The Effect of Foaming Agents on the Thermal Behavior of Aluminum Precursors
Abstract
1. Introduction
2. Materials and Methods
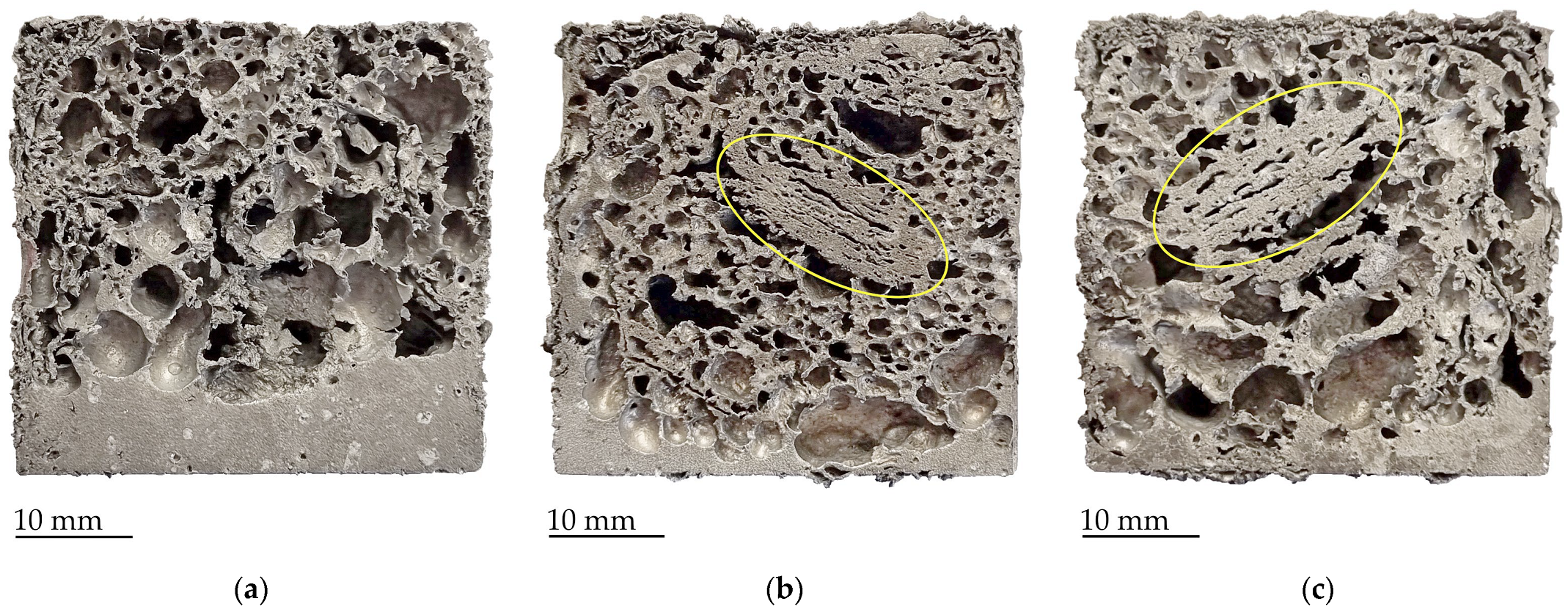
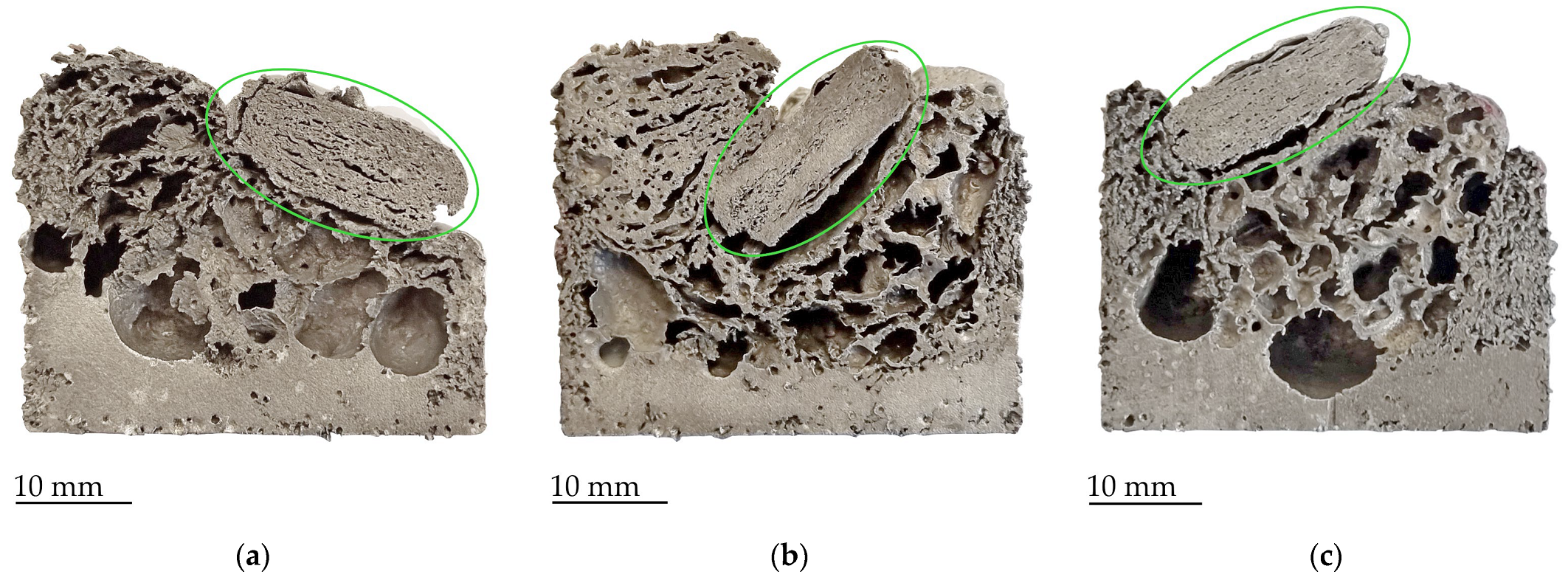
3. Results and Discussion
4. Conclusions
- (i)
- At a temperature of 700 °C, foaming of all precursors, except original (non-annealed) TiH2, was unsuccessful due to delayed pore nucleation. Too low of a temperature in the mold prevented foaming of the upper precursor pieces located near the top of the mold. Furthermore, at a temperature of 700 °C, the amount of gas released from the ZrH2 agent is too small for complete foaming.
- (ii)
- Calcium carbonate is the most effective foaming agent in the case of pure aluminum as it promotes rapid foaming through the development of carbon dioxide, which also helps to stabilize the cell walls. Thus, they create foams with a very large number of small cells due to the later onset of gas release from the foaming agent without drainage of the melt in the lower part of the sample.
- (iii)
- Unlike CaCO3, hydrides do not contribute to foam stabilization. The choice of hydride-based foaming agent for pure aluminum is more crucial. TiH2 proves to be the preferred option as it releases the most hydrogen stored in its structure up to 750 °C. The samples with the original TiH2 powder showed very good foaming properties. There was little drainage at the bottom of the samples and the foam cells were larger compared to the cells formed by the CaCO3 agent. But their size varied greatly due to the unstable structure because some cells start to merge and form larger ones.
- (iv)
- Heat-treated TiH2 agent has low foaming properties. This is the result of hydrogen loss during annealing, which is usually considered as the best approach for aluminum alloy foams, but not in the case of studied pure aluminum.
- (v)
- Samples with ZrH2 foaming agent have shown low foamability at lower temperatures. At 750 °C, the foaming was relatively good due to the higher hydrogen volume released, which makes the cells grow, but large melt drainage appears at the bottom of the sample.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- García-Moreno, F. Commercial Applications of Metal Foams: Their Properties and Production. Materials 2016, 9, 85. [Google Scholar] [CrossRef] [PubMed]
- Patrone, G.L.; Paffumi, E.; Otura, M.; Centurelli, M.; Ferrarese, C.; Jahn, S.; Brenner, A.; Thieringer, B.; Braun, D.; Hoffmann, T. Assessing the Energy Consumption and Driving Range of the QUIET Project Demonstrator Vehicle. Energies 2022, 15, 1290. [Google Scholar] [CrossRef]
- Kováčik, J.; Jerz, J.; Gopinathan, A.; Simančík, F.; Marsavina, L.; Linul, E. Effect of Sample Shape on Compression Behavior of Aluminum Foams. Mater. Today Proc. 2023, 78, 308–313. [Google Scholar] [CrossRef]
- Jerz, J.; Simančík, F.; Španielka, J.; Šebek, J.; Kováčik, J.; Tobolka, P.; Dvorák, T.; Orovčík, Ľ. Energy Demand Reduction in Nearly Zero-Energy Buildings by Highly Efficient Aluminium Foam Heat Exchangers. Mater. Sci. Forum 2018, 919, 236–245. [Google Scholar] [CrossRef]
- Parveez, B.; Jamal, N.A.; Maleque, A.; Yusof, F.; Jamadon, N.H.; Adzila, S. Review on Advances in Porous Al Composites and the Possible Way Forward. J. Mater. Res. Technol. 2021, 14, 2017–2038. [Google Scholar] [CrossRef]
- Lefebvre, L.-P.; Banhart, J.; Dunand, D.C. Porous Metals and Metallic Foams: Current Status and Recent Developments. Adv. Eng. Mater. 2008, 10, 775–787. [Google Scholar] [CrossRef]
- Demir, G.; Akyurek, D.; Hassoun, A.; Mutlu, I. Production of Biodegradable Metal Foams by Powder Metallurgy Method. Phys. Mesomech. 2023, 26, 196–208. [Google Scholar] [CrossRef]
- Gopinathan, A.; Jerz, J.; Kováčik, J.; Dvorák, T. Investigation of the Relationship Between Morphology and Thermal Conductivity of Powder Metallurgically Prepared Aluminium Foams. Materials 2021, 14, 3623. [Google Scholar] [CrossRef]
- Andreozzi, A.; Bianco, N.; Iasiello, M.; Naso, V. Natural Convection in a Vertical Channel with Open-Cell Foams. J. Phys. Conf. Ser. 2020, 1599, 012013. [Google Scholar] [CrossRef]
- Chen, J.; Yang, D.; Jiang, J.; Ma, A.; Song, D. Research Progress of Phase Change Materials (PCMs) Embedded with Metal Foam (a Review). Procedia Mater. Sci. 2014, 4, 389–394. [Google Scholar] [CrossRef]
- Banhart, J. Manufacture, Characterisation and Application of Cellular Metals and Metal Foams. Prog. Mater. Sci. 2001, 46, 559–632. [Google Scholar] [CrossRef]
- Goyal, B.; Pandey, A. Critical Review on Porous Material Manufacturing Techniques, Properties and Their Applications. Mater. Today Proc. 2021, 46, 8196–8203. [Google Scholar] [CrossRef]
- Parveez, B.; Jamal, N.A.; Anuar, H.; Ahmad, Y.; Aabid, A.; Baig, M. Microstructure and Mechanical Properties of Metal Foams Fabricated via Melt Foaming and Powder Metallurgy Technique: A Review. Materials 2022, 15, 5302. [Google Scholar] [CrossRef] [PubMed]
- Bisht, A.; Gangil, B.; Patel, V.K. Selection of Blowing Agent for Metal Foam Production: A Review. J. Met. Mater. Miner. 2020, 30, 1–10. [Google Scholar] [CrossRef]
- Wan, T.; Liu, Y.; Zhou, C.; Chen, X.; Li, Y. Fabrication, Properties, and Applications of Open-Cell Aluminum Foams: A Review. J. Mater. Sci. Technol. 2021, 62, 11–24. [Google Scholar] [CrossRef]
- Meenashisundaram, G.K.; Xu, Z.; Nai, M.L.S.; Lu, S.; Ten, J.S.; Wei, J. Binder Jetting Additive Manufacturing of High Porosity 316L Stainless Steel Metal Foams. Materials 2020, 13, 3744. [Google Scholar] [CrossRef]
- Maskery, I.; Aboulkhair, N.T.; Aremu, A.O.; Tuck, C.J.; Ashcroft, I.A.; Wildman, R.D.; Hague, R.J.M. A Mechanical Property Evaluation of Graded Density Al-Si10-Mg Lattice Structures Manufactured by Selective Laser Melting. Mater. Sci. Eng. A 2016, 670, 264–274. [Google Scholar] [CrossRef]
- Yan, C.; Hao, L.; Hussein, A.; Bubb, S.L.; Young, P.; Raymont, D. Evaluation of Light-Weight AlSi10Mg Periodic Cellular Lattice Structures Fabricated via Direct Metal Laser Sintering. J. Mater. Process. Technol. 2014, 214, 856–864. [Google Scholar] [CrossRef]
- Banhart, J. Light-Metal Foams—History of Innovation and Technological Challenges. Adv. Eng. Mater. 2013, 15, 82–111. [Google Scholar] [CrossRef]
- Paulin, I.; Šuštaršič, B.; Kevorkijan, V.; Škapin, S.D.; Jenko, M. Synthesis of Aluminium Foams by the Powder-Metallurgy Process: Compacting of Precursors. Mater. Tehnol. 2011, 45, 13–19. [Google Scholar]
- Gergely, V.; Curran, D.C.; Clyne, T.W. The FOAMCARP Process: Foaming of Aluminium MMCs by the Chalk-Aluminium Reaction in Precursors. Compos. Sci. Technol. 2003, 63, 2301–2310. [Google Scholar] [CrossRef]
- Bunjan, I.; Grilec, K.; Ćorić, D. Investigation and Statistical Evaluation of Reinforced Aluminum Foams. Processes 2021, 9, 315. [Google Scholar] [CrossRef]
- Krolo, J.; Lela, B.; Grgić, K.; Jozić, S. Production of Closed-Cell Foams Out of Aluminum Chip Waste: Mathematical Modeling and Optimization. Metals 2022, 12, 933. [Google Scholar] [CrossRef]
- Paulin, I. Synthesis and Characterization of Al Foams Produced by Powder Metallurgy Route Using Dolomite and Titanium Hydride as a Foaming Agents. Mater. Tehnol. 2014, 48, 943–947. [Google Scholar]
- Yang, D.; Chen, J.; Wang, H.; Jiang, J.; Ma, A.; Lu, Z.P. Effect of Decomposition Kinetics of Titanium Hydride on the Al Alloy Melt Foaming Process. J. Mater. Sci. Technol. 2015, 31, 361–368. [Google Scholar] [CrossRef]
- von Zeppelin, F.; Hirscher, M.; Stanzick, H.; Banhart, J. Desorption of Hydrogen from Blowing Agents Used for Foaming Metals. Compos. Sci. Technol. 2003, 63, 2293–2300. [Google Scholar] [CrossRef]
- Orovčík, Ľ.; Nosko, M.; Švec, P.; Nagy, Š.; Čavojský, M.; Simančík, F.; Jerz, J. Effect of the TiH2 Pre-Treatment on the Energy Absorption Ability of 6061 Aluminium Alloy Foam. Mater. Lett. 2015, 148, 82–85. [Google Scholar] [CrossRef]
- Lázaro, J.; Solórzano, E.; Rodríguez-Pérez, M.A.; Rämer, O.; García-Moreno, F.; Banhart, J. Heat Treatment of Aluminium Foam Precursors: Effects on Foam Expansion and Final Cellular Structure. Procedia Mater. Sci. 2014, 4, 287–292. [Google Scholar] [CrossRef]
- Cambronero, L.E.G.; Ruiz-Roman, J.M.; Corpas, F.A.; Ruiz Prieto, J.M. Manufacturing of Al–Mg–Si Alloy Foam Using Calcium Carbonate as Foaming Agent. J. Mater. Process. Technol. 2009, 209, 1803–1809. [Google Scholar] [CrossRef]
- Koizumi, T.; Kido, K.; Kita, K.; Mikado, K.; Gnyloskurenko, S.; Nakamura, T. Method of Preventing Shrinkage of Aluminum Foam Using Carbonates. Metals 2012, 2, 1–9. [Google Scholar] [CrossRef]
- Byakova, A.; Gnyloskurenko, S.; Nakamura, T. The Role of Foaming Agent and Processing Route in the Mechanical Performance of Fabricated Aluminum Foams. Metals 2012, 2, 95–112. [Google Scholar] [CrossRef]
- Paulin, I. Stability of Close-Cell Al Foams Depending on the Usage of Different Foaming Agents. Mater. Tehnol. 2015, 49, 983–988. [Google Scholar] [CrossRef]
- Singh, R.; Arora, R.; Sharma, J.D. Effect of Viscosity Enhancing Agents on Quasi-Static Compression Behavior of Aluminum Foams. Mater. Today Proc. 2021, 39, 1661–1666. [Google Scholar] [CrossRef]
- Geramipour, T.; Oveisi, H. Effects of Foaming Parameters on Microstructure and Compressive Properties of Aluminum Foams Produced by Powder Metallurgy Method. Trans. Nonferrous Met. Soc. China 2017, 27, 1569–1579. [Google Scholar] [CrossRef]
- Rodinger, T.; Ćorić, D.; Alar, Ž. The Influence of Foaming Agents on Aluminium Foam Cell Morphology. Metals 2023, 13, 1146. [Google Scholar] [CrossRef]
- Jaafar, A.H.; Al-Ethari, H.; Farhan, K. Modelling and Optimization of Manufacturing Calcium Carbonate-Based Aluminum Foam. Mater. Res. Express 2019, 6, 0865g1. [Google Scholar] [CrossRef]
- Haesche, M.; Lehmhus, D.; Weise, J.; Wichmann, M.; Magnabosco Mocellin, I.C. Carbonates as Foaming Agent in Chip-Based Aluminium Foam Precursor. J. Mater. Sci. Technol. 2010, 26, 845–850. [Google Scholar] [CrossRef]
- Wang, X.; Meng, Q.; Wang, T.; Chu, X.; Fan, A.; Wang, H. New Insights into Fabrication of Al-Based Foam with Homogeneous Small Pore-Structure Using MgCO3/Zn Composite Powder as a Foaming Agent. Metals 2022, 12, 786. [Google Scholar] [CrossRef]
- Matijasevic, B.; Görke, O.; Schubert, H.; Banhart, J. Zirconium Hydride—A Possible Blowing Agent for Making Aluminium Alloy Foams. In Porous Metals and Metal Foaming Technology; Nakajima, H., Kanetake, N., Eds.; The Japan Institute of Metals: Kyoto, Japan, 2005; pp. 107–110. [Google Scholar]
- Kamm, P.H.; García-Moreno, F.; Jiménez, C.; Banhart, J. Suitability of Various Complex Hydrides for Foaming Aluminum Alloys. J. Mater. Res. 2013, 28, 2436–2443. [Google Scholar] [CrossRef][Green Version]
- Hammond, C.R. Properties of the Elements and Inorganic Compounds. In CRC Handbook of Chemistry and Physics, 97th ed.; Haynes, W.M., Lide, D.R., Bruno, T.J., Eds.; CRC Press: Boca Raton, FL, USA, 2017; pp. 4-44–4-96. [Google Scholar]
- Nosko, M.; Simančík, F.; Florek, R. Reproducibility of Aluminum Foam Properties: Effect of Precursor Distribution on the Structural Anisotropy and the Collapse Stress and Its Dispersion. Mater. Sci. Eng. A 2010, 527, 5900–5908. [Google Scholar] [CrossRef]
- Gergely, V.; Clyne, T.W. Drainage in Standing Liquid Metal Foams: Modelling and Experimental Observations. Acta Mater. 2004, 52, 3047–3058. [Google Scholar] [CrossRef]
- Borchers, C.; Khomenko, T.I.; Leonov, A.V.; Morozova, O.S. Interrupted Thermal Desorption of TiH2. Thermochim. Acta 2009, 493, 80–84. [Google Scholar] [CrossRef]
- Ma, M.; Liang, L.; Tang, B.; Xiang, W.; Wang, Y.; Cheng, Y.; Tan, X. Decomposition Kinetics Study of Zirconium Hydride by Interrupted Thermal Desorption Spectroscopy. J. Alloys Compd. 2015, 645, S217–S220. [Google Scholar] [CrossRef]
- Banhart, J.; Ritter, C. Decomposition of Ti and Zr Hydrides Studied by Neutron Diffraction. In Proceedings of the 11th International Conference on Porous Metals and Metallic Foams (MetFoam 2019); Dukhan, N., Ed.; The Minerals, Metals & Materials Society: Dearborn, MI, USA, 2020; pp. 39–46. [Google Scholar] [CrossRef]
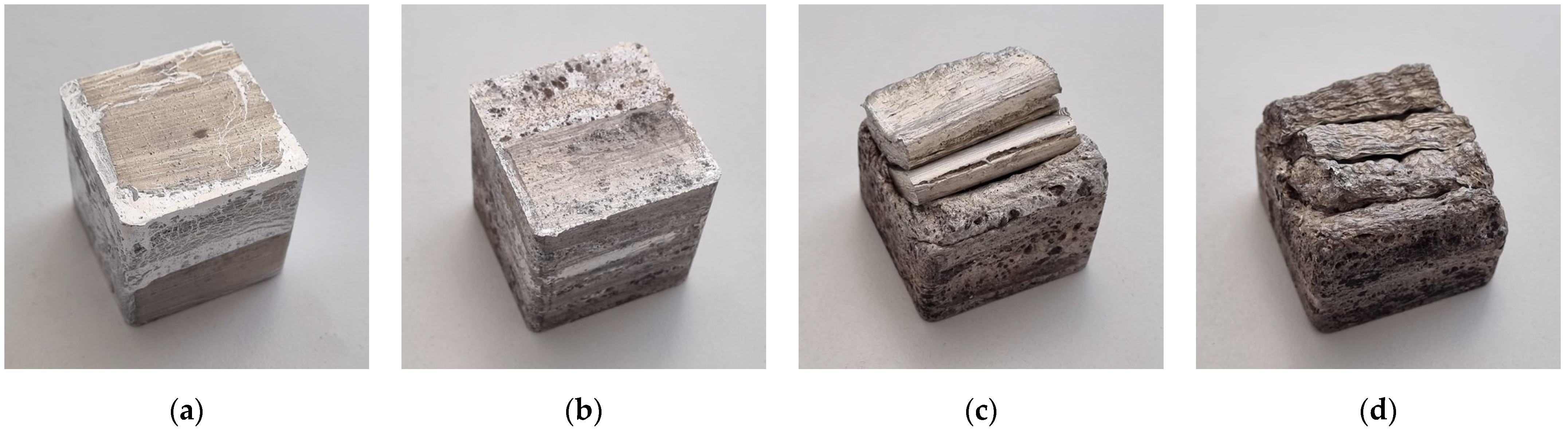
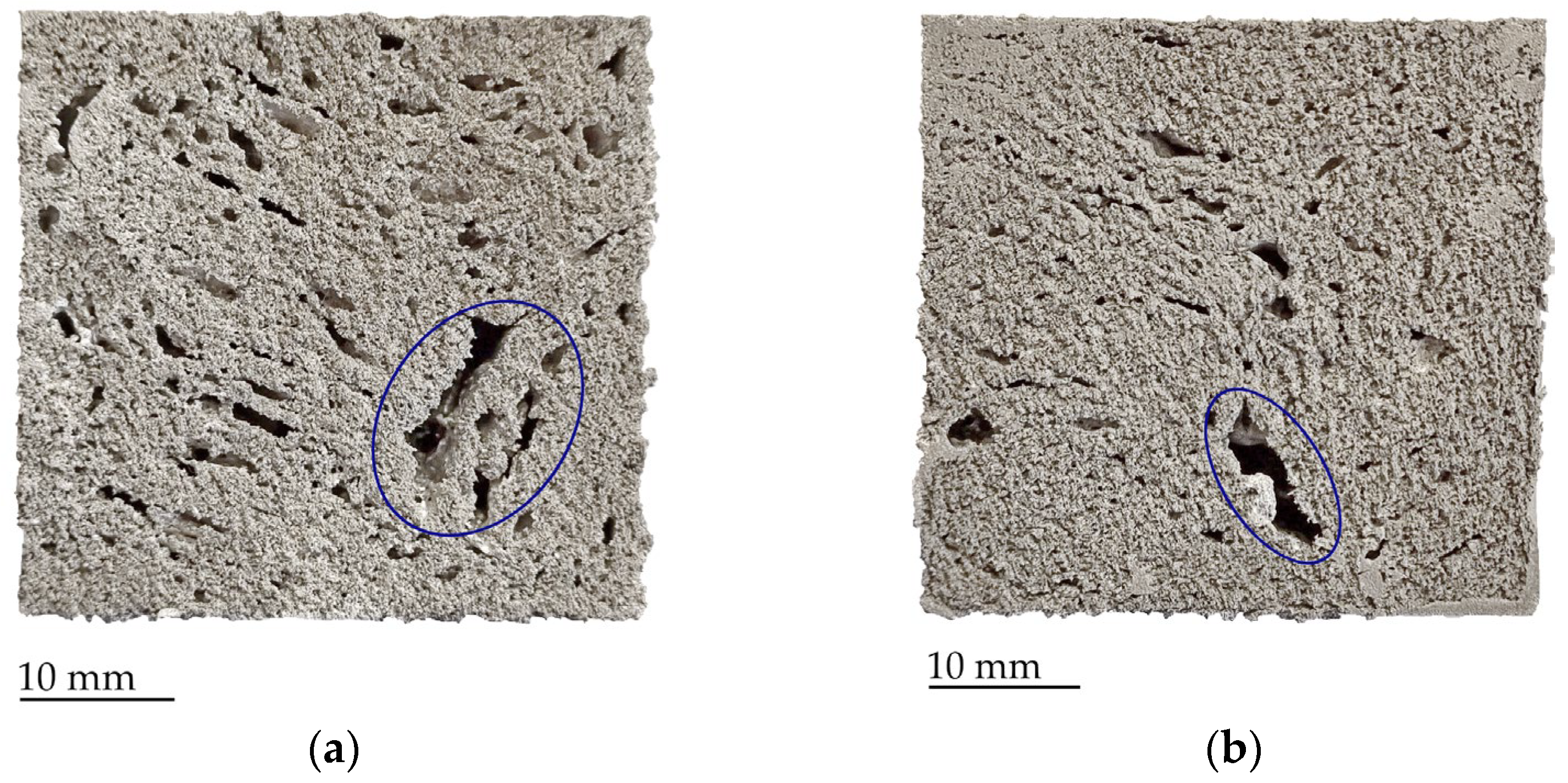
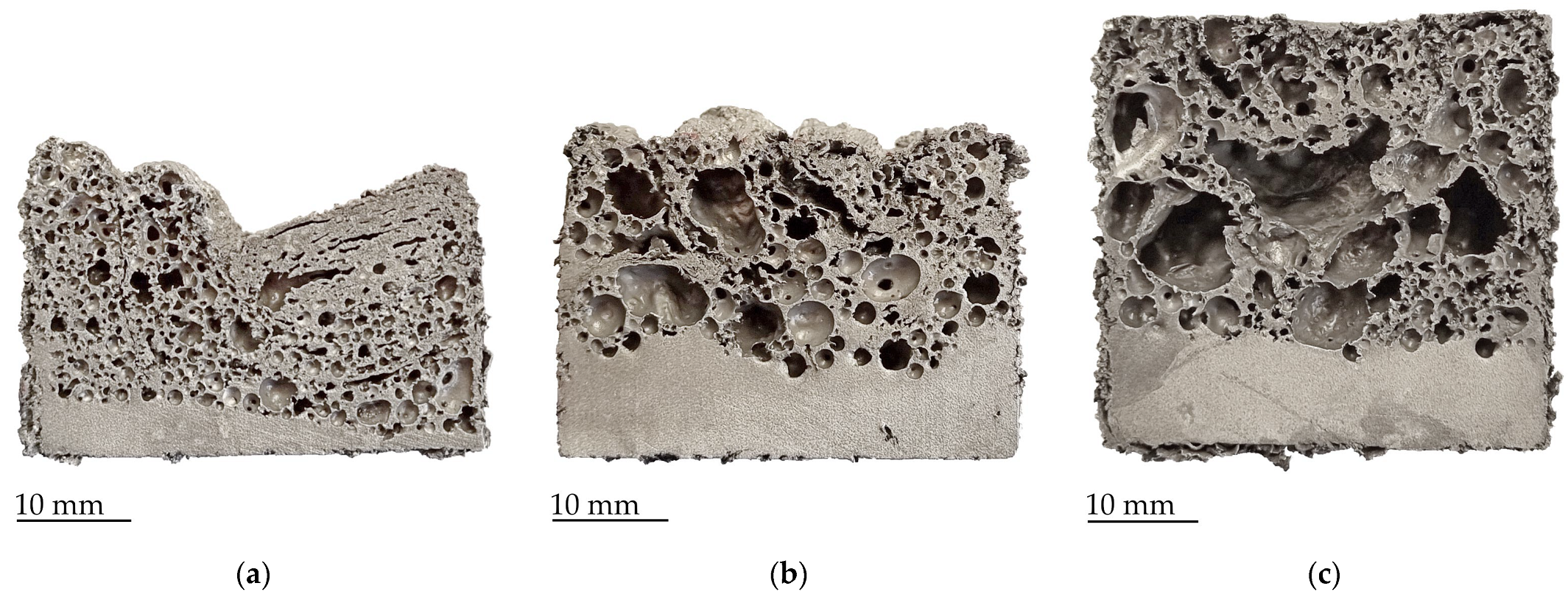
| Mixture | Chemical Composition |
|---|---|
| Al-CaCO3 | 99 wt.% Al + 1 wt.% CaCO3 |
| Al-TiH2 | 99 wt.% Al + 1 wt.% TiH2 |
| Al-TiH2 (HT) | 99 wt.% Al + 1 wt.% TiH2 (HT) |
| Al-ZrH2 | 99 wt.% Al + 1 wt.% ZrH2 |
| Precursor | Foaming Temperatures, °C | ||
|---|---|---|---|
| Al-CaCO3 | – | 720 | 750 |
| Al-TiH2 | 700 | 720 | 750 |
| Al-TiH2 (HT) | 700 | 720 | 750 |
| Al-ZrH2 | 700 | 720 | 750 |
| Material | d50, μm | d90, μm |
|---|---|---|
| Aluminum | 60.32 ± 0.57 | 102.48 ± 0.26 |
| CaCO3 | 2.26 ± 0.20 | 11.67 ± 0.80 |
| TiH2 | 17.34 ± 0.11 | 37.52 ± 0.01 |
| TiH2 (HT) | 16.29 ± 0.18 | 37.74 ± 0.46 |
| ZrH2 | 6.33 ± 0.04 | 17.46 ± 0.59 |
| Precursor | Measured Density, g/cm³ | Theoretical Density, g/cm³ | Relative Density, % |
|---|---|---|---|
| Al-CaCO3 | 2.6861 ± 0.0021 | 2.7001 | 99.48 |
| Al-TiH2 | 2.7013 ± 0.0057 | 2.7105 | 99.66 |
| Al-TiH2 (HT) | 2.7023 ± 0.0039 | 2.7105 | 99.70 |
| Al-ZrH2 | 2.7119 ± 0.0033 | 2.7290 | 99.37 |
| Sample | Foaming Temperature, °C | Number of Cells | Cell Size, mm2 | Foamability | ||
|---|---|---|---|---|---|---|
| Mean Size | Standard Deviation | Maximum Size | ||||
| Al-CaCO3 | 720 | 18,458 | 0.02315 | 0.34739 | 31.76052 | + |
| 750 | 12,451 | 0.01567 | 0.32500 | 33.29073 | + | |
| 700 | 762 | 1.16649 | 11.42545 | 245.42725 | + | |
| Al-TiH2 | 720 | 611 | 1.21161 | 9.81946 | 201.5518 | + |
| 750 | 476 | 1.71642 | 11.23842 | 181.45033 | + | |
| 700 | 359 | 1.37276 | 12.02811 | 168.69567 | − | |
| Al-TiH2 (HT) | 720 | 526 | 0.69720 | 7.13230 | 118.32997 | − |
| 750 | 807 | 0.62731 | 5.89777 | 141.79737 | − | |
| 700 | 535 | 0.64446 | 2.14417 | 23.79973 | − | |
| Al-ZrH2 | 720 | 200 | 1.58381 | 7.34641 | 92.04191 | − |
| 750 | 350 | 2.25070 | 15.94923 | 218.48624 | + | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodinger, T.; Ćorić, D.; Kováčik, J. The Effect of Foaming Agents on the Thermal Behavior of Aluminum Precursors. Materials 2024, 17, 710. https://doi.org/10.3390/ma17030710
Rodinger T, Ćorić D, Kováčik J. The Effect of Foaming Agents on the Thermal Behavior of Aluminum Precursors. Materials. 2024; 17(3):710. https://doi.org/10.3390/ma17030710
Chicago/Turabian StyleRodinger, Tomislav, Danko Ćorić, and Jaroslav Kováčik. 2024. "The Effect of Foaming Agents on the Thermal Behavior of Aluminum Precursors" Materials 17, no. 3: 710. https://doi.org/10.3390/ma17030710
APA StyleRodinger, T., Ćorić, D., & Kováčik, J. (2024). The Effect of Foaming Agents on the Thermal Behavior of Aluminum Precursors. Materials, 17(3), 710. https://doi.org/10.3390/ma17030710








