Towards Production of Cost-Effective Modification of SmCo5-Type Alloys Suitable for Permanent Magnets
Abstract
1. Introduction
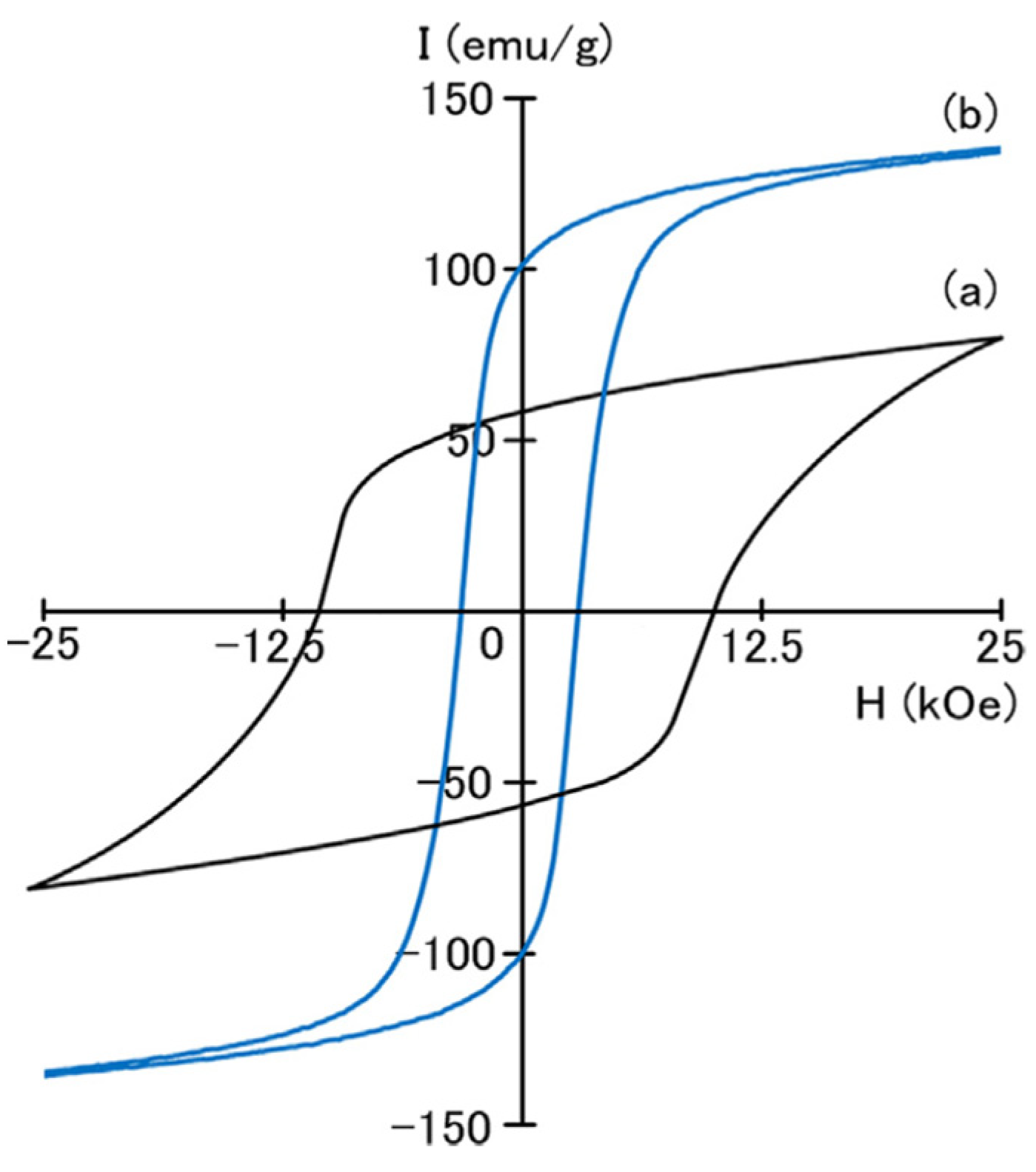
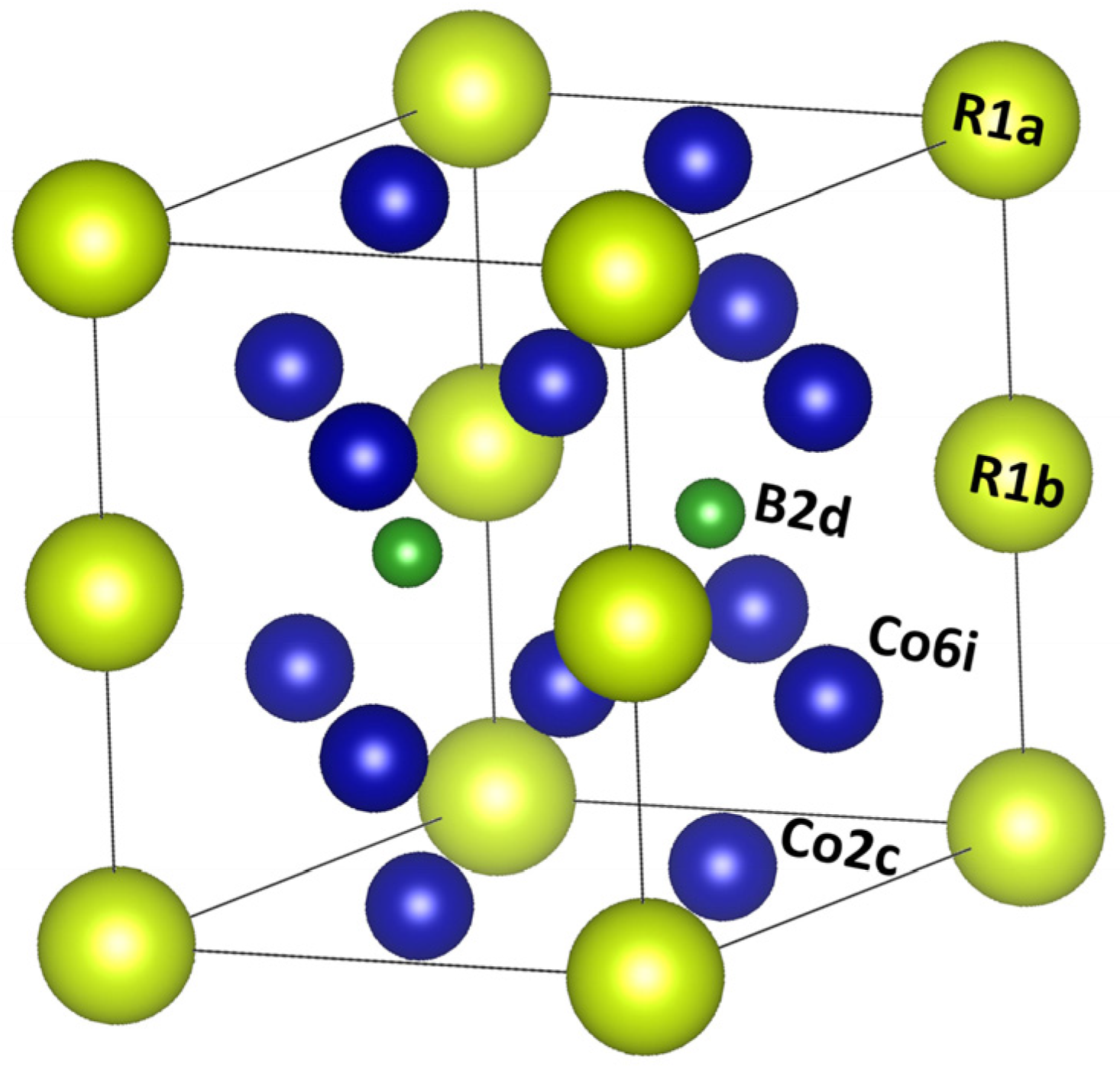
2. A Short View of Structure and Magnetic Properties of SmCo5 Magnets
3. SmCo5 Transition Metal Substitution (Fe, Cu, Ni, Zr, Ti, and Nb)
3.1. Substitution of Fe for Co
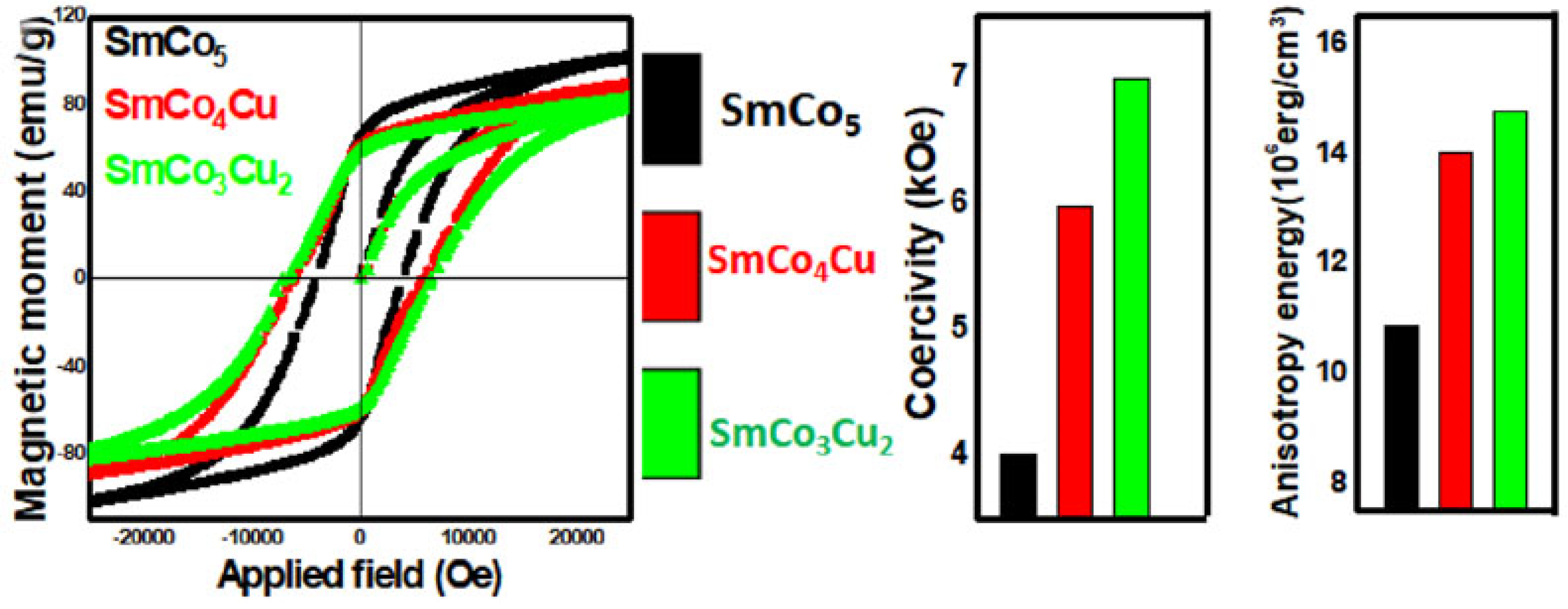
3.2. Substitution of Cu and Other Transition or Non-Transition Elements for Co on SmCo5 Alloys
3.3. Simultaneous Substitution of Two or More Transition Metals Like Fe and Cu for Co in SmCo5 Alloys
4. Substitution of p-Elements for Co
5. A New Promising Magnetic Material with CaCu5 Structure
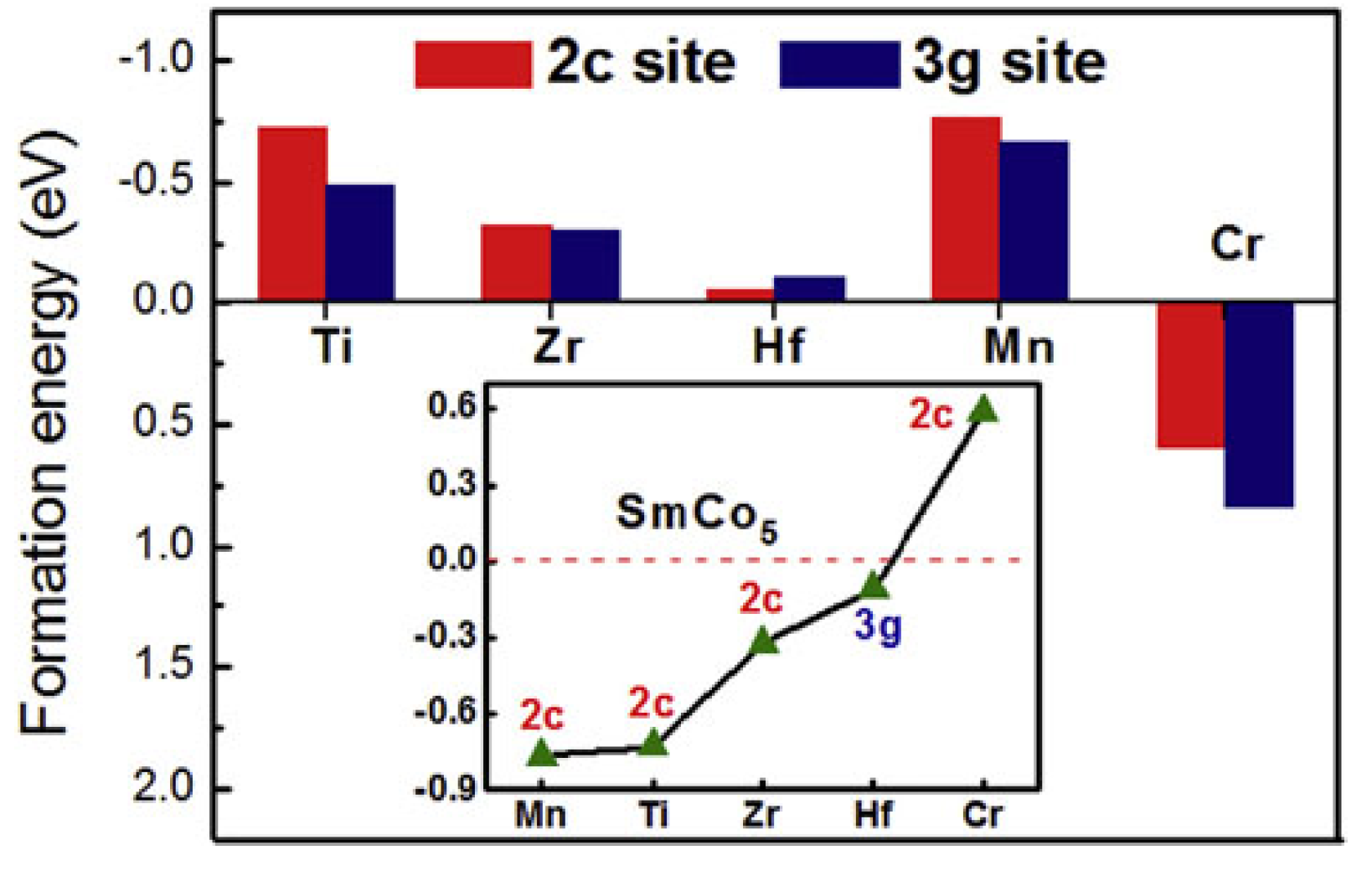
6. Recent Experimental and Theoretical Research Approaches on SmCo5 Alloys: The Case for Mm (Misch-Metal) Substitution for Sm on SmCo5 Alloys
7. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gauß, R.; Burkhardt, C.; Carencotte, F.; Gasparon, M.; Gutfleisch, O.; Higgins, I.; Karajić, M.; Klossek, A.; Mäkinen, M.; Schäfer, B.; et al. Rare Earth Magnets and Motors: A European Call for Action. In A Report by the Rare Earth Magnets and Motors Cluster of the European Raw Materials Alliance; ERMA: Berlin, Germany, 2021. [Google Scholar]
- Gutfleisch, O.; Willard, M.A.; Brück, E.; Chen, C.H.; Sankar, S.G.; Liu, J.P. Magnetic materials and devices for the 21st century: Stronger, lighter, and more energy efficient. Adv. Mater. 2011, 23, 821–842. [Google Scholar] [CrossRef]
- Du, X.; Graedel, T.E. Global Rare Earth in-Use Stocks in NdFeB Permanent Magnets. J. Ind. Ecol. 2011, 15, 836–843. [Google Scholar] [CrossRef]
- Skomski, R. Permanent magnets: History, current research, and outlook. In Springer Series in Materials Science; Springer: Cham, Switzerland, 2016; pp. 359–395. [Google Scholar]
- Coey, J.M.D. Magnetism and Magnetic Materials; Cambridge University Press: Cambridge, UK, 2009. [Google Scholar]
- Strnat, K.; Hoffer, G.; Olson, J.; Ostertag, W.; Becker, J.J. A Family of New Cobalt-Base Permanent Magnet Materials. J. Appl. Phys. 1967, 38, 1001–1002. [Google Scholar] [CrossRef]
- Strnat, K.J. Rare-earth magnets in present production and development. J. Magn. Magn. Mater. 1978, 7, 351. [Google Scholar] [CrossRef]
- Strnat, K.J.; Strnat, R.M.W. Rare earth-cobalt permanent magnets. J. Magn. Magn. Mater. 1991, 100, 38. [Google Scholar] [CrossRef]
- Kumar, K. RETM5 and RE2TM17 permanent magnets development. J. Appl. Phys. 1988, 63, R13–R57. [Google Scholar] [CrossRef]
- Walmer, M.H.; Liu, J.F.; Dent, P.C. Current status of permanent magnet industry in the United States. In Proceedings of the 20th International Workshop on “Rare Earth Permanent Magnets and Their Applications”, Knossos, Crete, 8–10 September 2008. [Google Scholar]
- Duerrschnabel, A.M.; Yi, M.; Uestuener, K.; Liesegang, M.; Katter, M.; Kleebe, H.-J.; Xu, B.; Gutfleisch, O.; Molina-Luna, L. Atomic structure and domain wall pinning in samarium-cobalt based permanent magnets. Nat. Commun. 2017, 8, 54. [Google Scholar] [CrossRef] [PubMed]
- Skokov, K.P.P.; Gutfleisch, O. Heavy rare earth free, free rare earth and rare earth free magnets—Vision and reality. Scr. Mater. 2018, 154, 289–294. [Google Scholar] [CrossRef]
- Coey, J.M.D. Perspective and Prospects for Rare Earth Permanent Magnets. Engineering 2020, 6, 119–131. [Google Scholar] [CrossRef]
- Yang, Y.; Walton, A.; Sheridan, R.; Güth, K.; Gauß, R.; Gutfleisch, O.; Buchert, M.; Steenari, B.-M.; Van Gerven, T.; Jones, P.T.; et al. REE Recovery from End-of-Life NdFeB Permanent Magnet Scrap: A Critical Review. J. Sustain. Metall. 2017, 3, 122–149. [Google Scholar] [CrossRef]
- Skomski, R.; Coey, J.M.D. Permanent Magnetism; Institute of Physics: Bristol, UK, 1999. [Google Scholar]
- Skomski, R. Nanomagnetics. J. Phys. Condens. Matter 2003, 15, R841. [Google Scholar] [CrossRef]
- Walmer, M.S.; Chen, C.H.; Walmer, M.H. A new class of Sm-TM magnets for operating temperatures up to 550/spldeg/C. IEEE Trans. Magn. 2000, 36, 3376–3381. [Google Scholar] [CrossRef]
- Skomski, R.; Manchanda, P.; Kumar, P.; Balamurugan, B.; Kashyap, A.; Sellmyer, D.J. Predicting the future of permanent-magnet materials (invited). IEEE Trans. Magn. 2013, 49, 3215. [Google Scholar] [CrossRef]
- London Metal Exchange Website. Available online: https://www.lme.com/Metals/EV/LME-Cobalt#Summary (accessed on 18 January 2024).
- World Bank Website. Commodity Markets. Available online: https://www.worldbank.org/en/research/commodity-markets (accessed on 18 January 2024).
- Muff, R.; Tamiraa, A. Rare Earths of Mongolia: Evaluation of Market Opportunities for the Principal Deposits of R. Mongolia; Technical Report; Mineral Resources Authority of Mongolia; Ulaanbaatar/Bundesanstalt für Geowissenschaften und Rohstoffe: Hannover, Germany, 2013; p. 63. [Google Scholar]
- Amato, A.; Becci, A.; Bollero, A.; del Mar Cerrillo-Gonzalez, M.; Cuesta-Lopez, S.; Ener, S.; Dirba, I.; Gutfleisch, O.; Innocenzi, V.; Montes, M.; et al. Life Cycle Assessment of Rare Earth Elements-Free Permanent Magnet Alternatives: Sintered Ferrite and Mn-Al-C. ACS Sustain. Chem. Eng. 2023, 11, 36. [Google Scholar] [CrossRef]
- IEA. Global EV Outlook 2023; License: CC BY 4.0; IEA: Paris, France, 2023; Available online: https://www.iea.org/reports/global-ev-outlook-2023 (accessed on 15 January 2024).
- Pavel, C.C.; Thiel, C.; Degreif, S.; Blagoeva, D.; Buchert, M.; Schüler, D.; Tzimas, E. Role of substitution in mitigating the supply pressure of rare earths in electric road transport applications. Sustain. Mater. Technol. 2017, 12, 62–72. [Google Scholar] [CrossRef]
- Pavel, C.C.; Lacal-Arántegui, R.; Marmier, A.; Schüler, D.; Tzimas, E.; Buchert, M.; Jenseit, W.; Blagoeva, D. Substitution strategies for reducing the use of rare earths in wind turbines. Resour. Policy 2017, 52, 349–357. [Google Scholar] [CrossRef]
- Fernandez, V. Rare-earth elements market: A historical and financial perspective. Resour. Policy 2017, 53, 26–45. [Google Scholar] [CrossRef]
- Li, Z.Z.; Meng, Q.; Zhang, L.; Lobont, O.R.; Shen, Y. How do rare earth prices respond to economic and geopolitical factors? Resour. Policy 2023, 85, 103853. [Google Scholar] [CrossRef]
- Geng, Y.; Sarkis, J.; Bleischwitz, R. How to build a circular economy for rare-earth elements. Nature 2023, 619, 248. [Google Scholar] [CrossRef] [PubMed]
- Ilankoon, I.M.S.K.; Dushyantha, N.P.; Mancheri, N.; Edirisinghe, P.; Neethling, S.; Ratnayake, N.; Rohitha, L.; Dissanayake, D.; Premasiri, H.; Abeysinghe, A.; et al. Constraints to rare earth elements supply diversification: Evidence from an industry survey. J. Clean. Prod. 2022, 331, 129932. [Google Scholar] [CrossRef]
- Wang, Q.C.; Chen, W.Q.; Wang, P.; Dai, T. Illustrating the supply chain of dysprosium in China through material flow analysis. Resour. Conserv. Recycl. 2022, 184, 106417. [Google Scholar] [CrossRef]
- Haque, N.; Hughes, A.; Lim, S.; Vernon, C. Rare Earth Elements: Overview of Mining, Mineralogy, Uses, Sustainability and Environmental Impact. Resources 2014, 3, 614–635. [Google Scholar] [CrossRef]
- Rosner, N.; Koudela, D.; Schwarz, U.; Handstein, A.; Hanfland, M.; Opahle, I.; Koepernik, K.; Kuz’Min, M.D.; Müller, K.-H.; Mydosh, J.A.; et al. Magneto-elastic lattice collapse in YCo5. Nature Phys. 2006, 2, 469–472. [Google Scholar] [CrossRef]
- Hua, G.; Song, X.; Tang, F.; Liu, D.; Wang, D.; Wang, H.; Liu, X. Modeling and experimental studies of Hf-doped nanocrystalline SmCo7 alloys. CrystEngComm 2016, 18, 8080–8088. [Google Scholar] [CrossRef]
- Strnat, K. The recent development of permanent magnet materials containing rare earth metals. IEEE Trans. Magn. 1970, 6, 182–190. [Google Scholar] [CrossRef]
- Coey, J.M.D. Hard magnetic materials: A perspective. IEEE Trans. Magn. 2011, 47, 4671. [Google Scholar] [CrossRef]
- Coey, J.M.D. Permanent magnets: Plugging the gap. Scr. Mater. 2012, 67, 524–529. [Google Scholar] [CrossRef]
- Miyazaki, T.; Takahashi, M.; Xingbo, Y.; Saito, H.; Takahashi, M. Formation and magnetic properties of metastable (TM)5Sm and (TM)7Sm2 (TM = Fe,Co) compounds. J. Magn. Magn. Mater. 1988, 75, 123–129. [Google Scholar] [CrossRef]
- Miyazaki, T.; Takahashi, M.; Yang, X.; Saito, H.; Takahashi, M. Formation of metastable compounds and magnetic properties in rapidly quenched Sm (Fe1−xCox)5 and Sm2(Fe1−xCox)7 alloy systems. J. Appl. Phys. 1988, 64, 5974. [Google Scholar] [CrossRef]
- Saito, T.; Nishio-Hamane, D. Magnetic properties of SmCo5−xFex (x = 0–4) melt-spun ribbon. J. Alloys Compd. 2014, 585, 423–427. [Google Scholar] [CrossRef]
- Das, B.; Choudhary, R.; Skomski, R.; Balasubramanian, B.; Pathak, A.K.; Paudyal, D.; Sellmyer, D.J. Anisotropy and orbital moment in Sm-Co permanent magnets. Phys. Rev. B 2019, 100, 024419. [Google Scholar] [CrossRef]
- Liu, X.B.; Altounian, Z. Magnetic moments and exchange interaction in Sm(Co, Fe)5 from first-principles. Comput. Mater. Sci. 2011, 50, 841–846. [Google Scholar] [CrossRef]
- Chouhan, R.K.; Pathak, A.K.; Paudyal, D. Review Articles: Understanding the origin of magneto-crystalline anisotropy in pure and Fe/Si substituted SmCo5. J. Magn. Magn. Mater. 2021, 522, 167549. [Google Scholar] [CrossRef]
- Nesbitt, E.A.; Willens, R.H.; Sherwood, R.C.; Buehler, E.; Wernick, J.H. New permanent magnet materials. Appl. Phys. Lett. 1968, 12, 361–362. [Google Scholar] [CrossRef]
- Hofer, F. Physical metallurgy and magnetic measurements of SmCo5-SmCu5 alloys. IEEE Trans. Magn. 1970, 6, 221–224. [Google Scholar] [CrossRef]
- Katayama, T.; Shibata, T. Annealing Effect on Magnetic Properties of Permanent Magnet Materials Based on Sm(Co, Cu)5. Jpn. J. Appl. Phys. 1973, 12, 319. [Google Scholar] [CrossRef]
- Kamino, K.; Kimura, Y.; Suzuki, T.; Itayama, Y. Variation of the Magnetic Properties of Sm(Co, Cu)5 Alloys with Temperature. Trans. Jpn. Inst. Met. 1973, 14, 135–139. [Google Scholar] [CrossRef][Green Version]
- Barbara, B.; Uehara, M. Anisotropy and coercivity in SmCo5-based compounds. IEEE Trans. Magn. 1976, 12, 997–999. [Google Scholar] [CrossRef]
- Mitchell, R.K.; McCurrie, R.A. Magnetic properties, microstructure, and domain structure of SmCo3.25Cu1.75. J. Appl. Phys. 1986, 59, 4113–4122. [Google Scholar] [CrossRef]
- Oesterreicher, H.; Parker, F.T.; Misroch, M. Giant intrinsic magnetic hardness in SmCo5−xCux. J. Appl. Phys. 1979, 50, 4273–4278. [Google Scholar] [CrossRef]
- Lectard, E.; Allibert, C.H.; Valignat, N. Coercivity in hard magnets based on Sm2Co17. In Proceedings of the 8th International Symposium on Magnetic Anisotropy in Rare Earth—Transition Metal Alloys, Birmingham, UK, 15 September 1994; Manwaring, C.A.F., Ed.; University of Birmingham: Birmingham, UK, 1994; p. 308. [Google Scholar]
- Téllez-Blanco, J.C.; Grössinger, R.; Turtelli, R.S. Structure and magnetic properties of SmCo5−xCux alloys. J. Alloys Compd. 1998, 281, 1–5. [Google Scholar] [CrossRef]
- Téllez-Blanco, J.C.; Turtelli, R.S.; Grössinger, R.; Estévez-Rams, E.; Fidler, J. Giant magnetic viscosity in SmCo5−xCux alloys. J. Appl. Phys. 1999, 86, 5157–5163. [Google Scholar] [CrossRef]
- Yamashita, O. Coercivity mechanism of Sm(Co, Cu)5. J. Phys. Chem. Solids 2004, 65, 907–912. [Google Scholar] [CrossRef]
- Nishida, I.; Uehara, M. Study of the crystal structure and stability of pseudo-binary compound SmCo5−xCux. J. Less Common Met. 1974, 34, 285–291. [Google Scholar] [CrossRef]
- Gabay, A.M.; Larson, P.; Mazi, I.I.; Hadjipanayis, G.C. Magnetic states and structural transformations in Sm(Co, Cu)5 and Sm(Co, Fe, Cu)5 permanent magnets. J. Phys. D Appl. Phys. 2005, 38, 1337–1341. [Google Scholar] [CrossRef]
- Haider, S.K.; Ngo, H.M.; Kim, D.; Kang, Y.S. Enhancement of anisotropy energy of SmCo5 by ceasing the coupling at 2c sites in the crystal lattice with Cu substitution. Sci. Rep. 2021, 11, 10063. [Google Scholar] [CrossRef] [PubMed]
- Oesterreicher, H.; McNeely, D. Low-temperature magnetic studies on various substituted rare earth (R)-transition metal (T) compounds RT5. J. Less Common Met. 1976, 45, 111–116. [Google Scholar] [CrossRef]
- Mao, F.; Lu, H.; Liu, D.; Guo, K.; Tang, F.; Song, X. Structural stability and magnetic properties of SmCo5 compounds doped with transition metal elements. J. Alloys Compd. 2019, 810, 151888. [Google Scholar] [CrossRef]
- Tawara, Y.; Senno, H. Bulk Hardened Magnet of Nonstoichiometric Rare-Earth Cobalt. Jpn. J. Appl. Phys. 1973, 12, 761. [Google Scholar] [CrossRef]
- Ojima, T.; Tomizawa, S.; Yoneyama, T.; Hori, T. Magnetic properties of a new type of rare-earth cobalt magnets Sm2(Co, Cu, Fe, M)17. IEEE Trans. Magn. 1977, 13, 1317–1319. [Google Scholar] [CrossRef]
- Hadjipanayis, G.C.; Gabay, A.M. Overview of the High-Temperature 2:17 Magnets. In Proceedings of the 18th International Workshop on High Performance Magnets and Their Applications, HPMA’04, Annecy, France, 29 August–2 September 2004; Volume 2, p. 590. [Google Scholar]
- Suresh, K.; Gopalan, R.; Bhikshamaiah, G.; Singh, A.; Rao, D.S.; Muraleedharan, K.; Chandrasekaran, V. Phase formation, microstructure and magnetic properties investigation in Cu and Fe substituted SmCo5 melt-spun ribbons. J. Alloys Compd. 2008, 463, 73–77. [Google Scholar] [CrossRef]
- Zhou, J.; Skomski, R.; Chen, C.; Hadjipanayis, G.C.; Sellmyer, D.J. Sm–Co–Cu–Ti high-temperature permanent magnets. Appl. Phys. Lett. 2000, 77, 1514–1516. [Google Scholar] [CrossRef]
- Chacon, C.; Isnard, O. Magnetic Properties of the RCo4B Compounds (R = Y, Pr, Nd, Sm, Gd, Tb). J. Solid State Chem. 2000, 154, 242–245. [Google Scholar] [CrossRef]
- Zlotea, C.; Isnard, O. Neutron powder diffraction and magnetic phase diagram of RCo4Ga compounds (R = Y and Pr). J. Alloys Compd. 2002, 346, 29–37. [Google Scholar] [CrossRef]
- Zlotea, C.; Isnard, O. Structural and magnetic properties of RCo4Al compounds (R = Y, Pr). J. Magn. Magn. Mater. 2002, 832, 242–245. [Google Scholar] [CrossRef]
- Zlotea, C.; Isnard, O. Neutron diffraction and magnetic investigations of the TbCo4M compounds (M = Al and Ga). J. Phys. Condens. Matter 2002, 14, 10211. [Google Scholar] [CrossRef]
- Kuz’ma, Y.B.; Bilonzhko, N.S. New Boride Structural Types in the Homologous Series Based on the CaCu5 and CeCo3B2 Types. Sov. Phys. Crystallogr. 1974, 18, 447. [Google Scholar]
- Momma, K.; Izumi, F. VESTA 3 for three-dimensional visualization of crystal, volumetric and morphology data. J. Appl. Crystallogr. 2011, 44, 1272–1276. [Google Scholar] [CrossRef]
- Laslo, A.; Colin, C.V.; Isnard, O.; Guillot, M. Effect of the M/Co substitution on magnetocrystalline anisotropy and magnetization in SmCo5−xMx compounds (M = Ga, Al). J. Appl. Phys. 2010, 107, 09A732. [Google Scholar] [CrossRef]
- Liu, T.-Y.; Chen, S.-Y.; Wang, S.; Wang, L.-Z.; Sun, J.-B.; Jiang, Y.-F. Effect of annealing on the structure and magnetic properties of SmCo5-based ribbons with Al-Cu-Fe addition. Mater. Lett. 2021, 286, 129237. [Google Scholar] [CrossRef]
- Dong, Z.; Wang, S.; Chen, S.; Cao, Y.; Ge, Z.; Sun, J. Effect of Al82.8Cu17Fe0.2 alloy doping on structure and magnetic properties of SmCo5-based ribbons. J. Rare Earths 2022, 40, 93–101. [Google Scholar] [CrossRef]
- Antoniou, E.; Sempros, G.; Gjoka, M.; Sarafidis, C.; Polatoglou, H.M.; Kioseoglou, J. Structural and magnetic properties of SmCo5−XNix intermetallic compounds. J. Alloys Compd. 2021, 882, 160699. [Google Scholar] [CrossRef]
- Saito, T.; Nishio-Hamane, D. Synthesis of SmFe5 intermetallic compound. AIP Adv. 2020, 10, 015311. [Google Scholar] [CrossRef]
- Söderlind, P.; Landa, A.; Locht, I.L.M.; Åberg, D.; Kvashnin, Y.; Pereiro, M.; Däne, M.; Turchi, P.E.A.; Antropov, V.P.; Eriksson, O. Prediction of the new efficient permanent magnet SmCoNiFe3. Phys. Rev. B 2017, 96, 100404(R). [Google Scholar] [CrossRef]
- Landa, A.; Söderlind, P.; Parker, D.; Åberg, D.; Lordi, V.; Perron, A.; Turchi, P.; Chouhan, R.; Paudyal, D.; Lograsso, T. Thermodynamics of SmCo5 compound doped with Fe and Ni: An ab initio study. J. Alloys Compd. 2018, 765, 659–663. [Google Scholar] [CrossRef]
- Guo, Q.; Kleppa, O.J. Standard enthalpies of formation for some samarium alloys, Sm + Me (Me = Ni, Rh, Pd, Pt), determined by high-temperature direct synthesis calorimetry. Met. Mater. Trans. B 1998, 29, 815–820. [Google Scholar] [CrossRef]
- Meyer-Liautaud, F.; Allibert, C.H.; Castanet, R. Enthalpies of formation of SmCo alloys in the composition range 10–22 at. % Sm. J. Less Common Met. 1987, 127, 243–250. [Google Scholar] [CrossRef]
- Gavrikov, I.S.; Karpenkov, D.Y.; Zheleznyi, M.V.; Kamynin, A.V.; Khotulev, E.S.; Bazlov, A.I. Effect of Ni doping on stabilization of Sm(Co1−xFex)5 compound: Thermodynamic calculation and experiment. J. Phys. Condens. Matter 2020, 32, 425803. [Google Scholar] [CrossRef]
- Sempros, G.; Giaremis, S. Cost effective modification of SmCo5-type alloys Special Collection: 15th Joint MMM-Intermag Conference. AIP Adv. 2022, 12, 035343. [Google Scholar] [CrossRef]
- Liu, S.-L.; Fan, H.-R.; Liu, X.; Meng, J.; Butcher, A.R.; Yann, L.; Yang, K.-F.; Li, X.-C. Global rare earth elements projects: New developments and supply chains. Ore Geol. Rev. 2023, 157, 105428. [Google Scholar] [CrossRef]
- Binnemans, K.; Jones, P.T.; Müller, T.; Yurramendi, L. Rare Earths and the Balance Problem: How to Deal with Changing Markets? J. Sustain. Met. 2018, 4, 126–146. [Google Scholar] [CrossRef]
- Li, Z.; Liu, W.; Zha, S.; Li, Y.; Wang, Y.; Zhang, D.; Yue, M.; Zhang, J. Effects of lanthanum substitution on microstructures and intrinsic magnetic properties of Nd-Fe-B alloy. J. Rare Earths 2015, 33, 961–964. [Google Scholar] [CrossRef]
- Tang, W.; Zhou, S.; Wang, R. Preparation, and microstructure of La-containing R-Fe-B permanent magnets. J. Appl. Phys. 1989, 65, 3142–3145. [Google Scholar] [CrossRef]
- Benz, M.G.; Martin, D.L. Cobalt Mischmetal Samarium Permanent Magnet Alloys. J. Appl. Phys. 1971, 42, 1534. [Google Scholar] [CrossRef]
- Gjoka, M.; Sempros, G.; Giaremis, S.; Kioseoglou, J.; Sarafidis, C. On Structural and Magnetic Properties of Substituted SmCo5 Materials. Materials 2023, 16, 547. [Google Scholar] [CrossRef]
- Gjoka, M.; Sarafidis, C.; Sempros, G.; Giaremis, S.; Kioseoglou, J. Effect of Mischmetal (MM) and transition metal elements (TM) doped on properties of the SmCo5 intermetallics. In Proceedings of the First International Conference of National Institute of Physics (NIP), 10–11 February 2022; Abstracts Book; Academy of Sciences of Albania: Tiranë, Albania, 2022; p. 24. [Google Scholar]
- Larson, P.; Mazin, I.I.; Papaconstantopoulos, D.A. Calculation of magnetic anisotropy energy in SmCo5. Phys. Rev. B 2003, 67, 214405. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865. [Google Scholar] [CrossRef]
- Giaremis, S.; Katsikas, G.; Sempros, G.; Gjoka, M.; Sarafidis, C.; Kioseoglou, J. Ab initio, artificial neural network predictions and experimental synthesis of mischmetal alloying in Sm–Co permanent magnets. Nanoscale 2022, 14, 5824–5839. [Google Scholar] [CrossRef]
- Cadogan, J.M.; Li, H.S.; Margarian, A.; Dunlop, J.; Ryan, D.H.; Collocott, S.J.; Davis, R.L. New rare-earth intermetallic phases R3(Fe,M)29−X: (R = Ce, Pr, Nd, Sm, Gd; M = Ti, V, Cr, Mn; and X = H, N, C). J. Appl. Phys. 1994, 76, 6138–6143. [Google Scholar] [CrossRef]
- Kresse, G.; Joubert, D. From ultrasoft pseudopotentials to the projector augmented-wave method. Phys. Rev. B 1999, 59, 1758–1775. [Google Scholar] [CrossRef]
- Irkhin, Y.P.; Irkhin, V.Y. The anion and cation effects in the magnetic anisotropy of rare-earth compounds: Charge screening by conduction electrons. Phys. Solid State 2000, 42, 1087–1093. [Google Scholar] [CrossRef]
- Chen, C.H.; Walmer, M.S.; Walmer, M.H.; Gong, W.; Ma, B.M. The relationship of thermal expansion to magnetocrystalline anisotropy, spontaneous magnetization, and Tc for permanent magnets. J. Appl. Phys. 1999, 85, 5669–5671. [Google Scholar] [CrossRef]
- Nguyen, M.C.; Yao, Y.; Wang, C.Z.; Ho, K.M.; Antropov, V.P. Magnetocrystalline anisotropy in cobalt based magnets: A choice of correlation parameters and the relativistic effects. J. Phys. Condens. Matter 2018, 30, 195801. [Google Scholar] [CrossRef] [PubMed]
- Zhu, J.X.; Janoschek, M.; Rosenberg, R.; Ronning, F.; Thompson, J.D.; Torrez, M.A.; Bauer, E.D.; Batista, C.D. LDA+DMFT Approach to Magnetocrystalline Anisotropy of Strong Magnets. Phys. Rev. X 2014, 4, 021027. [Google Scholar] [CrossRef]
- Patrick, C.E.; Staunton, J.B. Rare-earth/transition-metal magnets at finite temperature: Self-interaction-corrected relativistic density functional theory in the disordered local moment picture. Phys. Rev. B 2018, 97, 224415. [Google Scholar] [CrossRef]



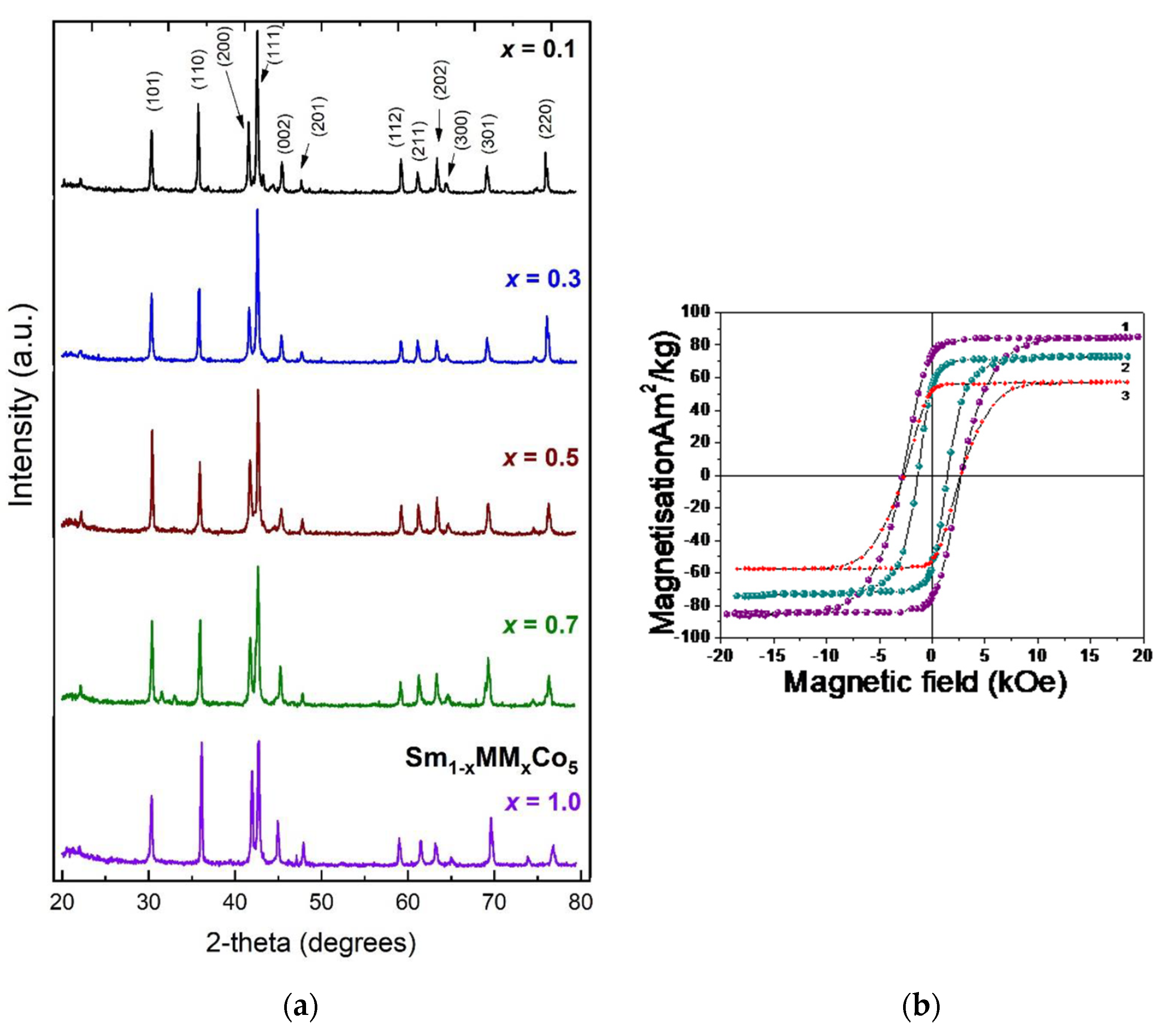
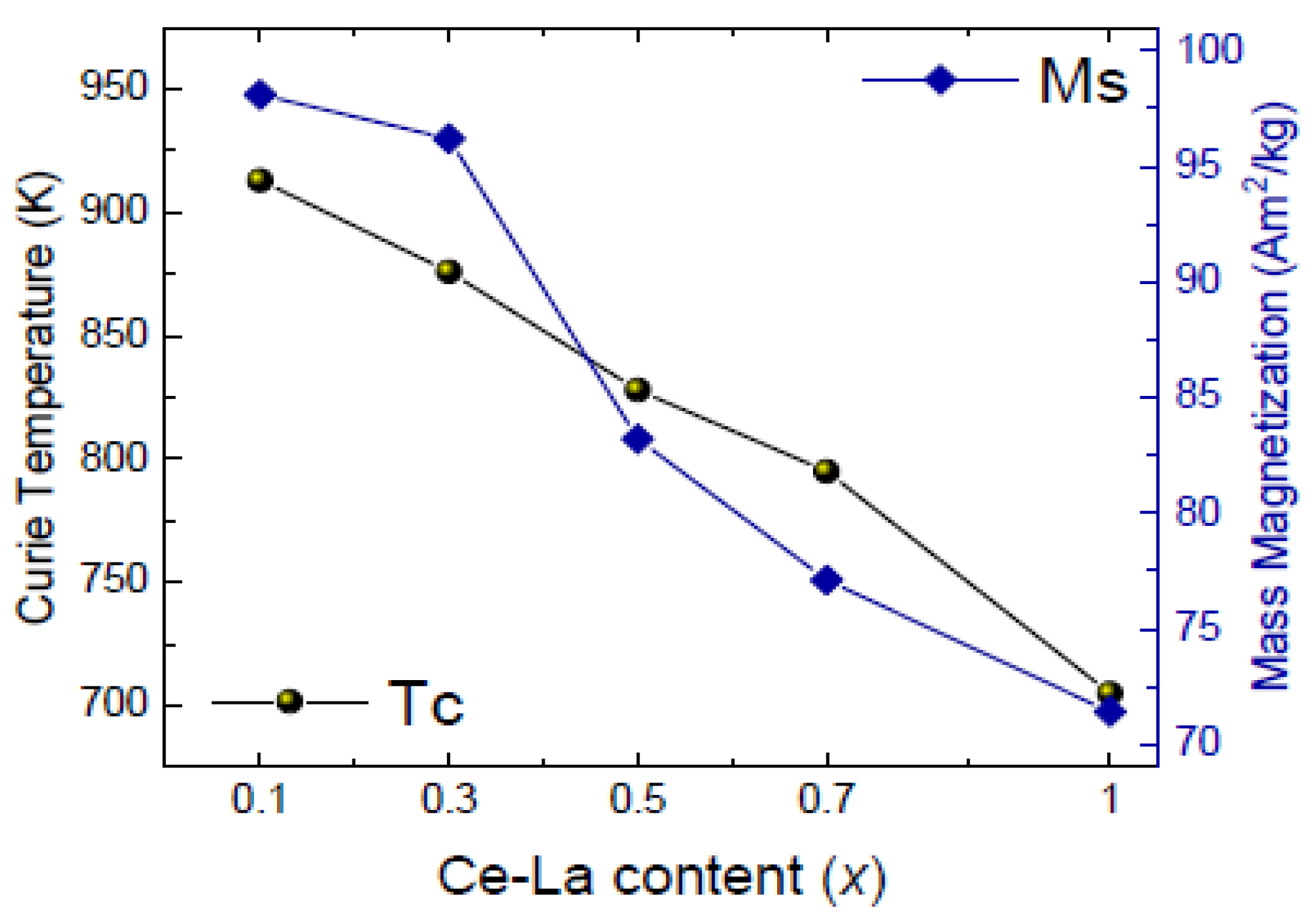
| Material | At 298 K | At 523 K | Tc (K) | ||||
|---|---|---|---|---|---|---|---|
| Ms | K1 | iHc | (BH)max | iHc | (BH)max | ||
| (kG) | (kOe) | (kOe) | (MGOe) | (kOe) | (MGOe) | ||
| Nd2Fe14B | 16.08 | 13.7 | 13.9 | 64.34 | 0.65 | 1.52 | 588 |
| Sm2(Co, Fe)17 based | 12.19 | 10.69 | 25 | 36.95 | 10.3 | 25.4 | 1078 |
| SmCo5 | 10.81 | 8.95 | 29 | 29.08 | 15.2 | 15. 8 | 1020 |
| Alnico 5 | 14.07 | 0.74 | 0.75 | 5.3 | 0.75 | 5.18 | 1210 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gjoka, M.; Sarafidis, C.; Giaremis, S. Towards Production of Cost-Effective Modification of SmCo5-Type Alloys Suitable for Permanent Magnets. Materials 2024, 17, 808. https://doi.org/10.3390/ma17040808
Gjoka M, Sarafidis C, Giaremis S. Towards Production of Cost-Effective Modification of SmCo5-Type Alloys Suitable for Permanent Magnets. Materials. 2024; 17(4):808. https://doi.org/10.3390/ma17040808
Chicago/Turabian StyleGjoka, Margarit, Charalampos Sarafidis, and Stefanos Giaremis. 2024. "Towards Production of Cost-Effective Modification of SmCo5-Type Alloys Suitable for Permanent Magnets" Materials 17, no. 4: 808. https://doi.org/10.3390/ma17040808
APA StyleGjoka, M., Sarafidis, C., & Giaremis, S. (2024). Towards Production of Cost-Effective Modification of SmCo5-Type Alloys Suitable for Permanent Magnets. Materials, 17(4), 808. https://doi.org/10.3390/ma17040808






