Experimental Studies on the Effect of Expired Amiodarone Drug (EAD) as a Corrosion Inhibitor on Mild Steel in 1 M HCl
Abstract
1. Introduction
2. Experimental Techniques
2.1. Preparation of Specimen
2.2. Preparation of Solution
2.3. Determination of Corrosion Rate (CR) via Weight Loss Method
2.4. Specimen Surface Area Determination
2.5. Potentiodynamic Polarization Study
2.6. AC Impedance Measurements
2.7. Surface Characterization Studies
2.8. Scanning Electron Microscopy (SEM)
2.9. Energy Dispersive Analysis of X-rays (EDAX)
2.10. Atomic Force Microscopy Characterization (AFM)
3. Results and Discussion
3.1. Weight Loss Method
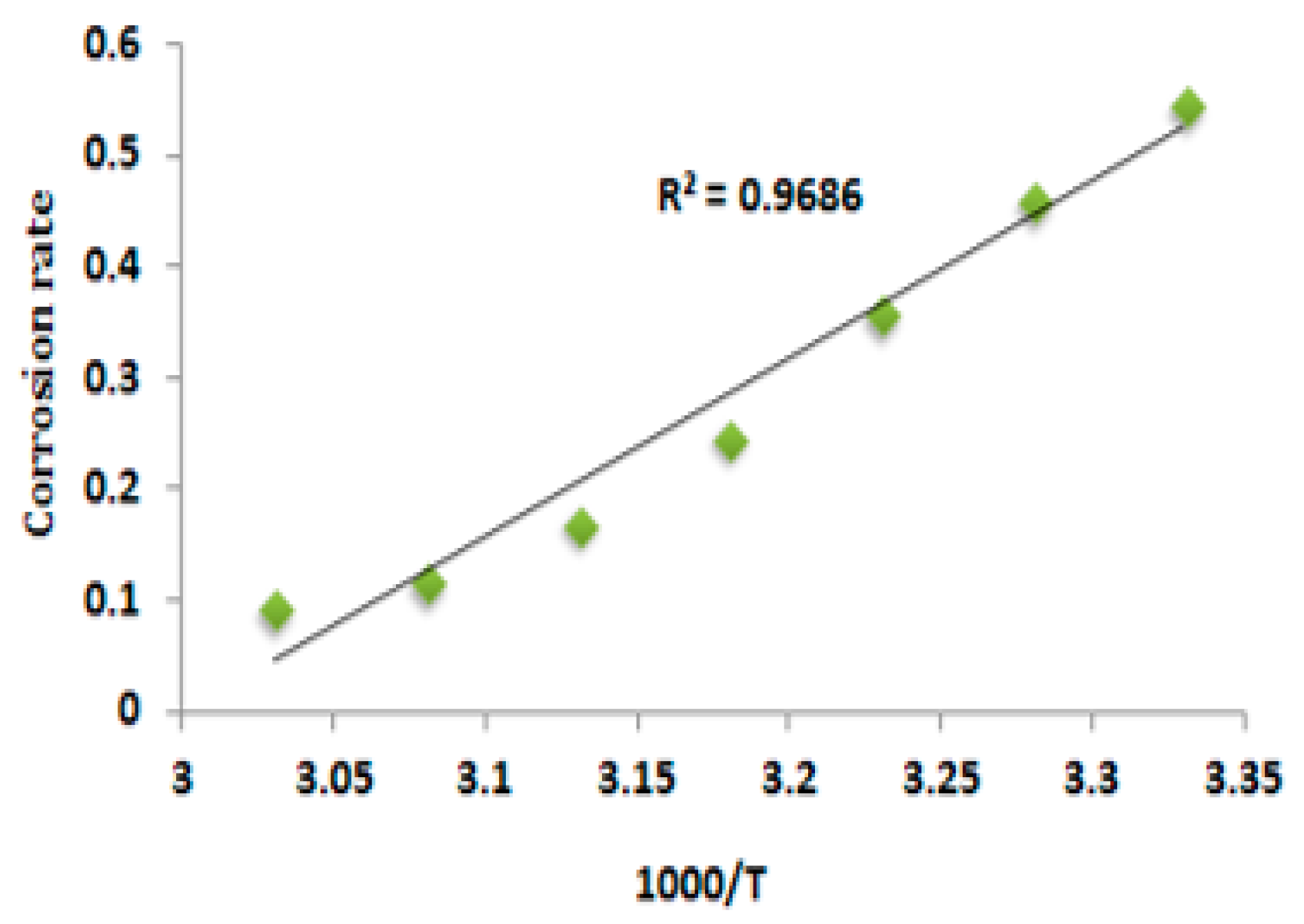
3.2. Effect of Temperature
3.3. Adsorption Isotherm
3.4. Temkin Adsorption Isotherm
3.5. Free Energy of Adsorption and Energy of Activation Parameters for Anti-Corrosion Process
3.5.1. Free Energy of Adsorption ()
3.5.2. Energy of Activation (Ea)
3.6. Electrochemical Investigation
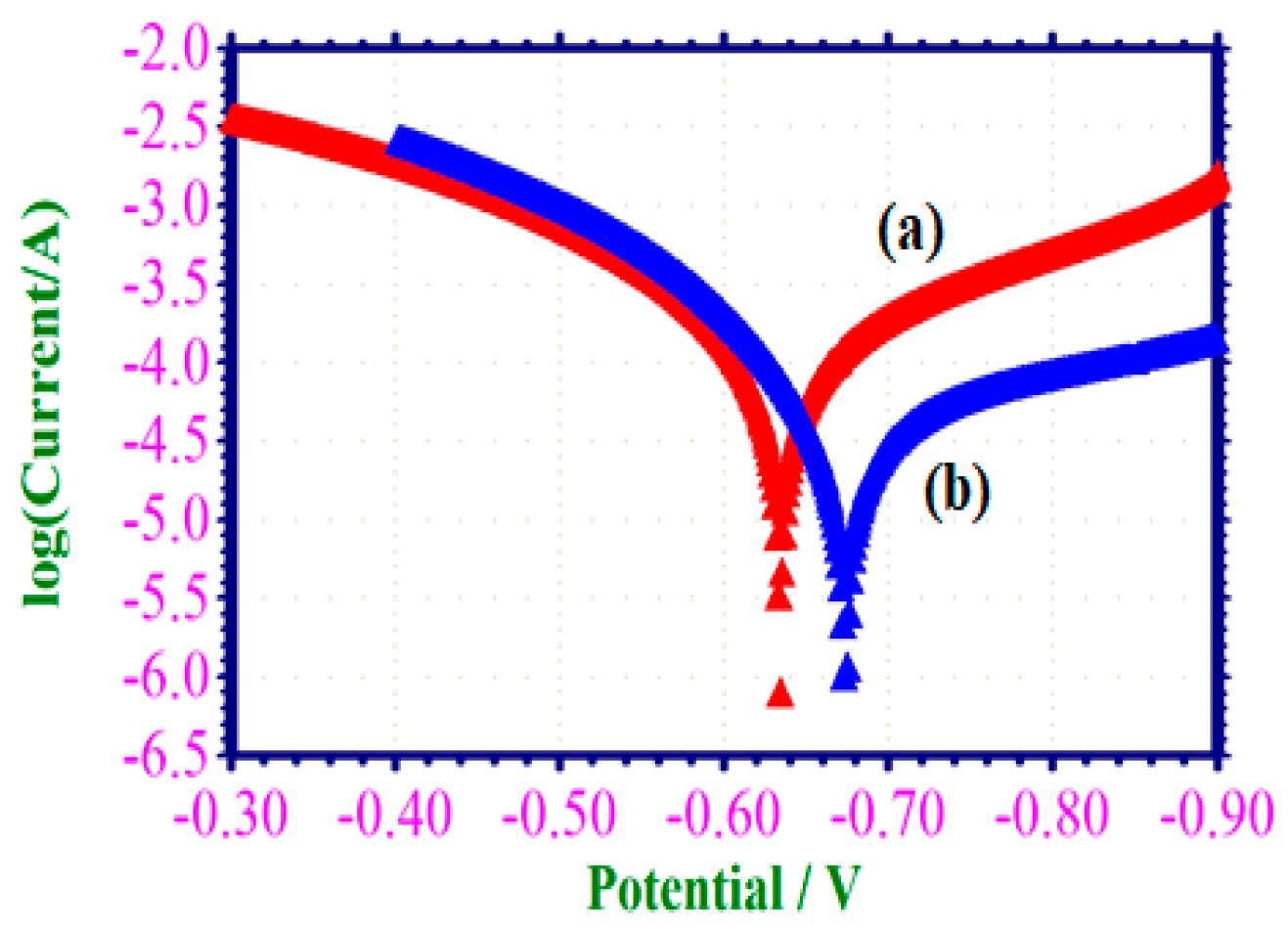
3.6.1. Potentiodynamic Polarization Study
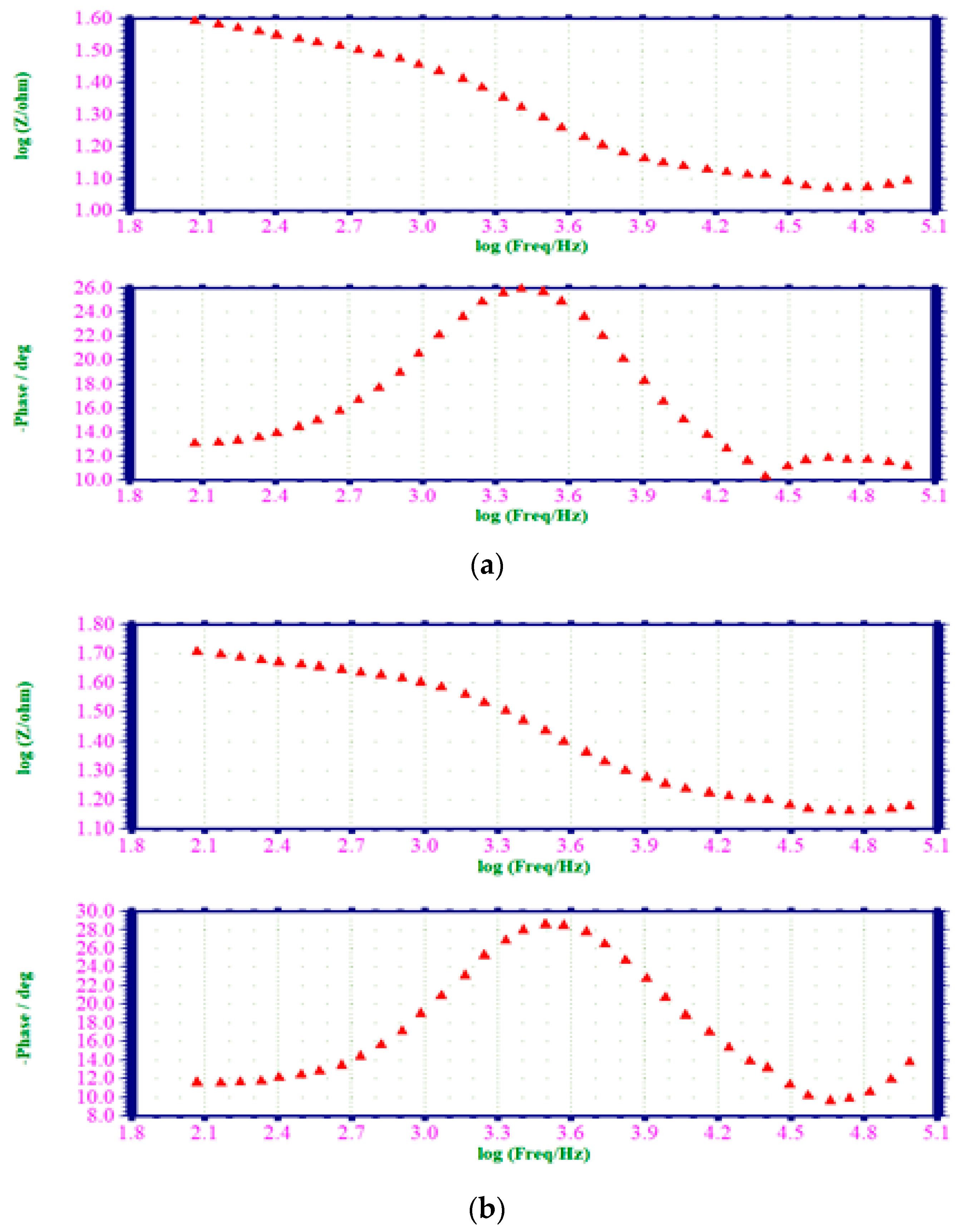
3.6.2. Alternating Current Impedance Spectra
3.7. Surface Analysis of Mild Steel by SEM
3.8. Surface Characterization of Mild Steel via Energy Dispersive Analysis (EDAX)
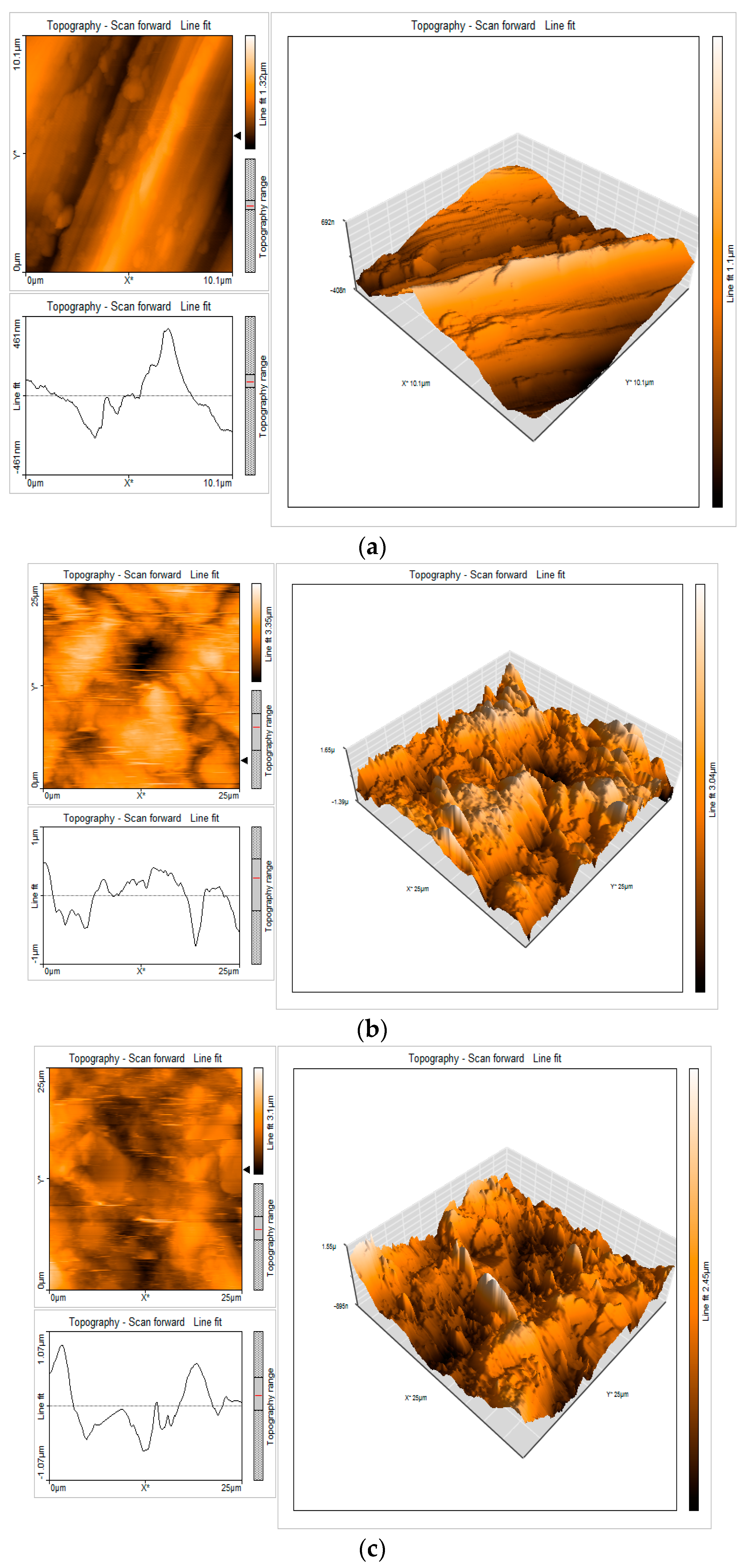
3.9. Surface Characterization of Mild Steel via Atomic Force Microscopy
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Medupin, R.O.; Ukoba, K.; Yoro, K.O.; Jen, T.C. Sustainable approach for corrosion control in mild steel using plant-based inhibitors: A review. Mater. Today Sustain. 2023, 22, 100373. [Google Scholar] [CrossRef]
- Kamal, C.; Sethuraman, M.G. Spirulina platensis—A novel green inhibitor for acid corrosion of mild steel. Arab. J. Chem. 2012, 5, 155–161. [Google Scholar] [CrossRef]
- Eddy, N.O.; Ekwumemgbo, P.A.; Mamza, P.A. Ethanol extract of Terminalia catappa as a green inhibitor for the corrosion of mild steel in H2SO4. Green Chem. Lett. Rev. 2009, 2, 223–231. [Google Scholar] [CrossRef]
- Rahimi, A.; Abdouss, M.; Farhadian, A.; Guo, L.; Neshati, J. Development of a novel thermally stable inhibitor based on furfuryl alcohol for mild steel corrosion in a 15% HCl medium for acidizing application. Ind. Eng. Chem. Res. 2021, 60, 11030–11044. [Google Scholar] [CrossRef]
- Hua, W.; Xu, X.; Zhang, X.; Yan, H.; Zhang, J. Progress in corrosion and anti-corrosion measures of phase change materials in thermal storage and management systems. J. Energy Storage 2022, 56, 105883. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Sustainable inhibitors for corrosion mitigation in aggressive corrosive media: A comprehensive study. J. Bio-Tribo-Corros. 2021, 7, 67. [Google Scholar] [CrossRef]
- Geethamani, P.; Narmatha, M.; Dhanalakshmi, R.; Aejitha, S.; Kasthuri, P.K. Corrosion inhibition and adsorption properties of mild steel in 1 M hydrochloric acid medium by expired ambroxol drug. J. Bio-Tribo-Corros. 2019, 5, 16. [Google Scholar] [CrossRef]
- Anadebe, V.C.; Onukwuli, O.D.; Abeng, F.E.; Okafor, N.A.; Ezeugo, J.O.; Okoye, C.C. Electrochemical-kinetics, MD-simulation and multi-input single-output (MISO)modeling using adaptive neuro-fuzzy inference system (ANFIS) prediction for dexamethasone drug as eco-friendly corrosion inhibitor for mild steel in 2 M HCl electrolyte. J. Taiwan Inst. Chem. Eng. 2020, 115, 251–265. [Google Scholar] [CrossRef]
- Aslam, R.; Mobin, M.; Aslam, J.; Aslam, A. Pharmaceutical drugs protecting metals in aggressive environments. In Eco-Friendly Corrosion Inhibitors; Elsevier: Amsterdam, The Netherlands, 2022; pp. 229–262. [Google Scholar] [CrossRef]
- Tanwer, S.; Shukla, S.K. Recent advances in the applicability of drugs as corrosion inhibitor on metal surface: A review. Curr. Res. Green Sustain. Chem. 2022, 5, 100227. [Google Scholar] [CrossRef]
- Bharatiya, U.; Gal, P.; Agrawal, A.; Shah, M.; Sircar, A. Effect of corrosion on crude oil and natural gas pipeline with emphasis on prevention by eco-friendly corrosion inhibitors: A comprehensive review. J. Bio-Tribo-Corros. 2019, 5, 1. [Google Scholar] [CrossRef]
- Fawzy, A.; El-Sayed, R.; Al Bahir, A.; Morad, M.; Althagafi, I.; Althagafy, K. Assessment of new designed surfactants as eco-friendly inhibitors for the corrosion of steel in acidic environment and evaluation of their biological and surface features: Thermodynamic, kinetic and mechanistic aspects. J. Adhes. Sci. Technol. 2022, 36, 1993–2019. [Google Scholar] [CrossRef]
- Chen, L.; Lu, D.; Zhang, Y. Organic compounds as corrosion inhibitors for carbon steel in HCl solution: A comprehensive review. Materials 2022, 15, 2023. [Google Scholar] [CrossRef] [PubMed]
- Priyanka, S.; Dheeraj, S.C.; Kritika, S.; Vandana, S.; Quraishi, M.A. Expired atorvastatin drug as corrosion inhibitor for mild steel in hydrochloric acid solution. Int. J. Ind. Chem. 2017, 8, 363–372. [Google Scholar] [CrossRef]
- Nicolae, V.; Valentin, O.; Alexandra, B. Corrosion inhibitors from expired drugs. Int. J. Pharm. 2012, 431, 241–244. [Google Scholar] [CrossRef]
- Su, P.; Li, L.; Li, W.; Huang, C.; Wang, X.; Liu, H.; Singh, A. Expired drug theophylline as potential corrosion inhibitor for 7075 aluminium alloy in 1M NaOH solution. Int. J. Electrochem. Sci. 2020, 15, 1412–1425. [Google Scholar] [CrossRef]
- Hameed, R.A.; Ismail, E.A.; Abu-Nawwas, A.H.; Al-Shafey, H.I. Expired Voltaren drugs as corrosion inhibitor for aluminium in hydrochloric acid. Int. J. Electrochem. Sci. 2015, 10, 2098–2109. [Google Scholar] [CrossRef]
- Dohare, P.; Chauhan, D.S.; Sorour, A.A.; Quraishi, M.A. DFT and experimental studies on the inhibition potentials of expired Tramadol drug on mild steel corrosion in hydrochloric acid. Mater. Discov. 2017, 9, 30–41. [Google Scholar] [CrossRef]
- Chaudhari, L.P.; Patel, S.N. Corrosion inhibition study of expired acetazolamide on mild steel in dilute hydrochloric acid solution. J. Bio-Tribo-Corros. 2019, 5, 20. [Google Scholar] [CrossRef]
- Tasić, Z.Z.; Mihajlović, M.B.P.; Simonović, A.T.; Radovanović, M.B.; Antonijević, M.M. Ibuprofen as a corrosion inhibitor for copper in synthetic acid rain solution. Sci. Rep. 2019, 9, 14710. [Google Scholar] [CrossRef]
- Mohammadi, H.S.; Asl, A.H.; Khajenoori, M. Determination of amiodarone hydrochloride solubility in pure and ethanol-modified subcritical water: Experimental data and modeling. J. Mol. Liq. 2022, 362, 119679. [Google Scholar] [CrossRef]
- Larson, J.; Rich, L.; Deshmukh, A.; Judge, E.C.; Liang, J.J. Pharmacologic management for ventricular arrhythmias: Overview of anti-arrhythmic drugs. J. Clin. Med. 2022, 11, 3233. [Google Scholar] [CrossRef]
- El-Shamy, A.M.; Mouneir, S.M. Medicinal materials as eco-friendly corrosion inhibi-tors for industrial applications: A review. J. Bio-Tribo-Corros. 2023, 9, 3. [Google Scholar] [CrossRef]
- Fouda, A.S.; El Morsi, M.A.; El Mogy, T. Studies on the inhibition of carbon steel corrosion in hydrochloric acid solution by expired Carvedilol drug. Green Chem. Lett. Rev. 2017, 10, 336–345. [Google Scholar] [CrossRef]
- Patel, N.S.; Jauhariand, S.; Mehta, G.N.; Al-Deyab, S.S.; Warad, I.; Hammouti, B. Mild steel corrosion inhibition by various plant extracts in 0.5 M sulphuric acid. Int. J. Electrochem. Sci. 2013, 8, 2635–2655. [Google Scholar]
- Geethamani, P.; Kasthuri, P.K. Adsorption and corrosion inhibition of mild steel in acidic media by expired pharmaceutical drug. Cogent Chem. 2015, 1, 1091558. [Google Scholar] [CrossRef]
- Chen, T.; Gan, H.; Chen, Z.; Chen, M.; Fu, C. Eco-friendly approach to corrosion inhibition of AA5083 aluminum alloy in HCl solution by the expired Vitamin B1 drugs. J. Mol. Struct. 2021, 1244, 130881. [Google Scholar] [CrossRef]
- Ganapathi Sundaram, R.; Vengatesh, G.; Thamaraiselvi, M.; Prabakaran, R.; Thailan, V.; Muthuvel, I.; Niraimathi, S. Experimental and computational approach of an expired antibiotic drug Kynurenic acid as an efficient corrosion inhibitor for mild steel in HNO3 medium. J. Iran. Chem. Soc. 2022, 19, 2311–2329. [Google Scholar] [CrossRef]
- Anaee, R.A.; Tomi, I.H.R.; Abdulmajeed, M.H.; Naser, S.A.; Kathem, M.M. Expired Etoricoxib as a corrosion inhibitor for steel in acidic solution. J. Mol. Liq. 2019, 279, 594–602. [Google Scholar] [CrossRef]
- Raghavendra, N. Expired lorazepam drug: A medicinal compound as green corrosion inhibitor for mild steel in hydrochloric acid system. Chem. Afr. 2019, 2, 463–470. [Google Scholar] [CrossRef]
- Motawea, M.M. Corrosion inhibition effect of expired levothyroxine drug on stainless steel 304L in 0.5 M H2SO4 solution. Int. J. Electrochem. Sci. 2021, 16, 21021. [Google Scholar] [CrossRef]
- El Haddad, M.N.; Fouda, A.E.A.S. Evaluation of curam drug as an ecofriendly corrosion inhibitor for protection of stainless steel304 in hydrochloric acid solution: Chemical, electrochemical, and surface morphology studies. J. Chin. Chem. Soc. 2021, 68, 826–836. [Google Scholar] [CrossRef]
- Geethamani, P.; Kasthuri, P.K. The inhibitory action of expired asthalin drug on the corrosion of mild steel in acidic media: A comparative study. J. Taiwan Inst. Chem. Eng. 2016, 63, 490–499. [Google Scholar] [CrossRef]
- Abdallah, M.; Fawzy, A.; Alfakeer, M. Inhibition potentials and adsorption performance of two sulfonylurea antibiotic expired drugs on the corrosion of mild steel in 0.5 M H2SO4. Int. J. Electrochem. Sci. 2020, 15, 10289–10303. [Google Scholar] [CrossRef]
- Fadila, B.; Sihem, A.; Sameh, A.; Kardas, G. A study on the inhibition effect of expired amoxicillin on mild steel corrosion in 1N HCl. Mater. Res. Express 2019, 6, 046419. [Google Scholar] [CrossRef]
- Fajobi, M.A.; Fayomi, O.S.I.; Akande, I.G.; Odunlami, O.A. Inhibitive performance of ibuprofen drug on mild steel in 0.5 M of H2SO4 acid. J. Bio-Tribo-Corros. 2019, 5, 79. [Google Scholar] [CrossRef]
- Matad, P.B.; Mokshanatha, P.B.; Hebbar, N.; Venkatesha, V.T.; Tandon, H.C. Ketosulfone drug as a green corrosion inhibitor for mild steel in acidic medium. Ind. Eng. Chem. Res. 2014, 53, 8436–8444. [Google Scholar] [CrossRef]
- Al Bahir, A. Evaluation of expired linezolid and norfloxacin drugs as proficient environmentally safe inhibitors for mitigation of aluminum corrosion in sodium chloride medium. Chem. Data Collect. 2022, 42, 100960. [Google Scholar] [CrossRef]
- Alqarni, N.; El-Gammal, B.; Fawzy, A.; Al Bahir, A.; Toghan, A. Investigation of expired ticarcillin and carbenicillin drugs for inhibition of aluminum corrosion in hydrochloric acid solution. Int. J. Electrochem. Sci. 2022, 17, 2212113. [Google Scholar] [CrossRef]
- Kalkhambkar, A.G.; Rajappa, S.K.; Manjanna, J.; Malimath, G.H. Effect of expired doxofylline drug on corrosion protection of soft steel in 1 M HCl: Electrochemical, quantum chemical and synergistic effect studies. J. Indian Chem. Soc. 2022, 99, 100639. [Google Scholar] [CrossRef]
- Zakaria, K.; Abbas, M.A.; Bedair, M.A. Herbal expired drug bearing glycosides and polysaccharides moieties as green and cost-effective oilfield corrosion inhibitor: Electrochemical and computational studies. J. Mol. Liq. 2022, 352, 118689. [Google Scholar] [CrossRef]
- Iroha, N.B.; Nnanna, L.A.; Maduelosi, N.J.; Anadebe, V.C.; Abeng, F.E. Evaluation of the anticorrosion performance of Tamsulosin as corrosion inhibitor for pipeline steel in acidic environment: Experimental and theoretical study. J. Taibah Univ. Sci. 2022, 16, 288–299. [Google Scholar] [CrossRef]
- Fouda, A.S.; Badawy, A.A. Adsorption and corrosion inhibition of Cu in nitric acid by expired simvastatin drug. Prot. Met. Phys. Chem. Surf. 2019, 55, 572–582. [Google Scholar] [CrossRef]
- Abeng, F.E.; Ikpi, M.E.; Ushie, O.A.; Anadebe, V.C.; Nyong, B.E.; Obeten, M.E.; Nkom, P.Y. Insight into corrosion inhibition mechanism of carbon steel in 2 M HCl electrolyte by eco-friendly based pharmaceutical drugs. Chem. Data Collect. 2021, 34, 100722. [Google Scholar] [CrossRef]
- Nathiya, R.S.; Perumal, S.; Murugesan, V.; Raj, V. Expired drugs: Environmentally safe inhibitors for aluminium corrosion in 1 M H2SO4. J. Bio-Tribo-Corros. 2018, 4, 4. [Google Scholar] [CrossRef]
- Tijani, S.K.U.; Odulanmi, O.A.; Fayomi, O.S.I.; Williams, A.B.; Daramola, M. Passive characteristics and Arrhenius responses of expired inhibitor drug on UNG1050 steel. IOP Conf. Ser. Mater. Sci. Eng. 2021, 1107, 012223. [Google Scholar] [CrossRef]
- Montaser, A.A.; El-Mahdy, M.S.; Mahmoud, E.E.; Fouda, A.S. Recycling of expired ciprofloxacin in synthetic acid rain (SAR) solution as a green corrosion inhibitor for copper: A theoretical and experimental evaluation. J. Appl. Electrochem. 2024, 54, 439–456. [Google Scholar] [CrossRef]
- Iroha, N.B.; Maduelosi, N.J.; Nnanna, L.A. The impact of tizanidine on E24 carbon steel corrosion inhibition in oilfield acidizing solution: Experimental and quantum chemical studies. Emergent Mater. 2023, 6, 137–146. [Google Scholar] [CrossRef]
- Narang, R.; Vashishth, P.; Bairagi, H.; Shukla, S.K.; Mangla, B. Electrochemical and surface study of an antibiotic drug as sustainable corrosion inhibitor on mild steel in 0.5 M H2SO4. J. Mol. Liq. 2023, 384, 122277. [Google Scholar] [CrossRef]
- Shetty, S.; Rao, P.; Kedimar, N.; Rao, S.A. Electrochemical and quantum chemical investigation to evaluate corrosion inhibition performance of therapeutic drug. Can. Metall. Q. 2023, 1–15. [Google Scholar] [CrossRef]
- Haruna, K.; Saleh, T.A.; Quraishi, M.A. Expired metformin drug as green corrosion inhibitor for simulated oil/gas well acidizing environment. J. Mol. Liq. 2020, 315, 113716. [Google Scholar] [CrossRef]
- Kamel, M.M.; Mohsen, Q.; Anwar, Z.M.; Sherif, M.A. An expired ceftazidime antibiotic as an inhibitor for disintegration of copper metal in pickling HCl media. J. Mater. Res. Technol. 2021, 11, 875–886. [Google Scholar] [CrossRef]
- Iroha, N.B.; Anadebe, V.C.; Maduelosi, N.J.; Nnanna, L.A.; Isaiah, L.C.; Dagdag, O.; Ebenso, E.E. Linagliptin drug molecule as corrosion inhibitor for mild steel in 1 M HCl solution: Electrochemical, SEM/XPS, DFT and MC/MD simulation approach. Colloids Surf. A Physicochem. Eng. Asp. 2023, 660, 130885. [Google Scholar] [CrossRef]
- Feng, L.; Zhang, S.; Zhou, Y.; Pan, R.; Du, H.; Liu, F.; Yang, Y. Expired Glucosamine Drugs as Green Corrosion Inhibitors for Carbon Steel in H2SO4 Solution and Synergistic Effect of Glucosamine Molecules with Iodide Ions: Combined Experimental and Theoretical Investigations. Crystals 2023, 13, 205. [Google Scholar] [CrossRef]
- Pour-Ali, S.; Hejazi, S. Tiazofurin drug as a new and non-toxic corrosion inhibitor for mild steel in HCl solution: Experimental and quantum chemical investigations. J. Mol. Liq. 2022, 354, 118886. [Google Scholar] [CrossRef]
- Abbar, J.C.; Swetha, G.A.; Sachin, H.P. Impact of an expired hemorheologic drug on the mitigation of zinc corrosion in acidic environment: Insights from chemical, electrochemical, and surface evaluation. Colloids Surf. A Physicochem. Eng. Asp. 2022, 650, 129518. [Google Scholar] [CrossRef]
- Sheit, H.M.K.; Mubarak, M.S.; Benitta, G. Anti-Corrosive Efficiency of Mild Steel in Sodium Chloride Solution Using 5-Acetyl-3-Phenyl-2,6-Dipyridin-2-Yltetra-Hydropyrimidin-4(1H)-1 Compound as an Inhibitor. J. Bio-Tribo-Corros. 2022, 8, 103. [Google Scholar] [CrossRef]
- Sheit, H.M.K.; Kani, S.M.; Sathiq, M.A.; Abuthahir, S.S.; Mohan, K.S.; Mary, S.B.; Gunavathy, K.V. Investigations on the effect of 2-[(furan-3ylmethylene)-amino]-benzenethiol on corrosion in carbon steel. Results Surf. Interfaces 2023, 12, 100143. [Google Scholar] [CrossRef]
- Zadeh, A.S.K.; Zandi, M.S.; Kazemipour, M. Corrosion protection of carbon steel in acidic media by expired bupropion drug; experimental and theoretical study. J. Indian Chem. Soc. 2022, 99, 100522. [Google Scholar] [CrossRef]
- Almashhadani, H.A.; Alshujery, M.K.; Khalil, M.; Kadhem, M.M.; Khadom, A.A. Corrosion inhibition behavior of expired diclofenac Sodium drug for Al 6061 alloy in aqueous media: Electrochemical, morphological, and theoretical investigations. J. Mol. Liq. 2021, 343, 117656. [Google Scholar] [CrossRef]




| Concentration of EAD Inhibitor (M) | Corrosion Rate (mm y−1) | Inhibition Efficiency (%) | Surface Coverage (θ) |
|---|---|---|---|
| - | 0.8386 | - | - |
| 0.0000001 | 0.3508 | 58.16 | 0.5816 |
| 0.000001 | 0.2738 | 67.35 | 0.6735 |
| 0.00001 | 0.2225 | 73.46 | 0.7346 |
| 0.0001 | 0.1540 | 81.63 | 0.8163 |
| 0.001 | 0.0941 | 88.77 | 0.8877 |
| Temperature (K) | Corrosion Rate (mm y−1) | Inhibition Efficiency (%) | Surface Coverage (θ) |
|---|---|---|---|
| 303 | 0.0941 | 88.77 | 0.8877 |
| 308 | 0.1198 | 85.71 | 0.8571 |
| 313 | 0.1711 | 79.59 | 0.7959 |
| 318 | 0.2481 | 70.41 | 0.7041 |
| 323 | 0.3594 | 57.14 | 0.5714 |
| 328 | 0.4621 | 44.89 | 0.4489 |
| 333 | 0.5476 | 34.70 | 0.3470 |
| Inhibitor System | (kJ/mol−1) | ||||||
|---|---|---|---|---|---|---|---|
| 303 K | 308 K | 313 K | 318 K | 323 K | 328 K | 333 K | |
| 1 M HCl + EAD inhibitor | 26.9287 | 26.6642 | 25.9733 | 25.0781 | 23.9208 | 22.9536 | 22.1205 |
| System | Ea (KJ/mol) |
|---|---|
| Blank | 20.127 |
| EAD inhibitor | 52.3865 |
| Concentration of the EAD Inhibitor (M) | Ecorr mV/SCE | Tafel Slope | Icorr A/cm2 | LPR /cm2 | |
|---|---|---|---|---|---|
| ba mV/dec | bc mV/dec | ||||
| Blank | −632 | 0.152 | 0.208 | 1.2730 × 10−4 | 291 |
| 0.001 | −672 | 0.110 | 0.322 | 4.6340 × 10−4 | 792 |
| Concentration of the EAD Inhibitor (M) | Nyquist Plot | Impedance ) | Phase Angle (Degree) | |
|---|---|---|---|---|
| /cm2 | Cdl F/cm2 | |||
| - | 23.69 | 42.3779 × 10−6 | 0.502 | 25.9 |
| 0.001 | 35.44 | 57.6733 × 10−6 | 0.532 | 28.4 |
| Samples | Sa (nm) | Sq (nm) | Sy (nm) | Sp (nm) |
|---|---|---|---|---|
| Mild steel surface | 136.47 | 168.68 | 892.96 | 476.88 |
| Mild steel surface immersed in 1 M HCl | 379.59 | 476.21 | 3295.40 | 1530.6 |
| Mild steel surface immersed in 1 M HCl + 0.001 M of EAD | 274.51 | 344.64 | 2639.30 | 1679.00 |
| Samples | Ra (nm) | Rq (nm) | Ry (nm) | Rp (nm) |
|---|---|---|---|---|
| Mild steel surface | 119.44 | 161.1 | 684.87 | 406.66 |
| Mild steel surface immersed in 1 M HCl | 344.72 | 393.18 | 1359.9 | 726.51 |
| Mild steel surface immersed in 1 M HCl + 0.001 M of EAD | 198.54 | 256.62 | 1099.60 | 662.49 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheit, H.M.K.; Kani, S.M.; Sathiq, M.A.; Abuthahir, S.S.S.; Subhapriya, P.; Nivedhitha, K.S.; Umarfarooq, M.A.; Badruddin, I.A.; Kamangar, S.; Shaik, A.S. Experimental Studies on the Effect of Expired Amiodarone Drug (EAD) as a Corrosion Inhibitor on Mild Steel in 1 M HCl. Materials 2024, 17, 751. https://doi.org/10.3390/ma17030751
Sheit HMK, Kani SM, Sathiq MA, Abuthahir SSS, Subhapriya P, Nivedhitha KS, Umarfarooq MA, Badruddin IA, Kamangar S, Shaik AS. Experimental Studies on the Effect of Expired Amiodarone Drug (EAD) as a Corrosion Inhibitor on Mild Steel in 1 M HCl. Materials. 2024; 17(3):751. https://doi.org/10.3390/ma17030751
Chicago/Turabian StyleSheit, H. Mohamed Kasim, S. Musthafa Kani, M. Anwar Sathiq, S. S. Syed Abuthahir, P. Subhapriya, K. S. Nivedhitha, M. A. Umarfarooq, Irfan Anjum Badruddin, Sarfaraz Kamangar, and Abdul Saddique Shaik. 2024. "Experimental Studies on the Effect of Expired Amiodarone Drug (EAD) as a Corrosion Inhibitor on Mild Steel in 1 M HCl" Materials 17, no. 3: 751. https://doi.org/10.3390/ma17030751
APA StyleSheit, H. M. K., Kani, S. M., Sathiq, M. A., Abuthahir, S. S. S., Subhapriya, P., Nivedhitha, K. S., Umarfarooq, M. A., Badruddin, I. A., Kamangar, S., & Shaik, A. S. (2024). Experimental Studies on the Effect of Expired Amiodarone Drug (EAD) as a Corrosion Inhibitor on Mild Steel in 1 M HCl. Materials, 17(3), 751. https://doi.org/10.3390/ma17030751







