Influence of the Original Concrete Strength and Initial Moisture Condition on the Properties Improvement of Recycled Coarse Aggregate via Accelerated Carbonation Reactions
Abstract
1. Introduction
2. Materials and Methods
2.1. Raw Materials and Mixture Proportions for the Original Concrete of RCA
2.2. RCA Production and Preparation
- (1)
- Complete drying state: the RCA sample was poured into a shallow plate and dried to a constant weight at 105 ± 5 °C in a blast oven.
- (2)
- Untreated state: the RCA after crushing and screening was used directly for reserve, without any treatment.
- (3)
- Complete wetting state: the RCA was soaked in water for 24 h for fully saturated water treatment and removed with a wet towel to dry the water on the surface of the sample to prepare a saturated surface dry sample for use.
2.3. CO2 Curing Treatment Method for RCA
2.4. Determination of the Properties of Coarse Aggregate
2.4.1. Apparent Density
2.4.2. Water Absorption
2.4.3. Moisture Content
2.4.4. Mass Variation and Carbonation Ratio of CRCA
3. Results and Analysis
3.1. Macroscopic Properties of RCA before and after Carbonation
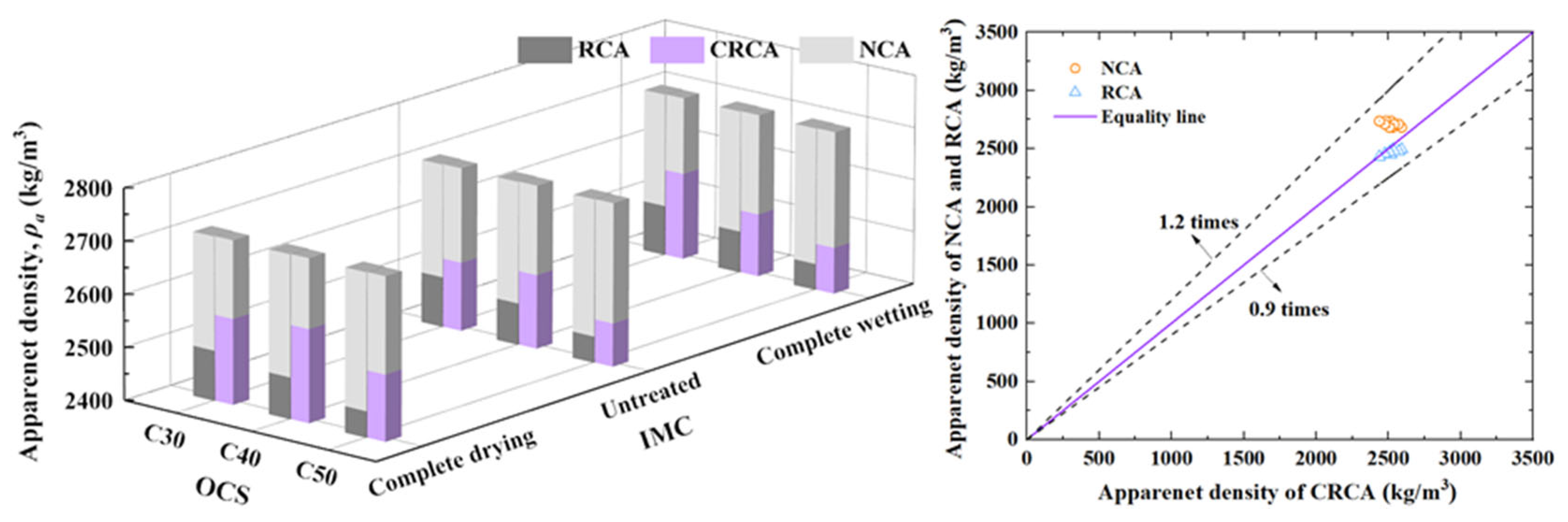
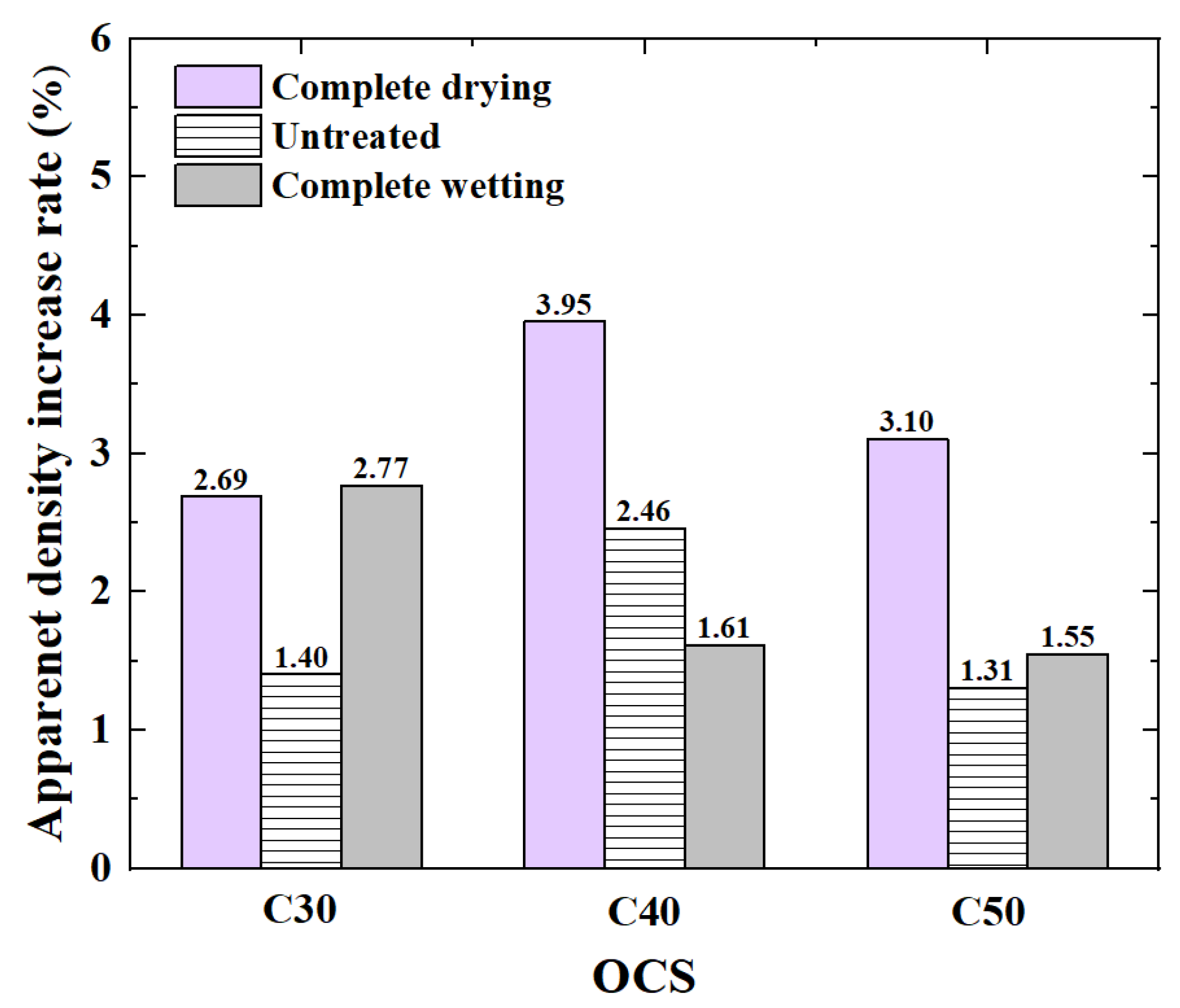
3.1.1. Apparent Density
- (1)
- The influence of OCS on apparent density
- (2)
- The influence of IMC on apparent density
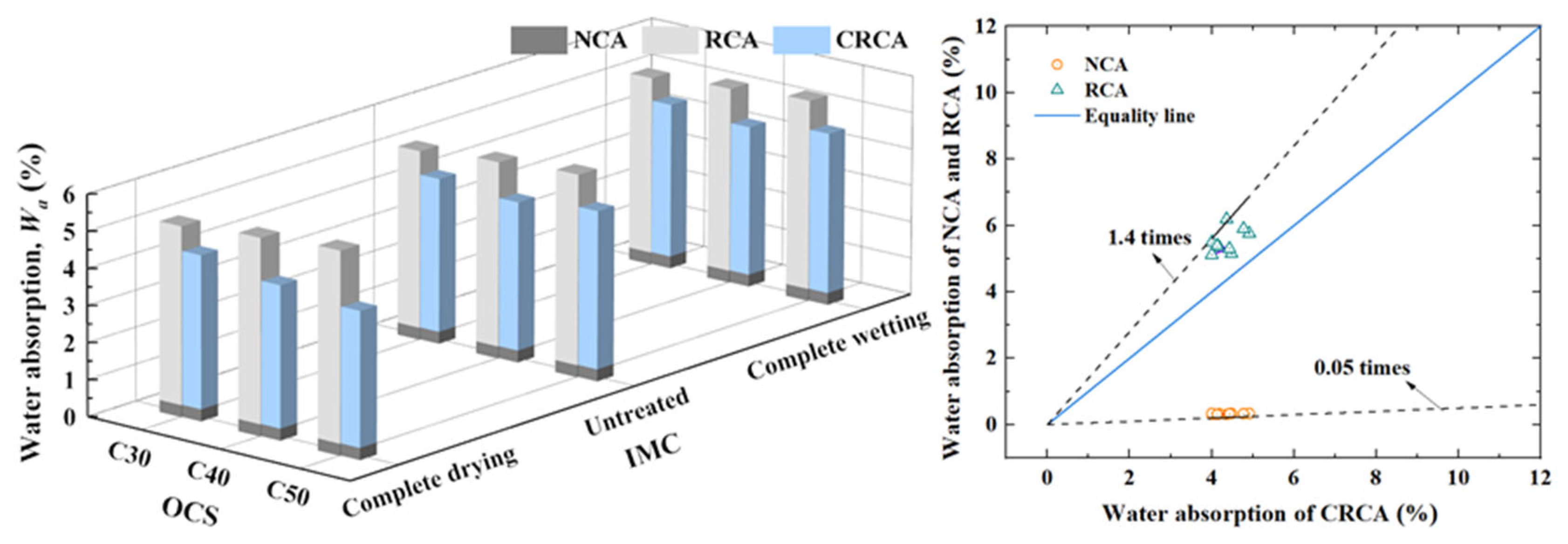
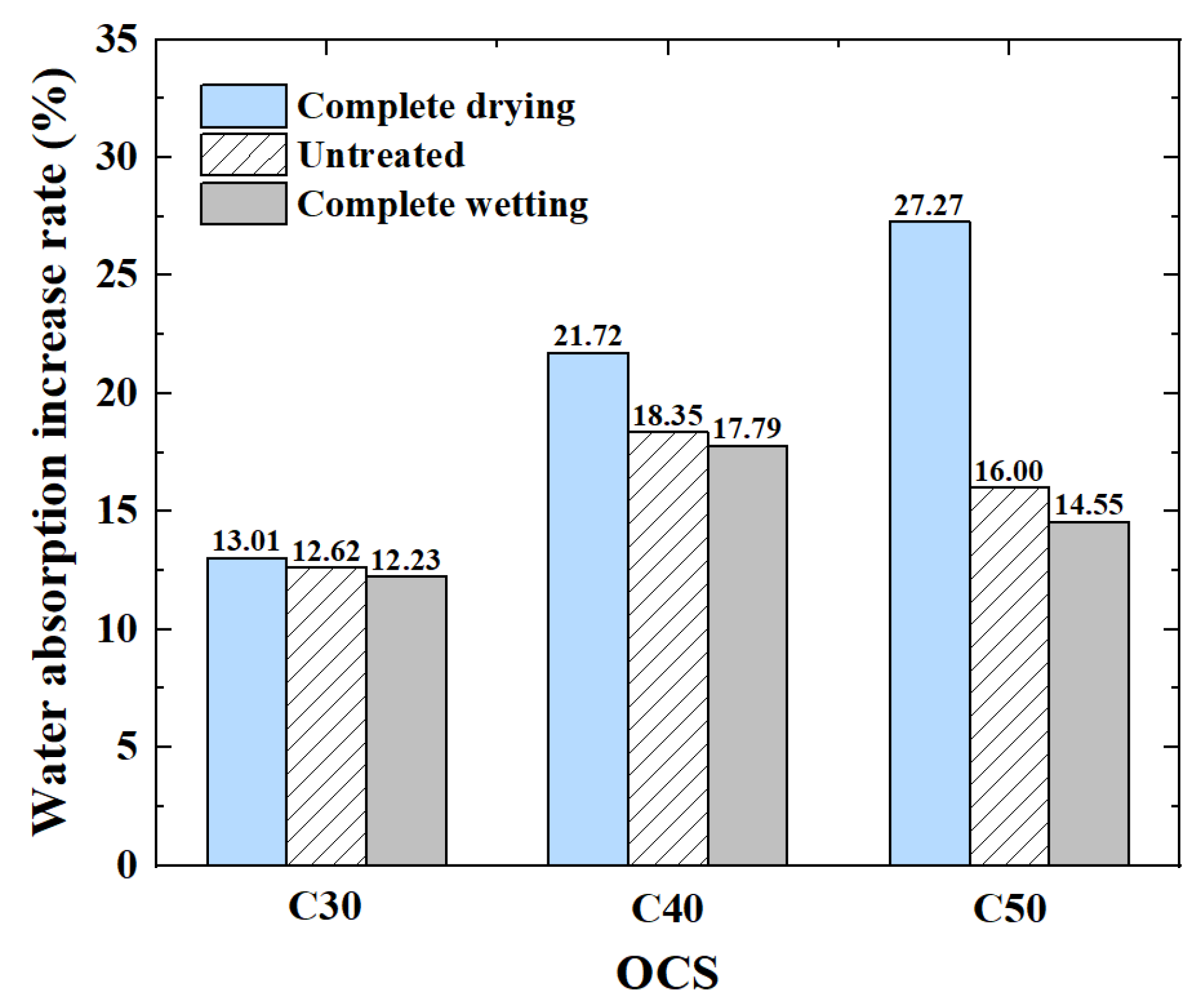
3.1.2. Water Absorption
- (1)
- The influence of OCS on water absorption
- (2)
- The influence of IMC on water absorption
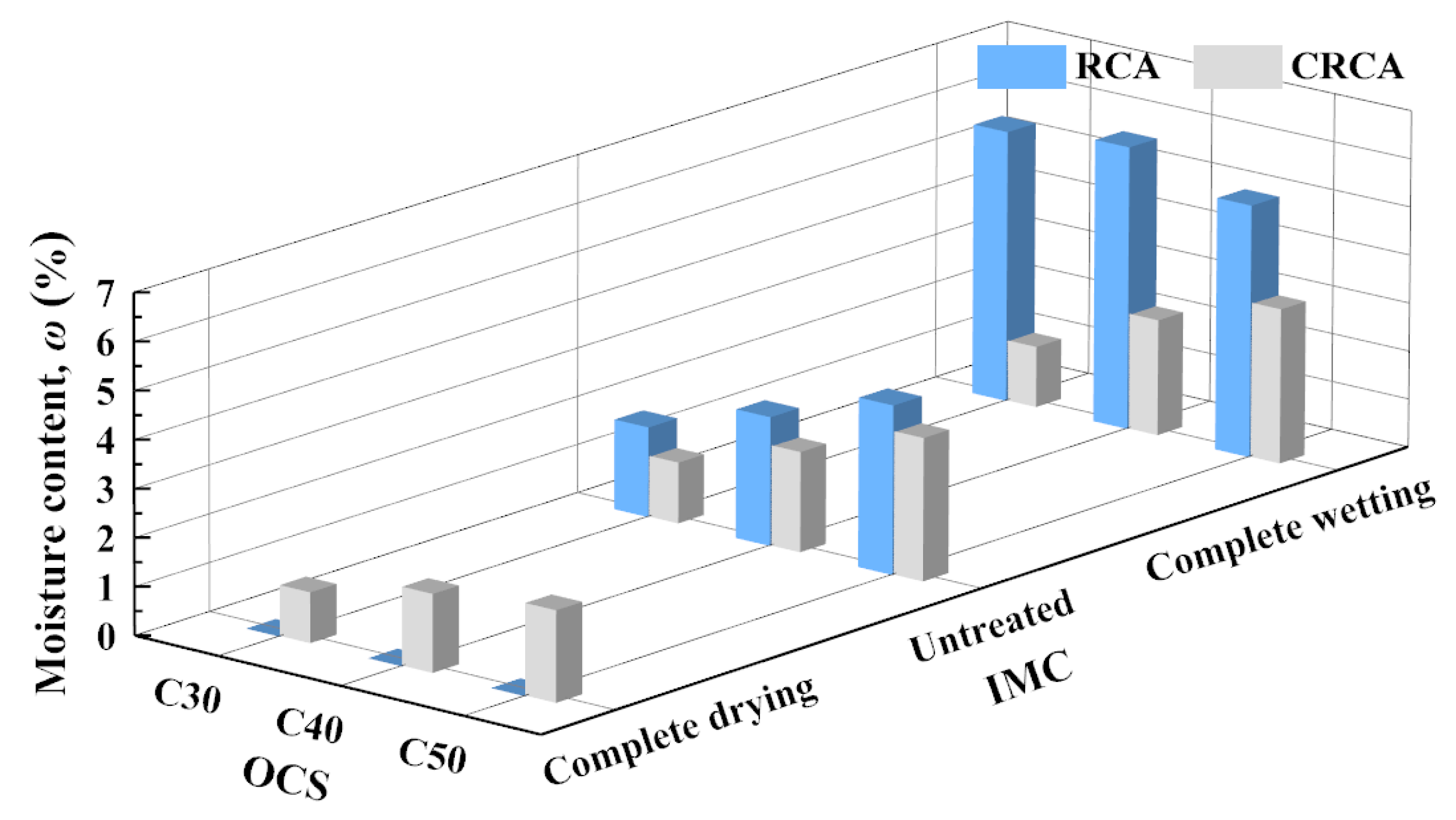
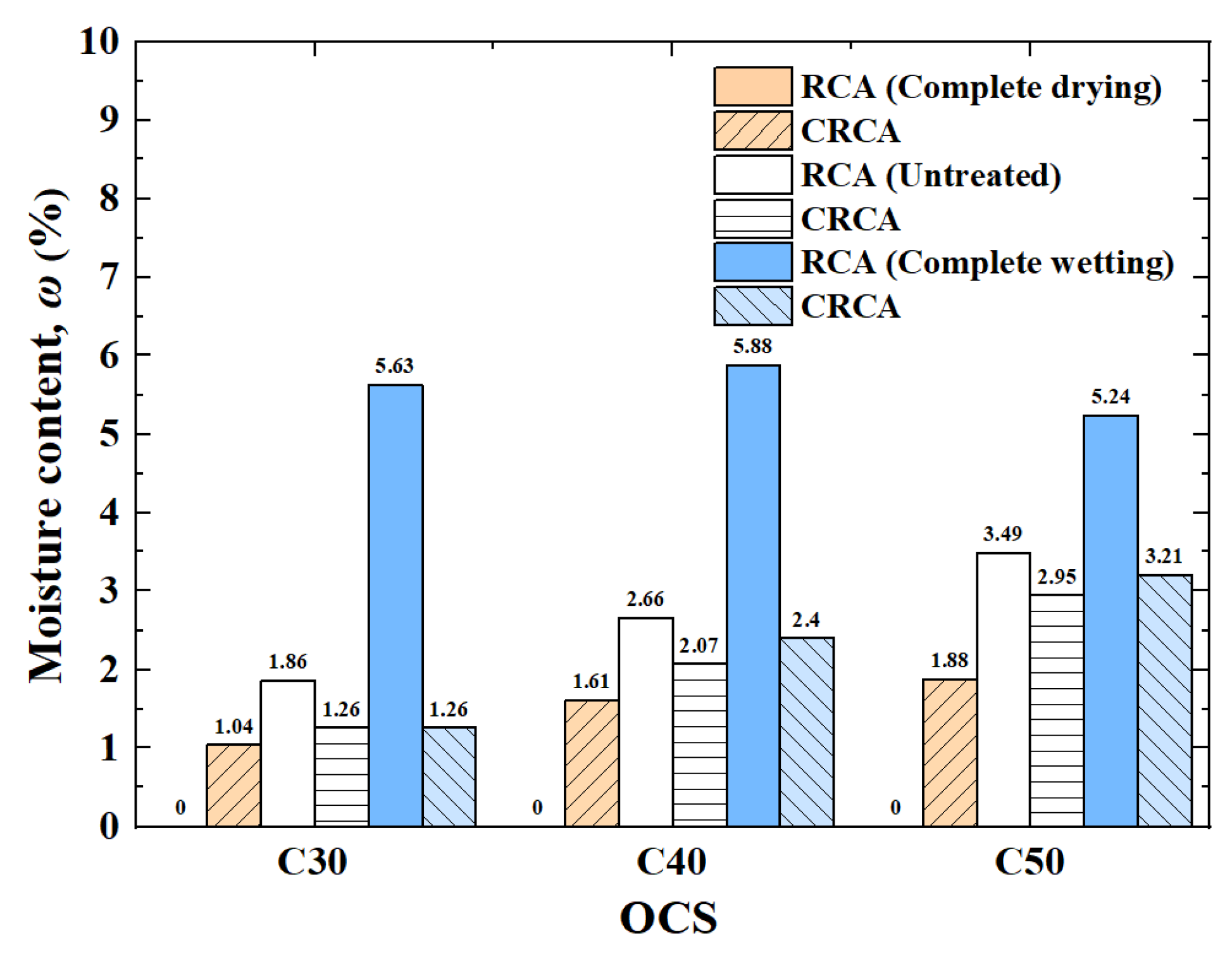
3.1.3. Moisture Content
- (1)
- The influence of OCS on moisture content
- (2)
- The influence of IMC on moisture content
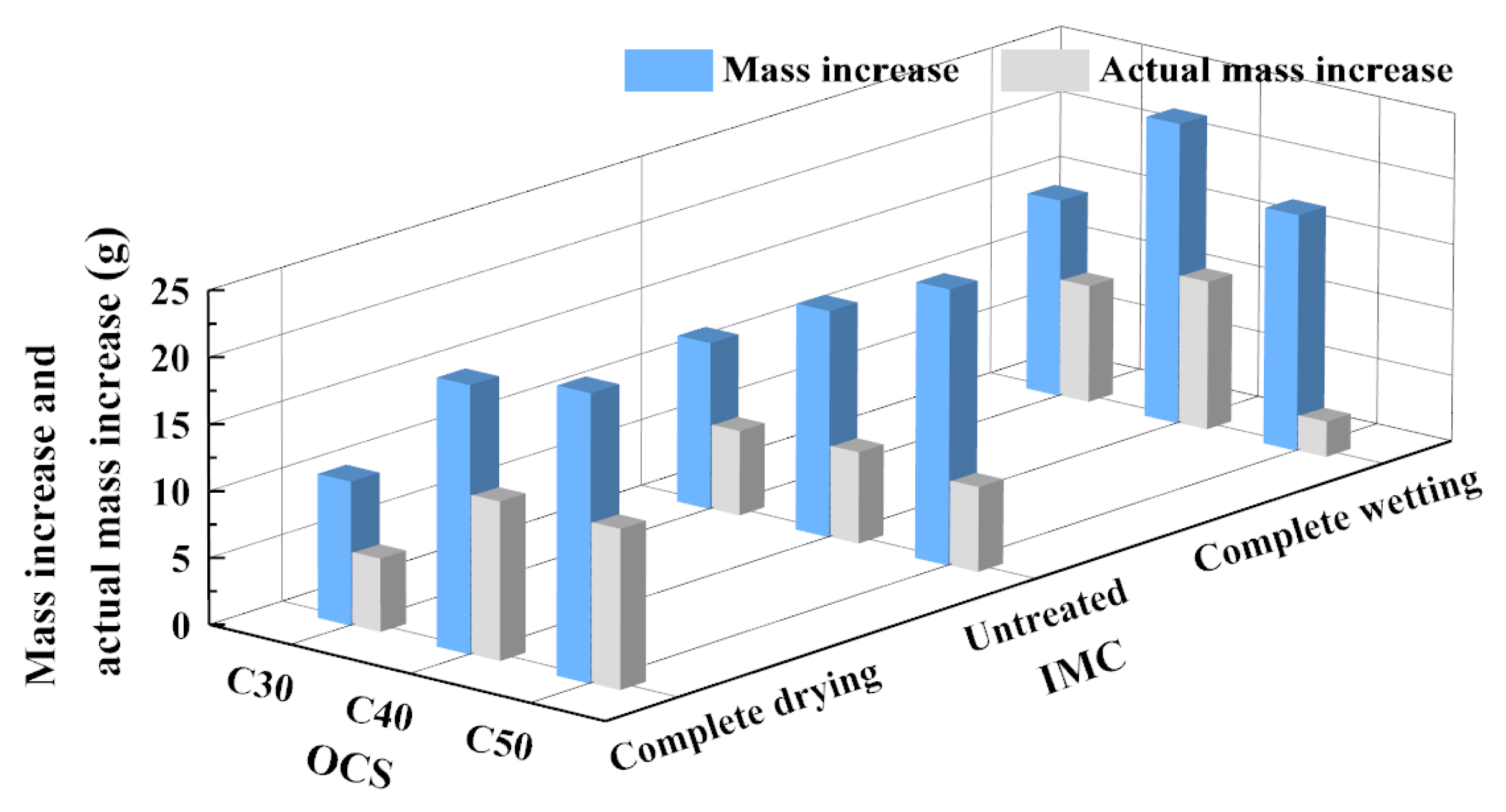
3.1.4. Mass Increase and Actual Mass Increase
- (1)
- The influence of OCS on mass increase
- (2)
- The influence of IMC on mass increase
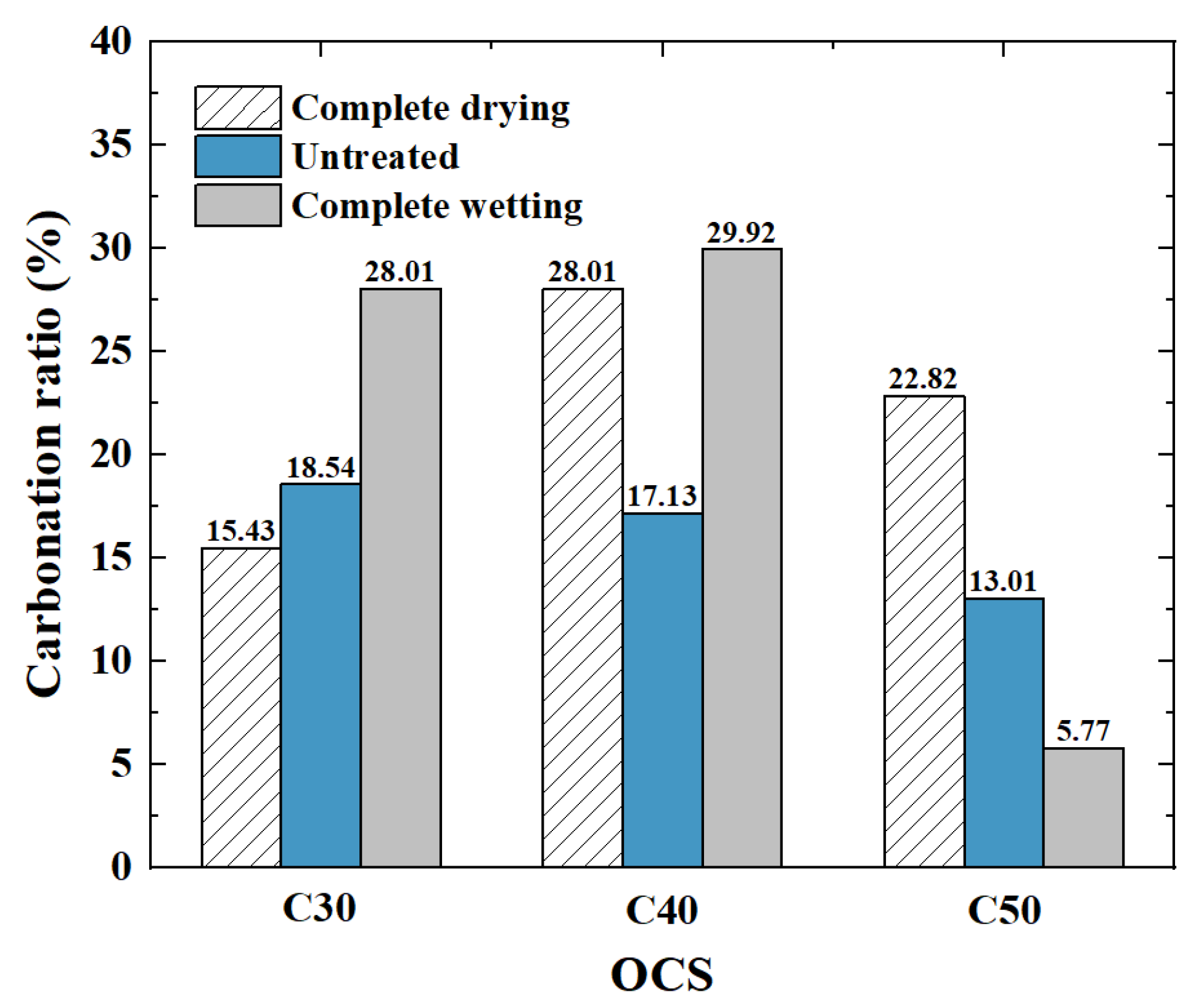
3.1.5. Carbonation Ratio
- (1)
- Influence of the OCS on the carbonation ratio
- (2)
- Influence of the IMC on the carbonation ratio
3.2. Microanalysis of RCAs before and after Carbonation
4. Discussion
5. Conclusions and Foresight
- (1)
- Compared with that of RCA, the apparent density of CRCA increased by 0.3–3.95%. The apparent density of dried RCAs with OCSs of C40 and C50 increased the most.
- (2)
- The water absorption of CRCA decreased by 7.98–29.56%. In particular, the dried RCAs with an OCS of C50 decreased the most.
- (3)
- The actual mass increase of CRCA increased by 0.54–2.66%, and the actual mass increase of the dried RCAs with an OCS of C50 was the most significant.
- (4)
- The carbonation rates of the completely dried and untreated RCAs with an OCS of C40 were the highest, with values of 29.92%.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wang, X.; Wang, A.; Zhang, Z.; Dai, J.-G.; Liu, K.; Chu, Y.; Guan, Y.; Sun, D. Enhancing the performance of alkali-activated material based coral concrete through microbubble aeration clean technology. Compos. Part B Eng. 2023, 252, 110519. [Google Scholar] [CrossRef]
- Lu, S.; Xia, W.; Bai, E.; Ling, L.; Du, Y. Interfacial modification: The dynamic compression properties and enhancement mechanism of concrete added with micro-nano hierarchical carbon-based fiber. Compos. Part B Eng. 2022, 247, 110340. [Google Scholar] [CrossRef]
- Li, C. Mechanical and transport properties of recycled aggregate concrete modified with limestone powder. Compos. Part B Eng. 2020, 197, 108189. [Google Scholar] [CrossRef]
- Li, Z. Rheological model of fresh concrete considering granular characteristics. Compos. Part B Eng. 2022, 244, 110148. [Google Scholar] [CrossRef]
- Qin, J.; Dai, F.; Ma, H.; Dai, X.; Li, Z.; Jia, X.; Qian, J. Development and characterization of magnesium phosphate cement based ultra-high performance concrete. Compos. Part B Eng. 2022, 234, 109694. [Google Scholar] [CrossRef]
- Xie, J.; Wang, J.; Rao, R.; Wang, C.; Fang, C. Effects of combined usage of GGBS and fly ash on workability and mechanical properties of alkali activated geopolymer concrete with recycled aggregate. Compos. Part B Eng. 2019, 164, 179–190. [Google Scholar] [CrossRef]
- Nour, A.I.; Güneyisi, E.M. Prediction model on compressive strength of recycled aggregate concrete filled steel tube columns. Compos. Part B Eng. 2019, 173, 106938. [Google Scholar] [CrossRef]
- Zhang, H.; Wu, J.; Jin, F.; Zhang, C. Effect of corroded tension reinforcements on flexural performance of reinforced recycled aggregate concrete beams strengthened with CFRP. Compos. Part B Eng. 2019, 162, 589–599. [Google Scholar] [CrossRef]
- Khedmati, M.; Kim, Y.-R.; Turner, J.A. Investigation of the interphase between recycled aggregates and cementitious binding materials using integrated microstructural-nanomechanical-chemical characterization. Compos. Part B Eng. 2019, 158, 218–229. [Google Scholar] [CrossRef]
- Ouyang, K.; Liu, J.; Liu, S.; Song, B.; Guo, H.; Li, G.; Shi, C. Influence of pre-treatment methods for recycled concrete aggregate on the performance of recycled concrete: A review. Resour. Conserv. Recycl. 2023, 188, 106717. [Google Scholar] [CrossRef]
- Pu, Y.; Li, L.; Wang, Q.; Shi, X.; Fu, L.; Zhang, G.; Luan, C.; Abomohra, A.E.-F. Accelerated carbonation treatment of recycled concrete aggregates using flue gas: A comparative study towards performance improvement. J. CO2 Util. 2021, 43, 101362. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Soomro, M.; Evangelista, A.C.J. A review of recycled aggregate in concrete applications (2000–2017). Constr. Build. Mater. 2018, 172, 272–292. [Google Scholar] [CrossRef]
- Guo, H.; Shi, C.; Guan, X.; Zhu, J.; Ding, Y.; Ling, T.-C.; Zhang, H.; Wang, Y. Durability of recycled aggregate concrete—A review. Cem. Concr. Compos. 2018, 89, 251–259. [Google Scholar] [CrossRef]
- Xing, W.; Tam, V.W.Y.; Le, K.N.; Butera, A.; Hao, J.L.; Wang, J. Effects of mix design and functional unit on life cycle assessment of recycled aggregate concrete: Evidence from CO2 concrete. Constr. Build. Mater. 2022, 348, 128712. [Google Scholar] [CrossRef]
- Liang, C.; Pan, B.; Ma, Z.; He, Z.; Duan, Z. Utilization of CO2 curing to enhance the properties of recycled aggregate and prepared concrete: A review. Cem. Concr. Compos. 2020, 105, 103446. [Google Scholar] [CrossRef]
- Neves Junior, A.; Ferreira, S.R.; Toledo Filho, R.D.; Fairbairn, E.d.M.R.; Dweck, J. Effect of early age curing carbonation on the mechanical properties and durability of high initial strength Portland cement and lime-pozolan composites reinforced with long sisal fibres. Compos. Part B Eng. 2019, 163, 351–362. [Google Scholar] [CrossRef]
- Tam, V.W.Y.; Butera, A.; Le, K.N. An investigation of the shrinkage, concrete shrinkage reversibility and permeability of CO2-treated concrete. Constr. Build. Mater. 2023, 365, 130120. [Google Scholar] [CrossRef]
- Tino Balestra, C.E.; Garcez, L.R.; Couto da Silva, L.; Veit, M.T.; Jubanski, E.; Nakano, A.Y.; Pietrobelli, M.H.; Schneider, R.; Ramirez Gil, M.A. Contribution to low-carbon cement studies: Effects of silica fume, fly ash, sugarcane bagasse ash and acai stone ash incorporation in quaternary blended limestone-calcined clay cement concretes. Environ. Dev. 2023, 45, 100792. [Google Scholar] [CrossRef]
- Zhang, J.; Shi, C.; Li, Y.; Pan, X.; Poon, C.-S.; Xie, Z. Performance Enhancement of Recycled Concrete Aggregates through Carbonation. J. Mater. Civ. Eng. 2015, 27, 04015029. [Google Scholar] [CrossRef]
- Wang, C.; Xiao, J.; Zhang, G.; Li, L. Interfacial properties of modeled recycled aggregate concrete modified by carbonation. Constr. Build. Mater. 2016, 105, 307–320. [Google Scholar] [CrossRef]
- Pu, Y.; Li, L.; Wang, Q.; Shi, X.; Luan, C.; Zhang, G.; Fu, L.; El-Fatah Abomohra, A. Accelerated carbonation technology for enhanced treatment of recycled concrete aggregates: A state-of-the-art review. Constr. Build. Mater. 2021, 282, 122671. [Google Scholar] [CrossRef]
- Ying, J. 6—Research on improving the properties and functionalities of recycled aggregate concrete. In Multi-Functional Concrete with Recycled Aggregates; Xu, Y., Jin, R., Eds.; Woodhead Publishing: Sawston, UK, 2023; pp. 127–143. [Google Scholar] [CrossRef]
- Zhan, M.; Pan, G.; Wang, Y.; Fu, M.; Lu, X. Effect of presoak-accelerated carbonation factors on enhancing recycled aggregate mortars. Mag. Concr. Res. 2017, 69, 838–849. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S.; Shi, C.J. Effect of curing parameters on CO2 curing of concrete blocks containing recycled aggregates. Cem. Concr. Compos. 2016, 71, 122–130. [Google Scholar] [CrossRef]
- Fang, X.; Xuan, D.; Poon, C.S. Empirical modelling of CO2 uptake by recycled concrete aggregates under accelerated carbonation conditions. Mater. Struct. 2017, 50, 200. [Google Scholar] [CrossRef]
- Pan, G.; Zhan, M.; Fu, M.; Wang, Y.; Lu, X. Effect of CO2 curing on demolition recycled fine aggregates enhanced by calcium hydroxide pre-soaking. Constr. Build. Mater. 2017, 154, 810–818. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S. Development of a new generation of eco-friendly concrete blocks by accelerated mineral carbonation. J. Clean. Prod. 2016, 133, 1235–1241. [Google Scholar] [CrossRef]
- Hyvert, N.; Sellier, A.; Duprat, F.; Rougeau, P.; Francisco, P. Dependency of C–S–H carbonation rate on CO2 pressure to explain transition from accelerated tests to natural carbonation. Cem. Concr. Res. 2010, 40, 1582–1589. [Google Scholar] [CrossRef]
- Li, L.; Xiao, J.; Xuan, D.; Poon, C.S. Effect of carbonation of modeled recycled coarse aggregate on the mechanical properties of modeled recycled aggregate concrete. Cem. Concr. Compos. 2018, 89, 169–180. [Google Scholar] [CrossRef]
- Zhan, B.J.; Xuan, D.X.; Poon, C.S. Enhancement of recycled aggregate properties by accelerated CO2 curing coupled with limewater soaking process. Cem. Concr. Compos. 2018, 89, 230–237. [Google Scholar] [CrossRef]
- Etxeberria, M.; Vázquez, E.; Marí, A.; Barra, M. Influence of amount of recycled coarse aggregates and production process on properties of recycled aggregate concrete. Cem. Concr. Res. 2007, 37, 735–742. [Google Scholar] [CrossRef]
- Williams, M.L. CRC Handbook of Chemistry and Physics, 76th edition. Occup. Environ. Med. 1996, 53, 504. [Google Scholar] [CrossRef]
- Bertos, M.F.; Simons, S.; Hills, C.D.; Carey, P.J. A review of accelerated carbonation technology in the treatment of cement-based materials and sequestration of CO2. J. Hazard. Mater. 2004, 112, 193–205. [Google Scholar]
- Tang, B.; Fan, M.; Yang, Z.; Sun, Y.; Yuan, L. A comparison study of aggregate carbonation and concrete carbonation for the enhancement of recycled aggregate pervious concrete. Constr. Build. Mater. 2023, 371, 130797. [Google Scholar] [CrossRef]
- Luo, S.; Lin, Q.; Lin, T.; Wang, D.; Wang, S. Effects of pressurized carbonation with presoaking in calcium hydroxide solution on the fracture behaviours of recycled coarse aggregate concrete. Constr. Build. Mater. 2023, 397, 132386. [Google Scholar] [CrossRef]
- Xuan, D.; Zhan, B.; Poon, C.S.; Zheng, W. Carbon dioxide sequestration of concrete slurry waste and its valorisation in construction products. Constr. Build. Mater. 2016, 113, 664–672. [Google Scholar] [CrossRef]
- JTS 202-2011; Specifications for Concrete Construction of Port and Waterway Engineering. Chinese Ministry of Communications: Beijing, China, 2011. (In Chinese)
- JTS 151-2011; Design Code for Concrete Structures of Port and Waterway Engineering. Chinese Ministry of Communications: Beijing, China, 2011. (In Chinese)
- JGJ 55-2011; Specification for Mix Proportion Design of Ordinary Concrete. Standards Press of China: Beijing, China, 2011. (In Chinese)
- GB/T 14685-2011; Pebble and Crushed Stone for Construction. Standards Press of China: Beijing, China, 2011. (In Chinese)
- Zhan, B.; Poon, C.S.; Liu, Q.; Kou, S.; Shi, C. Experimental study on CO2 curing for enhancement of recycled aggregate properties. Constr. Build. Mater. 2014, 67, 3–7. [Google Scholar] [CrossRef]



| Strength | W/C | Water (kg/m3) | Cement (kg/m3) | River Sand (kg/m3) | Crush Stone (kg/m3) |
|---|---|---|---|---|---|
| C30 | 0.6 | 195 | 325 | 639 | 1241 |
| C40 | 0.5 | 195 | 390 | 617 | 1198 |
| C50 | 0.4 | 195 | 488 | 584 | 1134 |
| Material Factors | Original Concrete Strength, OCS (MPa) | Initial Moisture Condition, IMC |
|---|---|---|
| MF-30-D | C30 | Complete drying |
| MF-30-U | Untreated | |
| MF-30-W | Complete wetting | |
| MF-40-D | C40 | Complete drying |
| MF-40-U | Untreated | |
| MF-40-W | Complete wetting | |
| MF-50-D | C50 | Complete drying |
| MF-50-U | Untreated | |
| MF-50-W | Complete wetting |
| Types of CA | OCS (MPa) | ρa (kg/m3) | Wa (%) |
|---|---|---|---|
| NCA | — | 2710 | 0.33 |
| RCA | C30 | 2495 | 5.15 |
| C40 | 2479 | 5.34 | |
| C50 | 2450 | 5.50 |
| OCS (MPa) | IMC | ωi (%) |
|---|---|---|
| C30 | Complete drying | 0.00 |
| Untreated | 1.86 | |
| Complete wetting | 5.63 | |
| C40 | Complete drying | 0.00 |
| Untreated | 2.66 | |
| Complete wetting | 5.88 | |
| C50 | Complete drying | 0.00 |
| Untreated | 3.49 | |
| Complete wetting | 5.24 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ju, X.; Wu, L.; Liu, M.; Jiang, H.; Zhang, W.; Guan, L.; Chen, X.; Fan, X. Influence of the Original Concrete Strength and Initial Moisture Condition on the Properties Improvement of Recycled Coarse Aggregate via Accelerated Carbonation Reactions. Materials 2024, 17, 706. https://doi.org/10.3390/ma17030706
Ju X, Wu L, Liu M, Jiang H, Zhang W, Guan L, Chen X, Fan X. Influence of the Original Concrete Strength and Initial Moisture Condition on the Properties Improvement of Recycled Coarse Aggregate via Accelerated Carbonation Reactions. Materials. 2024; 17(3):706. https://doi.org/10.3390/ma17030706
Chicago/Turabian StyleJu, Xueli, Linjian Wu, Mingwei Liu, Han Jiang, Wenxiao Zhang, Li Guan, Xiang Chen, and Xinhui Fan. 2024. "Influence of the Original Concrete Strength and Initial Moisture Condition on the Properties Improvement of Recycled Coarse Aggregate via Accelerated Carbonation Reactions" Materials 17, no. 3: 706. https://doi.org/10.3390/ma17030706
APA StyleJu, X., Wu, L., Liu, M., Jiang, H., Zhang, W., Guan, L., Chen, X., & Fan, X. (2024). Influence of the Original Concrete Strength and Initial Moisture Condition on the Properties Improvement of Recycled Coarse Aggregate via Accelerated Carbonation Reactions. Materials, 17(3), 706. https://doi.org/10.3390/ma17030706






