Characterization and Analysis of Corrosion Resistance of Rubber Materials for Downhole Tools in a High-Stress Environment with Coupled H2S-CO2
Abstract
1. Introduction
2. Materials and Methods
2.1. Corrosion of Experimental Rubber Material
2.2. Corrode the Experimental Medium
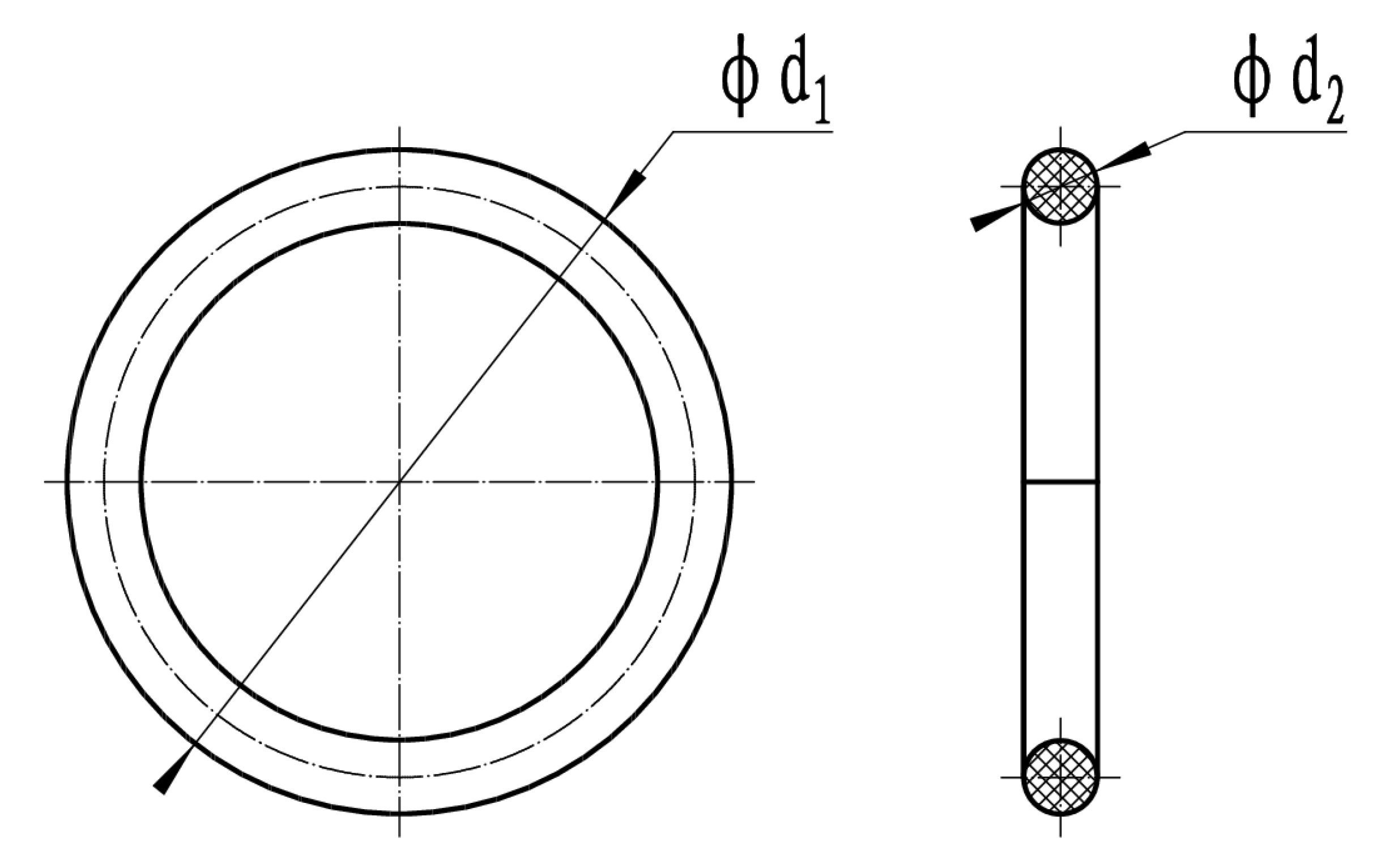
2.3. Sample Processing and Size Requirements for the Experiment
- (1)
- O-ring sample
- (2)
- Hardness sample
- (3)
- Dumbbell-type sample
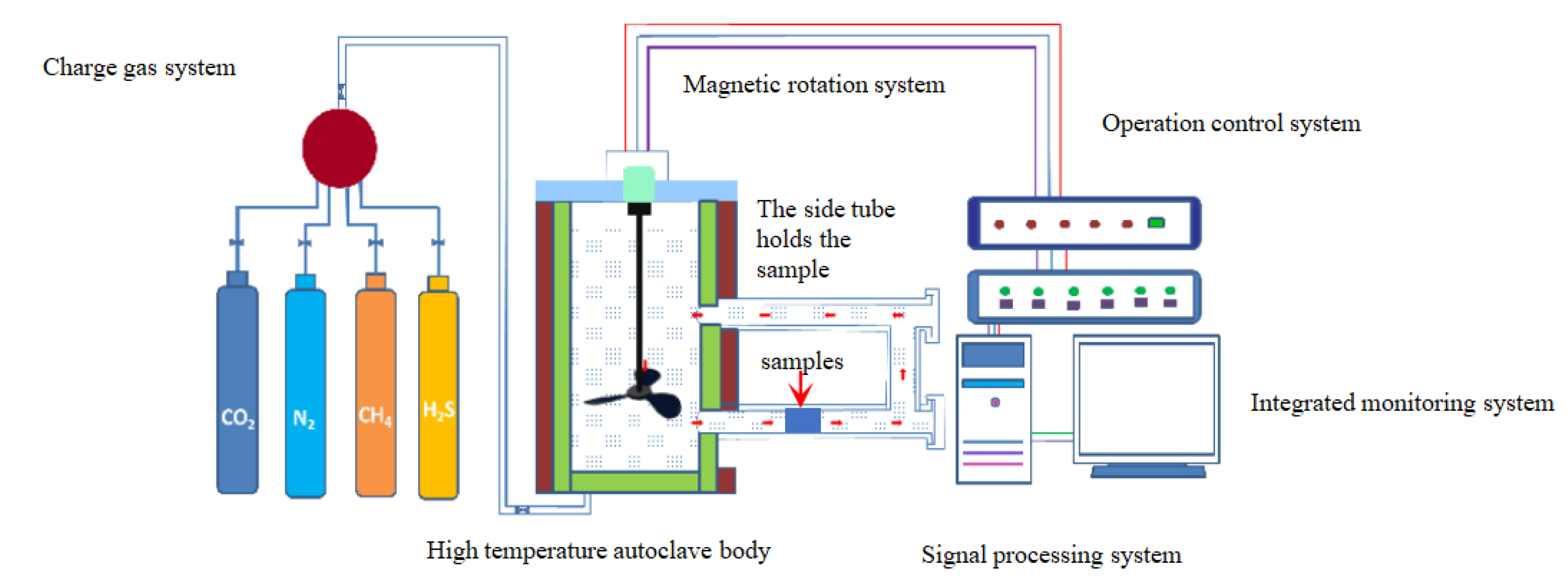
2.4. Instruments for Corrosion Experiments
- (1)
- Calculation formula for permanent deformation of tensile break:
- (2)
- Tensile strength (MPa):
- (3)
- Elongation at break (%):
2.5. Corrosion Test Procedure
3. Results and Discussion
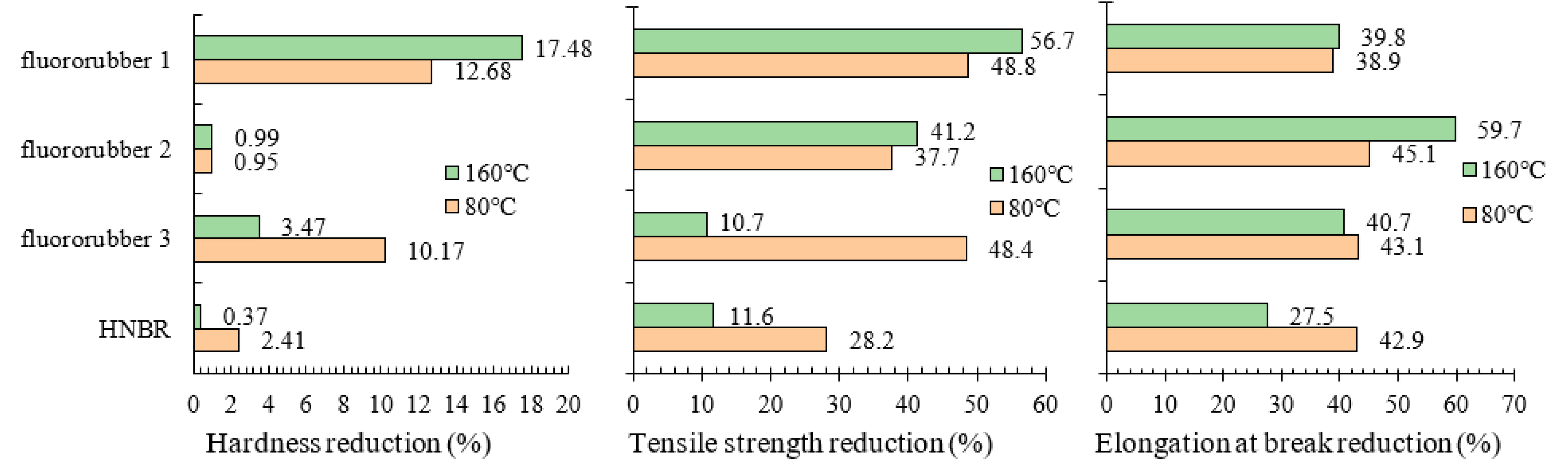
3.1. Hardness Properties of Different Rubber Materials
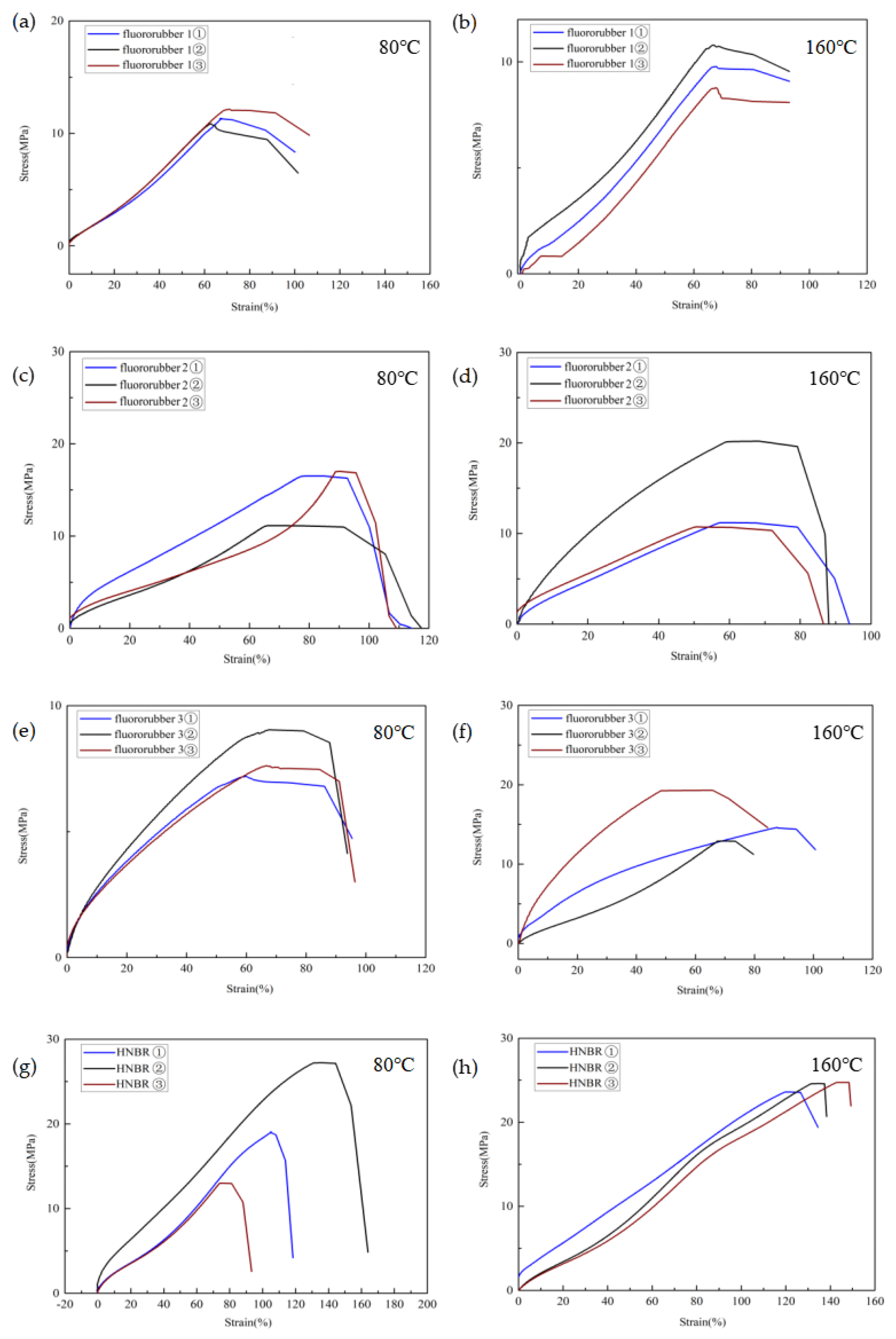
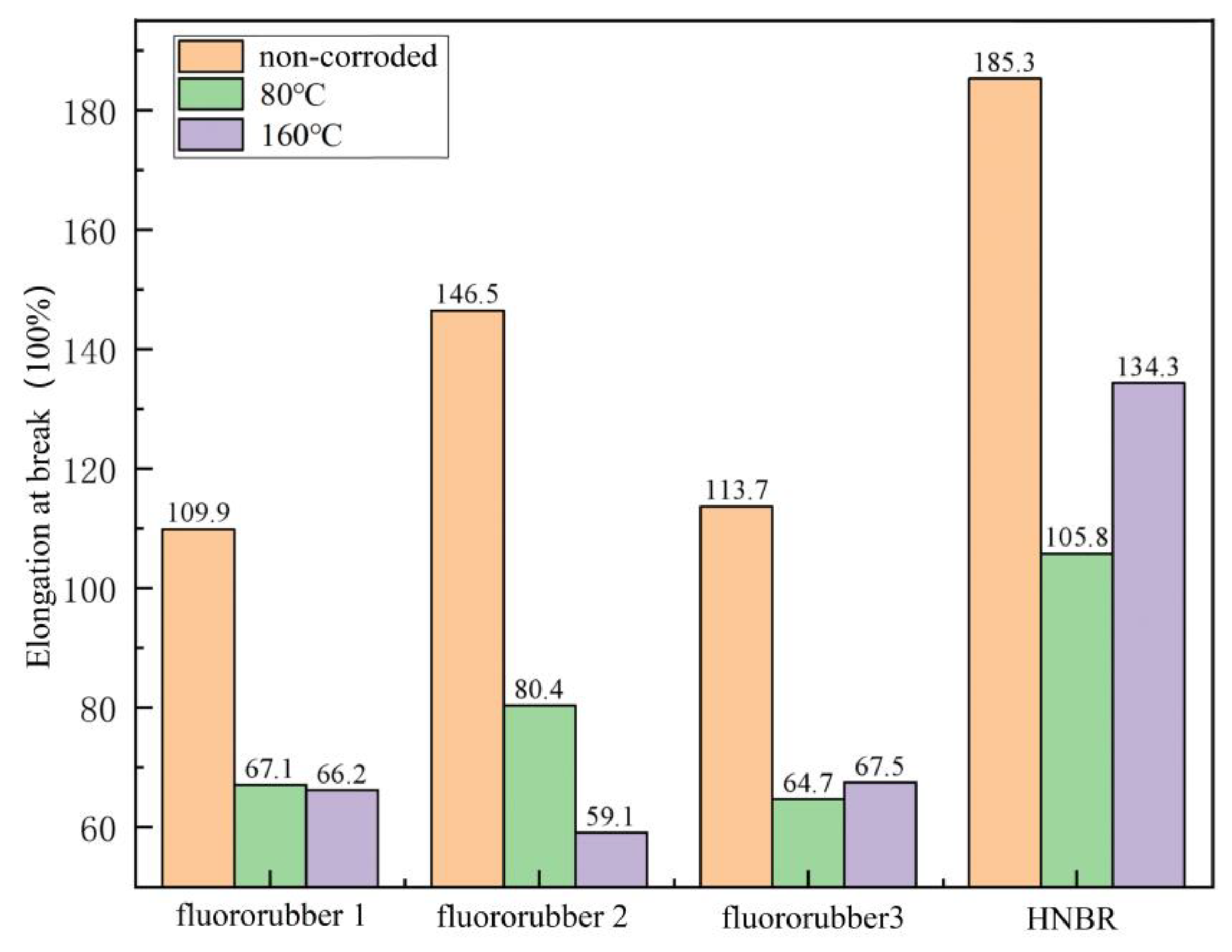
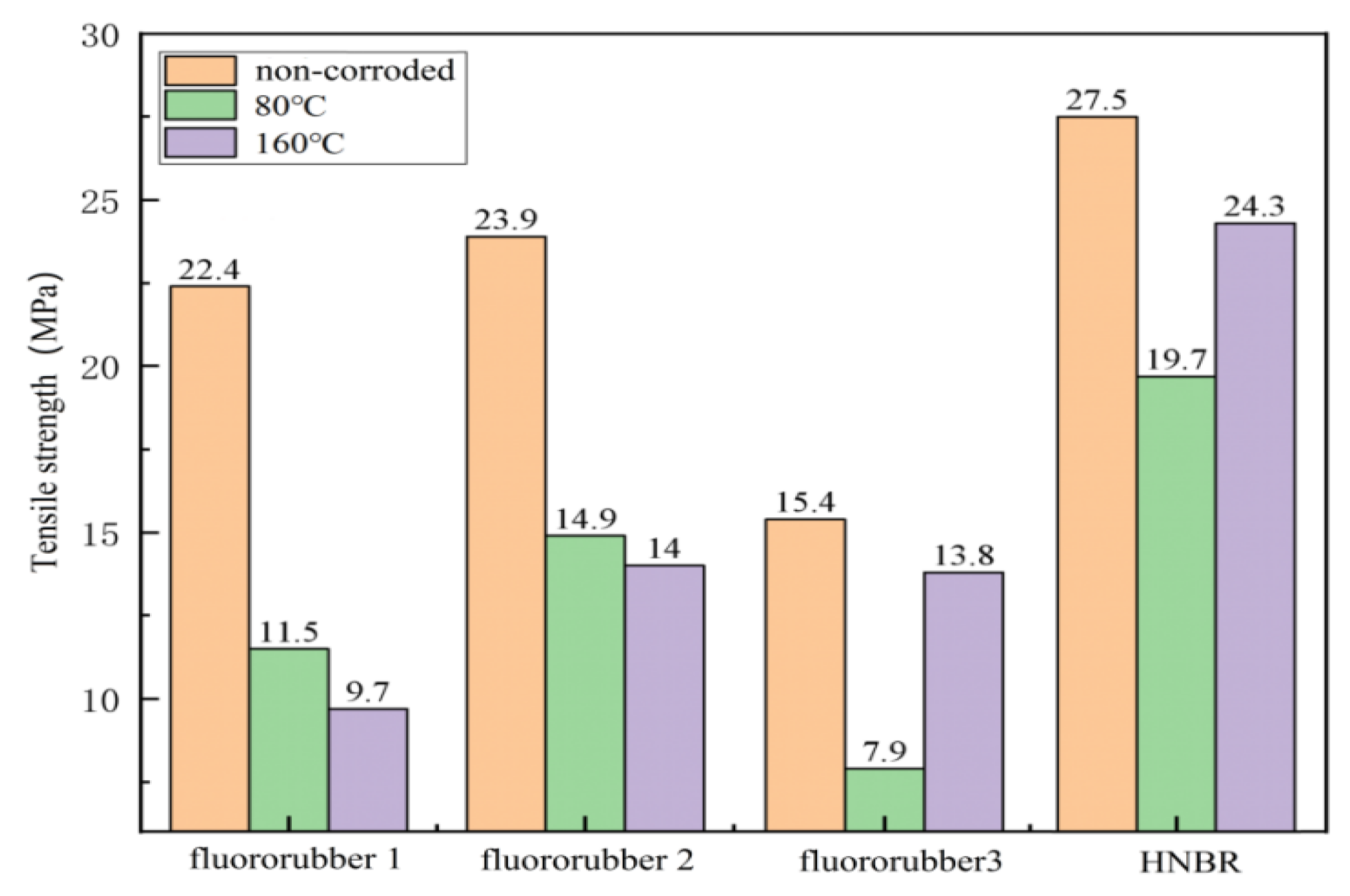
3.2. Tensile Properties of Different Rubber Materials
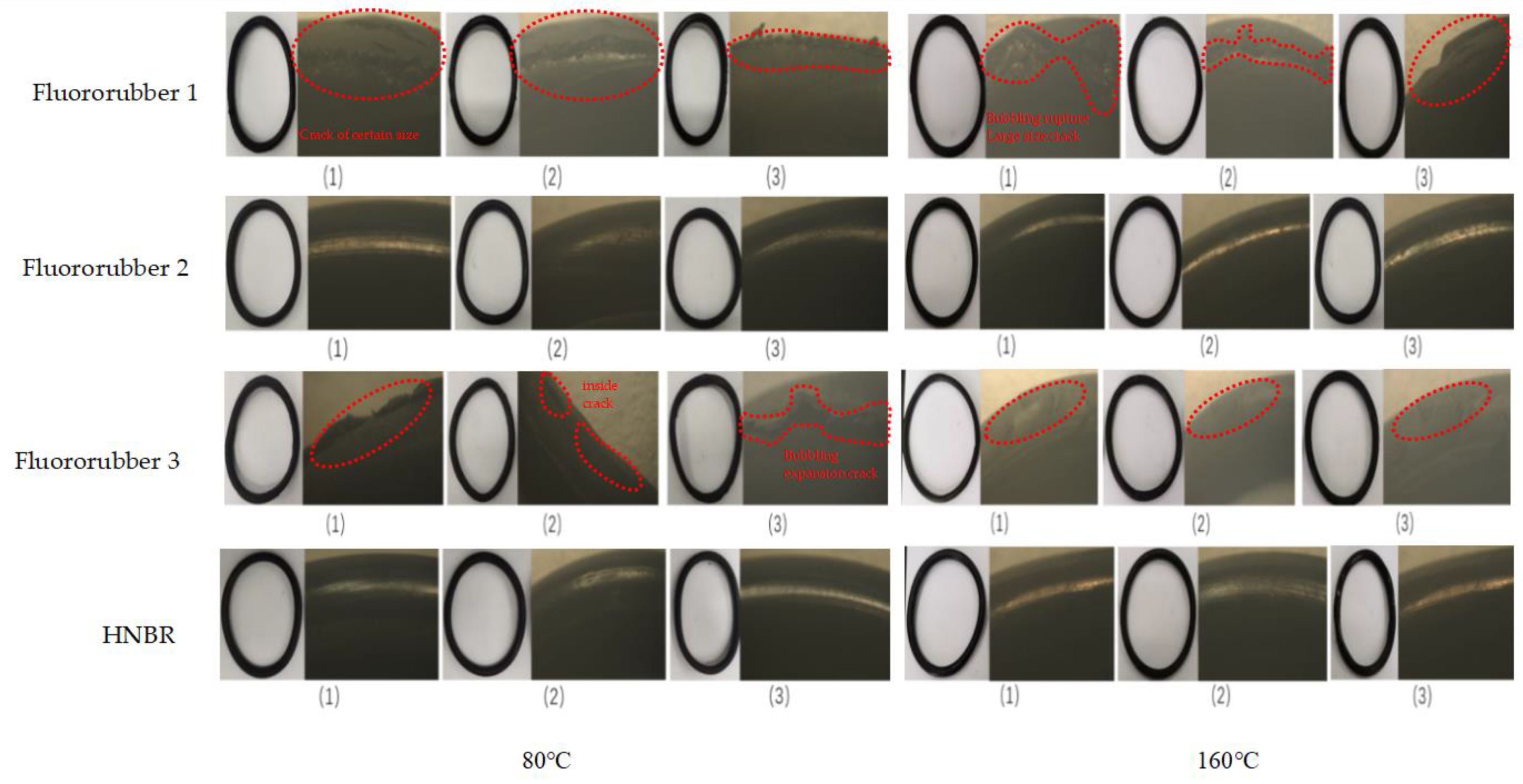
3.3. Sealing Performance of Different Rubber O-Rings
3.4. Experimental Results of Corrosion Resistance of Rubber Material
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhai, X.; Chen, X.; Zheng, F.; Han, D.; Zheng, J.; Ye, X.; Li, X.; Zhang, L. Designing a Coupling Agent with Aliphatic Polyether Chain and Exploring Its Effect on Silica/Natural Rubber Nanocomposites under the Action of Non-Rubber Contents. Polymers 2023, 15, 674. [Google Scholar] [CrossRef]
- Burelo, M.; Gutiérrez, S.; Treviño-Quintanilla, C.D.; Cruz-Morales, J.A.; Martínez, A.; López-Morales, S. Synthesis of Biobased Hydroxyl-Terminated Oligomers by Metathesis Degradation of Industrial Rubbers SBS and PB: Tailor-Made Unsaturated Diols and Polyols. Polymers 2022, 14, 4973. [Google Scholar] [CrossRef]
- Cruz-Morales, J.A.; Gutiérrez-Flores, C.; Zárate-Saldaña, D.; Burelo, M.; García-Ortega, H.; Gutiérrez, S. Synthetic Polyisoprene Rubber as a Mimic of Natural Rubber: Recent Advances on Synthesis, Nanocomposites, and Applications. Polymers 2023, 15, 4074. [Google Scholar] [CrossRef]
- Müller, M.; Šleger, V.; Čedík, J.; Pexa, M. Research on the Material Compatibility of Elastomer Sealing O-Rings. Polymers 2022, 14, 3323. [Google Scholar] [CrossRef]
- Yu, H.; Zheng, W.; Zhang, C.; Chen, S.; Tian, G.; Wang, T. Dual Network Co-Crosslinked HNBR Composites with Enhanced Tribological Properties under Water Lubrication. Lubricants 2023, 11, 534. [Google Scholar] [CrossRef]
- Cheng, K.; Yuan, M.; Zhang, Y.; Sun, N.; Peng, B. Application and Properties of Polyglycolic Acid as a Degradation Agent in MPU/HNBR Degradable Elastomer Composites for Dissolvable Frac Plugs. Polymers 2024, 16, 181. [Google Scholar] [CrossRef]
- Dong, B.; Liu, W.; Cheng, L.; Gong, J.; Wang, Y.; Gao, Y.; Dong, S.; Zhao, Y.; Fan, Y.; Zhang, T.; et al. Investigation on mechanical properties and corrosion behavior of rubber for packer in CO2-H2S gas well. Eng. Fail. Anal. 2021, 124, 105364. [Google Scholar] [CrossRef]
- Hasan, A.R.; Izgec, B.; Kabir, C.S. Sustaining Production by Managing Annular-Pressure Buildup. SPE Prod. Oper. 2010, 25, 195–203. [Google Scholar] [CrossRef]
- Zeng, D.; Yang, X.; Zhu, D.; Zhang, Z.; Cao, D.; Chong, X.; Shi, T. Corrosion Property Testing of AFLAS Rubber under The Simulation modes of High Acid Gas Wells. Energy Procedia 2012, 16, 822–827. [Google Scholar] [CrossRef]
- Liu, J.; Wei, W.; Han, B.; Wang, H. Corrosion resistance of tetrapropyl fluororubber and hydrogenated nitrile butadiene rubber to CO2. Corros. Prot. 2017, 38, 702–704. [Google Scholar]
- Wang, J.; Deng, X.; Zeng, D.; Yu, Z.; Li, T.; Qi, Y. Research on corrosion damage of tetrapropyl fluoro-rubber O-ring under simulated wellbore conditions. Oil Gas Chem. Ind. 2018, 47, 77–82. [Google Scholar]
- Zhang, R.; Li, P.; Feng, L.; Cong, C.; Liao, H.; Ma, L. Corrosion resistance of tetrapropyl fluororubbers in acidic environment containing H2S and CO2. Corros. Prot. 2018, 39, 582–586. [Google Scholar]
- Zhu, D.; Lin, Y.; Ma, H.; Zhang, H.; Li, Y.; Zhang, L.; Deng, K. Experimental studies on CO2 corrosion of rubber materials for packer under compressive stress in gas wells. Eng. Fail. Anal. 2017, 80, 11–23. [Google Scholar] [CrossRef]
- Jin, L.; Li, S.; Cheng, Y.; Liu, J. A time-dependent Yeoh model to predict the corrosion effect of supercritical CO2 on the HNBR sealing rubber. J. Mech. Sci. Technol. 2022, 36, 2461–2470. [Google Scholar] [CrossRef]
- Ma, Y.Y.; Zhang, M.; Gong, N.; Zhang, B.; Zhang, Z.; Li, Y.; Zhong, X.; Huo, H. Adaptability of rubber sealing materials for packer in acidic environment containing H2S and CO2. Lubr. Seal. 2019, 48, 172–177. [Google Scholar]
- Li, S.; Ge, J.; Yin, Q.; Shi, S.; Xu, J.; Zhao, Q. Influence of gas well corrosion Environment on rubber Material of Packer casing. Surf. Technol. 2018, 47, 51–59. [Google Scholar]
- Smejda-Krzewicka, A.; Mrozowski, K.; Strzelec, K. Interelastomer Reactions Occurring during the Cross-Linking of Hydrogenated Acrylonitrile-Butadiene (HNBR) and Chloroprene (CR) Rubbers Blends in the Presence of Silver(I) Oxide (Ag2O) and Mechanical Properties of Cured Products. Materials 2023, 16, 4573. [Google Scholar] [CrossRef]
- Shaw, B.; Ramier, J.; Busfield, J.J. The Effect of Thermal Ageing on the Fatigue Resistance of Hydrogenated Acrylonitrile Butadiene Rubber (HNBR) Compounds. Adv. Polym. Sci. 2023, 289, 143–165. [Google Scholar]
- Cui, Z.; Li, X.; Feng, W.; Wei, L.; Liu, Y.; Du, A. Effect of crosslinking agent dosage on the morphology and properties of thermoplastic vulcanizates based on hydrogenated acrylonitrile butadiene rubber and thermoplastic polyester elastomer. Polymer 2023, 287, 126420. [Google Scholar] [CrossRef]
- Cui, Z.; Wang, J.; Wei, L.; Liu, Y.; Du, A. Effect of dynamic vulcanization time on the performance of thermoplastic vulcanizates based on hydrogenated acrylonitrile butadiene rubber/thermoplastic polyester elastomer. J. Appl. Polym. Sci. 2023, 140, 54703. [Google Scholar] [CrossRef]
- Xun, W.; Wang, B.; Yang, L. Application of rate-transient analysis in Yuanba Gas Field. Reserv. Eval. Dev. 2021, 11, 652–658. [Google Scholar]
- Deng, L.; Huang, C.; Pan, D. Completion technology for high temperature sour gas reservoir in Anyue Gas Field. Well Test. 2019, 28, 52–59. [Google Scholar]
- Wang, J. Application of coiled tubing driven jet acidification in Puguang high sulfur gasfield. Complex Hydrocarb. Reserv. 2021, 14, 94–97. [Google Scholar]
- Liu, X.; Li, C.; Yu, F. Study of corrosion behavior of carbon steel in H2S-saturated NaCl solution. Corros. Prot. Petrochem. Ind. 2009, 26, 32–35. [Google Scholar]
- Wang, X.B.; Yu, H.Y.; Guan, W.; Sun, D.B. CO2 corrosion behavior of X52 steel in conditions of service of Puguang Gas Field. J. Mater. Eng. 2014, 4, 72–78. [Google Scholar]
- Huang, Z.; Zhou, Z. Research and application of development technology in high sulfur gas field of Zhongba area. Drill. Prod. Technol. 2012, 35, 67–69. [Google Scholar]
- GB/T 528-2009; China Rubber Group Shenyang Rubber Research and Design Institute. Rubber, Vulcanized or Thermoplastic—Determination of Tensile Stress-Strain Properties. General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of China: Beijing, China.
- GB/T 531.1-2008; Guangdong Academy of Metrology. Rubber, Vulcanized or Thermoplastic—Determination of Indentation Hardness—Part 1:Duromerer Method (Shore Hardness). General Administration of Quality Supervision, Inspection and Quarantine of the People's Republic of China, Standardization Administration of China: Beijing, China.
- Gao, K.; Shang, S.; Zhang, Z.; Gao, Q.; Ma, J.; Liu, W. Effect of Temperature on Corrosion Behavior and Mechanism of S135 and G105 Steels in CO2/H2S Coexisting System. Metals 2022, 12(11), 1848. [Google Scholar] [CrossRef]
- Li, X. Effect of Temperature on Mechanical Properties of Rubber and Its Mechanism. Master’s thesis, Qingdao University of Science and Technology, Qingdao, China, 2020. [Google Scholar]
- Zhao, S.; Zhou, H.; Cheng, W.; Cai, H.; Man, Z.; Wang, J. Effect of CO2-H2S and high temperature environment on rubber material properties of completion tools. Offshore Oil 2019, 41, 31–36. [Google Scholar]
- Zou, D. Research on Sealing Performance of Rubber Casing Packer in CARBON dioxide Flooding Gas Well. Master’s thesis, Southwest Petroleum University, Chengdu, China, 2014. [Google Scholar]
- Xu, Z.; Kang, H.; Li, D.; Li, Z.; Fang, H. Relationship between crosslinking density and crystallinity of natural rubber/Gutta-percha and its Influence on properties. Rubber Ind. 2019, 70, 163–169. [Google Scholar]
- Zou, H. Research on Mechanical Properties and Oil Swelling Resistance of Hydrogenated Nitrile Butadiene Rubber. Master’s thesis, Qingdao University of Science and Technology, Qingdao, China, 2022. [Google Scholar]





| Name | Basic Formula (Mass Fraction) | Supplier |
|---|---|---|
| Fluororubber 1 | Tetrapropyl fluororubber is 100, carbon black is 40, Bisphenol AF is 5, BPP is 2. | Shanghai Truthful sealing Technology Co., Ltd. (Shanghai, China) |
| Fluororubber 2 | Fluorine rubber is 100, carbon black is 40, Bisphenol AF is 2, BPP is 2. | Shanghai Truthful sealing Technology Co., Ltd. (Shanghai, China) |
| Fluororubber 3 | Perflurane rubber is 7, tetrapropyl fluorine rubber is 48, fluorine silicone rubber is 48, BIBP is 2, Cycloxy promoter is 2. | Hebei Cheneng Technology Co., Ltd. (Hebei, China) |
| HNBR | HNBR is 100, carbon black is 60, Stearic acid is 2, Zinc oxide is 2. | Shanghai Truthful sealing Technology Co., Ltd. (Shanghai, China) |
| Serial Number | Ion Species | Ion Content, mg/L | Serial Number | Ion Species | Ion Content, mg/L |
|---|---|---|---|---|---|
| 1 | K+ + Na+ | 14,940 | 4 | Ba2+ | 865 |
| 2 | Ca2+ | 9227 | 5 | Sr2+ | 131 |
| 3 | Mg2+ | 2023 | 6 | Cl− | 46,728 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gong, L.; Chen, Y.; Cai, M.; Li, J.; Lu, Q.; Hou, D. Characterization and Analysis of Corrosion Resistance of Rubber Materials for Downhole Tools in a High-Stress Environment with Coupled H2S-CO2. Materials 2024, 17, 863. https://doi.org/10.3390/ma17040863
Gong L, Chen Y, Cai M, Li J, Lu Q, Hou D. Characterization and Analysis of Corrosion Resistance of Rubber Materials for Downhole Tools in a High-Stress Environment with Coupled H2S-CO2. Materials. 2024; 17(4):863. https://doi.org/10.3390/ma17040863
Chicago/Turabian StyleGong, Leilei, Yulin Chen, Meng Cai, Junliang Li, Qiuyu Lu, and Duo Hou. 2024. "Characterization and Analysis of Corrosion Resistance of Rubber Materials for Downhole Tools in a High-Stress Environment with Coupled H2S-CO2" Materials 17, no. 4: 863. https://doi.org/10.3390/ma17040863
APA StyleGong, L., Chen, Y., Cai, M., Li, J., Lu, Q., & Hou, D. (2024). Characterization and Analysis of Corrosion Resistance of Rubber Materials for Downhole Tools in a High-Stress Environment with Coupled H2S-CO2. Materials, 17(4), 863. https://doi.org/10.3390/ma17040863






