Shrinkage Reduction in Nanopore-Rich Cement Paste Based on Facile Organic Modification of Montmorillonite
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Mix Design and Preparation
2.3. Test Methods
3. Results and Discussion
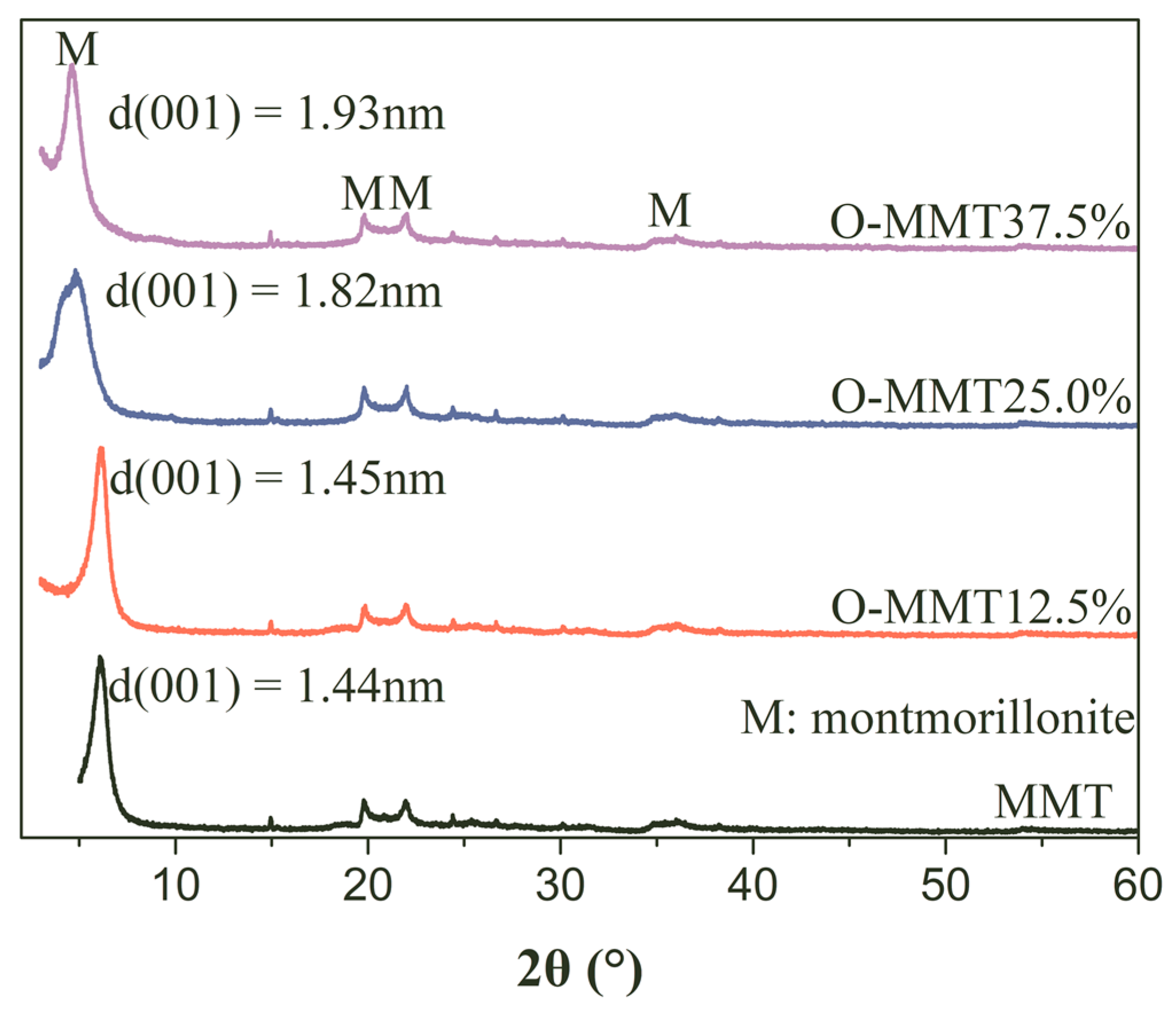
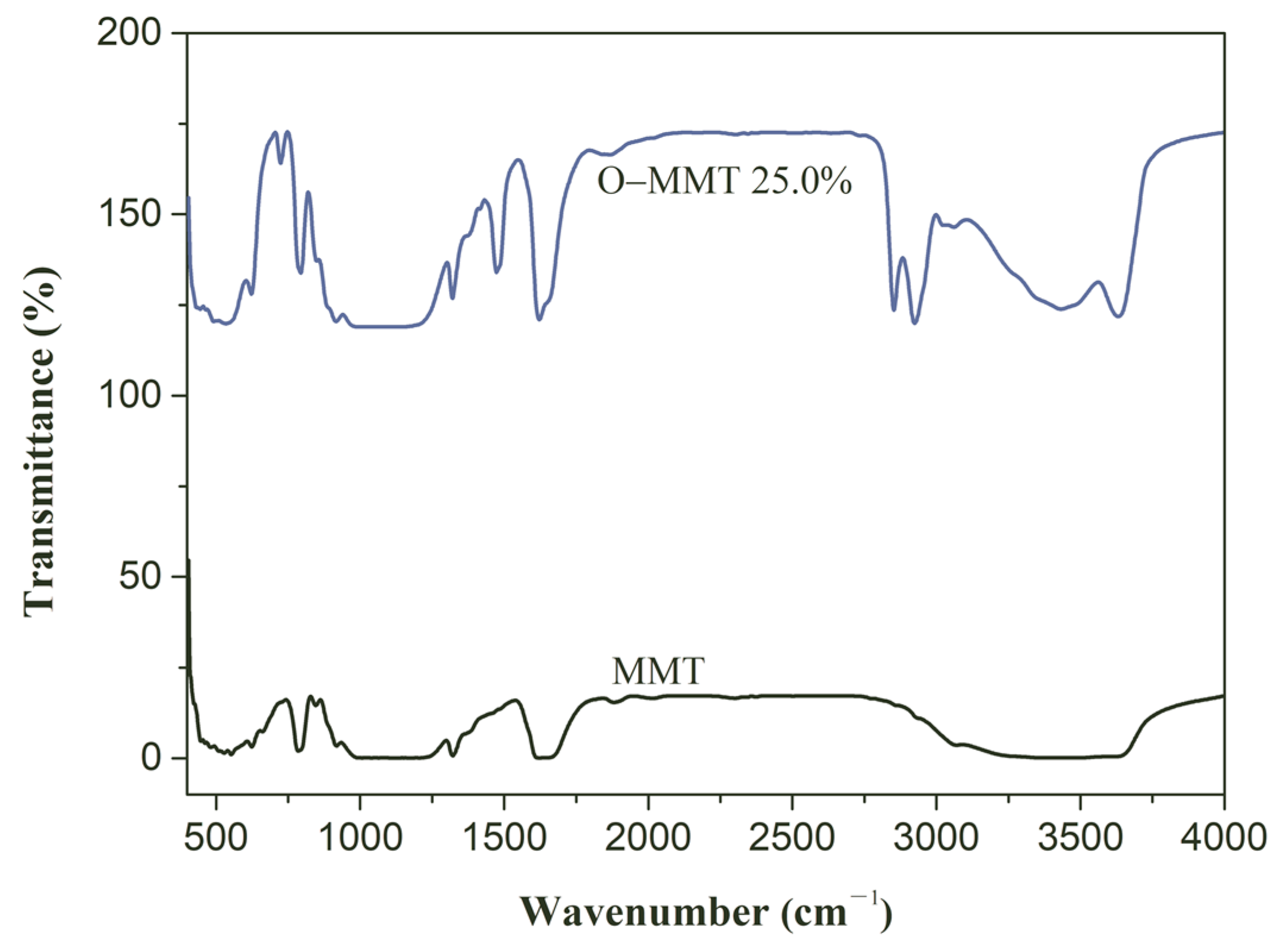
3.1. Montmorillonite Modification
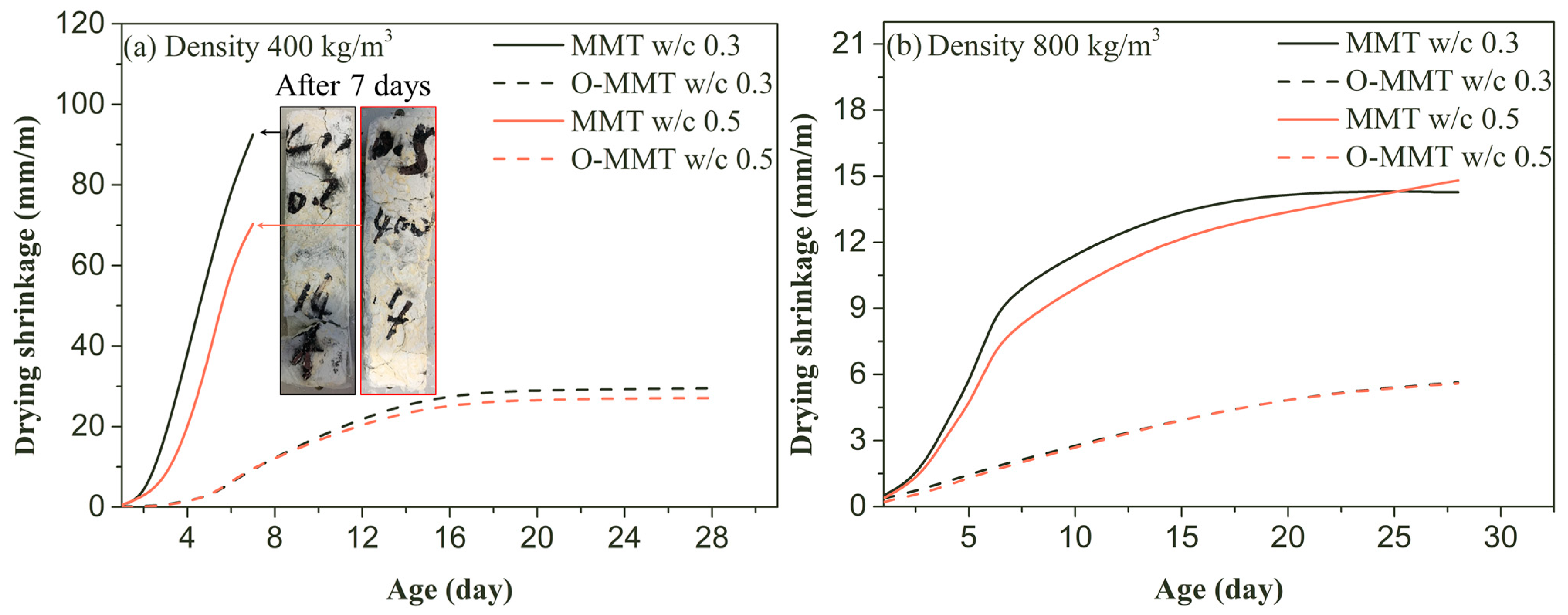
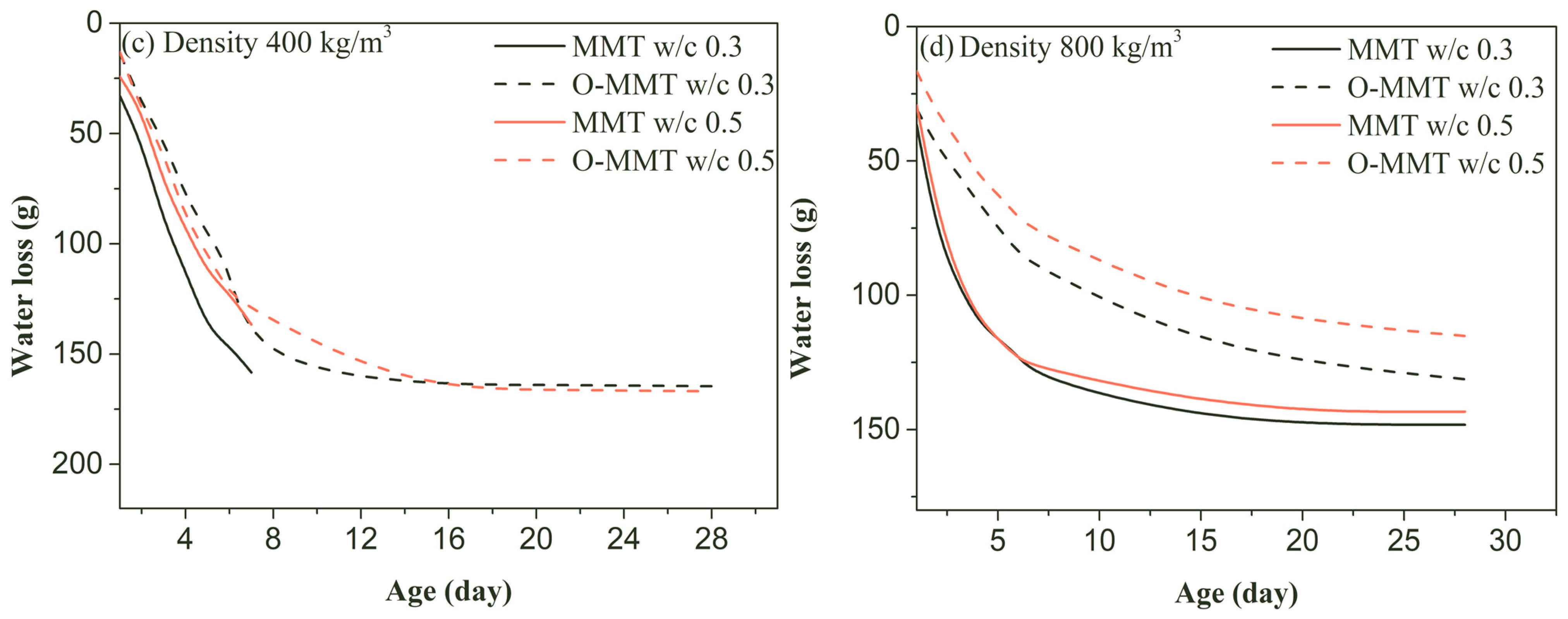
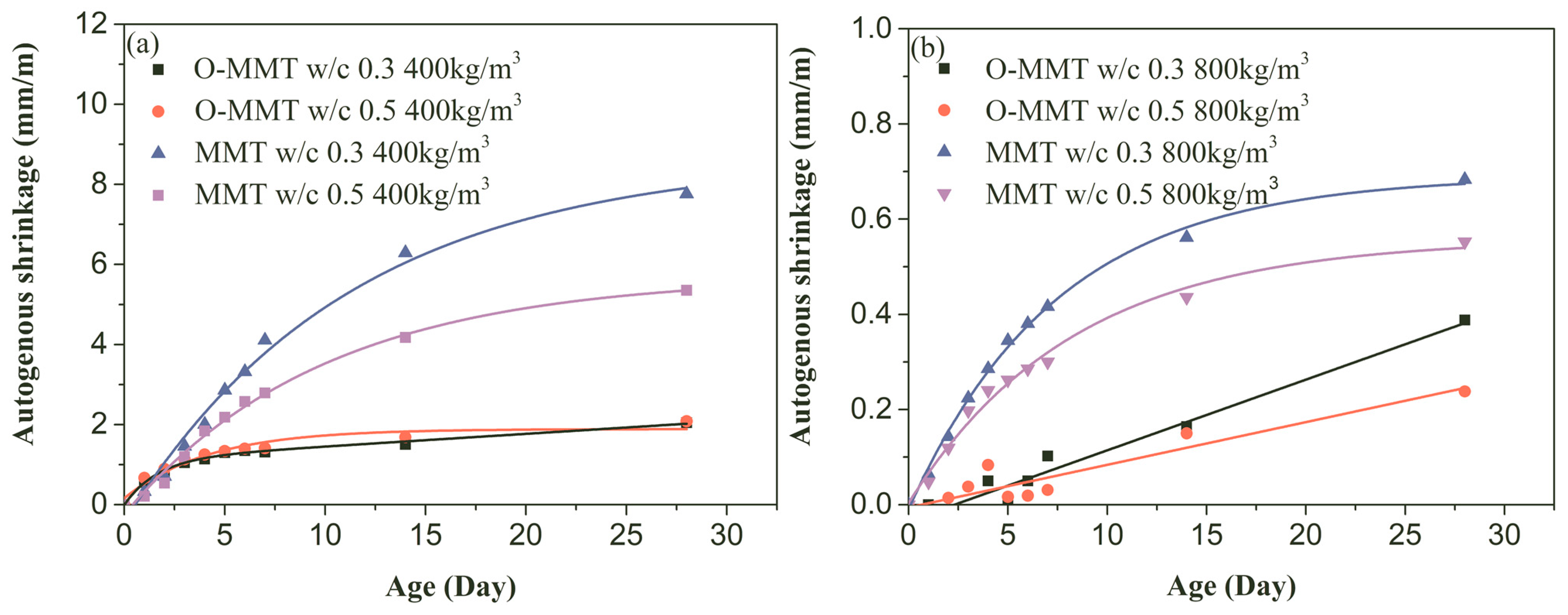
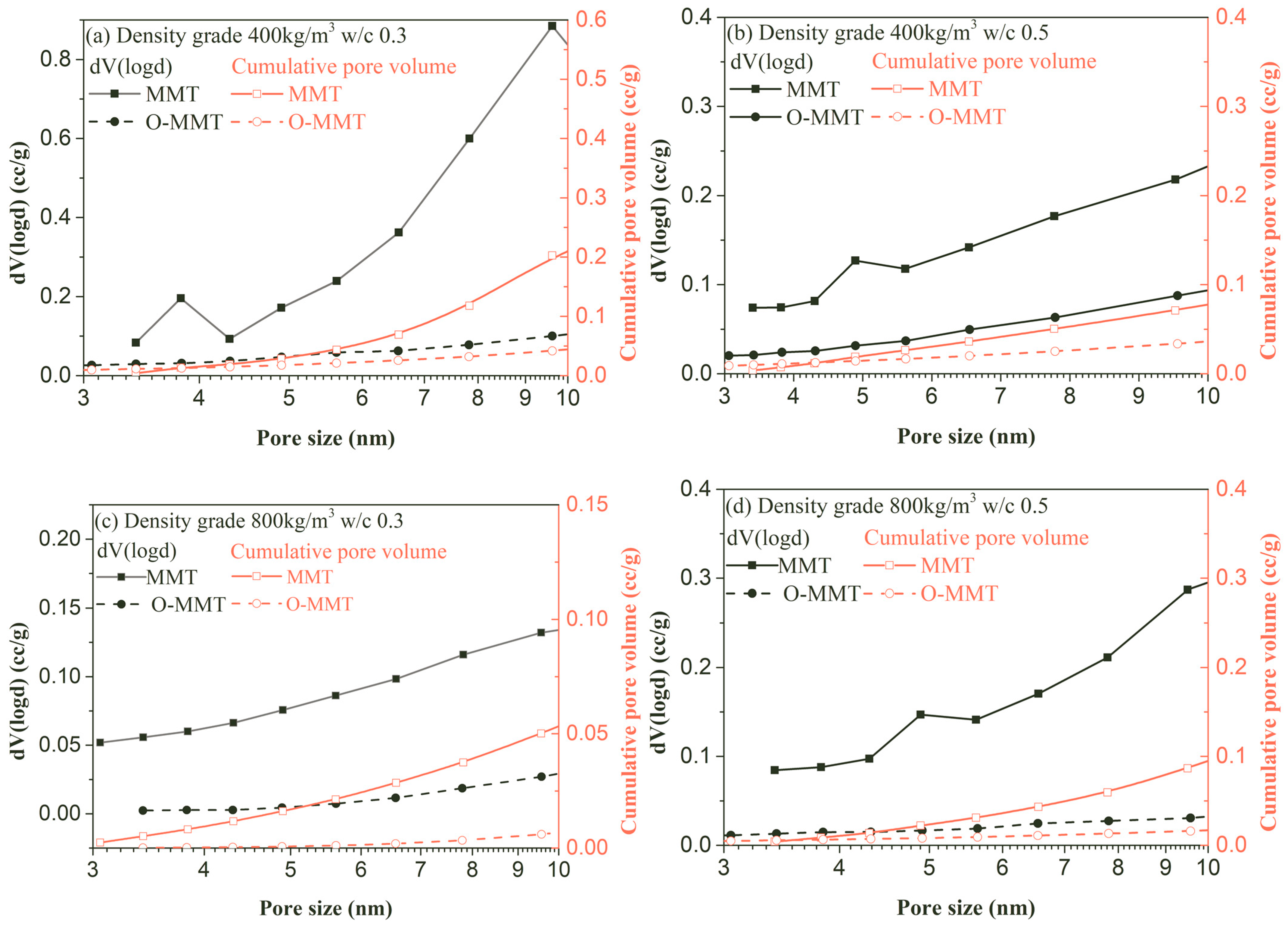
3.2. Drying and Autogenous Shrinkage
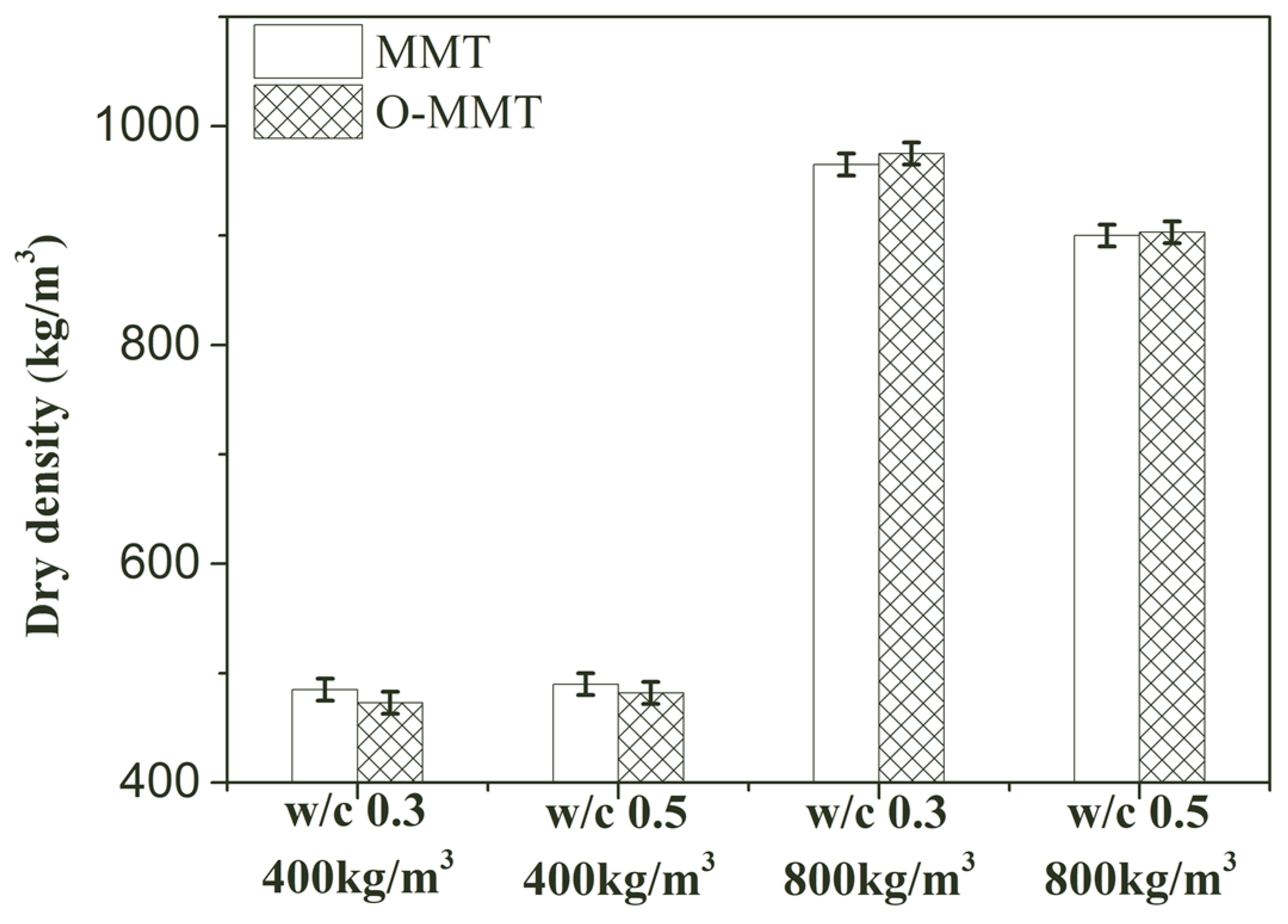
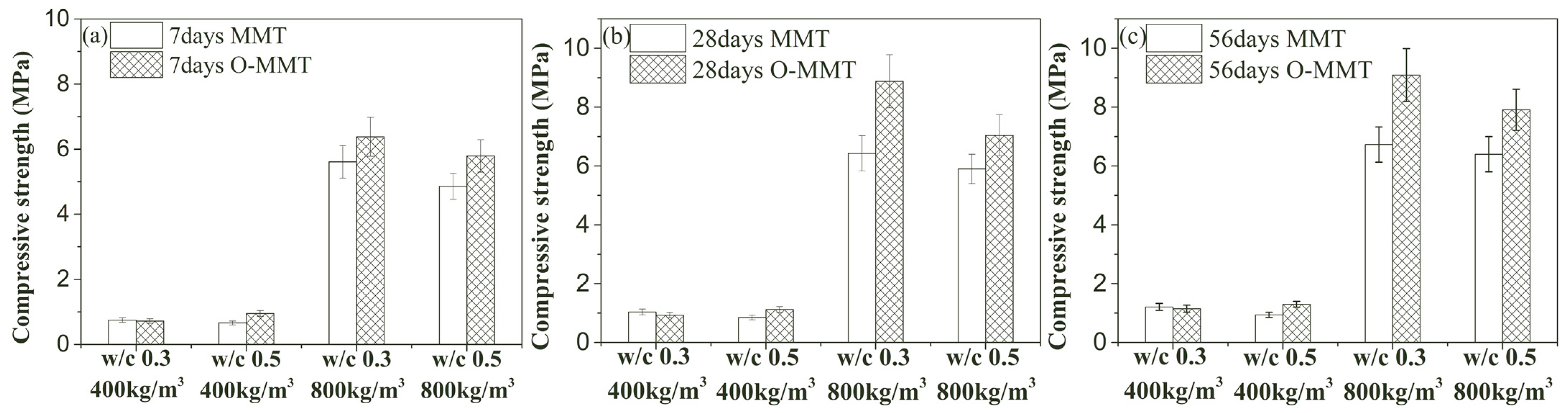
3.3. Fundamental Performance
4. Conclusions
- (1)
- Montmorillonite can be modified by using cetyltrimethyl ammonium bromide at 80 °C for 2 h, successfully achieving organic modification, which enlarges the interlayer pores and brings the hydrophobic chain into interlamination, hindering the penetration of water molecules.
- (2)
- Autogenous and drying shrinkage were significantly reduced when organic-modified montmorillonite was used to replace original montmorillonite. The autogenous 28-day shrinkages at design density values of 400 kg/m3 and 800 kg/m3 were reduced to 2.05 mm/m and 0.24 mm/m, respectively, and the highest reduction percentages for the 28-day drying shrinkage were increased to at least 68.1% and 62.2%, respectively.
- (3)
- Organic-modified montmorillonite has a minor influence on the dry density and thermal conductivity of MNCP, but it contributed to enhancing the strength of MNCP. The 56-day compressive strength of modified MNCP could be improved to 38.3% and 35.1% at densities of 400 kg/m3 and 800 kg/m3.
- (4)
- The significant reduction in shrinkage, the enhancement of the mechanical performance, and the minor influence on the insulation performance showed that this process has great potential for applications in external building envelopes, and further research on the applications of nanopore-rich cement paste in building insulation will be necessary in the future.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Abhilasha, K.R.; Lakhani, R.; Mishra, R.K.; Khan, S. Utilization of solid waste in the production of autoclaved aerated concrete and their effects on its physio-mechanical and microstructural properties: Alternative sources, characterization, and performance insights. Int. J. Concr. Struct. Mater. 2023, 17, 6. [Google Scholar] [CrossRef]
- Park, B.; Choi, Y.C. Investigation of carbon-capture property of foam concrete using stainless steel AOD slag. J. Clean. Prod. 2021, 288, 125621. [Google Scholar] [CrossRef]
- Shah, S.N.; Mo, K.H.; Yap, S.P.; Yang, J.; Ling, T.C. Lightweight foamed concrete as a promising avenue for incorporating waste materials: A review. Resour. Conserv. Recycl. 2021, 164, 105103. [Google Scholar] [CrossRef]
- Shrestha, S.S.; Tiwari, J.; Rai, A.; Hun, D.E.; Howard, D.; Desjarlais, A.O.; Feng, T. Solid and gas thermal conductivity models improvement and validation in various porous insulation materials. Int. J. Therm. Sci. 2023, 187, 108164. [Google Scholar] [CrossRef]
- Pasztory, Z. An overview of factors influencing thermal conductivity of building insulation materials. J. Build. Eng. 2021, 44, 102604. [Google Scholar]
- Awoyera, P.O.; Althoey, F.; Ajinomisan, H.; Othuman Mydin, M.A.; Bheel, N.; Sabri Sabri, M.M.; Ahmad, M. Potential of natural rubber latex in cement mortar for thermal insulating material in buildings. Front. Mater. 2023, 10, 1152492. [Google Scholar] [CrossRef]
- Majdoubi, H.; Haddaji, Y.; Bourzik, O.; Nadi, M.; Ziraoui, J.; Alomayri, T.S.; Hannache, H. Enhancing thermal insulation with phosphate washing sludge waste as an inorganic foaming agent in porous acid-based geopolymers: Formulation and processing optimization. Constr. Build. Mater. 2023, 407, 133486. [Google Scholar] [CrossRef]
- Seker, B.S.; Gokçe, M.; Toklu, K. Investigation of the effect of silica fume and synthetic foam additive on cell structure in ultra-low density foam concrete. Case Stud. Constr. Mater. 2022, 16, e01062. [Google Scholar]
- Mydin, M.A.O.; Sor, N.H.; Althoey, F.; Özkılıç, Y.O.; Abdullah, M.M.A.B.; Isleem, H.F.; Tawfik, T.A. Performance of lightweight foamed concrete partially replacing cement with industrial and agricultural wastes: Microstructure characteristics, thermal conductivity, and hardened properties. Ain Shams Eng. J. 2023, 14, 102546. [Google Scholar] [CrossRef]
- Owolabi, O.S.B.; Abas, N.F. The impact of density and porosity on thermal properties of kernelrazzo concrete floor finish. J. Adv. Res. Appl. Sci. Eng. Tech. 2023, 29, 291–303. [Google Scholar]
- Golaszewski, J.; Klemczak, B.; Smolana, A.; Golaszewska, M.; Cygan, G.; Mankel, C.; Koenders, E.A. Effect of foaming agent, binder and density on the compressive strength and thermal conductivity of ultra-light foam concrete. Buildings 2022, 12, 1176. [Google Scholar] [CrossRef]
- Janssen, H.; Van De Walle, W. The impact of pore structure parameters on the thermal conductivity of porous building blocks. Constr. Build. Mater. 2022, 324, 126681. [Google Scholar] [CrossRef]
- Ahmida, F.; Sayah, G.M.; Zineb, D.; Queneudect Kint, M. Experimental study on the effect of lime and aluminium content on porosity, introduced porosity, compressive strength and thermal conductivity of a lightweight cellular concrete based on limestone sand. Constr. Build. Mater. 2023, 392, 131552. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Z.; Li, J.; Xie, Y.; Luo, K.; Niu, Y. Preparation and properties of nanopore-rich lightweight cement paste based on swelled bentonite. Constr. Build. Mater. 2019, 199, 72–81. [Google Scholar] [CrossRef]
- Zambotti, A.; Ionescu, E.; Gargiulo, N.; Caputo, D.; Vakifahmetoglu, C.; Santhosh, B.; Soraru, G.D. Processing of polymer-derived, aerogel-filled, SiC foams for high-temperature insulation. J. Am. Ceram. Soc. 2023, 106, 4891–4901. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Pontinha, A.D.R.; Alves, P.; Santos, P.; Durães, L. Progress in silica aerogel-containing materials for buildings’ thermal insulation. Constr. Build. Mater. 2021, 286, 122815. [Google Scholar] [CrossRef]
- Shohan, A.A.A.; Zaid, O.; Arbili, M.M.; Alsulamy, S.H.; Ibrahim, W.M. Development of novel ultra-high-performance lightweight concrete modified with dehydrated cement powder and aerogel. J. Sustain. Cem.-Based Mater. 2023, 13, 1–24. [Google Scholar] [CrossRef]
- Gencel, O.; Nodehi, M.; Bayraktar, O.Y.; Kaplan, G.; Benli, A.; Gholampour, A.; Ozbakkaloglu, T. Basalt fiber-reinforced foam concrete containing silica fume: An experimental study. Constr. Build. Mater. 2022, 326, 126861. [Google Scholar] [CrossRef]
- Gencel, O.; Bayraktar, O.Y.; Kaplan, G.; Benli, A.; Martinez-Barrera, G.; Brostow, W.; Bodur, B. Characteristics of hemp fibre reinforced foam concretes with fly ash and Taguchi optimization. Constr. Build. Mater. 2021, 294, 123607. [Google Scholar] [CrossRef]
- Aghaee, K.; Khayat, K.H. Design and performance of fiber-reinforced shrinkage compensating eco-friendly concrete. Constr. Build. Mater. 2023, 408, 133803. [Google Scholar] [CrossRef]
- Tran, N.P.; Gunasekara, C.; Law, D.W.; Houshyar, S.; Setunge, S.; Cwirzen, A. A critical review on drying shrinkage mitigation strategies in cement-based materials. J. Build. Eng. 2021, 38, 102210. [Google Scholar] [CrossRef]
- Kurup, D.S.; Mohan, M.K.; Van Tittelboom, K.; De Schutter, G.; Santhanam, M.; Rahul, A.V. Early-age shrinkage assessment of cementitious materials: A critical review. Cem. Concr. Comp. 2023, 145, 105343. [Google Scholar] [CrossRef]
- Ghanem, H.; Ramadan, R.; Khatib, J.; Elkordi, A. A Review on chemical and autogenous shrinkage of cementitious systems. Material 2024, 17, 283. [Google Scholar] [CrossRef]
- Mindess, S.; Young, J.F.; Darwin, D. Concrete, 2nd ed.; Pearson Education, North America: Boston, MA, USA, 2005. [Google Scholar]
- Salami, B.A.; Ibrahim, M.; Al-Osta, M.A.; Nasir, M.; Ali, M.R.; Bahraq, A.A.; Wasiu, A. Engineered and green natural pozzolan-nano silica-based alkali-activated concrete: Shrinkage characteristics and life cycle assessment. Environ. Sci. Pollut. R. 2023, 30, 17840–17853. [Google Scholar] [CrossRef]
- Melo, V.F.; Salata, R.; Abate, G.; Azevedo, A.C.; Kummer, L. Characterization and manipulation of montmorillonite properties towards technological and environmental applications. Rev. Bras. Cienc. Solo 2021, 45, e0200149. [Google Scholar] [CrossRef]
- Dhar, A.K.; Himu, H.A.; Bhattacharjee, M.; Mostufa, M.G.; Parvin, F. Insights on applications of bentonite clays for the removal of dyes and heavy metals from wastewater: A review. Environ. Sci. Pollut. Res. 2023, 30, 5440–5474. [Google Scholar] [CrossRef] [PubMed]
- Archibong, F.N.; Orakwe, L.C.; Ogah, O.A.; Mbam, S.O.; Ajah, S.A.; Okechukwu, M.E.; Ikelle, I.I. Emerging progress in montmorillonite rubber/polymer nanocomposites: A review. J. Mater. Sci. 2023, 58, 2396–2429. [Google Scholar] [CrossRef]
- Penchev, H.; Abdelhamid, A.E.; Ali, E.A.; Budurova, D.; Grancharov, G.; Ublekov, F.; Khalil, A.M. Novel electrospun composite membranes based on polyhydroxybutyrate and poly (vinyl formate) loaded with protonated montmorillonite for organic dye removal: Kinetic and isotherm studies. Membranes 2023, 13, 582. [Google Scholar] [CrossRef] [PubMed]
- Costanza-Robinson, M.S.; Payne, E.M.; Dellinger, E.; Fink, K.; Bunt, R.C.; Littlefield, M.; Wilcox, E.H. Influence of water saturation on interlayer properties of HDTMA-, HDTMP-, and HDPy-modified montmorillonite organoclays. Appl. Clay Sci. 2024, 247, 107188. [Google Scholar] [CrossRef]
- Bangar, S.P.; Chaudhary, V.; Akhila, P.P.; Sunooj, K.V. Recent functionality developments in montmorillonite as a nanofiller in food packaging. Trends Food. Sci. Tech. 2023, 140, 104148. [Google Scholar] [CrossRef]
- Olszewski, A.; Lawniczak, A.; Kosmela, P.; Strąkowski, M.; Mielewczyk-Gryn, A.; Hejna, A.; Piszczyk, L. Influence of surface-modified montmorillonite clays on the properties of elastomeric thin layer nanocomposites. Materials 2023, 16, 1703. [Google Scholar] [CrossRef]
- GB 175-2007; Common Portland Cement. China National Standardization Administration: Beijing, China, 2007.
- Jiang, J.; Liu, T.; Tong, L.; Yang, Y.; Guan, X.; Lei, L.; Ding, T.; Lu, Z.; Li, J. Effect of EPS on shrinkage of porous bentonite/cement composites. Non-Met. Mines 2022, 45, 27–30. [Google Scholar]
- ISO 679:2009; Cement: Test Methods—Determination of Strength. ISO: Geneva, Switzerland, 2009.
- ISO 22007-2:2022; Plastics: Determination of Thermal Conductivity and Thermal Diffusivity—Part 2: Transient Plane Heat Source (Hot Disc) Method. ISO: Geneva, Switzerland, 2022.
- JC/T 603-2004; Standard Test Method for Drying Shinkage of Mortar. China National Standardization Administration: Beijing, China, 2004.
- Gurses, A.; Guneş, K. Preparation of polyethylene clay composites via melt intercalation using hydrophobic and superhydrophobic organoclays and comparison of their textural, mechanical and thermal properties. Polymers 2024, 16, 272. [Google Scholar] [CrossRef]
- Mohammed, A.K.; Saadoon, S.M.; Abd Ali, Z.T.; Rashid, I.M.; Sbani, N.H.A. Removal of amoxicillin from contaminated water using modified bentonite as a reactive material. Heliyon 2024, 10, e24916. [Google Scholar] [CrossRef]
- Ristic, M.; Samarzija-Jovanovic, S.; Jovanovic, T.; Jovanovic, V.; Kostic, M.; Markovic, G.; Marinovic-Cincovic, M. Organically modified montmorillonite as an environmental adsorbent of pollutants: Formaldehyde from urea-formaldehyde resin and acid red 183 dye from the aqueous solution. J. Environ. Chem. Eng. 2024, 12, 111828. [Google Scholar] [CrossRef]
- Mennas, N.; Lahreche, S.; Chouli, F.; Sabantina, L.; Benyoucef, A. Adsorption of methylene blue dye by cetyltrimethylammonium bromide intercalated polyaniline-functionalized montmorillonite clay nanocomposite: Kinetics, isotherms, and mechanism study. Polymers 2023, 15, 3518. [Google Scholar] [CrossRef]
- Arabmofrad, S.; Jafari, S.M.; Lazzara, G.; Ziaiifar, A.M.; Shahiri Tabarestani, H.; Bahlakeh, G.; Nasiri Sarvi, M. Preparation and characterization of surface-modified montmorillonite by cationic surfactants for adsorption purposes. J. Therm. Anal. Calorim. 2023, 7, 103243. [Google Scholar] [CrossRef]
- Rezala, H.; Boukhatem, H.; Boudechiche, N.; Romero, A. Methyl orange adsorption by modified montmorillonite nanomaterials: Characterization, kinetic, isotherms and thermodynamic studies. Indian J. Chem. Technol. 2023, 30, 85–93. [Google Scholar]
- Jaberizadeh, M.M.; Danoglidis, P.A.; Shah, S.P.; Konsta-Gdoutos, M.S. Eco-efficient cementitious composites using waste cellulose fibers: Effects on autogenous shrinkage, strength and energy absorption capacity. Constr. Build. Mater. 2023, 408, 1133504. [Google Scholar] [CrossRef]
- ASTM C1698; Standard Test Method for Autogenous Strain of Cement Paste and Mortar. ASTM: West Conshohocken, PA, USA, 2019.
- Zhang, D.; Zhou, J.; Shi, H.; Wang, J.; Ni, X.; Zhang, J. Research progress of organoclays for oil-based drilling fluids. Mod. Chem. Res. 2022, 11, 168–170. [Google Scholar]
- Liu, C.; Lian, L.; Liu, Y.; Li, Q. Fabrication, characterization of ultra-low-density bulk nanoporous gold with uniform structure and volume shrinkage control during drying. Rare Metal Mater. Eng. 2018, 47, 2322–2327. [Google Scholar]
- Wang, L. Synthesis and Properties of Doubly Cross-Linked Polyorganosiloxane Aerogels. Master’s Thesis, National University of Defense Technology, China, 2019. [Google Scholar]
- Raman, S.; Gurikov, P.; Smirnova, I. Hybrid alginate based aerogels by carbon dioxide induced gelation: Novel technique for multiple applications. J. Supercrit. Fluid. 2015, 16, 23–33. [Google Scholar] [CrossRef]
- Zhao, Z.; Qu, X.; Pang, J.; Yang, X.; Wen, H.; Yu, C.; Zhao, S. Numerical simulation of pore structure and heat transfer behavior in aerated concrete. Constr. Build. Mater. 2023, 364, 129934. [Google Scholar] [CrossRef]
- Gu, X.; Wang, S.; Liu, J.; Wang, H.; Xu, X.; Wang, Q.; Zhu, Z. Effect of hydroxypropyl methyl cellulose (HPMC) as foam stabilizer on the workability and pore structure of iron tailings sand autoclaved aerated concrete. Constr. Build. Mater. 2023, 376, 130979. [Google Scholar] [CrossRef]




| Mix ID | Cement | Water | Water-to-Cement Ratio (W/C) | Nanopore-Forming Agent |
|---|---|---|---|---|
| MMT 400 0.3 | 212.1 | 63.9 | 0.3 | 909.6 (unmodified) |
| O-MMT 400 0.3 | 212.1 | 63.9 | 0.3 | 909.6 (modified) |
| MMT 400 0.5 | 219.1 | 109.6 | 0.5 | 856.6 (unmodified) |
| O-MMT 400 0.5 | 219.1 | 109.6 | 0.5 | 856.6 (modified) |
| MMT 800 0.3 | 577.5 | 173.2 | 0.3 | 671.0 (unmodified) |
| O-MMT 800 0.3 | 577.5 | 173.2 | 0.3 | 671.0 (modified) |
| MMT 800 0.5 | 596.4 | 298.2 | 0.5 | 527.1 (unmodified) |
| O-MMT 800 0.5 | 596.4 | 298.2 | 0.5 | 527.1 (modified) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, F.; Yang, Y.; Chen, S.; Jin, C.; Jiang, J.; Liu, T.; Lv, F.; Yang, C.; Lu, Z.; Li, J. Shrinkage Reduction in Nanopore-Rich Cement Paste Based on Facile Organic Modification of Montmorillonite. Materials 2024, 17, 922. https://doi.org/10.3390/ma17040922
Yang F, Yang Y, Chen S, Jin C, Jiang J, Liu T, Lv F, Yang C, Lu Z, Li J. Shrinkage Reduction in Nanopore-Rich Cement Paste Based on Facile Organic Modification of Montmorillonite. Materials. 2024; 17(4):922. https://doi.org/10.3390/ma17040922
Chicago/Turabian StyleYang, Fengyuan, Ying Yang, Shaoyou Chen, Chao Jin, Jun Jiang, Tie Liu, Fei Lv, Chenxi Yang, Zhongyuan Lu, and Jun Li. 2024. "Shrinkage Reduction in Nanopore-Rich Cement Paste Based on Facile Organic Modification of Montmorillonite" Materials 17, no. 4: 922. https://doi.org/10.3390/ma17040922
APA StyleYang, F., Yang, Y., Chen, S., Jin, C., Jiang, J., Liu, T., Lv, F., Yang, C., Lu, Z., & Li, J. (2024). Shrinkage Reduction in Nanopore-Rich Cement Paste Based on Facile Organic Modification of Montmorillonite. Materials, 17(4), 922. https://doi.org/10.3390/ma17040922






