Large-Scale Green Synthesis of Magnesium Whitlockite from Environmentally Benign Precursor
Abstract
1. Introduction
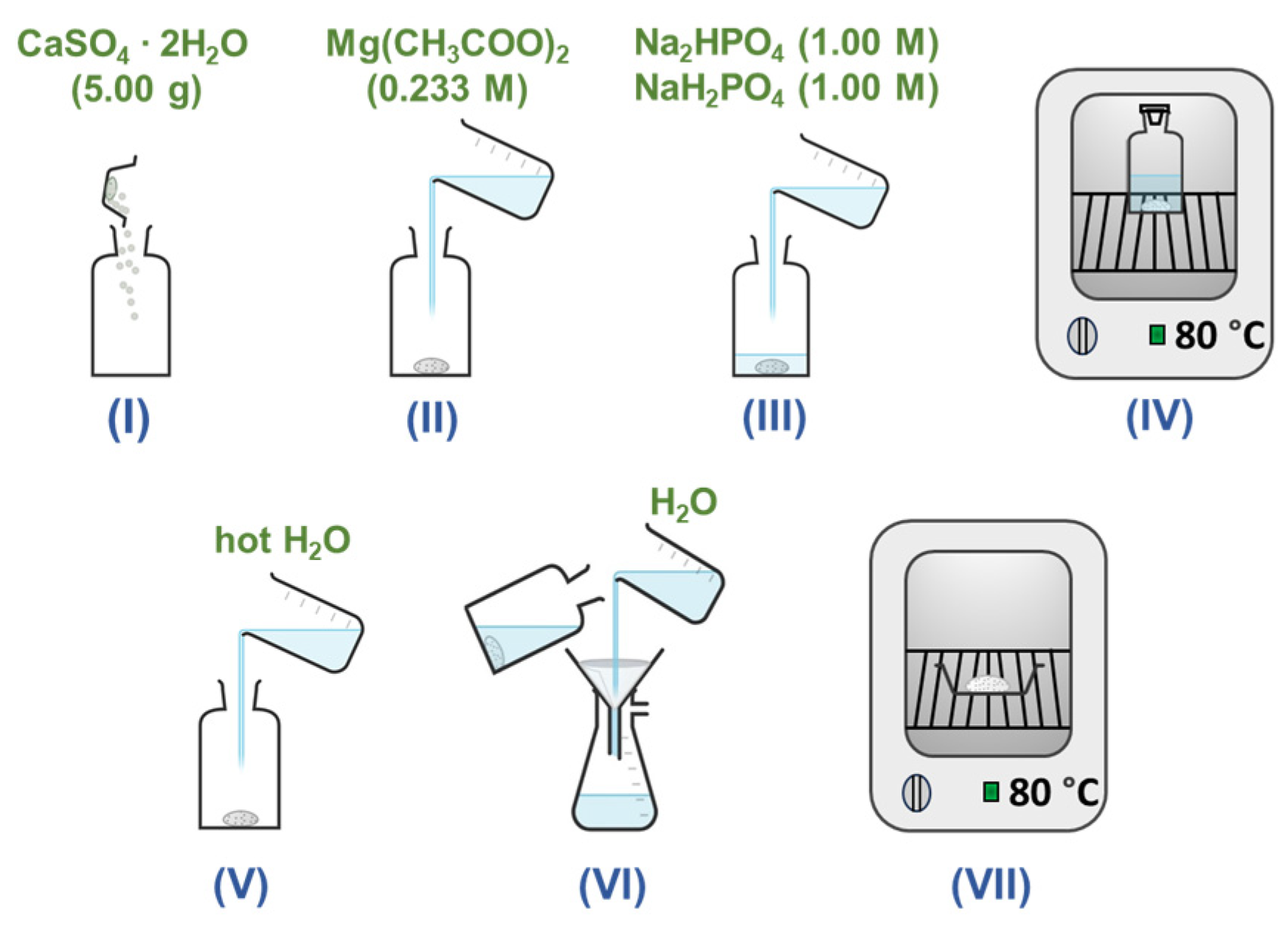
2. Materials and Methods
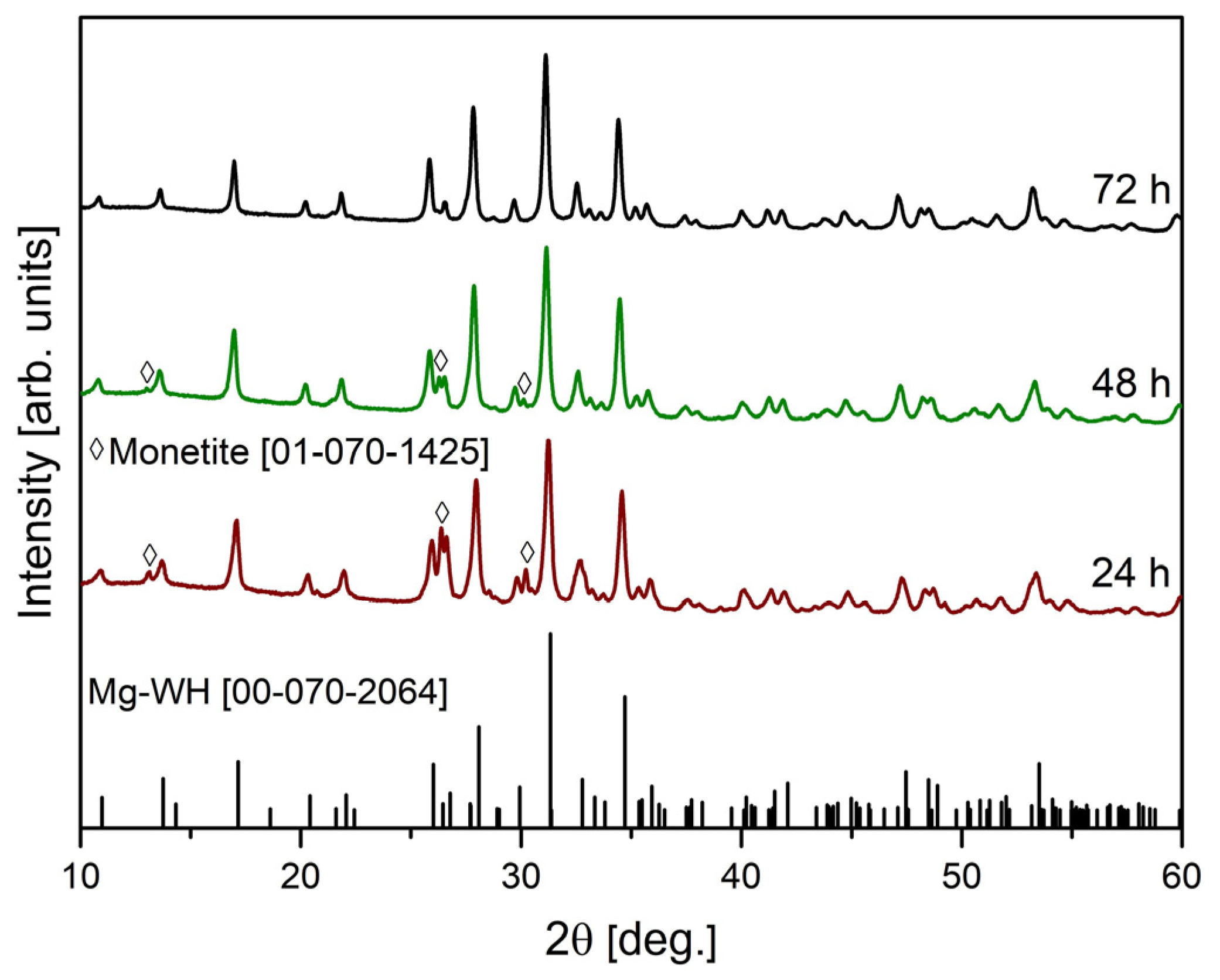
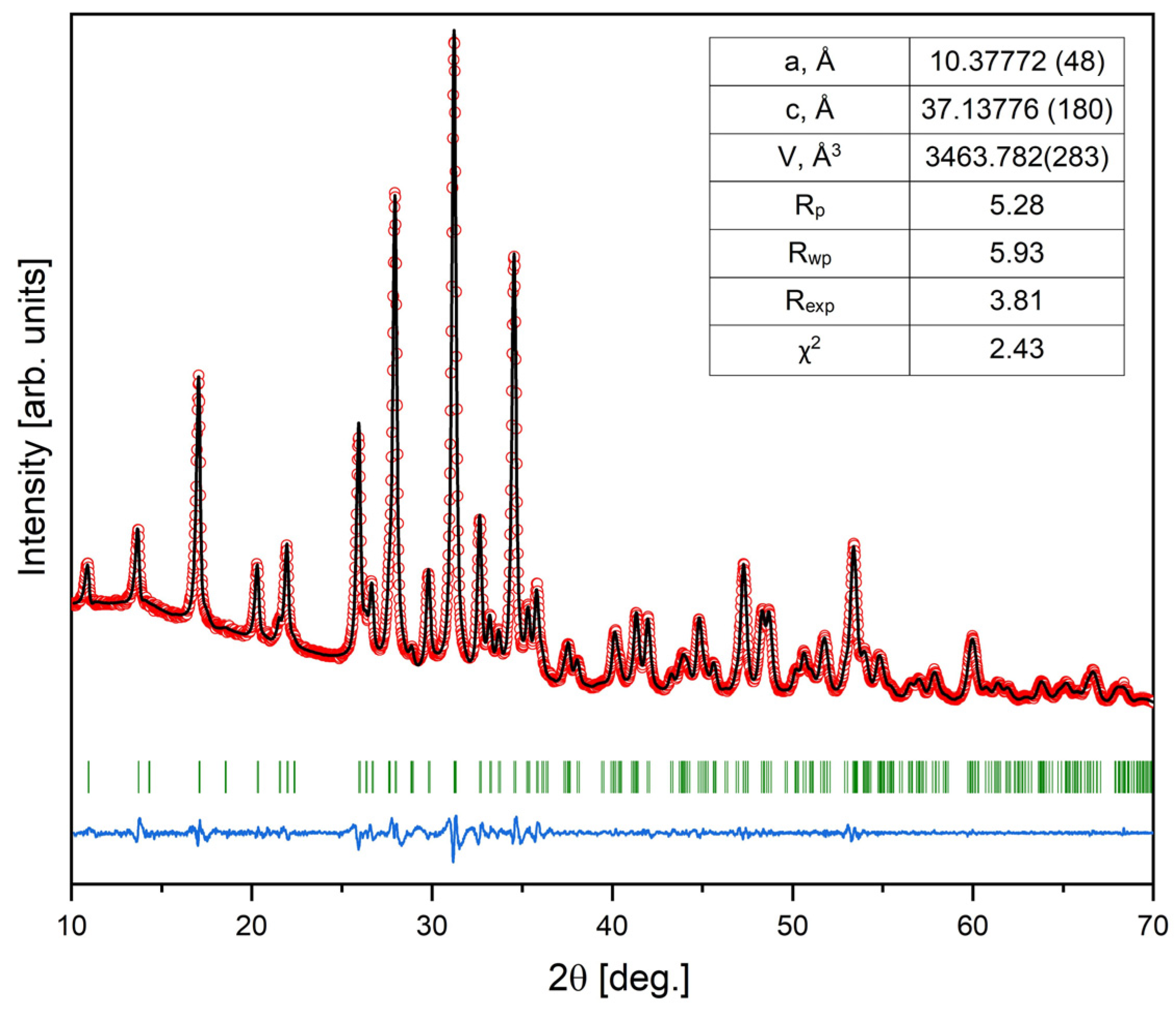
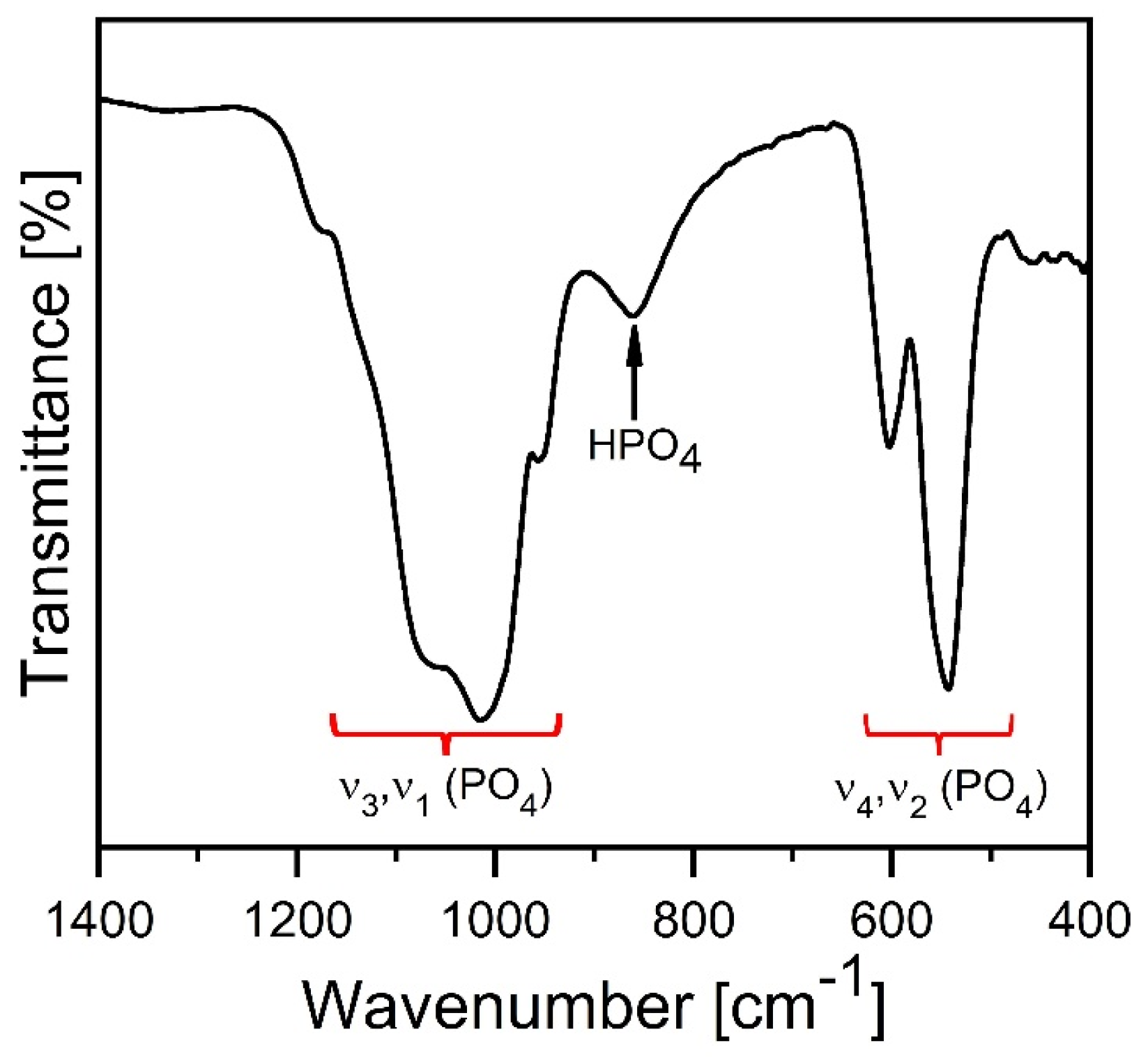
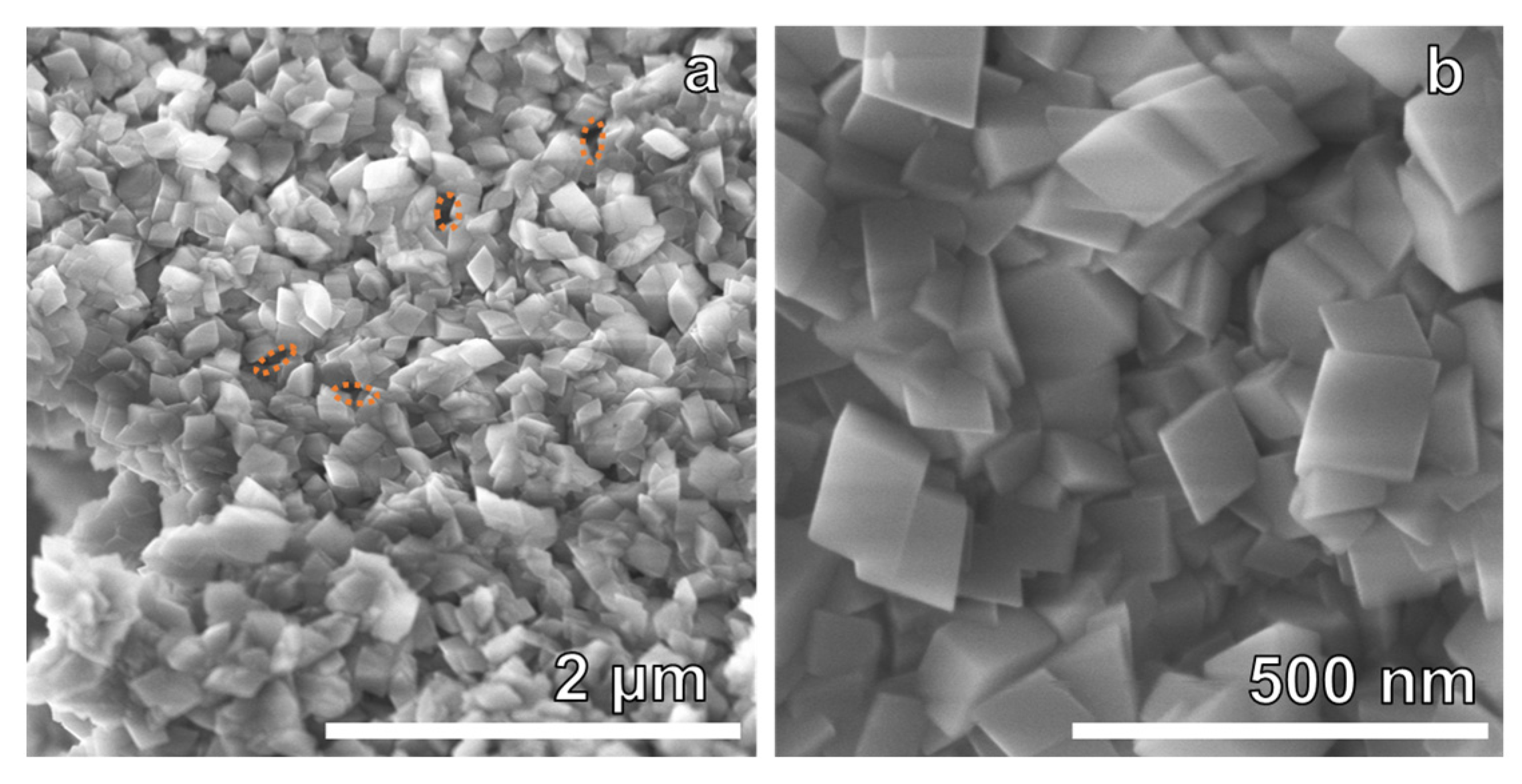
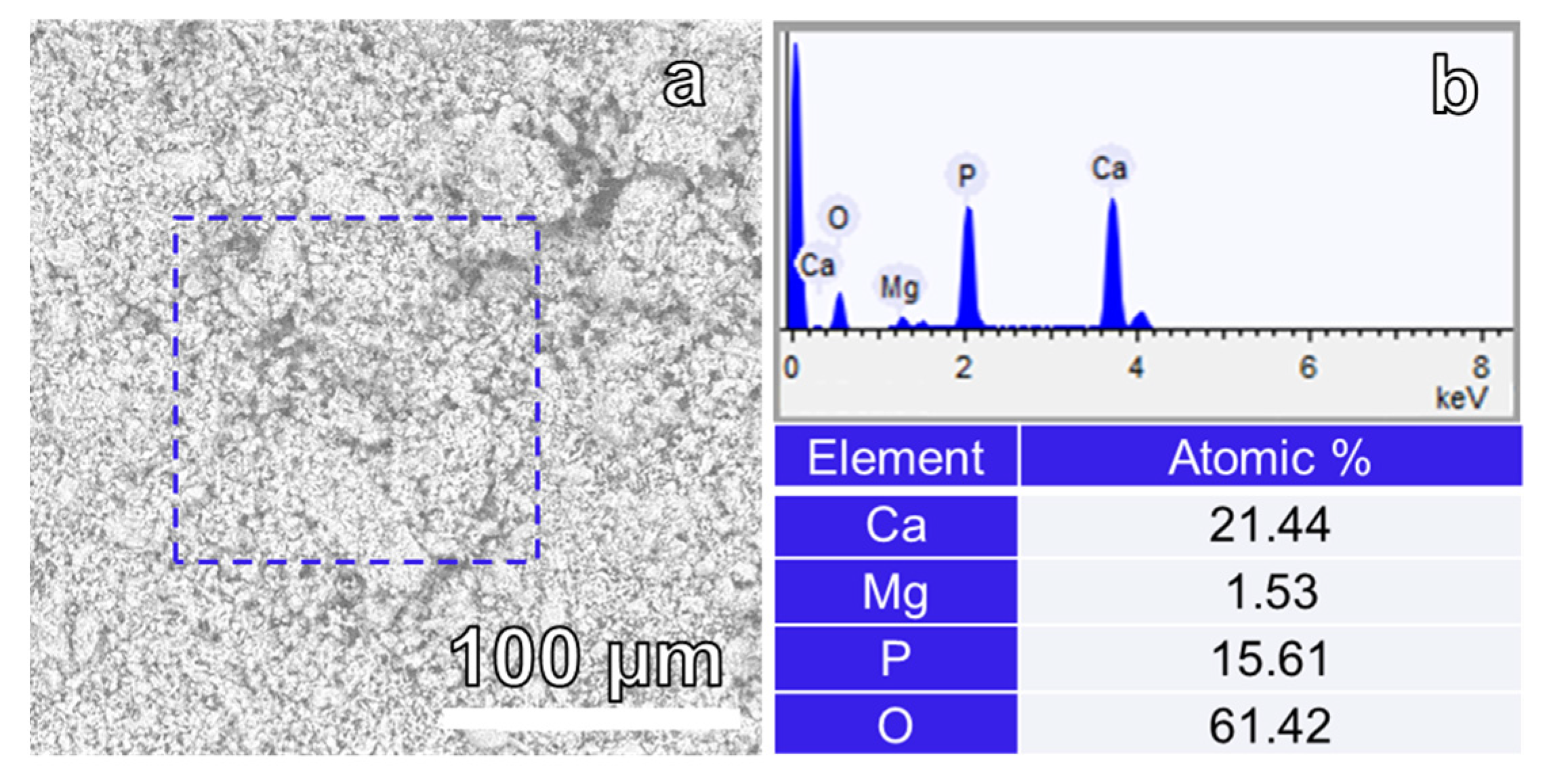
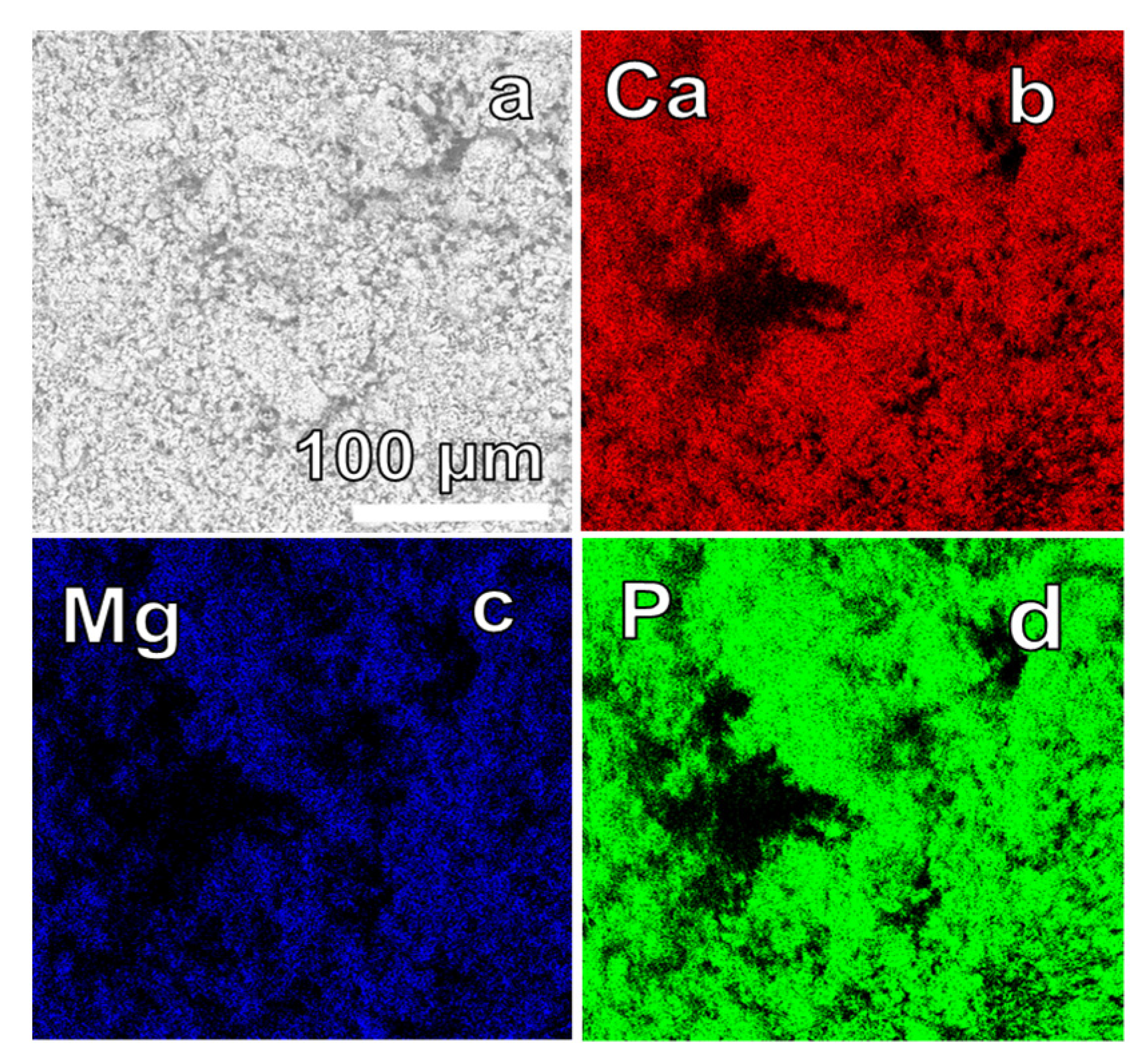
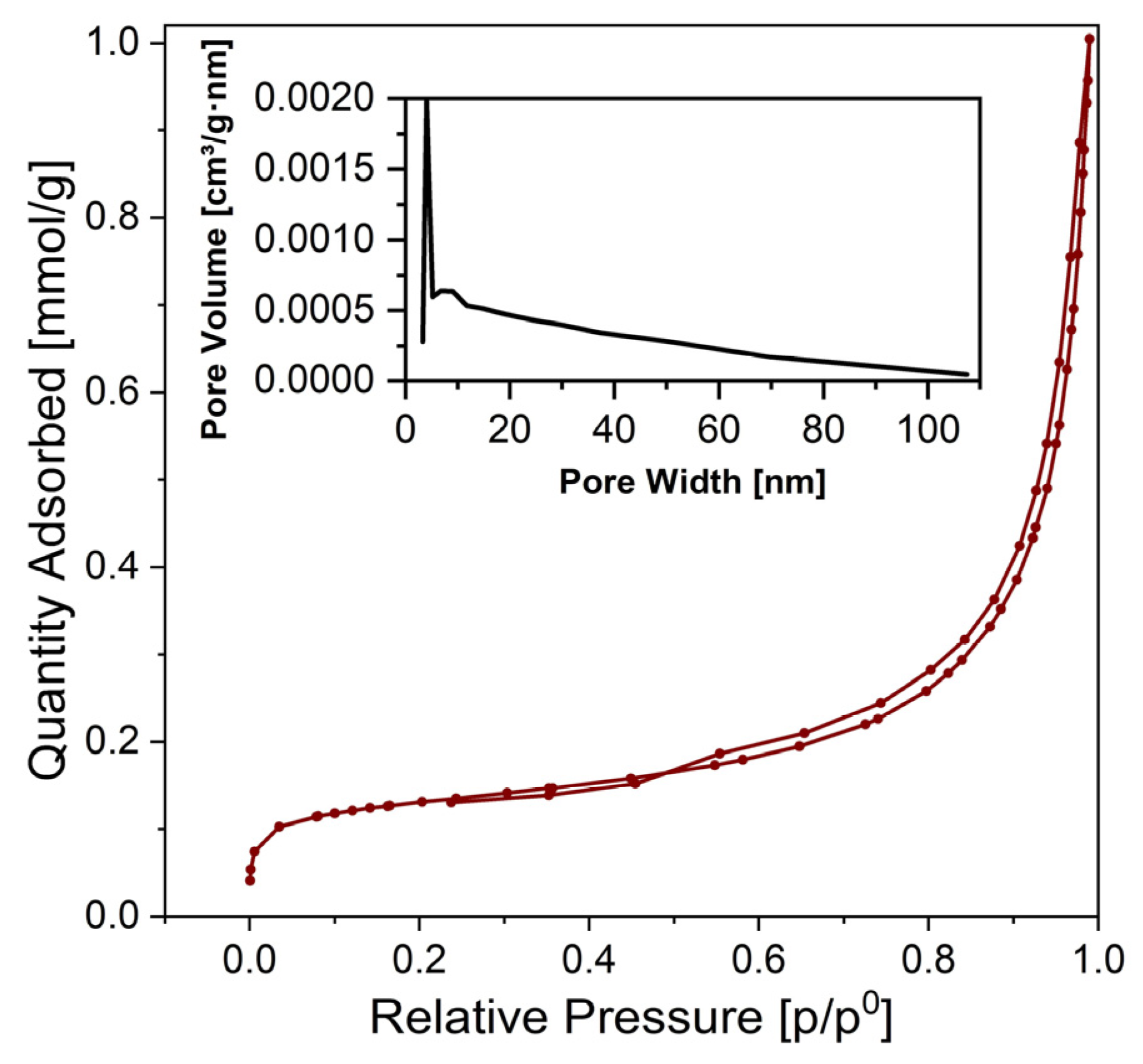
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lu, J.; Yu, H.; Chen, C. Biological properties of calcium phosphate biomaterials for bone repair: A review. RSC Adv. 2018, 8, 2015–2033. [Google Scholar] [CrossRef]
- García-Gareta, E.; Coathup, M.J.; Blunn, G.W. Osteoinduction of bone grafting materials for bone repair and regeneration. Bone 2015, 81, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Sari, M.; Hening, P.; Chotimah; Ana, I.D.; Yusuf, Y. Porous structure of bioceramics carbonated hydroxyapatite-based honeycomb scaffold for bone tissue engineering. Mater. Today Commun. 2021, 26, 102135. [Google Scholar] [CrossRef]
- Li, Y.; Chen, S.-K.; Li, L.; Qin, L.; Wang, X.-L.; Lai, Y.-X. Bone defect animal models for testing efficacy of bone substitute biomaterials. J. Orthop. Transl. 2015, 3, 95–104. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Yang, G.; Johnson, B.N.; Jia, X. Three-dimensional (3D) printed scaffold and material selection for bone repair. Acta Biomater. 2019, 84, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhou, B.; Wu, H.; Zheng, L.; Zhao, J. Effect of apatite formation of biphasic calcium phosphate ceramic (BCP) on osteoblastogenesis using simulated body fluid (SBF) with or without bovine serum albumin (BSA). Mater. Sci. Eng. C 2017, 70, 955–961. [Google Scholar] [CrossRef]
- Lu, T.; Yuan, X.; Zhang, L.; He, F.; Wang, X.; Zhang, Y.; Ye, J. High throughput synthesis and screening of zinc-doped biphasic calcium phosphate for bone regeneration. Appl. Mater. Today 2021, 25, 101225. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Wang, X.; Yu, Y.; Ji, L.; Geng, Z.; Wang, J.; Liu, C. Calcium phosphate-based materials regulate osteoclast-mediated osseointegration. Bioact. Mater. 2021, 6, 4517–4530. [Google Scholar] [CrossRef]
- Lee, W.-B.; Wang, C.; Lee, J.-H.; Jeong, K.-J.; Jang, Y.-S.; Park, J.-Y.; Ryu, M.H.; Kim, U.-K.; Lee, J.; Hwang, D.-S. Whitlockite Granules on Bone Regeneration in Defect of Rat Calvaria. ACS Appl. Bio Mater. 2020, 3, 7762–7768. [Google Scholar] [CrossRef]
- Jeong, J.; Shim, J.H.; Koo, B.M.; Choy, Y.B.; Heo, C.Y. Synergistic Effect of Whitlockite Scaffolds Combined with Alendronate to Promote Bone Regeneration. J. Tissue Eng. Reg. Med. 2022, 19, 83–92. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.A. Magnesium whitlockite—Omnipresent in pathological mineralisation of soft tissues but not a significant inorganic constituent of bone. Acta Biomater. 2021, 125, 72–82. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Pang, Y.; Tang, Y.; Wang, X.; Zhang, D.; Zhang, X.; Yu, Y.; Yang, X.; Cai, Q. A biomimetic piezoelectric scaffold with sustained Mg2+ release promotes neurogenic and angiogenic differentiation for enhanced bone regeneration. Bioact. Mater. 2023, 25, 399–414. [Google Scholar] [CrossRef] [PubMed]
- Bauer, L.; Antunović, M.; Rogina, A.; Ivanković, M.; Ivanković, H. Bone-mimetic porous hydroxyapatite/whitlockite scaffolds: Preparation, characterization and interactions with human mesenchymal stem cells. J. Mater. Sci. 2021, 56, 3947–3969. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, H.; Yang, H.; Zhao, Y.; Guo, J.; Yin, X.; Ma, T.; Liu, X.; Li, L. Magnesium-Based Whitlockite Bone Mineral Promotes Neural and Osteogenic Activities. ACS Biomater. Sci. Eng. 2020, 6, 5785–5796. [Google Scholar] [CrossRef]
- Jang, H.L.; Lee, H.K.; Kyoungsuk, J.; Ahn, H.-Y.; Lee, H.-E.; Nam, K.T. Phase transformation from hydroxyapatite to the secondary bone mineral, whitlockite. J. Mater. Chem. B 2015, 3, 1342–1349. [Google Scholar] [CrossRef]
- Wang, C.; Jeong, K.-J.; Park, H.J.; Lee, M.; Ryu, S.-C.; Hwang, D.Y.; Nam, K.H.; Han, I.H.; Lee, J. Synthesis and formation mechanism of bone mineral, whitlockite nanocrystals in tri-solvent system. J. Colloid Interface Sci. 2020, 569, 1–11. [Google Scholar] [CrossRef]
- Kizalaite, A.; Klimavicius, V.; Balevicius, V.; Niaura, G.; Salak, A.N.; Yang, J.-C.; Cho, S.H.; Goto, T.; Sekino, T.; Zarkov, A. Dissolution–precipitation synthesis and thermal stability of magnesium whitlockite. CrystEngComm 2023, 25, 4370–4379. [Google Scholar] [CrossRef]
- Ishikawa, K. Bone Substitute Fabrication Based on Dissolution-Precipitation Reactions. Materials 2010, 3, 1138–1155. [Google Scholar] [CrossRef]
- Griesiute, D.; Kizalaite, A.; Dubnika, A.; Klimavičius, V.; Kalendra, V.; Tyrpekl, V.; Cho, S.; Goto, T.; Sekino, T.; Zarkov, A. Copper-Containing Analog of the Biomineral Whitlockite: Dissolution-Precipitation Synthesis, Structural and Biological Properties. Dalton Trans. 2024, 53, 1722–1734. [Google Scholar] [CrossRef] [PubMed]
- Jang, H.L.; Zheng, G.B.; Park, J.; Kim, H.D.; Baek, H.-R.; Lee, H.K.; Lee, K.; Han, H.N.; Lee, C.-K.; Hwang, N.S.; et al. In Vitro and In Vivo Evaluation of Whitlockite Biocompatibility: Comparative Study with Hydroxyapatite and β-Tricalcium Phosphate. Adv. Healthc. Mater. 2016, 5, 128–136. [Google Scholar] [CrossRef]
- Kaliannagounder, V.K.; Raj, N.P.M.J.; Unnithan, A.R.; Park, J.; Park, S.S.; Kim, S.-J.; Park, C.H.; Kim, C.S.; Sasikala, A.R.K. Remotely controlled self-powering electrical stimulators for osteogenic differentiation using bone inspired bioactive piezoelectric whitlockite nanoparticles. Nano Energy 2021, 85, 105901. [Google Scholar] [CrossRef]
- Roisnel, T.; Rodriquez-Carvajal, J. WinPLOTR: A windows tool for powder diffraction pattern analysis. Mater. Sci. Forum 2001, 378–381, 118–123. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J.; Roisnel, T. Line broadening analysis using FullProf: Determination of microstructural properties. Mater. Sci. Forum 2004, 443–444, 123–126. [Google Scholar] [CrossRef]
- Hsu, H.-C.; Tuan, W.-H.; Lee, H.-Y. In-situ observation on the transformation of calcium phosphate cement into hydroxyapatite. Mater. Sci. Eng. C 2009, 29, 950–954. [Google Scholar] [CrossRef]
- El Hazzat, M.; El Hamidi, A.; Halim, M.; Arsalane, S. Complex evolution of phase during the thermal investigation of Brushite-type calcium phosphate CaHPO4·2H2O. Materialia 2021, 16, 101055. [Google Scholar] [CrossRef]
- Mhla, E.; Koutsoukos, P.G. Heterogeneous crystallization of calcium hydrogen phosphate anhydrous (monetite). Coll. Surf. A Physicochem. Eng. Asp. 2017, 513, 125–135. [Google Scholar] [CrossRef]
- Nigar, F.; Johnston, A.-L.; Smith, J.; Oakley, W.; Islam, M.T.; Felfel, R.; Grant, D.; Lester, E.; Ahmed, I. Production of Nano Hydroxyapatite and Mg-Whitlockite from Biowaste-Derived products via Continuous Flow Hydrothermal Synthesis: A Step towards Circular Economy. Materials 2023, 16, 2138. [Google Scholar] [CrossRef] [PubMed]
- Capitelli, F.; Bosi, F.; Capelli, S.C.; Radica, F.; Della Ventura, G. Neutron and XRD single-crystal diffraction study and vibrational properties of whitlockite, the natural counterpart of synthetic tricalcium phosphate. Crystals 2021, 11, 225. [Google Scholar] [CrossRef]
- Baino, F.; Caddeo, S.; Vitale-Brovarone, C. Sintering effects of bioactive glass incorporation in tricalcium phosphate scaffolds. Mater. Lett. 2020, 274, 128010. [Google Scholar] [CrossRef]
- Fulmer, M.T.; Ison, I.C.; Hankermayer, C.R.; Constantz, B.R.; Ross, J. Measurements of the solubilities and dissolution rates of several hydroxyapatites. Biomaterials 2002, 23, 751–755. [Google Scholar] [CrossRef] [PubMed]
- Stähli, C.; Thüring, J.; Galea, L.; Tadier, S.; Bohner, M.; Döbelin, N. Hydrogen-substituted β-tricalcium phosphate synthesized in organic media. Acta Cryst. 2016, B72, 875–884. [Google Scholar] [CrossRef]
- Carrodeguas, R.G.; De Aza, S. α-Tricalcium phosphate: Synthesis, properties and biomedical applications. Acta Biomater. 2011, 7, 3536–3546. [Google Scholar] [CrossRef] [PubMed]
- Afonina, A.; Dubauskas, A.; Klimavicius, V.; Zarkov, A.; Kareiva, A.; Grigoraviciute, I. Phase transformations during the dissolution-precipitation synthesis of magnesium whitlockite nanopowders from gypsum. Ceram. Int. 2023, 49, 38157–38164. [Google Scholar] [CrossRef]
- Myszka, B.; Schüßler, M.; Hurle, K.; Demmert, B.; Detsch, R.; Boccaccini, A.R.; Wolf, S.E. Phase-specific bioactivity and altered Ostwald ripening pathways of calcium carbonate polymorphs in simulated body fluid. RSC Adv. 2019, 9, 18232–18244. [Google Scholar] [CrossRef]
- Kizalaite, A.; Klimavicius, V.; Versockiene, J.; Lastauskiene, E.; Murauskas, T.; Skaudzius, R.; Yokoi, T.; Kawashita, M.; Goto, T.; Sekino, T.; et al. Peculiarities of the formation, structural and morphological properties of zinc whitlockite (Ca18Zn2(HPO4)2(PO4)12) synthesized via a phase transformation process under hydrothermal conditions. CrystEngComm 2022, 24, 5068–5079. [Google Scholar] [CrossRef]
- Ishikawa, K.; Garskaite, E.; Kareiva, A. Sol-gel synthesis of calcium phosphate-based biomaterials–A review of environmentally benign, simple and effective synthesis routes. J. Sol-Gel Sci. Technol. 2020, 94, 551–572. [Google Scholar] [CrossRef]
- Afonina, A.; Kizalaite, A.; Zarkov, A.; Drabavicius, A.; Goto, T.; Sekino, T.; Kareiva, A.; Grigoraviciute-Puroniene, I. Synthesis of whitlockite nanopowders with different magnesium content. Ceram. Int. 2022, 48, 32125–32130. [Google Scholar] [CrossRef]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Retajczyk, M.; Wróblewska, A.; Szymańska, A.; Michalkiewicz, B. Isomerization of limonene over natural zeolite-clinoptilolite. Clay Miner. 2019, 54, 121–129. [Google Scholar] [CrossRef]

| Synthesis Time (h) | Phase Composition (%) | |
|---|---|---|
| DCPA | Mg-WH | |
| 24 | 35 | 65 |
| 48 | 22 | 78 |
| 72 | - | 100 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Raiseliene, R.; Linkaite, G.; Zarkov, A.; Kareiva, A.; Grigoraviciute, I. Large-Scale Green Synthesis of Magnesium Whitlockite from Environmentally Benign Precursor. Materials 2024, 17, 788. https://doi.org/10.3390/ma17040788
Raiseliene R, Linkaite G, Zarkov A, Kareiva A, Grigoraviciute I. Large-Scale Green Synthesis of Magnesium Whitlockite from Environmentally Benign Precursor. Materials. 2024; 17(4):788. https://doi.org/10.3390/ma17040788
Chicago/Turabian StyleRaiseliene, Ruta, Greta Linkaite, Aleksej Zarkov, Aivaras Kareiva, and Inga Grigoraviciute. 2024. "Large-Scale Green Synthesis of Magnesium Whitlockite from Environmentally Benign Precursor" Materials 17, no. 4: 788. https://doi.org/10.3390/ma17040788
APA StyleRaiseliene, R., Linkaite, G., Zarkov, A., Kareiva, A., & Grigoraviciute, I. (2024). Large-Scale Green Synthesis of Magnesium Whitlockite from Environmentally Benign Precursor. Materials, 17(4), 788. https://doi.org/10.3390/ma17040788








