Synthesis, Purification, and Characterization of Molten Salt Fuel for the SALIENT-03 Irradiation Experiment
Abstract
1. Introduction
1.1. Molten Salt Reactor for the Sustainable Energy Production
1.2. Irradiation Experiments of Molten Salt Reactor Fuel Candidates
1.3. Preparation of the Fuel for the SALIENT-03 Experiment
1.3.1. Synthesis of the Pure Fluorides for the SALIENT-03 Fuel
1.3.2. Preparation of the Fuel Salts from the End Members
2. Experimental
2.1. Fuel Salts Specification
2.2. Irradiation Capsules
2.3. Experimental Set-Up for the Synthesis
2.4. Analytical Scheme
3. Results
3.1. Synthesis of the End-Members
- The dissolution of app. 20 g of 7LiOH⋅H2O in water to a concentration of app. 2 M (concentration is estimated as the initial material contained traces of 7Li2CO3 and moisture);
- The filtration of the undissolved 7Li2CO3, which was partly formed in the delivered package, likely due to CO2 intake during open handling of the material between manufacturing and delivery;
- The reaction of the 7LiOH solution with hydrofluoric acid solution (HF, 49 wt.%, Sigma-Aldrich, Nuremberg, Germany, p.a. plus, diluted to 2 M concentration), controlled online by a commercial pH meter with a calibrated pH electrode (InLab Routine, Mettler Toledo GmbH, Gießen, Germany), until it reached pH ~4–5;
- The filtration under a vacuum of the formed solid, 7LiF;
- The rinsing of the 7LiF with pure ethanol (p.a., Sigma-Aldrich, Nuremberg, Germany);
- The drying of the product at 150 °C for 2 h in air;
- The drying of the product at 350 °C under argon in the glove box described above in Section 2.3
3.2. Fabrication of Fuels-1, -2 and -3
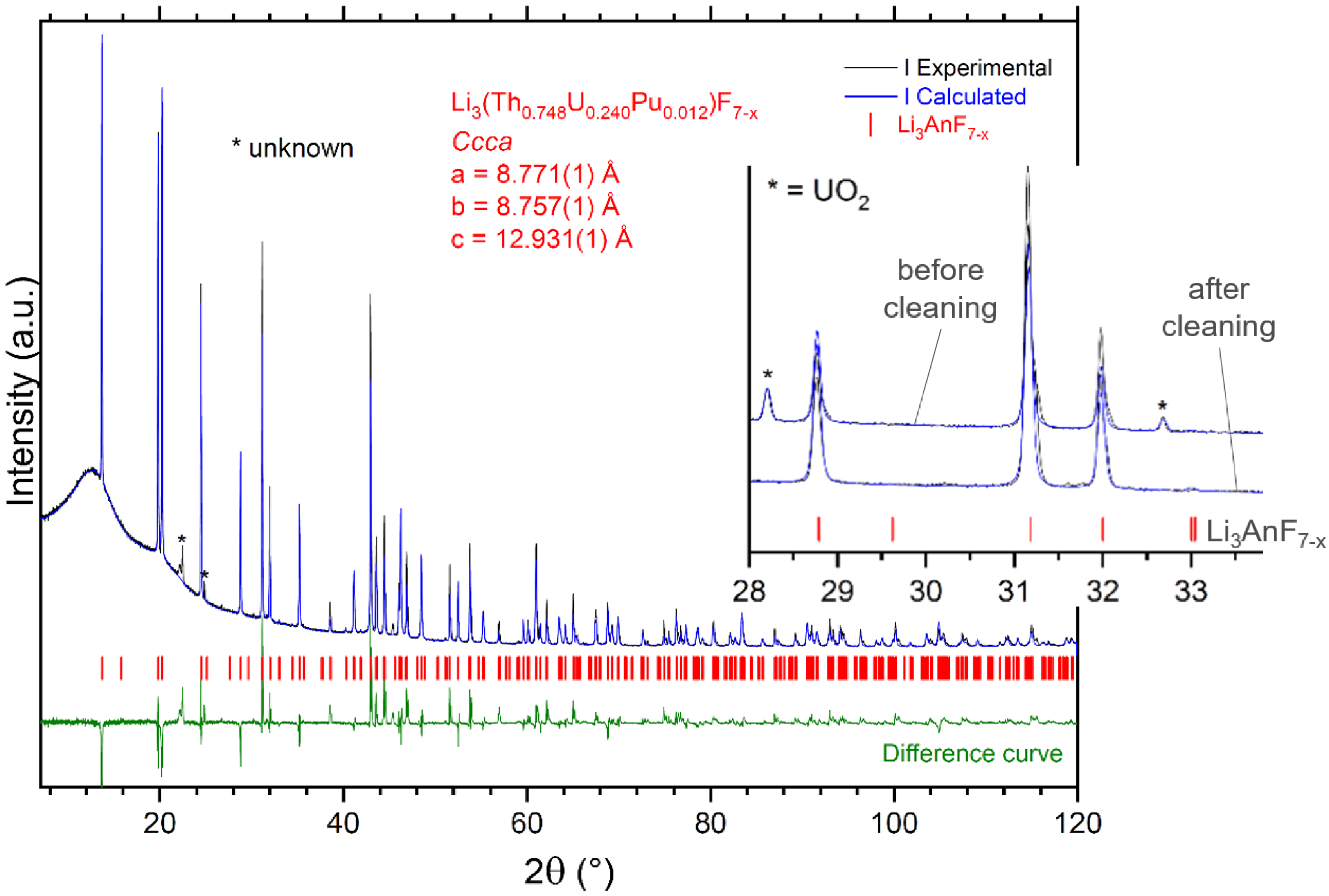
3.3. Fabrication of Fuel-4
4. Discussion
4.1. Outcomes from the Synthesis of Actinide Fluoride End-Members
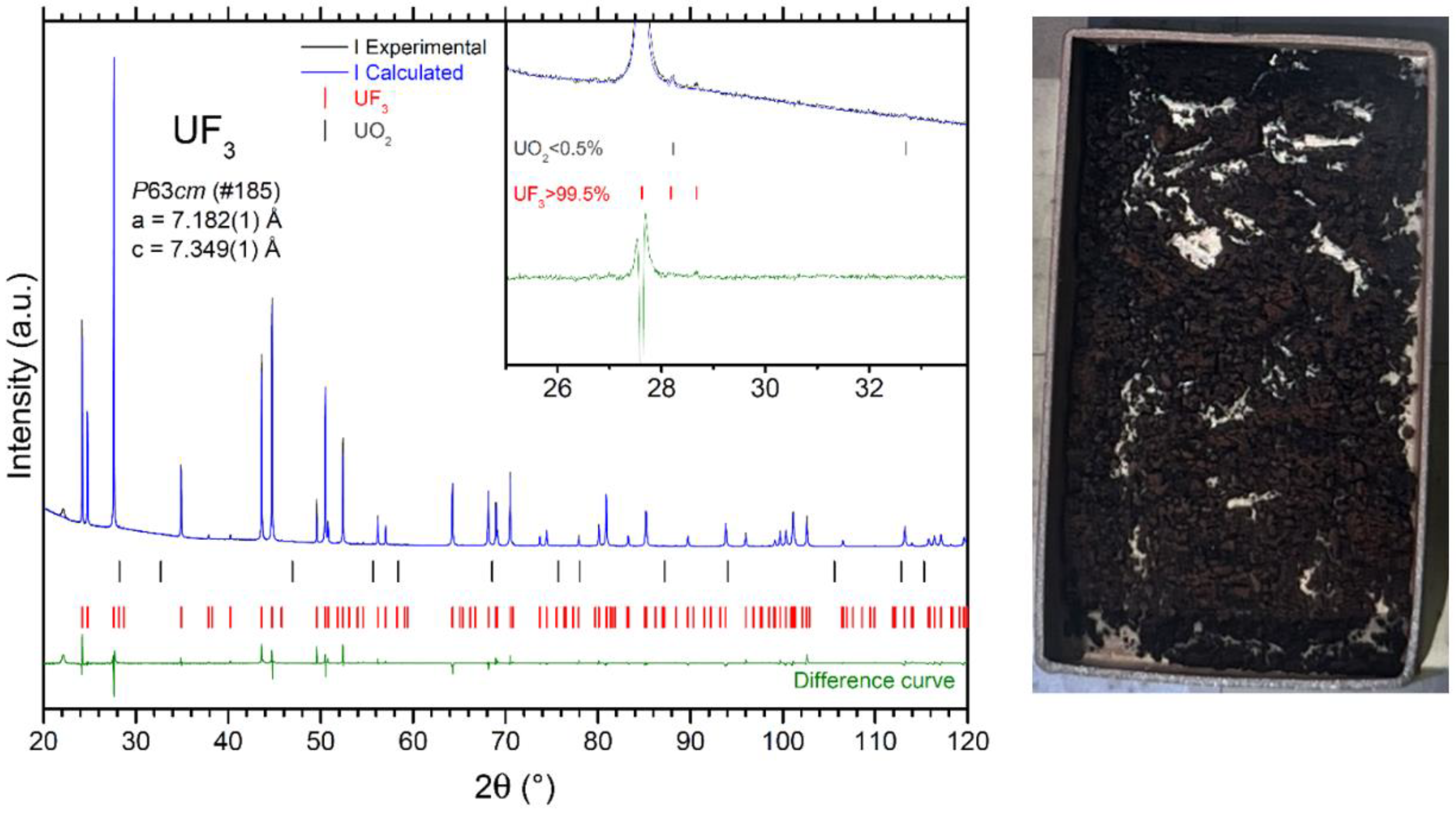
4.2. Outcomes from the Synthesis of UF3
- The reaction was not quantitative up to a temperature of 800 °C, as some residual UF4 was always present at lower temperatures, specifically at 600, 700, and 750 °C;
- Using a flow rate of H2/Ar gas lower than 600 mL/min led to disproportionation of the product at 800 °C;
- The reaction kinetics at 800 °C, with a H2/Ar gas flow rate of 600 mL/min, were still very slow, requiring 40 h to complete the conversion;
- The commercially available mixture of Ar/H2 (6% H2, Linde, 99.9999% purity) was insufficient to prevent the formation of trace amounts of UO2 in the product (<0.5 wt.%), likely due to oxygen or moisture impurities in this reaction gas.
4.3. Outcomes from Production of Solid Melt Ingots
5. Conclusions, Implications, and Future Research
- The fuels for the SALIENT-03 irradiation experiment have been successfully synthesized with the required composition, purity, and mass;
- The fuel ingots have been inserted into irradiation capsules, sealed in a gas-tight manner by orbital welding, and transported from JRC Karlsruhe to NRG Petten, where they have been accepted for the irradiation campaign;
- The achieved purity of all end-members synthesized during this work exceeded 99.0%, based on the detection limit and uncertainty of the applied analytical techniques;
- No impurities have been detected in the end-members, except for UF3, where trace amounts of oxide impurity (<0.5%) were observed;
- The synthesis campaign detailed herein has demonstrated the capability of JRC laboratories to prepare fluoride salts of high purity, including actinide fluorides, on a 100 g scale, since the total mass of the three fuels created for irradiation is 40.6 g, and the mass of the fuel for the out-of-pile electrochemical experiments is 53.8 g.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Decarbonising our Energy System to Meet our Climate Goals. European Commission Website. In Publications: Delivering the European Green Deal; European Union: Brussels, Belgium, 2021; ISBN 978-92-76-39782-3. Available online: https://ec.europa.eu/info/publications/delivering-european-green-deal_en (accessed on 18 December 2024).
- Schlömer, S.; Bruckner, T.; Fulton, L.; Hertwich, E.; McKinnon, A.; Perczyk, D.; Roy, J.; Schaeffer, R.; Sims, R.; Smith, P.; et al. Annex III: Technology-specific cost and performance parameters. In Climate Change 2014: Mitigation of Climate Change, Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Krey, V.; Masera, O.; Blanford, G.; Bruckner, T.; Cooke, R.; Fisher-Vanden, K.; Haberl, H.; Hertwich, E.; Kriegler, E.; Mueller, D.; et al. Annex II: Metrics & Methodology. In Climate Change 2014: Mitigation of Climate Change”, Contribution of Working Group III to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Technology Roadmap Update for Generation IV Nuclear Energy Systems, in Generation IV International Forum; OECD Nuclear Energy Agency: Paris, France. 2014. Available online: www.gen-4.org (accessed on 18 December 2024).
- Allibert, M.; MAufiero; Brovchenko, M.; Delpech, S.; Ghetta, V.; Heuer, D.; Laureau, A.; Merle-Lucotte, E. 7—Molten salt fast reactors. In Handbook of Generation IV Nuclear Reactors; Pioro, I.L., Ed.; Woodhead Publishing: Cambridge, UK, 2016; pp. 157–188. [Google Scholar]
- Serp, J.; Allibert, M.; Beneš, O.; Delpech, S.; Feynberg, O.; Ghetta, V.; Heuer, D.; Holcomb, D.; Ignatiev, V.; Kloosterman, J.L.; et al. The molten salt reactor (MSR) in generation IV: Overview and perspectives. Prog. Nucl. Energy 2014, 77, 308–319. [Google Scholar] [CrossRef]
- Elsheikh, B.M. Safety assessment of molten salt reactors in comparison with light water reactors. J. Radiat. Res. Appl. Sci. 2013, 6, 63–70. [Google Scholar] [CrossRef]
- Brovchenko, M.; Heuer, D.; Merle-Lucotte, E.; Allibert, M.; Ghetta, V.; Laureau, A.; Rubiolo, P. Design-Related Studies for the Preliminary Safety Assessment of the Molten Salt Fast Reactor. Nucl. Sci. Eng. 2013, 175, 329–339. [Google Scholar] [CrossRef]
- Paviet, P. Molten Salt Reactor Campaign. In 2021 Virtual Molten Salt Reactor (MSR) Workshop; Oak Ridge National Laboratory (ORNL): Oak Ridge, TN, USA, 2021; Available online: https://www.youtube.com/watch?v=tqZTZV4py-8&list=PLisa2eqmVBFaAI8_AFle1QxA26k3FTWB6&index=4 (accessed on 18 December 2024).
- Mallapaty, S. China prepares to test thorium-fuelled nuclear reactor. Nature 2021, 597, 311–312. [Google Scholar] [CrossRef]
- Hania, P.R.; Boomstra, D.A.; Benes, O.; Soucek, P.; de Koning, A.J.; Bobeldijk, I.; de Groot, S.; Konings, R.J.M.; Capelli, E.; Naji, M.; et al. Irradiation of thorium-bearing molten fluoride salt in graphite crucibles. Nucl. Eng. Des. 2021, 375, 111094. [Google Scholar] [CrossRef]
- D’Agata, E.; Hania, R.; Benes, O.; Konings, R.; Kottrup, K.; Soucek, P.; Boomstra, D.A.; Baas, P.J.; Charpin-Jacobs, F.F.; Uitslag-Doolaard, H.J.; et al. SALIENT-03: Irradiation of Molten Salt Fuel in Ni-Based Alloy Capsules at the High Flux Reactor. In Proceedings of the Global 2019, Seattle, WA, USA, 22–27 September 2019; pp. 279–284. [Google Scholar]
- Krepel, J. SAMOFAR—A paradigm shift in reactor safety with the molten salt fast reactor. In Proceedings of the ThEC15—International Thorium Energy Conference, Mumbai, India, 12–15 October 2015. [Google Scholar]
- Kloosterman, J.L. 20—Safety assessment of the molten salt fast reactor (SAMOFAR). In Molten Salt Reactors and Thorium Energy; Dolan, T.J., Ed.; Woodhead Publishing: Cambridge, UK, 2017; pp. 565–570. [Google Scholar]
- European Commission Web Page. 2019. Available online: https://cordis.europa.eu/project/id/847527 (accessed on 3 August 2022).
- Bellinger, S.L.; Fronk, R.G.; McNeil, W.J.; Sobering, T.J.; McGregor, D.S. Improved High Efficiency Stacked Microstructured Neutron Detectors Backfilled with Nanoparticle 6LiF. IEEE Trans. Nucl. Sci. 2012, 59, 167–173. [Google Scholar] [CrossRef]
- Suarez, D.S.; Pinna, E.G.; Rosales, G.D.; Rodriguez, M.H. Synthesis of Lithium Fluoride from Spent Lithium Ion Batteries. Minerals 2017, 7, 81. [Google Scholar] [CrossRef]
- Goodenough, R.D. Lithium Fluoride Production; U.S.P. Office, Ed.; Dow Chemical Co: Midland, MI, USA, 1966. [Google Scholar]
- Vallejo Hernandez, M.; Sosa, M.A.; Rivera, E.; Azorín, J.C.; Bernal, J.; Navarro, R.; Encarnación, E.K.; Díaz-Torres, L.A. Effect of Crystal Size and Ag Concentration on the Thermoluminescent Response of Pure and Ag-Doped LiF Cubes. Nano 2015, 11, 1650041. [Google Scholar] [CrossRef]
- Souček, P.; Beneš, O.; Claux, B.; Capelli, E.; Ougier, M.; Tyrpekl, V.; Vigier, J.-F.; Konings, R.J.M. Synthesis of UF4 and ThF4 by HF gas fluorination and re-determination of the UF4 melting point. J. Fluor. Chem. 2017, 200, 33–40. [Google Scholar] [CrossRef]
- Tosolin, A.; Souček, P.; Beneš, O.; Vigier, J.F.; Luzzi, L.; Konings, R.J.M. Synthesis of plutonium trifluoride by hydro-fluorination and novel thermodynamic data for the PuF3-LiF system. J. Nucl. Mater. 2018, 503, 171–177. [Google Scholar] [CrossRef]
- Roy, K.N.; Prasad, R.; Bhupathy, M.; Venugopal, V.; Singh, Z.; Sood, D.D. Preparation of uranium trifluoride and studies on its disproportionation. Thermochim. Acta 1981, 43, 333–338. [Google Scholar] [CrossRef]
- Domingues, J.T.H. The Kinetics of the Reduction of Uranium Fluoride by Magnesium. Ph.D. Thesis, University of London, London, UK, 1964. [Google Scholar]
- Runnalls, O.J.C. A New Synthesis of Uranium Trifluoride. Can. J. Chem. 1953, 31, 694–696. [Google Scholar] [CrossRef]
- Gilpatrick, L.O.R.; Baldock, J.R. Sites Mass Spectrometer Investigation of UF3. In ORNL-1736; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1952. [Google Scholar]
- Workshop on the Chemistry of Fuel Cycles for Molten Salt Reactor Technologies. 2023. Available online: https://conferences.iaea.org/event/361/ (accessed on 18 December 2024).
- Paviet, P. The Fuel Cycle of a Molten Salt Reactor. In Regulatory Information Conference W19 Molten Salt Reactors: Rethinking the Fuel Cycle; U.S. Nuclear Regulatory Commission (NRC): Rockville, MD, USA, 2022. [Google Scholar]
- Reed, W.; Carlson, J.; Iyengar, R. Technical Considerations for the Molten Salt Reactor Fuel Cycle. In Regulatory Information Conference (RIC) W19 on Molten Salt Reactors: Rethinking the Fuel Cycle; U.S. Nuclear Regulatory Commission (NRC): Rockville, MD, USA, 2022. [Google Scholar]
- Rose, M.A.; Ezell, D. Molten Salt Reactor Fuel Cycle Chemistry Workshop—Report for the US Department of Energy; ANL/CFCT-23/50; Office of Nuclear Energy, Argonne National Laboratory: Argonne, IL, USA, 2023. [Google Scholar]
- Fredrickson, G.; Cao, G.; Gakhar, R.; Yoo, T.S. Molten Salt Reactor Salt Processing—Technology Status; INL/EXT-18-51033; Idaho National Laboratory: Idaho Falls, ID, USA, 2018. [Google Scholar]
- Flanagan, G.F.; Holcomb, D.E.; Poore, I.W.P. MSR Fuel Qualification Consideration and Challenges; ORNL/LTR-2018/1045; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 2018. [Google Scholar]
- Souček, P.; Beneš, O.; Tosolin, A.; Konings, R. Chemistry of Molten Salt Reactor Fuel Salt Candidates. Trans. Am. Nucl. Soc. 2018, 118, 114–117. [Google Scholar]
- Souček, P.; Fucina, M.; Beneš, O.; Capelli, E.; Rodrigues, A.; Konings, R.J.M.; Rondinella, V. Synthesis and measurements of thermal properties of the molten salt reactor fuel candidates. In Proceedings of the Global 2024, Tokyo, Japan, 6–10 October 2024. [Google Scholar]
- Souček, P.; Fucina, M.; Beneš, O.; Vigier, J.F.; Rodrigues, A.; Walter, O.; Konings, R.J.M. Synthesis of actinide chlorides for Molten Salt Reactor fuels. In Proceedings of the 16th Information Exchange Meeting on Actinide and Fission Product Partitioning and Transmutation (16IEMPT), Boulogne-Billancourt, France, 24–27 October 2023. [Google Scholar]
- Malcolm, W.C., Jr. NIST-JANAF Thermochemical Tables, 4th ed.; American Chemical Society: Washington, DC, USA; American Institute of Physics for the National Institute of Standards and Technology: New York, NY, USA, 1998. [Google Scholar]
- Rand, M.; Fuger, J.; Grenthe, I.; Neck, V.; Raiof, D. Chemical thermodynamics of Thorium. Chem. Thermodyn. Ser. 2009, 11, 198. [Google Scholar]
- Sheil, R.J. The System UF4-UO2. In ORNL-2061; Oak Ridge National Laboratory: Oak Ridge, TN, USA, 1956; p. 71. [Google Scholar]
- Kumar, R.; Gupta, S.; Wajhal, S.; Satpati, S.K.; Sahu, M. Effect of process parameters on the recovery of thorium tetrafluoride prepared by hydrofluorination of thorium oxide, and their optimization. Nucl. Eng. Technol. 2022, 54, 1560–1569. [Google Scholar] [CrossRef]
- Fisher, R.W. The Preparation of Thorium Oxide and Thorium Fluoride from Thorium Nitrate. In Progress in Nuclear Energy, Series III; Process Chemistry; Pergamon Press: London, UK, 1958; Volume 2. [Google Scholar]
- Correa, H.S.; Costa, E.C. Dry-way Preparation of Thorium Tetrafluoride. In Publicação IEA No. 185; Instituto de Energia Atômica: Sao Paulo, Brazil, 1969. [Google Scholar]
- Wani, B.N.; Patwe, S.J.; Rao UR, K.; Venkateswarlu, K.S. Fluorination of oxides of uranium and thorium by ammonium hydrogenfluoride. J. Fluor. Chem. 1989, 44, 177–185. [Google Scholar] [CrossRef]
- D’Eye, R.W.M.; Booth, G.W.; Harper, E.A. The Preparation of Thorium Tetrafluoride by the Thermal Degradation of the Hydrate; Gt. Brit. Atomic Energy Research Establishment: Harwell, UK, 1955; p. 5. [Google Scholar]
- Dell, R.M.; Wheeler, V.J. Chemical reactivity of uranium trioxide. Part 1.—Conversion to U3O8, UO2 and UF4. Trans. Faraday Soc. 1962, 58, 1590–1607. [Google Scholar] [CrossRef]
- Ellis, W.P. Hydrofluorination kinetics of doped uranium dioxide. J. Nucl. Mater. 1966, 19, 212–214. [Google Scholar] [CrossRef]
- Nicole, C.; Patisson, F.; Ablitzer, D.; Houzelot, J.L. A thermogravimetric study of the kinetics of hydrofluorination of uranium dioxide. Chem. Eng. Sci. 1996, 51, 5213–5222. [Google Scholar] [CrossRef]
- Tomlinson, L.; Morrow, S.A.; Graves, S. Kinetics of the hydrofluorination of uranium dioxide. Trans. Faraday Soc. 1961, 57, 1008–1018. [Google Scholar] [CrossRef]
- Eykens, R.; Pauwels, J.; Van Audenhove, J. The hydrofluorination of uranium and plutonium. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 1985, 236, 497–499. [Google Scholar] [CrossRef]
- Kim, E.H.; Hwang, D.S.; Park, J.H. Fluorination of UO2 and CeO2 under the atmosphere of HF and H2. J. Ind. Eng. Chem. 2002, 8, 98–102. [Google Scholar]
- Dawson, J.K.; Elliott, R.M.; Hurst, R.; Truswell, A. The preparation and some properties of plutonium fluorides. J. Chem. Soc. (Resumed) 1954, 558–564. [Google Scholar] [CrossRef]
- Reavis, J.G.; Leary, J.A. Partial phase diagram of the PuCl3-PuOCl system. J. Inorg. Nucl. Chem. 1966, 28, 1205–1208. [Google Scholar] [CrossRef]
- Scheele, R.; McNamara, B.; Casella, A.M.; Kozelisky, A. On the use of thermal NF3 as the fluorination and oxidation agent in treatment of used nuclear fuels. J. Nucl. Mater. 2012, 424, 224–236. [Google Scholar] [CrossRef]
- Gibson, J.K.; Haire, R.G. High-temperature fluorination studies of uranium, neptunium, plutonium and americium. J. Alloys Compd. 1992, 181, 23–32. [Google Scholar] [CrossRef]
- Claux, B.; Beneš, O.; Capelli, E.; Souček, P.; Meier, R. On the fluorination of plutonium dioxide by ammonium hydrogen fluoride. J. Fluor. Chem. 2016, 183, 10–13. [Google Scholar] [CrossRef]
- Berndt, U.; Erdmann, B. Darstellung von ultrareinem UF3. Radiochim. Acta 1973, 19, 45–46. [Google Scholar] [CrossRef]
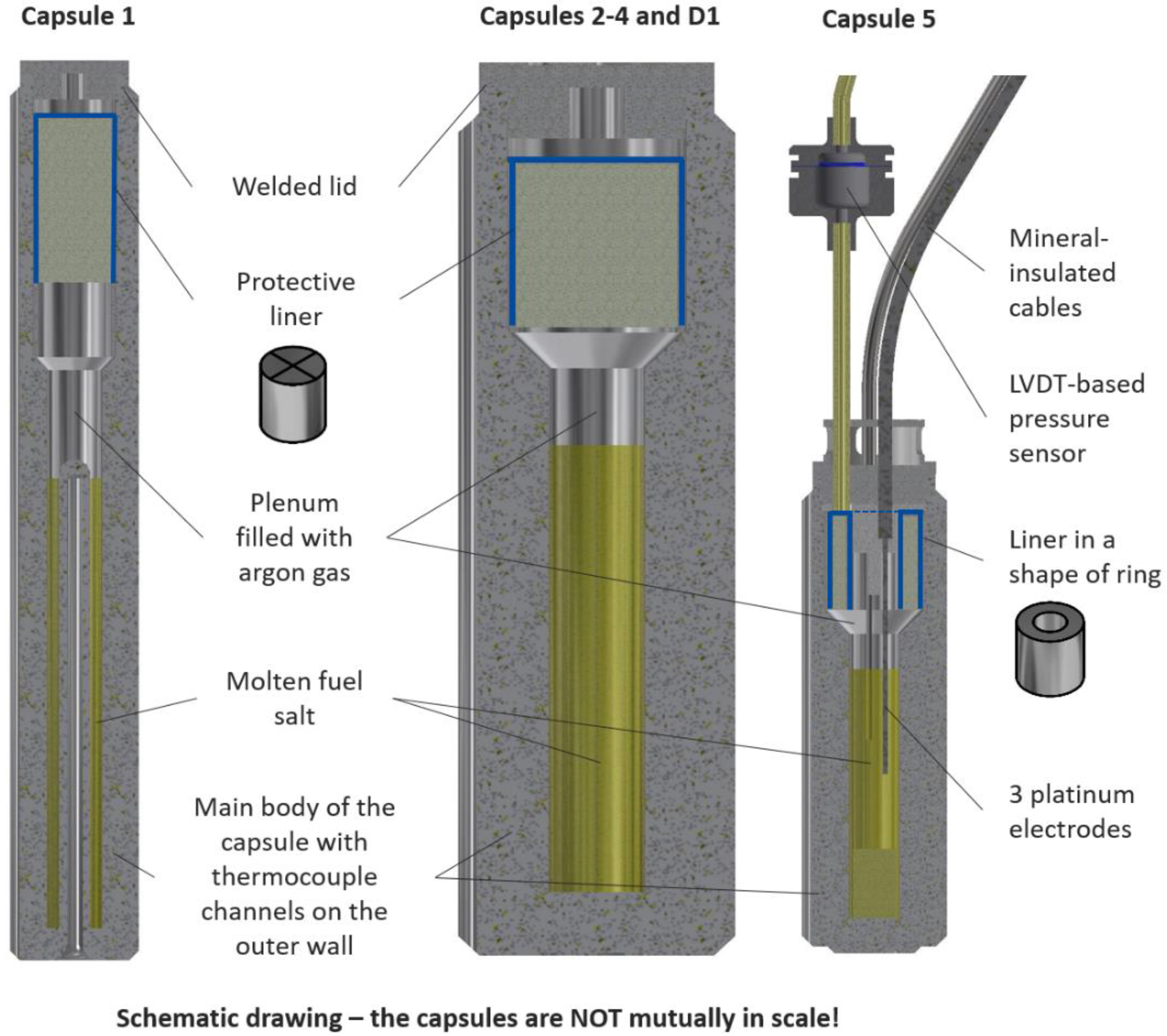




| Salt Composition (mol.%) | Mass (g) | Acronym |
|---|---|---|
| 75.07LiF—18.7ThF4—6.0UF4—0.3PuF3 | 23.151 | Fuel-1 |
| 75.07LiF—18.7ThF4—5.7UF4—0.3UF3—0.3PuF3 | 11.584 | Fuel-2 |
| 74.67LiF—18.6ThF4—6.0UF4—0.4CrF3 -0.3PuF3 | 5.792 | Fuel-3 |
| 75.0LiF—23.0ThF4—2.0UF4—0.1UF3 | 50.000 | Fuel-4 |
| End-Member | 7LiF | ThF4 | UF4 | UF3 | PuF3 |
|---|---|---|---|---|---|
| Mass required (g) | 10.075 | 66.423 | 12.811 | 0.282 | 0.459 |
| Pin | Material | Salt | Mass (g) |
|---|---|---|---|
| 1 | Hastelloy N | Fuel-1 | 11.567 |
| 2 | Hastelloy N | Fuel-2 | 5.792 |
| 3 | Hastelloy N | Fuel-1 | 5.792 |
| 4 | Hastelloy N | Fuel-3 | 5.792 |
| 5 | GH3535 | Fuel-2 | 5.792 |
| D1 | Hastelloy N | Fuel-1 | 5.792 |
| Batch | AnO2 Mass (g) | T (°C) | Time (h) | mAnFx (g) | Conversion Efficiency | XRD | DSC (m.p.°C) | mAnFx_FINAL (g) |
|---|---|---|---|---|---|---|---|---|
| ThF4-1 | 15.4910 | 600 | 5:15 | 17.8071 | 99.1% | phase pure | 1117.0 | 17.5865 |
| ThF4-2 | 16.0160 | 600 | 5:40 | 18.3997 | 98.4% | phase pure | 1118.6 | 18.2528 |
| ThF4-3 | 15.5905 | 600 | 5:45 | 17.2990 | n/a 1 | phase pure | 1119.4 2 | 17.1193 |
| ThF4-4 | 15.8677 | 600 | 5:15 | 18.5115 | 98.5% | phase pure | 1119.4 2 | 17.3325 |
| UF4-1 3 | 7.5841 | 450 | 6:30 | 8.7385 | 99.1% | phase pure | 1029.5 | 8.5123 |
| UF4-2 3 | 9.6630 | 450 | 7:00 | 11.1562 | 99.3% | phase pure | 1016.5 | 10.7598 |
| PuF3 4 | 2.8163 | 550 | 2:10 | n/a 5 | n/a 5 | n/a 5 | n/a 5 | n/a 5 |
| 600 | 15:00 | 3.0708 | 99.8% | phase pure | n/a 6 | 2.9239 |
| Batch | UF4 Mass (g) | T (°C) | Time (h) | UF3 Mass (g) | Conversion Efficiency | XRD | DSC (m.p.°C) | Final Mass (g) |
|---|---|---|---|---|---|---|---|---|
| UF3 03/19 | 1.0807 | 800 | 40:00 | 0.9797 | 96.5% 1 | >99.5% UF3<0.5% UO2 | n/a 2 | 0.7841 |
| Fuel | 7LiF (g) | ThF4 (g) | UF4 (g) | UF3 (g) | PuF3 (g) | CrF3 (g) | mFUEL (g) | mFINAL (g) |
|---|---|---|---|---|---|---|---|---|
| 1 | 5.6451 | 16.6662 | 5.4535 | - | 0.2571 | - | 27.7669 | 27.7135 |
| 2 | 3.0270 | 8.9347 | 2.7844 | 0.1308 | 0.1378 | - | 15.0148 | 14.9957 |
| 3 | 1.4030 | 4.1426 | 1.3613 | - | 0.0644 | 0.0351 | 7.0048 | 6.9930 |
| 4 | 11.0803 1 | 40.3503 | 3.5446 | 0.0333 | - | - | 55.0085 | 54.9448 |
| ID | mFINAL (g) | ΔmMELTING (g/%) | mBLACK_L (g) | Target Pin | Composition (mol.%) |
|---|---|---|---|---|---|
| 1-1 | 2.9324 | 0.0086/0.29 | 0.0171 | 1 | 75.07LiF—18.7ThF4 6.0UF4—0.3PuF3 |
| 1-2 | 2.9806 | 0.0094/0.31 | 0.0095 | ||
| 1-3 | 2.9599 | 0.0088/0.30 | 0.0103 | ||
| 1-4 | 2.7222 | 0.0079/0.29 | 0.0059 | ||
| 2-1 | 1.6925 | n/a | 0.0053 | 2 | 75.07LiF—18.7ThF4—5.7UF4—0.3UF3—0.3PuF3 |
| 2-2 | 1.3953 | 0.0035/0.25 | 0.0061 | ||
| 2-3 | 1.3455 | 0.0027/0.20 | 0.0024 | ||
| 2-4 | 1.3891 | 0.0032/0.23 | 0.0022 | ||
| 3-1 | 1.8507 | 0.0030/0.16 | 0.0079 | 3 | 75.07LiF—18.7ThF4 6.0UF4—0.3PuF3 |
| 3-2 | 1.3526 | 0.0039/0.29 | 0.0028 | ||
| 3-3 | 1.3227 | n/a | 0.0023 | ||
| 3-4 | 1.2800 | 0.0036/0.28 | 0.0020 | ||
| 4-1 | 1.4837 | 0.0044/0.30 | 0.0020 | 4 | 74.67LiF—18.6ThF4—6.0UF4—0.4CrF3—0.3PuF3 |
| 4-2 | 1.5049 | 0.0039/0.26 | 0.0033 | ||
| 4-3 | 1.4736 | 0.0043/0.29 | 0.0023 | ||
| 4-4 | 1.3569 | 0.0039/0.29 | 0.0033 | ||
| 5-1 | 1.5086 | 0.0034/0.22 | 0.0034 | 5 | 75.07LiF—18.7ThF4—5.7UF4—0.3UF3—0.3PuF3 |
| 5-2 | 1.3070 | n/a | 0.0017 | ||
| 5-3 | 1.1841 | n/a | 0.0020 | ||
| D-1 | 1.6061 | 0.0063/0.39 | 0.0057 | D | 75.07LiF—18.7ThF4 6.0UF4—0.3PuF3 |
| D-2 | 1.5822 | 0.0016/0.10 | 0.0004 | ||
| D-3 | 1.4849 | 0.0043/0.29 | 0.0030 | ||
| D-4 | 1.1511 | 0.0031/0.27 | 0.0017 | ||
| GC-1 | 0.4271 | 0.0012/0.28 | 0.0014 | 5 | 75.07LiF—18.7ThF4—5.7UF4—0.3UF3—0.3PuF3 |
| GC-2 | 0.3974 | 0.0003/0.08 | 0.0001 | ||
| GC-3 | 0.4153 | 0.0015/0.36 | 0.0010 | ||
| GC-4 | 0.4014 | n/a | 0.0009 | ||
| GC-5 | 0.4365 | 0.0010/0.23 | 0.0004 | QA | |
| GC-6 | 0.4093 | 0.0009/0.22 | 0.0010 |
| Capsule 1 | Sub-Ingot 1-1 | Sub-Ingot 1-2 | Sub-Ingot 1-3 | Sub-Ingot 1-4 |
|---|---|---|---|---|
| mSALT (g) | 2.9581 | 2.9995 | 2.9790 | 2.7360 |
| mINGOT (g) | 2.9495 | 2.9901 | 2.9702 | 2.7281 |
| ΔmMELTING (g/%) | 0.0086/0.29 | 0.0094/0.31 | 0.0088/0.30 | 0.0079/0.29 |
| mFINAL (g) | 2.9324 | 2.9806 | 2.9599 | 2.7222 |
| mBLACK_L (g/%) | 0.0171/0.58 | 0.0095/0.32 | 0.0103/0.35 | 0.0059/0.22 |
| ΔmLINER (g/%) | 0.0002/0.002 | 0.0002/0.001 | 0.0002/0.002 | 0.0002/0.002 |
| Photo |  |  |  |  |
| Target Capsule | mFINAL (g) | ΔmMELTING (g/%) | mBLACK_L (g) | ΔmREQUEST (%) | Density (g/cm3) |
|---|---|---|---|---|---|
| 1 | 11.5850 | 0.0021/0.02 | 0.0119 | 0.16 | 4.85 |
| 2 | 5.8039 | 0.0011/0.02 | 0.0076 | 0.21 | 4.40 |
| 3 | 5.7987 | 0.0017/0.03 | 0.0119 | 0.12 | 4.49 |
| 4 | 5.8182 | 0.0029/0.05 | 0.0030 | 0.45 | 4.55 |
| 5 | 5.7947 1 | 0.0022/0.031 | 0.0031 1 | 0.05 | 4.30 |
| D1 | 5.8047 | 0.0010/0.02 | 0.0037 | 0.22 | 4.35 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Souček, P.; Beneš, O.; Hania, P.R.; Kottrup, K.G.; D’Agata, E.; Rodrigues, A.; Uitslag-Doolaard, H.J.; Konings, R.J.M. Synthesis, Purification, and Characterization of Molten Salt Fuel for the SALIENT-03 Irradiation Experiment. Materials 2024, 17, 6215. https://doi.org/10.3390/ma17246215
Souček P, Beneš O, Hania PR, Kottrup KG, D’Agata E, Rodrigues A, Uitslag-Doolaard HJ, Konings RJM. Synthesis, Purification, and Characterization of Molten Salt Fuel for the SALIENT-03 Irradiation Experiment. Materials. 2024; 17(24):6215. https://doi.org/10.3390/ma17246215
Chicago/Turabian StyleSouček, Pavel, Ondřej Beneš, Pieter Ralph Hania, Konstantin Georg Kottrup, Elio D’Agata, Alcide Rodrigues, Helena Johanna Uitslag-Doolaard, and Rudy J. M. Konings. 2024. "Synthesis, Purification, and Characterization of Molten Salt Fuel for the SALIENT-03 Irradiation Experiment" Materials 17, no. 24: 6215. https://doi.org/10.3390/ma17246215
APA StyleSouček, P., Beneš, O., Hania, P. R., Kottrup, K. G., D’Agata, E., Rodrigues, A., Uitslag-Doolaard, H. J., & Konings, R. J. M. (2024). Synthesis, Purification, and Characterization of Molten Salt Fuel for the SALIENT-03 Irradiation Experiment. Materials, 17(24), 6215. https://doi.org/10.3390/ma17246215







