Experimental Study of the Moisture Resistance of Cement Mortar Using Pozzolan Materials and Calcium Stearate
Abstract
1. Introduction
2. Materials and Methods of the Experiment
2.1. Materials
2.2. Overview and Methods of the Experiment
2.2.1. Preparation of Hydrophobic Powder
2.2.2. Method of Specimen Production and Experiment
3. Experimental Results
3.1. Thermal Analysis Measurement
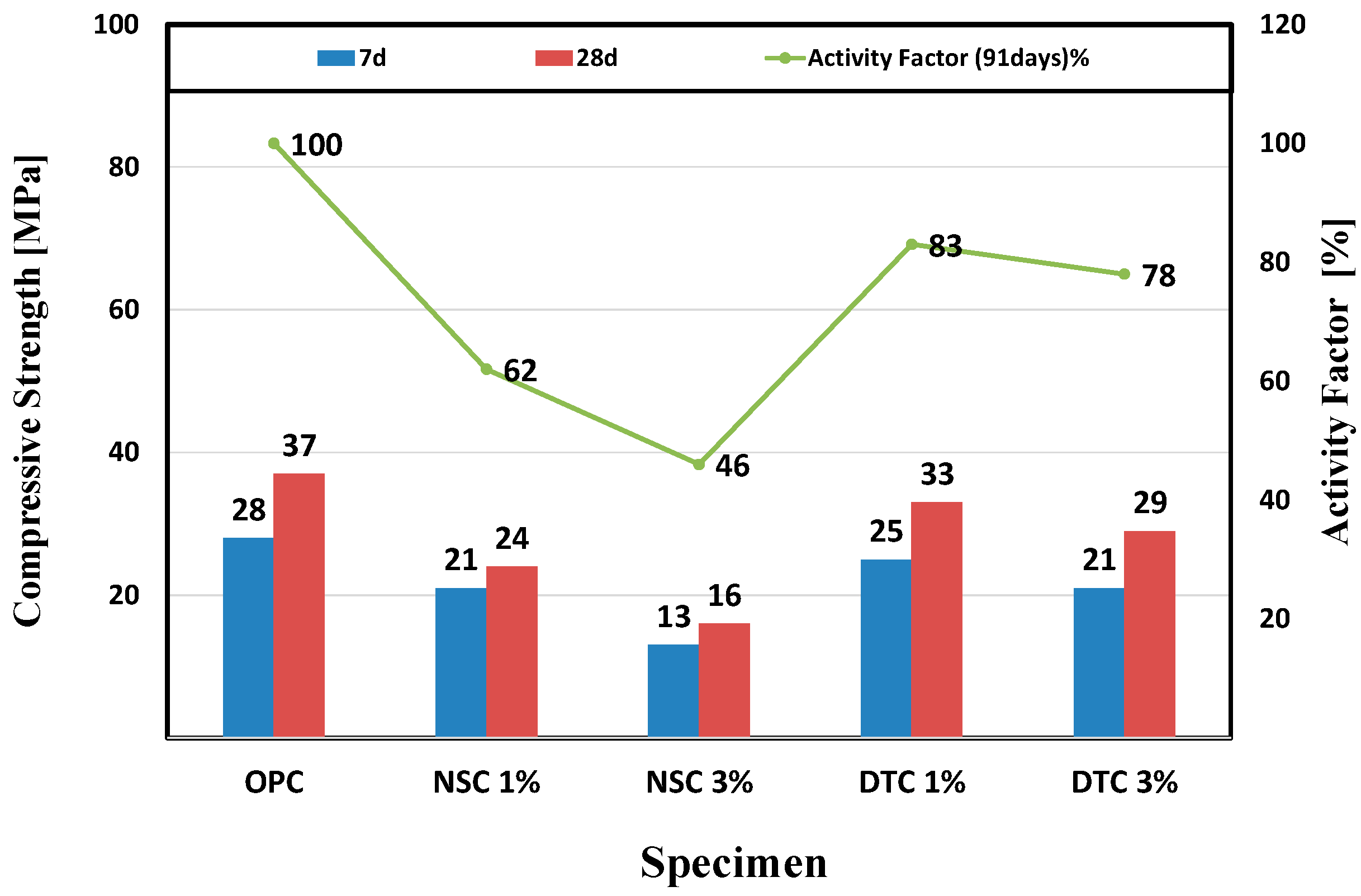
3.2. Compressive Strength and Activity Factor
- As: activity factor (%);
- C1: average compressive strength of the specimen replacing admixture (MPa);
- C2: average compressive strength of OPC specimen (MPa).
3.3. Water Absorption Measurement
- W0: mass before measurement (g);
- W1: mass after measurement (g).
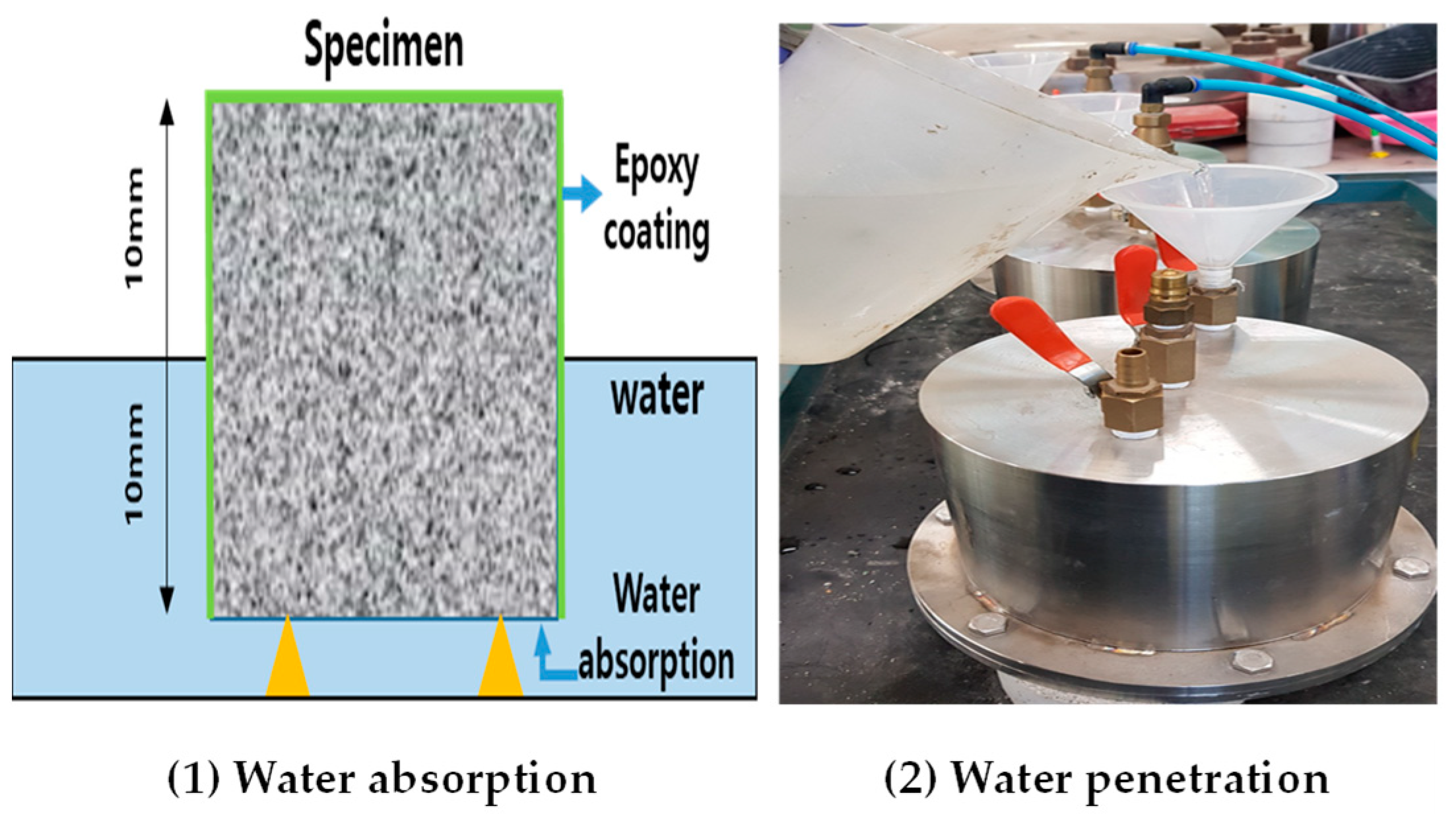
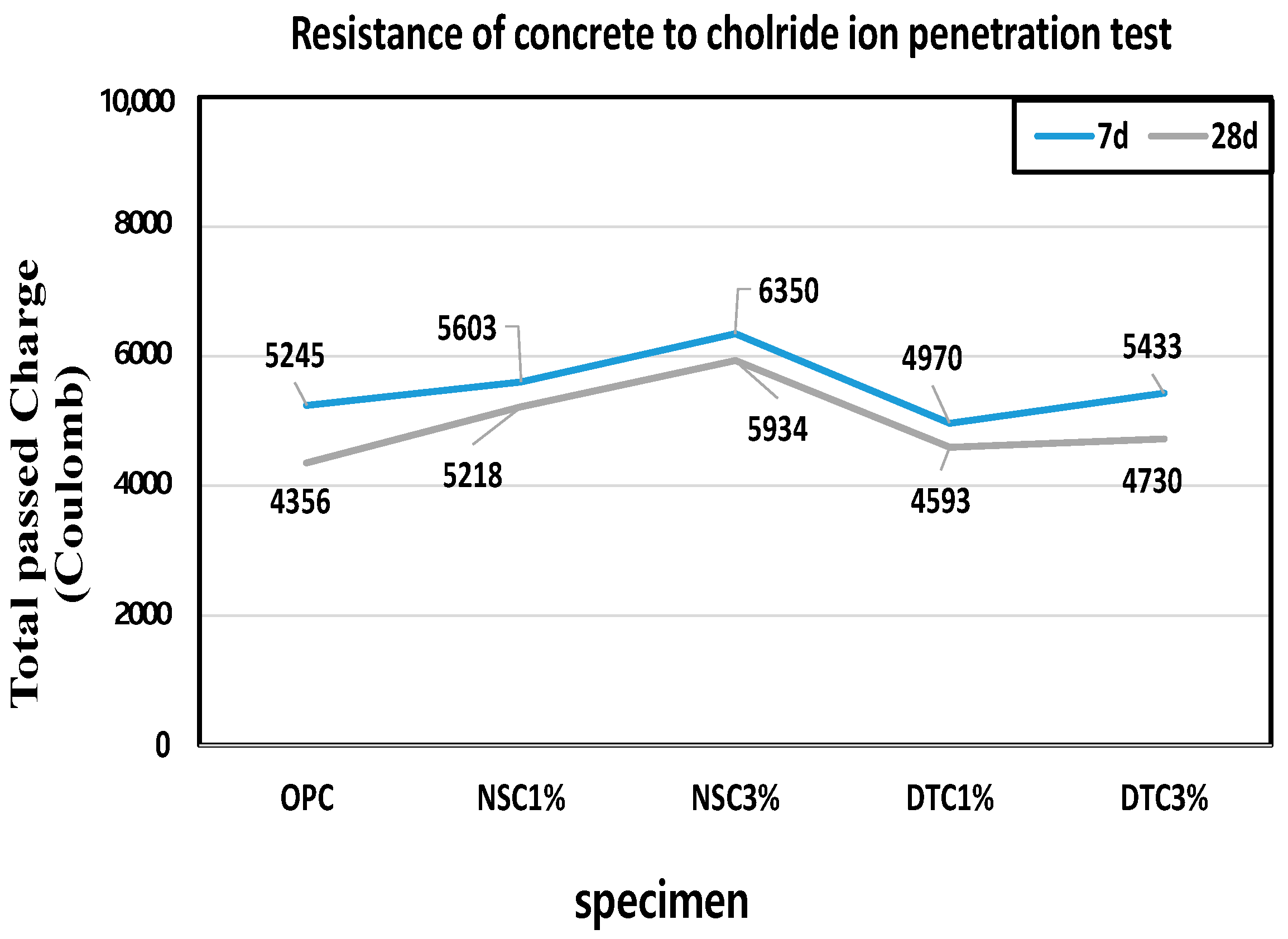
3.4. Measurement of Chloride Ion Penetration Resistance
- Q: total passing charge;
- I0: current immediately after starting test with applied voltage;
- I360: current 360 min after applying voltage.
| Coulombs (C) | Permeability |
|---|---|
| >4000 | High |
| 2000–4000 | Normal |
| 1000–2000 | Low |
| 100–1000 | Very low |
| <100 | Negligible |
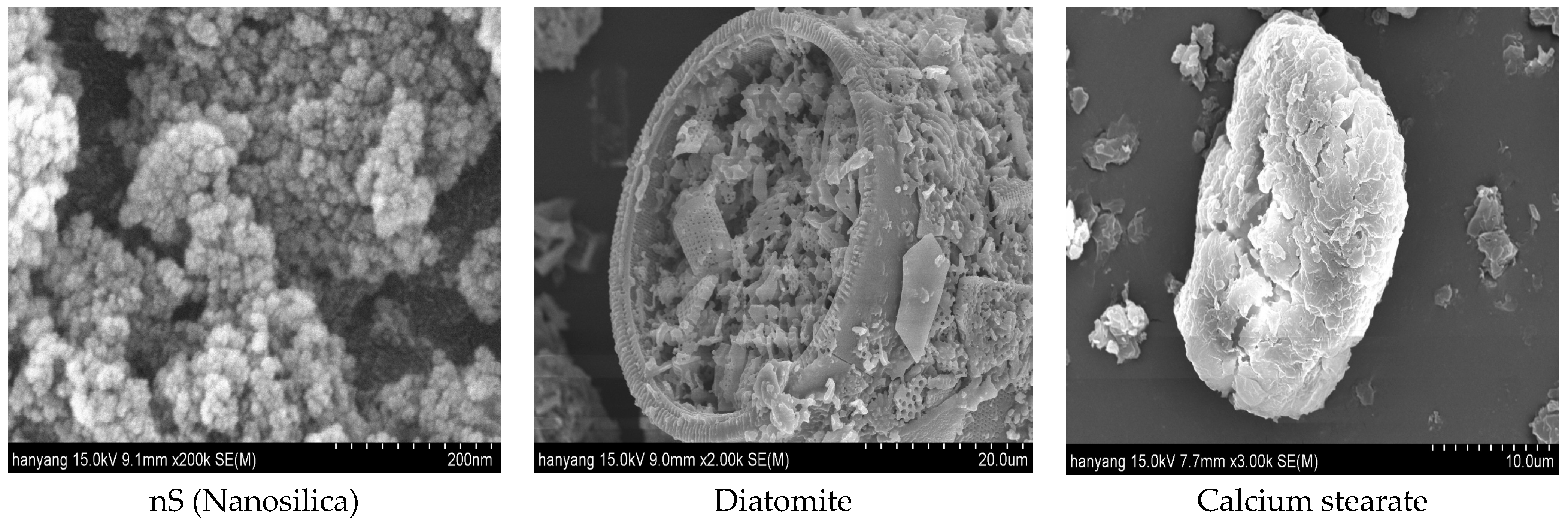
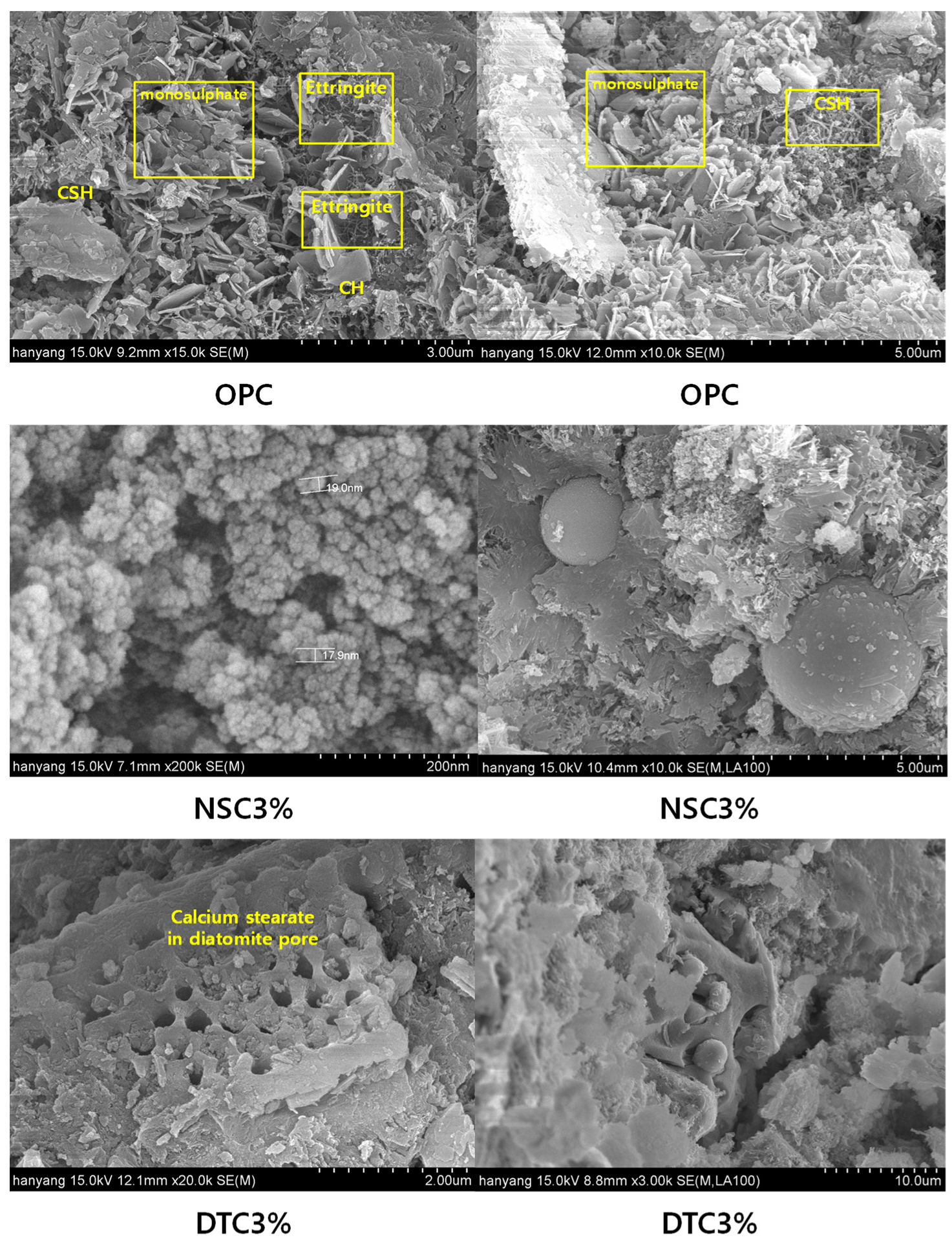
3.5. Scanning Electron Microscope (SEM)
4. Conclusions
- (1)
- The results of the literature review showed that mixing in only nanosilica can increase compressive strength. However, the fine particles of nanosilica are coated with liquefied calcium stearate, thereby inhibiting the pozzolanic reaction under the conditions of this experiment.
- (2)
- The moisture resistance measurement results were the best for the NCS3% specimen, which was rich in calcium stearate. It exhibited the best results under hydrostatic pressure, whereas its permeability was the highest under pressurization. As a simple filler, it can resist some moisture; however, water tightness cannot be expected due to the chemical reaction of nanosilica.
- (3)
- The chloride ion penetration resistance measurements also demonstrated that the increase in the amount of mixing admixture to which calcium stearate was attached led to an increase in the total charge that passed.
- (4)
- Diatomite, with its atypical particles and porosity, seems to have secured water tightness better than nanosilica because of the pozzolanic reaction in particles to which calcium stearate was not attached.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Shim, H.B.; Lee, M.S. An Experimental Study on Water Resistance of Penetrating Water Repellency of Emulsified Silicon Type Exposed to The Outdoor Environment. J. Korea Concr. Inst. 2014, 16, 477–484. [Google Scholar] [CrossRef]
- Maravelaki-Kalaitzaki, P. Hydraulic Lime Mortars with Siloxane for Waterproofing Historic Masonry. Cem. Concr. Res. 2007, 37, 283–290. [Google Scholar] [CrossRef]
- Moradllo, M.; Sudbrink, B.; Tyler Ley, M. Determining the Effective Service Life of Silane Treatments in Concrete Bridge Decks. Constr. Build. Mater. 2016, 116, 121–127. [Google Scholar] [CrossRef]
- Zhang, P.; Wittmann, F.; Vogel, M.; Muller, H.; Zhao, T. Influence of Freeze-thaw Cycles on Capillary Absorption and Chloride Penetration into Concrete. Cem. Concr. Res. 2017, 100, 60–67. [Google Scholar] [CrossRef]
- Yoon, C.B.; Lee, H.S. Selection of the Optimum Carrier for Manufacturing Water-Repellent Concrete and Durability Evaluation of Cement Mortar Using It. Appl. Sci. 2020, 10, 9097. [Google Scholar] [CrossRef]
- Naseroleslami, R.; Chari, M.N. The effects of calcium stearate on mechanical and durability aspects of self-consolidating concretes incorporating silica fume/natural zeolite. Constr. Build. Mater. 2019, 225, 384–400. [Google Scholar] [CrossRef]
- Yao, N.; Zhang, P.; Song, L.; Kang, M.; Lu, Z.; Zheng, R. Stearic acid coating on circulating fluidized bed combustion fly ashes and its effect on the mechanical performance of polymer composites. Appl. Surf. Sci. 2013, 279, 109–115. [Google Scholar] [CrossRef]
- Spathi, C.; Young, N.; Heng, J.Y.; Vandeperre, L.J.; Cheeseman, C.R. A simple method for preparing super-hydrophobic powder from paper sludge ash. Mater. Lett. 2015, 142, 80–83. [Google Scholar] [CrossRef]
- Liu, W.; Xie, Z.; Yang, X.; Wu, Y.; Jia, C.; Bo, T.; Wang, L. Surface modification mechanism of stearic acid to zirconia powders induced by ball milling for water-based injection molding. J. Am. Ceram. Soc. 2011, 94, 1327–1330. [Google Scholar] [CrossRef]
- Alonso, M.M.; Palacios, M.; Puertas, F. Compatibility between polycarboxylate-based admixtures and blended-cement pastes. Cem. Concr. Compos. 2013, 35, 151–162. [Google Scholar] [CrossRef]
- Choi, S.S.; Chung, H.S.; Joo, Y.T.; Yang, K.M.; Lee, S.H. Appearance contamination of EPDM article from water solution. Elastomers Compos. 2010, 45, 100–105. [Google Scholar]
- Abhilash, P.P.; Nayak, D.K.; Sangoju, B.; Kumar, R.; Kumar, V. Effect of nano-silica in concrete; a review. Constr. Build. Mater. 2021, 278, 122347. [Google Scholar]
- Mondal, P.; Shah, S.P.; Marks, L.D.; Gaitero, J.J. Comparative study of the effects of microsilica and nanosilica in concrete. Transp. Res. Rec. 2010, 2141, 6–9. [Google Scholar] [CrossRef]
- Ren, J.; Lai, Y.; Gao, J. Exploring the influence of SiO2 and TiO2 nanoparticles on the mechanical properties of concrete. Constr. Build. Mater. 2018, 175, 277–285. [Google Scholar] [CrossRef]
- Khan, K.; Ahmad, W.; Amin, M.N.; Nazar, S. Nano-silica-modified concrete: A bibliographic analysis and comprehensive review of material properties. Nanomaterials 2022, 12, 1989. [Google Scholar] [CrossRef]
- Chekravarty, D.S.V.S.M.R.K.; Mallika, A.; Sravana, P.; Rao, S. Effect of using nano silica on mechanical properties of normal strength concrete. Mater. Today Proc. 2022, 51, 2573–2578. [Google Scholar] [CrossRef]
- Ji, T. Preliminary study on the water permeability and microstructure of concrete incorporating nano-SiO2. Cem. Concr. Res. 2005, 35, 1943–1947. [Google Scholar] [CrossRef]
- Gaitero, J.J.; Campillo, I.; Guerrero, A. Reduction of the calcium leaching rate of cement paste by addition of silica nanoparticles. Cem. Concr. Res. 2008, 38, 1112–1118. [Google Scholar] [CrossRef]
- Jeong, G.Y. Quantitative Determination of Cristobalite Content in Diatomite and Filtered Food. J. Mineral. Soc. Korea 2019, 32, 313–321. [Google Scholar] [CrossRef]
- Zhang, X.; Liu, X.; Meng, G. Sintering Kinetics of Porous Ceramics from Natural Diatomite. J. Am. Soc. Ceram. 2005, 88, 1826–1830. [Google Scholar] [CrossRef]
- Sarıdemir, M.; Çelikten, S.; Yıldırım, A. Mechanical and microstructural properties of calcined diatomite powder modified high strength mortars at ambient and high temperatures. Adv. Powder Technol. 2020, 31, 3004–3017. [Google Scholar] [CrossRef]
- Degirmenci, N.; Yilmaz, A. Use of diatomite as partial replacement for Portland cement in cement mortars. Constr. Build. Mater. 2009, 23, 284–288. [Google Scholar] [CrossRef]
- Aydin, A.C.; Gül, R. Influence of volcanic originated natural materials as additives on the setting time and some mechanical properties of concrete. Constr. Build. Mater. 2007, 21, 1277–1281. [Google Scholar] [CrossRef]
- Posi, P.; Lertnimoolchai, S.; Sata, V.; Chindaprasirt, P. Pressed lightweight concrete containing calcined diatomite aggregate. Constr. Build. Mater. 2013, 47, 896–901. [Google Scholar] [CrossRef]
- Du, H.; Du, S.; Liu, X. Durability performances of concrete with nano-silica. Constr. Build. Mater. 2014, 73, 705–712. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, C.; Zhou, F.; Ma, S.; Zheng, H. The effect of nano-scale calcium stearate emulsion on the integral waterproof performance and chloride resistance of cement mortar. Constr. Build. Mater. 2022, 317, 125903. [Google Scholar] [CrossRef]
- KSL5201; Portland Cement. Korean Industrial Standards: Seoul, Republic of Korea, 2017.
- ASTM A C150; Standard Specification for Portland Cement. ASTM International: West Conshohocken, PA, USA, 2001.
- KS L ISO 679; Cement-Test Methods-Determination of Strength (Second edition 2009-05-01). Korean Industrial Standards: Seoul, Republic of Korea, 2009.
- Park, J.H.; Yoon, C.B. Properties and durability of cement mortar using calcium stearate and natural pozzolan for concrete surface treatment. Mater. Sci. 2022, 15, 5762. [Google Scholar] [CrossRef] [PubMed]
- KSL5109; Practice for Mechanical Mixing of Hydraulic Cement Pastes and Mortar of Plastic Consistency. Korean Industrial Standards: Seoul, Republic of Korea, 2017.
- KSL5105; Test Method for Compressive Strength of Hydraulic Cement Mortar. Korean Industrial Standards: Seoul, Republic of Korea, 2022.
- KSL5405; Fly AHY. Korean Industrial Standards: Seoul, Republic of Korea, 2018.
- KSL4919; Cement-Polymer Modified Waterproof Coatings. Korean Industrial Standards: Seoul, Republic of Korea, 2017.
- ASTM C 1202; Electrical Indication of Concrete’s Ability to Resist Chloride Ion Penetration. Annual Book of American Society for Testing Materials Standards, Vol. C04.02. ASTM International: West Conshohocken, PA, USA, 1993.
- KS F 2711; Standard Test Method for Resistance of Concrete to Chloride Ion Penetration by Electrical Conductance. Korean Industrial Standards: Seoul, Republic of Korea, 2017.
- Fernández, J.M.; Duran, A.; Navarro-Blasco, I.; Lanas, J.; Sirera, R.; Alvarez, J.I. Influence of nanosilica and a polycarboxylate ether superplasticizer on the performance of lime mortars. Cem. Concr. Res. 2013, 43, 12–24. [Google Scholar] [CrossRef]
- Shih, J.Y.; Chang, T.P.; Hsiao, T.C. Effect of nanosilica on characterization of Portland cement composite. Mater. Sci. Eng. A 2006, 424, 266–274. [Google Scholar] [CrossRef]
- Li, H.; Zhang, M.H.; Ou, J.P. Abrasion resistance of concrete containing nano-particles for pavement. Wear 2006, 260, 1262–1266. [Google Scholar] [CrossRef]
- Nemati Chari, M.; Naseroleslami, R.; Shekarchi, M. The impact of calcium stearate on characteristics of concrete. Asian J. Civ. Eng. 2019, 20, 1007–1020. [Google Scholar] [CrossRef]
- Lee, W.G.; Yoon, C.B. Experimental Study on Physical Properties and Water Absorption Resistance Evaluation of Cement Mortar Incorporating Inorganic Metal Salt-based Water Repellent Powder. J. Korean Recycl. Constr. Resour. Inst. 2021, 9, 609–616. [Google Scholar]
- Azarhomayun, F.; Haji, M.; Kioumarsi, M.; Shekarchi, M. Effect of calcium stearate and aluminum powder on free and restrained drying shrinkage, crack characteristic and mechanical properties of concrete. Cem. Concr. Compos. 2022, 125, 104276. [Google Scholar] [CrossRef]


| Name | Chemical Composition (%) | |||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | MgO | SO3 | K2O | L.O.I | |
| OPC | 19.29 | 5.16 | 2.87 | 61.68 | 4.17 | 2.53 | 0.89 | |
| Diatomite | 91.56 | 1.34 | 3.83 | 1.0 | 0.2 | 0.01 | 0.06 | |
| NanoSilica | 99.46 | 0.03 | 0.02 | 0.07 | 0.01 | 0.24 | 0.01 | 0.17 |
| Name | Chemical Composition of Metallic Salts | ||||
|---|---|---|---|---|---|
| Chemical Formula | Density (g/cm3) | pH | Melting Point | Molecular Weight (g/mol) | |
| Calcium stearate | C36H70CaO4 | 1.10 | 7−9 | 147−149 | 607 |
| Specimen | W/B | C | S | W | NSC | DTC |
|---|---|---|---|---|---|---|
| OPC | 50% | 510 | 1530 | 255 | - | |
| NSC 1% | 257.5 | 5.1 | ||||
| NSC 3% | 260.1 | 10.2 | ||||
| DTC 1% | 257.5 | 5.1 | ||||
| DTC 3% | 260.1 | 10.2 |
| Specimen [28 Days] | |||||
|---|---|---|---|---|---|
| OPC | NSC 1% | NSC 3% | DTC 1% | DTC 3% | |
| 0 H (g, W0) | 133.4 | 137.7 | 131.5 | 134.9 | 139.5 |
| 24 H (g, W1) | 136.1 | 140.1 | 133.1 | 137.6 | 141.8 |
| Absorption (g) | 2.7 | 2.4 | 1.6 | 2.7 | 2.3 |
| Specimen [28 Days] | |||||
|---|---|---|---|---|---|
| OPC | NSC 1% | NSC 3% | DTC 1% | DTC 3% | |
| Weight (before testing) | 855.8 | 869.5 | 850.7 | 862.8 | 860.4 |
| Weight (after the test) | 870.9 | 888.4 | 875.3 | 879.1 | 876.1 |
| Absorption (g) | 15.1 | 18.9 | 24.6 | 16.3 | 15.7 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Park, J.H.; Yoon, C.B. Experimental Study of the Moisture Resistance of Cement Mortar Using Pozzolan Materials and Calcium Stearate. Materials 2024, 17, 1014. https://doi.org/10.3390/ma17051014
Park JH, Yoon CB. Experimental Study of the Moisture Resistance of Cement Mortar Using Pozzolan Materials and Calcium Stearate. Materials. 2024; 17(5):1014. https://doi.org/10.3390/ma17051014
Chicago/Turabian StylePark, Jang Hyun, and Chang Bok Yoon. 2024. "Experimental Study of the Moisture Resistance of Cement Mortar Using Pozzolan Materials and Calcium Stearate" Materials 17, no. 5: 1014. https://doi.org/10.3390/ma17051014
APA StylePark, J. H., & Yoon, C. B. (2024). Experimental Study of the Moisture Resistance of Cement Mortar Using Pozzolan Materials and Calcium Stearate. Materials, 17(5), 1014. https://doi.org/10.3390/ma17051014






