efficient Oil/Water Separation. J. Water Process Eng. 2023, 54, 104015. [Google Scholar] [CrossRef]
- Farsad, S.; Hamou, A.B.; Chaoui, A.; Amjlef, A.; Lhanafi, S.; Et-Taleb, S.; Alem, N.E. Maximizing Bio-Methane Potential from Municipal Landfill Leachate through Ultrasonic Pretreatment. Heliyon 2023, 9, e21347. [Google Scholar] [CrossRef]
- Mazkad, D.; El Idrissi, A.; Marrane, S.E.; Lazar, N.; El Ouardi, M.; Dardari, O.; Channab, B.-E.; Ait Layachi, O.; Farsad, S.; Baqais, A.; et al. An Innovative Diatomite-Polypyrrole Composite for Highly Efficient Cr (VI) Removal through Optimized Adsorption via Surface Response Methodology. Colloids Surf. Physicochem. Eng. Asp. 2024, 685, 133172. [Google Scholar] [CrossRef]
- Marrane, S.E.; Dânoun, K.; Essamlali, Y.; Aboulhrouz, S.; Sair, S.; Amadine, O.; Jioui, I.; Rhihil, A.; Zahouily, M. Fixed-Bed Adsorption of Pb(II) and Cu(II) from Multi-Metal Aqueous Systems onto Cellulose-g-Hydroxyapatite Granules: Optimization Using Response Surface Methodology. RSC Adv. 2023, 13, 31935–31947. [Google Scholar] [CrossRef]
- Amjlef, A.; Farsad, S.; Chaoui, A.; Ben Hamou, A.; Ezzahery, M.; Et-Taleb, S.; El Alem, N. Effective Adsorption of Orange G Dye Using Chitosan Cross-Linked by Glutaraldehyde and Reinforced with Quartz Sand. Int. J. Biol. Macromol. 2023, 239, 124373. [Google Scholar] [CrossRef] [PubMed]
- Marrane, S.E.; Dänoun, K.; Allouss, D.; Sair, S.; Channab, B.-E.; Rhihil, A.; Zahouily, M. A Novel Approach to Prepare Cellulose-g-Hydroxyapatite Originated from Natural Sources as an Efficient Adsorbent for Heavy Metals: Batch Adsorption Optimization via Response Surface Methodology. ACS Omega 2022, 7, 28076–28092. [Google Scholar] [CrossRef]
- Ait Ahsaine, H.; BaQais, A.; Arab, M.; Bakiz, B.; Benlhachemi, A. Synthesis and Electrocatalytic Activity of Bismuth Tungstate Bi2WO6 for Rhodamine B Electro-Oxidation. Catalysts 2022, 12, 1335. [Google Scholar] [CrossRef]
- El Ouardi, M.; Ait Layachi, O.; Amaterz, E.; El Idrissi, A.; Taoufyq, A.; Bakiz, B.; Benlhachemi, A.; Arab, M.; BaQais, A.; Ait Ahsaine, H. Photo-Electrochemical Degradation of Rhodamine B Using Electrodeposited Mn3(PO4)2.3H2O Thin Films. J. Photochem. Photobiol. Chem. 2023, 444, 115011. [Google Scholar] [CrossRef]
- El ouardi, M.; Idrissi, A.; Ahsaine, H.; BaQais, A.; Saadi, M.; Arab, M. Current Advances on Nanostructured Oxide Photoelectrocatalysts for Water Splitting: A Comprehensive Review. Surf. Interfaces 2024, 45, 103850. [Google Scholar] [CrossRef]
- Mazkad, D.; Lazar, N.; Benzaouak, A.; Moussadik, A.; El Habib Hitar, M.; Touach, N.; El Mahi, M.; Lotfi, E.M. Photocatalytic Properties Insight of Sm-Doped LiNbO3 in Ferroelectric Li1− xNbSm1/3xO3 System. J. Environ. Chem. Eng. 2023, 11, 109732. [Google Scholar] [CrossRef]
- Lotfi, S.; El Ouardi, M.; Ait Ahsaine, H.; Madigou, V.; BaQais, A.; Assani, A.; Saadi, M.; Arab, M. Low-Temperature Synthesis, Characterization and Photocatalytic Properties of Lanthanum Vanadate LaVO4. Heliyon 2023, 9, e17255. [Google Scholar] [CrossRef]
- Elaouni, A.; El Ouardi, M.; BaQais, A.; Arab, M.; Saadi, M.; Ahsaine, H.A. Bismuth Tungstate Bi2WO6: A Review on Structural, Photophysical and Photocatalytic Properties. RSC Adv. 2023, 13, 17476–17494. [Google Scholar] [CrossRef] [PubMed]
- Jaspal, D.; Malviya, A.; El Allaoui, B.; Zari, N.; Bouhfid, R.; Qaiss, A.e.K.; Bhagwat, S. Emerging Advances of Composite Membranes for Seawater Pre-Treatment: A Review. Water Sci. Technol. 2023, 88, 408–429. [Google Scholar] [CrossRef] [PubMed]
- Channab, B.-E.; Ouardi, M.E.; Marrane, S.E.; Layachi, O.A.; Idrissi, A.E.; Farsad, S.; Mazkad, D.; BaQais, A.; Lasri, M.; Ahsaine, H.A. Alginate@ZnCO2O4 for Efficient Peroxymonosulfate Activation towards Effective Rhodamine B Degradation: Optimization Using Response Surface Methodology. RSC Adv. 2023, 13, 20150–20163. [Google Scholar] [CrossRef]
- El Allaoui, B.; Benzeid, H.; Zari, N.; Qaiss, A.e.K.; Bouhfid, R. Cellulose Beads Supported CoFe2O4: A Novel Heterogeneous Catalyst for Efficient Rhodamine B Degradation via Advanced Oxidation Processes. Int. J. Biol. Macromol. 2023, 259, 128893. [Google Scholar] [CrossRef] [PubMed]
- Daskalaki, V.M.; Antoniadou, M.; Li Puma, G.; Kondarides, D.I.; Lianos, P. Solar Light-Responsive Pt/CdS/TiO2 Photocatalysts for Hydrogen Production and Simultaneous Degradation of Inorganic or Organic Sacrificial Agents in Wastewater. Environ. Sci. Technol. 2010, 44, 7200–7205. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Shi, X.; Liu, X.; Li, X. Synthesis of Novel, Visible-Light Driven S, N-Doped NaTaO3 Catalysts with High Photocatalytic Activity. Appl. Surf. Sci. 2020, 508, 145306. [Google Scholar] [CrossRef]
- Chang, W.K.; Rao, K.K.; Kuo, H.C.; Cai, J.F.; Wong, M.S. A Novel Core–Shell like Composite In2O3@ CaIn2O4 for Efficient Degradation of Methylene Blue by Visible Light. Appl. Catal. Gen. 2007, 321, 1–6. [Google Scholar] [CrossRef]
- Wang, X.; Gu, F.; Li, L.; Fang, G.; Wang, X. A Facile Mixed-Solvothermal Route to γ-Bi2MoO6 Nanoflakes and Their Visible-Light-Responsive Photocatalytic Activity. Mater. Res. Bull. 2013, 48, 3761–3765. [Google Scholar] [CrossRef]
- Shi, B.; Yin, H.; Gong, J.; Nie, Q. A Novel Pn Heterojunction of Ag2O/Bi4Ti3O12 Nanosheet with Exposed (0 0 1) Facets for Enhanced Visible-Light-Driven Photocatalytic Activity. Mater. Lett. 2017, 201, 74–77. [Google Scholar] [CrossRef]
- Zhou, L.; Jin, C.; Yu, Y.; Chi, F.; Ran, S.; Lv, Y. Molten Salt Synthesis of Bi2WO6 Powders with Enhanced Visible-Light-Induced Photocatalytic Activities. J. Alloys Compd. 2016, 680, 301–308. [Google Scholar] [CrossRef]
- Ait Ahsaine, H. UV-Light Photocatalytic Properties of the Bismuth Lutetium Tungstate System Bi2-xLuxWO6 (0 ≤ x ≤ 1). Mater. Lett. 2020, 276, 128221. [Google Scholar] [CrossRef]
- Ahsaine, H.A.; Jaouhari, A.E.; Slassi, A.; Ezahri, M.; Benlhachemi, A.; Bakiz, B.; Guinneton, F.; Gavarri, J.-R. Electronic Band Structure and Visible-Light Photocatalytic Activity of Bi2WO6: Elucidating the Effect of Lutetium Doping. RSC Adv. 2016, 6, 101105–101114. [Google Scholar] [CrossRef]
- Lv, N.; Li, Y.; Huang, Z.; Li, T.; Ye, S.; Dionysiou, D.D.; Song, X. Synthesis of GO/TiO2/Bi2WO6 Nanocomposites with Enhanced Visible Light Photocatalytic Degradation of Ethylene. Appl. Catal. B Environ. 2019, 246, 303–311. [Google Scholar] [CrossRef]
- Ait Ahsaine, H.; Ezahri, M.; Benlhachemi, A.; Bakiz, B.; Guinneton, F.; Gavarri, J.-R. Electrical impedance spectroscopy analyses and optical properties of the bismuth lutetium tungstate BiLuWO6. Ferroelectrics 2017, 515, 112–119. [Google Scholar] [CrossRef]
- Ait Ahsaine, H.; Ezahri, M.; Benlhachemi, A.; Bakiz, B.; Villain, S.; Guinneton, F.; Gavarri, J.-R. Novel Lu-doped Bi2WO6 nanosheets: Synthesis, growth mechanisms and enhanced photocatalytic activity under UV-light irradiation. Ceram. Int. 2016, 42, 8552–8558. [Google Scholar] [CrossRef]
- Wang, L.; Guo, C.; Chen, F.; Ning, J.; Zhong, Y.; Hu, Y. pH-Induced Hydrothermal Synthesis of Bi2WO6 Nanoplates with Controlled Crystal Facets for Switching Bifunctional Photocatalytic Water Oxidation/Reduction Activity. J. Colloid Interface Sci. 2021, 602, 868–879. [Google Scholar] [CrossRef]
- Basaleh, A.S.; El-Hout, S.I. Sol-Gel Synthesis of Photoactive PtO/Bi2WO6 Nanocomposites for Improved Photoreduction of Hg (II) Ions under Visible Illumination. Mol. Catal. 2023, 547, 113413. [Google Scholar] [CrossRef]
- Hoang, L.H.; Phu, N.D.; Peng, H.; Chen, X.-B. High Photocatalytic Activity N-Doped Bi2WO6 Nanoparticles Using a Two-Step Microwave-Assisted and Hydrothermal Synthesis. J. Alloys Compd. 2018, 744, 228–233. [Google Scholar] [CrossRef]
- Alfaro, S.O.; Martínez-De La Cruz, A. Synthesis, Characterization and Visible-Light Photocatalytic Properties of Bi2WO6 and Bi2W2O9 Obtained by Co-Precipitation Method. Appl. Catal. Gen. 2010, 383, 128–133. [Google Scholar] [CrossRef]
- Hao, N.; Chen, S.; Qian, J.; Zhang, Y.; Liu, Q.; Zhang, X.; Wang, K. A Sensitive Photoelectrochemical (PEC) Platform Fabricated with Nitrogen-Doped Graphene Quantum Dots Decorated Bi2WO6 for Detection of Pentachlorophenol. J. Electroanal. Chem. 2017, 801, 410–415. [Google Scholar] [CrossRef]
- Al-Harahsheh, M.; Shawabkeh, R.; Al-Harahsheh, A.; Tarawneh, K.; Batiha, M.M. Surface Modification and Characterization of Jordanian Kaolinite: Application for Lead Removal from Aqueous Solutions. Appl. Surf. Sci. 2009, 255, 8098–8103. [Google Scholar] [CrossRef]
- Shi, R.; Huang, G.; Lin, J.; Zhu, Y. Photocatalytic Activity Enhancement for Bi2WO6 by Fluorine Substitution. J. Phys. Chem. C 2009, 113, 19633–19638. [Google Scholar] [CrossRef]
- Zhu, Y.; Wang, Y.; Ling, Q.; Zhu, Y. Enhancement of Full-Spectrum Photocatalytic Activity over BiPO4/Bi2WO6 Composites. Appl. Catal. B Environ. 2017, 200, 222–229. [Google Scholar] [CrossRef]
- Kong, X.Y.; Choo, Y.Y.; Chai, S.P.; Soh, A.K.; Mohamed, A.R. Oxygen Vacancies Induced Bi2WO6 for Realization of Full Solar Spectrum Photocatalytic CO2 Reduction: From UV to NIR Region. Chem. Commun. 2016, 52, 14242–14245. [Google Scholar] [CrossRef]
- Shannon, R.D. Handbook of Chemistry and Physics. Acta Crystallogr. 1976, 32, 751. [Google Scholar] [CrossRef]
- Tauc, J.; Grigorovici, R.; Vancu, A. Optical Properties and Electronic Structure of Amorphous Germanium. Phys. Status Solidi B 1966, 15, 627–637. [Google Scholar] [CrossRef]
- Mahanty, S.; Ghose, J. Preparation and Optical Studies of Polycrystalline Bi2WO6. Mater. Lett. 1991, 11, 254–256. [Google Scholar] [CrossRef]
- Liu, Y.; Wei, B.; Xu, L.; Gao, H.; Zhang, M. Generation of Oxygen Vacancy and OH Radicals: A Comparative Study of Bi2WO6 and Bi2WO6−x Nanoplates. ChemCatChem 2015, 7, 4076–4084. [Google Scholar] [CrossRef]
- Jansi Rani, B.; Ravi, G.; Yuvakkumar, R.; Praveenkumar, M.; Ravichandran, S.; Muthu Mareeswaran, P.; Hong, S.I. Bi2WO6 and FeWO4 Nanocatalysts for the Electrochemical Water Oxidation Process. ACS Omega 2019, 4, 5241–5253. [Google Scholar] [CrossRef]
- Wu, L.; Bi, J.; Li, Z.; Wang, X.; Fu, X. Rapid Preparation of Bi2WO6 Photocatalyst with Nanosheet Morphology via Microwave-Assisted Solvothermal Synthesis. Catal. Today 2008, 131, 15–20. [Google Scholar] [CrossRef]
- Wang, J.; Tang, L.; Zeng, G.; Deng, Y.; Dong, H.; Liu, Y.; Wang, L.; Peng, B.; Zhang, C.; Chen, F. 0D/2D Interface Engineering of Carbon Quantum Dots Modified Bi2WO6 Ultrathin Nanosheets with Enhanced Photoactivity for Full Spectrum Light Utilization and Mechanism Insight. Appl. Catal. B Environ. 2018, 222, 115–123. [Google Scholar] [CrossRef]
- Liang, H.; Sun, H.; Zhu, Y.; Fan, L.; Xu, Q. Bi/Bi2WO6 Plasmonic Composites with Enhanced Photocatalytic Activity for Degradation of Gasphase Toluene. Catal. Lett. 2023, 153, 559–569. [Google Scholar] [CrossRef]
- Huang, Y.; Kang, S.; Yang, Y.; Qin, H.; Ni, Z.; Yang, S.; Li, X. Facile Synthesis of Bi/Bi2WO6 Nanocomposite with Enhanced Photocatalytic Activity under Visible Light. Appl. Catal. B Environ. 2016, 196, 89–99. [Google Scholar] [CrossRef]
- Elaouni, A.; Ouardi, M.E.; Zbair, M.; BaQais, A.; Saadi, M.; Ahsaine, H.A. ZIF-8 Metal Organic Framework Materials as a Superb Platform for the Removal and Photocatalytic Degradation of Organic Pollutants: A Review. RSC Adv. 2022, 12, 31801–31817. [Google Scholar] [CrossRef] [PubMed]
- Bahsaine, K.; Benzeid, H.; El Allaoui, B.; Zari, N.; El Mahdi, M.; Qaiss, A.e.K.; Bouhfid, R. Porous Polyvinyl Fluoride Coated Cellulose Beads for Efficient Removal of Cd(II) from Phosphoric Acid. Int. J. Biol. Macromol. 2024, 254, 127867. [Google Scholar] [CrossRef]
- Yang, Z.; Xia, X.; Shao, L.; Wang, L.; Liu, Y. Efficient Photocatalytic Degradation of Tetracycline under Visible Light by Z-Scheme Ag3PO4/Mixed-Valence MIL-88A(Fe) Heterojunctions: Mechanism Insight, Degradation Pathways and DFT Calculation. Chem. Eng. J. 2021, 410, 128454. [Google Scholar] [CrossRef]
- Azmi, S.; Layachi, O.A.; Ouardi, M.E.; Khoumri, E.M.; Moujib, A.; Brouzi, A.E.; Nohair, M.; Pezzato, L.; Dabala, M. Growth of Cu2ZnSnS4 Thin Film Absorber Layer on Transparent Conductive Oxides and Molybdenum Substrates by Electrodeposition for Photovoltaic Application. Optik 2022, 250, 168320. [Google Scholar] [CrossRef]
- Ait Layachi, O.; Azmi, S.; Moujib, A.; Nohair, M.; Khoumri, E. Investigation of Nucleation and Growth Mechanism of Cu2ZnSnS4 Absorber Layer Electrodeposition on Indium Tin Oxide Coated Glass. Thin Solid Films 2023, 782, 140019. [Google Scholar] [CrossRef]
- Boudouma, A.; Ait Layachi, O.; Hrir, H.; Khoumri, E. A One-Step Electrodeposition Method Was Used to Produce Monoclinic Cu2SnS3 Thin Films for the Development of Solar Cells. J. Mater. Sci. Mater. Electron. 2023, 34, 1903. [Google Scholar] [CrossRef]
- Ganeshbabu, M.; Kannan, N.; Venkatesh, P.S.; Paulraj, G.; Jeganathan, K.; MubarakAli, D. Synthesis and Characterization of BiVO4 Nanoparticles for Environmental Applications. RSC Adv. 2020, 10, 18315–18322. [Google Scholar] [CrossRef]
- Wang, Q.; Qian, X.; Xu, H.; He, G.; Chen, H. Enriched Surface Oxygen Vacancies of Bi2WO6/NH2-MIL-68(In) Z-Scheme Heterojunction with Boosted Visible-Light Photocatalytic Degradation for Levofloxacin: Performance, Degradation Pathway and Mechanism Insight. Sep. Purif. Technol. 2023, 306, 122577. [Google Scholar] [CrossRef]
- Zhou, W.; Jia, J.; Lu, J.; Yang, L.; Hou, D.; Li, G.; Chen, S. Recent Developments of Carbon-Based Electrocatalysts for Hydrogen Evolution Reaction. Nano Energy 2016, 28, 29–43. [Google Scholar] [CrossRef]
- Cai, T.; Zeng, W.; Liu, Y.; Wang, L.; Dong, W.; Chen, H.; Xia, X. A Promising Inorganic-Organic Z-Scheme Photocatalyst Ag3PO4/PDI Supermolecule with Enhanced Photoactivity and Photostability for Environmental Remediation. Appl. Catal. B Environ. 2020, 263, 118327. [Google Scholar] [CrossRef]
- Jiang, Z.; Wan, W.; Li, H.; Yuan, S.; Zhao, H.; Wong, P.K. A Hierarchical Z-Scheme α-Fe2O3/g-C3N4 Hybrid for Enhanced Photocatalytic CO2 Reduction. Adv. Mater. 2018, 30, 1706108. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Guo, L.; Zhen, Y.; Yue, L.; Xue, G.; Fu, F. AgBr Quantum Dots Decorated Mesoporous Bi2WO6 Architectures with Enhanced Photocatalytic Activities for Methylene Blue. J. Mater. Chem. A 2014, 2, 11716–11727. [Google Scholar] [CrossRef]
- Lotfi, S.; Ouardi, M.E.; Ahsaine, H.A.; Assani, A. Recent Progress on the Synthesis, Morphology and Photocatalytic Dye Degradation of BiVO4 Photocatalysts: A Review. Catal. Rev. 2022, 66, 1–45. [Google Scholar] [CrossRef]
- Wang, Q.; Li, H.; Yu, X.; Jia, Y.; Chang, Y.; Gao, S. Morphology Regulated Bi2WO6 Nanoparticles on TiO2 Nanotubes by Solvothermal Sb3+ Doping as Effective Photocatalysts for Wastewater Treatment. Electrochim. Acta 2020, 330, 135167. [Google Scholar] [CrossRef]
- Liu, Z.; Wang, Q.; Rong, W.; Jin, R.; Cui, Y.; Gao, S. CTAB Assisted Hydrothermal Preparation of Bi2WO6WO3 Nanosheets on TiO2 Nanotube Arrays for Photoelectrocatalytic Applications. Sep. Purif. Technol. 2018, 200, 191–197. [Google Scholar] [CrossRef]
- Silva, R.M.; Batista Barbosa, D.A.; de Jesus Silva Mendonça, C.; de Oliveira Lima, J.R.; Silva, F.C.; Longo, E.; Maciel, A.P.; de Araujo Paschoal, C.W.; Almeida, M.A.P. Morphological Evolution and Visible Light-Induced Degradation of Rhodamine 6G by Nanocrystalline Bismuth Tungstate Prepared Using a Template-Based Approach. J. Phys. Chem. Solids 2016, 96–97, 83–91. [Google Scholar] [CrossRef]
- Shang, Y.; Cui, Y.; Shi, R.; Yang, P. Effect of Acetic Acid on Morphology of Bi2WO6 with Enhanced Photocatalytic Activity. Mater. Sci. Semicond. Process. 2019, 89, 240–249. [Google Scholar] [CrossRef]
- Tahmasebi, N.; Maleki, Z.; Farahnak, P. Enhanced Photocatalytic Activities of Bi2WO6/BiOCl Composite Synthesized by One-Step Hydrothermal Method with the Assistance of HCl. Mater. Sci. Semicond. Process. 2019, 89, 32–40. [Google Scholar] [CrossRef]
- Hu, T.; Li, H.; Du, N.; Hou, W. Iron-Doped Bismuth Tungstate with an Excellent Photocatalytic Performance. ChemCatChem 2018, 10, 3040–3048. [Google Scholar] [CrossRef]
- Phu, N.D.; Hoang, L.H.; Chen, X.-B.; Kong, M.-H.; Wen, H.-C.; Chou, W.C. Study of Photocatalytic Activities of Bi2WO6 Nanoparticles Synthesized by Fast Microwave-Assisted Method. J. Alloys Compd. 2015, 647, 123–128. [Google Scholar] [CrossRef]
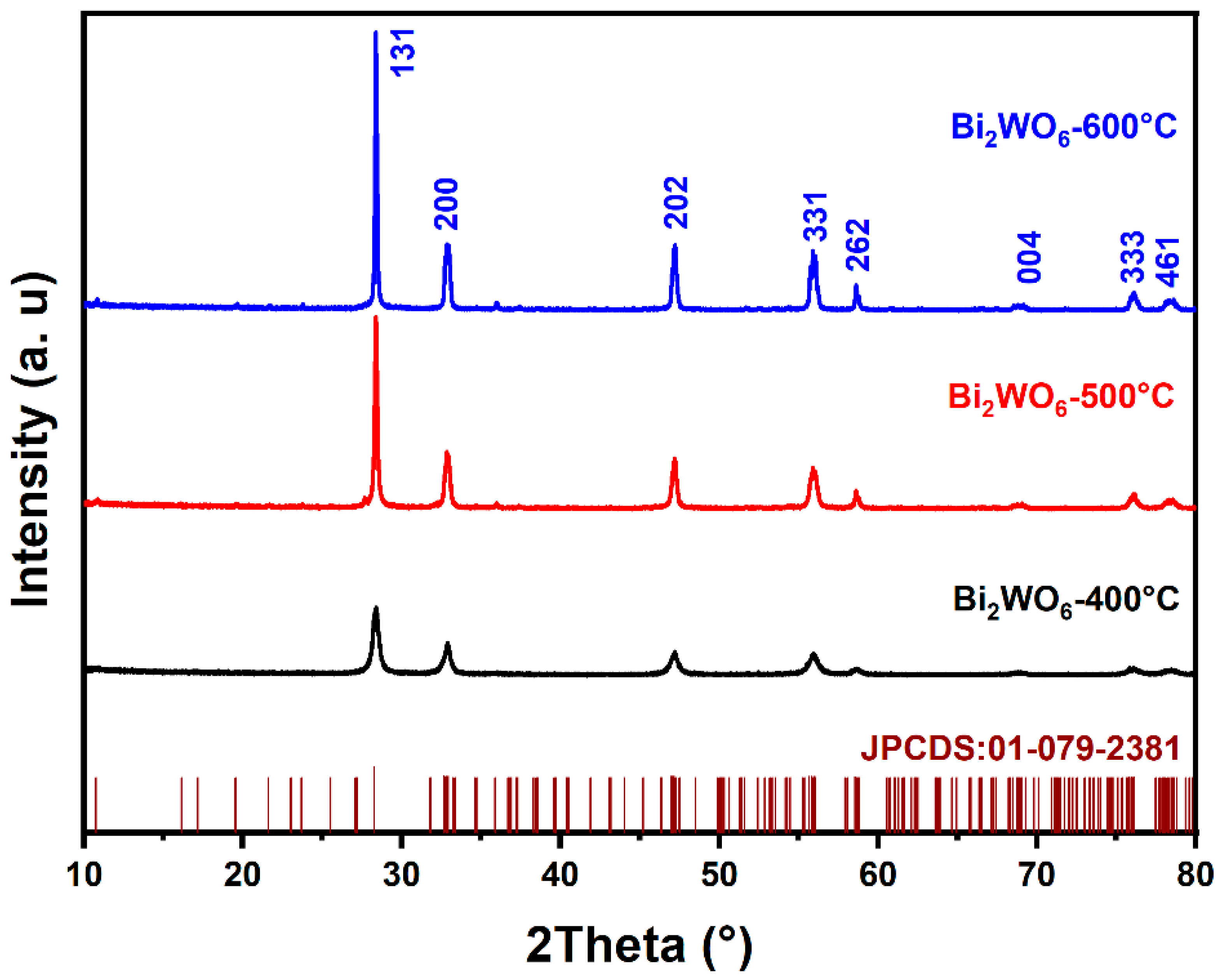





| Treatment Temperature | a (Å) | b (Å) | c (Å) | Unit Cell Volume (Å)3 |
|---|---|---|---|---|
| JCPDS card No. 01-079-2381 | 5.4373 | 16.4302 | 5.4584 | 487.6312 |
| 400 °C | 5.4430 | 16.3134 | 5.4486 | 483.8021 |
| 500 °C | 5.4428 | 16.3141 | 5.4474 | 483.6985 |
| 600 °C | 5.4419 | 16.3204 | 5.4457 | 483.6543 |
| Treatment Temperature | Raman Stretching Frequency (cm−1) | Bond Length (Å) |
|---|---|---|
| 400 °C | 820 | 1.7003 |
| 500 °C | 828 | 1.6952 |
| 600 °C | 828 | 1.6852 |
| Photocatalyst | Pollutants | Concentration (mg/L) | Light Source (W) | Efficiency (%) | Ref. |
|---|---|---|---|---|---|
| Sb3+/Bi2WO6 | RhB and MO | 6.5 | Solar light | 80.58 and 77.23 | [69] |
| Bi2WO6/WO3 | MO | - | Solar light | 75.65 | [70] |
| Bi2WO6 | RhB | 5 | 500 W Xe | 95 | [71] |
| Bi2WO6 | RhB | 5 | 500 W Xe | 92 | [72] |
| Bi2WO6/BiOCl | MO | 10 | Xe lamp | 68 | [73] |
| Fe-Bi2WO6 | RhB | 10 | 500 W Xe | 93 | [74] |
| Bi2WO6 | RhB | 4.8 | 300 W Xe | 90 | [75] |
| BWO400 | RhB and MO | 5 | Philips lamps (300 W) | 97 and 92 | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
El Aouni, A.; El Ouardi, M.; Arab, M.; Saadi, M.; Haspel, H.; Kónya, Z.; Ben Ali, A.; Jada, A.; BaQais, A.; Ait Ahsaine, H. Design of Bismuth Tungstate Bi2WO6 Photocatalyst for Enhanced and Environmentally Friendly Organic Pollutant Degradation. Materials 2024, 17, 1029. https://doi.org/10.3390/ma17051029
El Aouni A, El Ouardi M, Arab M, Saadi M, Haspel H, Kónya Z, Ben Ali A, Jada A, BaQais A, Ait Ahsaine H. Design of Bismuth Tungstate Bi2WO6 Photocatalyst for Enhanced and Environmentally Friendly Organic Pollutant Degradation. Materials. 2024; 17(5):1029. https://doi.org/10.3390/ma17051029
Chicago/Turabian StyleEl Aouni, Aicha, Mohamed El Ouardi, Madjid Arab, Mohamed Saadi, Henrik Haspel, Zoltán Kónya, Abdelkader Ben Ali, Amane Jada, Amal BaQais, and Hassan Ait Ahsaine. 2024. "Design of Bismuth Tungstate Bi2WO6 Photocatalyst for Enhanced and Environmentally Friendly Organic Pollutant Degradation" Materials 17, no. 5: 1029. https://doi.org/10.3390/ma17051029
APA StyleEl Aouni, A., El Ouardi, M., Arab, M., Saadi, M., Haspel, H., Kónya, Z., Ben Ali, A., Jada, A., BaQais, A., & Ait Ahsaine, H. (2024). Design of Bismuth Tungstate Bi2WO6 Photocatalyst for Enhanced and Environmentally Friendly Organic Pollutant Degradation. Materials, 17(5), 1029. https://doi.org/10.3390/ma17051029








