Mesoporous Carbon Composites Containing Carbon Nanostructures: Recent Advances in Synthesis and Applications in Electrochemistry
Abstract
1. Introduction
2. Definition of Mesoporous Materials and Composites
3. Physical and Chemical Properties of Mesoporous Materials with Relevance in Electrochemical Processes
4. Preparation of Mesoporous Carbon/CN Composites
4.1. Possible Modification Approaches of CNs
4.2. Methods of Synthesis of Mesoporus Carbon Composites
4.3. Factors Affecting the Size of the Pores
4.4. Heteroatom-Doped Mesoporous Carbon/CN Composites
5. Application of Mesoporous Carbon/CN Composites: Catalysis and Electrochemistry
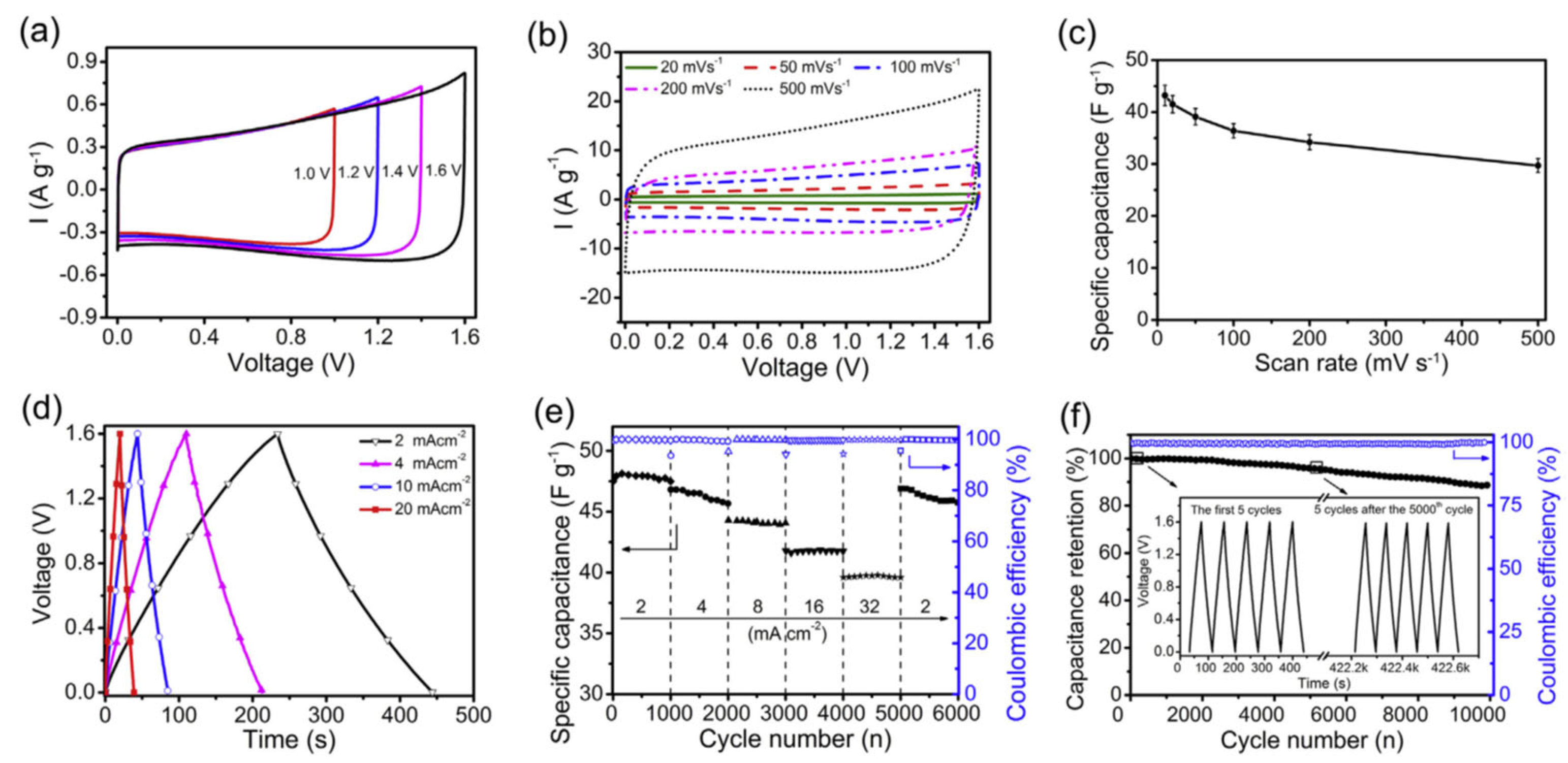
5.1. Electrochemical Applications of Mesoporous Carbon/CN Composites
| Carbon Nanostructure | Precursor of Mesoporous Carbon | Template | Pore-Forming Agent | T (°C) | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | Heteroatom (%) | Cs (F g−1) | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Graphene | Resol, urea | Hard | F127, TEOS | 850 | 1348 | 1.18 | 3.6 | N (3) | 246 | [46] |
| Graphene | 3-Aminophenol, HCHO, ethylenediamine | Hard | TEOS | 700 | 989 | 1.82 | 10.6 | N (7) | 249 | [43] |
| Graphene | Pyrrole, (NH4)2S2O8 | Hard | P123, TEOS | 900 | 383 | - | 2–5 | N (3.9) | 196.5 | [47] |
| Graphene | CCl4, ethylenediamine | Hard | SBA-15 | 600 | 362 | 0.43 | 4–22 | N (10) | 240 | [39] |
| Graphene | Phenol, HCHO, S | Hard | F127, TEOS | 800 | 1709 | 1.89 | 1.41 4.54 | S (0.1) | 314 | [44] |
| Graphene | Resol, dicyandiamide | Hard | F127, TEOS | 700 | 1569 | 1.38 | 0.56–6.4 | N (6.25) | 377 | [45] |
| Fullerene C60 | Chlorinated naphthalene | Hard | SBA-15 | 900 | 680 | 0.85 | 4.5–10.6 | - | 116 | [40] |
| Graphene CNTs | Aniline, (NH4)2S2O8 | Hard | SiO2 | 900 | 785.7 | 1.66 | 7–22 | N (7.3) | 112 | [35] |
| Fullerene C70 | Chlorinated naphthalene | Hard | SBA-15 | 900 | 585.8 | 0.79 | 2.7–10.1 | - | 172 | [41] |
| Fullerene C60 | Sucrose | Hard | SBA-15 | 900 | 808 | 1.5 | 3.6 | - | 213 | [42] |
| Graphene | 2,6-Diaminopyridine | Soft | PS-b-PEO | 700 | 324 | - | 8–25 | N (19) | 256 | [76] |
| Graphene | resorcinol, hexamine | Soft | F127 | 800 | 1072 | - | 2.7 | - | 209 | [55] |
| Graphene | phenol, HCHO | Soft | F127 | 800 | 2109 | 1.24 | 3.41 | - | 329.5 | [51] |
| Graphene | Resorcinol, mesitylene, hexamine | Soft | F127 | 800 | 1040 | 0.73 | 1.4–3.6 | N (5.24) | 304 | [56] |
| Graphene | Phenol, HCHO | Soft | F127 | 800 | 1309 | 0.89 | 0.71–4.75 | - | 332.5 | [52] |
| CNOs | ‘Star’ polymer, HCHO | Block copolymer | PMA chains | 800 | 247 | 0.139 | 4–8 | N (0.7) | 139 | [30] |
| CNOs | ‘Star’ polymer | Block copolymer | PMA chains | 800 | 74 | 1.44 | 3–14 | N (8.0) | 83 | [34] |
| Graphene | Pyrrole, (NH4)2S2O8 | - | - | 700 | - | - | - | N (9) | 296 | [77] |
| Graphene | Glucose, graphene | - | - | 800 | 763 | 3.06 | - | 305.5 | [78] | |
| Graphene | Ethylenediamine, phytic acid | - | - | 900 | 596 | 0.55 | 3.7 | N (3.6) P (0.3) | 201 | [79] |
| Graphene | Resorcinol, HCHO | - | - | 800 | 534 | 1.28 | 7.2 | - | 120 | [74] |
| Graphene | CTAB, aqueous mesophase pitch | - | - | 900 | 1151 | 0.86 | 4.3–17 | - | 356.3 | [80] |
| Graphene | Polyacrylonitrile | - | - | 800 | 389 | - | 15–65 | N (10) | 431.9 | [81] |
| Graphene | Urea, pyrrole | - | - | 550 | 158 | - | 3.2 | N (-) | 803 | [75] |
| Graphene CNTs | Activated carbon | - | - | 450 | 953 | 1.075 | 4.5 | N (7.38) | 750 | [82] |
| CNOs | Resorcinol, HCHO | Soft | F127 | 800 | 723 | 0.659 | 5.5–6.5 | - | 85 | [83] |
| CNOs | Resorcinol, HCHO, melamine | Soft | F127 | 800 | 923 | 1.242 | 8–11 | N (2.3) | 160 | [83] |
5.2. Catalytic and Electrocatalytic Properties of Mesoporous Carbon/CN Composites
5.3. Other Applications of Mesoporous Carbon/CN Composites
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Poothanari, M.A.; Pottathara, Y.B.; Thomas, S. Carbon Nanostructures for Electromagnetic Shielding Applications. In Industrial Applications of Nanomaterials; Elsevier: Amsterdam, The Netherlands, 2019; pp. 205–223. ISBN 978-0-12-815749-7. [Google Scholar]
- Wang, N.; Pandit, S.; Ye, L.; Edwards, M.; Mokkapati, V.R.S.S.; Murugesan, M.; Kuzmenko, V.; Zhao, C.; Westerlund, F.; Mijakovic, I.; et al. Efficient Surface Modification of Carbon Nanotubes for Fabricating High Performance CNT Based Hybrid Nanostructures. Carbon 2017, 111, 402–410. [Google Scholar] [CrossRef]
- Slepičková Kasálková, N.; Slepička, P.; Švorčík, V. Carbon Nanostructures, Nanolayers, and Their Composites. Nanomaterials 2021, 11, 2368. [Google Scholar] [CrossRef] [PubMed]
- Noonan, O.; Liu, Y.; Huang, X.; Yu, C. Layered Graphene/Mesoporous Carbon Heterostructures with Improved Mesopore Accessibility for High Performance Capacitive Deionization. J. Mater. Chem. A 2018, 6, 14272–14280. [Google Scholar] [CrossRef]
- Wang, G.; Dong, Q.; Wu, T.; Zhan, F.; Zhou, M.; Qiu, J. Ultrasound-Assisted Preparation of Electrospun Carbon Fiber/Graphene Electrodes for Capacitive Deionization: Importance and Unique Role of Electrical Conductivity. Carbon 2016, 103, 311–317. [Google Scholar] [CrossRef]
- Javanbakht, S.; Namazi, H. Doxorubicin Loaded Carboxymethyl Cellulose/Graphene Quantum Dot Nanocomposite Hydrogel Films as a Potential Anticancer Drug Delivery System. Mater. Sci. Eng. C 2018, 87, 50–59. [Google Scholar] [CrossRef]
- Mohamed, M.G.; Atayde, E.C.; Matsagar, B.M.; Na, J.; Yamauchi, Y.; Wu, K.C.-W.; Kuo, S.-W. Construction Hierarchically Mesoporous/Microporous Materials Based on Block Copolymer and Covalent Organic Framework. J. Taiwan Inst. Chem. Eng. 2020, 112, 180–192. [Google Scholar] [CrossRef]
- Eftekhari, A.; Fan, Z. Ordered Mesoporous Carbon and Its Applications for Electrochemical Energy Storage and Conversion. Mater. Chem. Front. 2017, 1, 1001–1027. [Google Scholar] [CrossRef]
- Petkovich, N.D.; Stein, A. Controlling Macro- and Mesostructures with Hierarchical Porosity through Combined Hard and Soft Templating. Chem. Soc. Rev. 2013, 42, 3721–3739. [Google Scholar] [CrossRef]
- Xin, W.; Song, Y. Mesoporous Carbons: Recent Advances in Synthesis and Typical Applications. RSC Adv. 2015, 5, 83239–83285. [Google Scholar] [CrossRef]
- Tanaka, S.; Nishiyam, N. Morphology Control of Ordered Mesoporous Carbon Using Organic-Templating Approach. In Progress in Molecular and Environmental Bioengineering—From Analysis and Modeling to Technology Applications; Carpi, A., Ed.; InTech: Houston, TX, USA, 2011; ISBN 978-953-307-268-5. [Google Scholar]
- Wang, H.; Shao, Y.; Mei, S.; Lu, Y.; Zhang, M.; Sun, J.; Matyjaszewski, K.; Antonietti, M.; Yuan, J. Polymer-Derived Heteroatom-Doped Porous Carbon Materials. Chem. Rev. 2020, 120, 9363–9419. [Google Scholar] [CrossRef]
- Lin, Z.; Zeng, Z.; Gui, X.; Tang, Z.; Zou, M.; Cao, A. Carbon Nanotube Sponges, Aerogels, and Hierarchical Composites: Synthesis, Properties, and Energy Applications. Adv. Energy Mater. 2016, 6, 1600554. [Google Scholar] [CrossRef]
- Lamy-Mendes, A.; Silva, R.F.; Durães, L. Advances in Carbon Nanostructure–Silica Aerogel Composites: A Review. J. Mater. Chem. A 2018, 6, 1340–1369. [Google Scholar] [CrossRef]
- Hu, Y.; Shenderova, O.A.; Hu, Z.; Padgett, C.W.; Brenner, D.W. Carbon Nanostructures for Advanced Composites. Rep. Prog. Phys. 2006, 69, 1847–1895. [Google Scholar] [CrossRef]
- Dai, L.; Mau, A.W.H. Controlled Synthesis and Modification of Carbon Nanotubes and C60: Carbon Nanostructures for Advanced Polymeric Composite Materials. Adv. Mater. 2001, 13, 899–913. [Google Scholar] [CrossRef]
- Park, S.-H.; Bae, J. Polymer Composite Containing Carbon Nanotubes and Their Applications. Recent Pat. Nanotechnol. 2017, 11, 109–115. [Google Scholar] [CrossRef]
- Rahman, M.M.; Ara, M.G.; Alim, M.A.; Uddin, M.S.; Najda, A.; Albadrani, G.M.; Sayed, A.A.; Mousa, S.A.; Abdel-Daim, M.M. Mesoporous Carbon: A Versatile Material for Scientific Applications. Int. J. Mol. Sci. 2021, 22, 4498. [Google Scholar] [CrossRef]
- Sattler, K.D. Carbon Nanomaterials Sourcebook: Nanoparticles, Nanocapsules, Nanofibers, Nanoporous Structures, and Nanocomposites, Volume II; CRC Press: Boca Raton, FL, USA, 2018; ISBN 978-1-315-36244-1. [Google Scholar]
- Beck, J.S.; Vartuli, J.C.; Roth, W.J.; Leonowicz, M.E.; Kresge, C.T.; Schmitt, K.D.; Chu, C.T.W.; Olson, D.H.; Sheppard, E.W.; McCullen, S.B.; et al. A New Family of Mesoporous Molecular Sieves Prepared with Liquid Crystal Templates. J. Am. Chem. Soc. 1992, 114, 10834–10843. [Google Scholar] [CrossRef]
- Kresge, C.T.; Leonowicz, M.E.; Roth, W.J.; Vartuli, J.C.; Beck, J.S. Ordered Mesoporous Molecular Sieves Synthesized by a Liquid-Crystal Template Mechanism. Nature 1992, 359, 710–712. [Google Scholar] [CrossRef]
- Ryoo, R.; Joo, S.H.; Jun, S. Synthesis of Highly Ordered Carbon Molecular Sieves via Template-Mediated Structural Transformation. J. Phys. Chem. B 1999, 103, 7743–7746. [Google Scholar] [CrossRef]
- Liu, C.; Chu, J.; Liu, Y.; Lyu, Y.; Guo, B. The Synergistic Effect of Carbon Coating and CNTs Compositing on the Hard Carbon Anode for Sodium Ion Batteries. RSC Adv. 2019, 9, 21667–21670. [Google Scholar] [CrossRef]
- Liu, B.; Liu, L.; Yu, Y.; Zhang, Y.; Chen, A. Synthesis of Mesoporous Carbon with Tunable Pore Size for Supercapacitors. New J. Chem. 2020, 44, 1036–1044. [Google Scholar] [CrossRef]
- Liu, R.; Wang, X.; Zhao, X.; Feng, P. Sulfonated Ordered Mesoporous Carbon for Catalytic Preparation of Biodiesel. Carbon 2008, 46, 1664–1669. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, S.; Luo, Y.; Pan, S.; Ding, H.; Li, G. Synthesis of Mesoporous Carbon Capsules Encapsulated with Magnetite Nanoparticles and Their Application in Wastewater Treatment. J. Mater. Chem. 2011, 21, 3664. [Google Scholar] [CrossRef]
- Srivastava, I.; Singh, P.K.; Gupta, T.; Sankararamakrishnan, N. Preparation of Mesoporous Carbon Composites and Its Highly Enhanced Removal Capacity of Toxic Pollutants from Air. J. Environ. Chem. Eng. 2019, 7, 103271. [Google Scholar] [CrossRef]
- Kerimkulova, A.R.; Azat, S.; Mansurov, Z.A.; Gilmanov, M.K.; Ibragimova, S.A.; Adekenov, S.M.; Rachimova, B.B. Mesoporous Nano Carbon Sorbents for Separating Different Biomolecules. Adv. Mater. Res. 2012, 535–537, 284–288. [Google Scholar] [CrossRef]
- Stankovich, S.; Dikin, D.A.; Dommett, G.H.B.; Kohlhaas, K.M.; Zimney, E.J.; Stach, E.A.; Piner, R.D.; Nguyen, S.T.; Ruoff, R.S. Graphene-Based Composite Materials. Nature 2006, 442, 282–286. [Google Scholar] [CrossRef]
- Siemiaszko, G.; Hryniewicka, A.; Breczko, J.; Delgado, O.F.; Markiewicz, K.H.; Echegoyen, L.; Plonska-Brzezinska, M.E. Polymeric Network Hierarchically Organized on Carbon Nano-Onions: Block Polymerization as a Tool for the Controlled Formation of Specific Pore Diameters. ACS Appl. Polym. Mater. 2022, 4, 2442–2458. [Google Scholar] [CrossRef]
- Reyhani, R.; Zadhoush, A.; Tabrizi, N.S.; Nazockdast, H.; Naeimirad, M. Synthesis and Characterization of Powdered CNT-Doped Carbon Aerogels. J. Non-Cryst. Solids 2021, 571, 121058. [Google Scholar] [CrossRef]
- Li, S.; Liu, Y.; Gong, H.; Wu, K.-H.; Ba, H.; Duong-Viet, C.; Jiang, C.; Pham-Huu, C.; Su, D. N-Doped 3D Mesoporous Carbon/Carbon Nanotubes Monolithic Catalyst for H2S Selective Oxidation. ACS Appl. Nano Mater. 2019, 2, 3780–3792. [Google Scholar] [CrossRef]
- Fulvio, P.F.; Mayes, R.T.; Wang, X.; Mahurin, S.M.; Bauer, J.C.; Presser, V.; McDonough, J.; Gogotsi, Y.; Dai, S. “Brick-and-Mortar” Self-Assembly Approach to Graphitic Mesoporous Carbon Nanocomposites. Adv. Funct. Mater. 2011, 21, 2208–2215. [Google Scholar] [CrossRef]
- Siemiaszko, G.; Hryniewicka, A.; Breczko, J.; Brzezinski, K.; Plonska-Brzezinska, M.E. Carbon Nano-Onion Induced Organization of Polyacrylonitrile-Derived Block Star Polymers to Obtain Mesoporous Carbon Materials. Chem. Commun. 2022, 58, 6829–6832. [Google Scholar] [CrossRef] [PubMed]
- Tu, S.; Su, H.; Sui, D.; He, Y.; Cheng, M.; Bai, P.; Zhang, C.; Sun, P.; Wang, C.; Jiang, J.; et al. Mesoporous Carbon Nanomaterials with Tunable Geometries and Porous Structures Fabricated by a Surface-Induced Assembly Strategy. Energy Storage Mater. 2021, 35, 602–609. [Google Scholar] [CrossRef]
- Xie, Y.; Kocaefe, D.; Chen, C.; Kocaefe, Y. Review of Research on Template Methods in Preparation of Nanomaterials. J. Nanomater. 2016, 2016, 2302595. [Google Scholar] [CrossRef]
- Kong, Y.; Nanjundan, A.K.; Liu, Y.; Song, H.; Huang, X.; Yu, C. Modulating Ion Diffusivity and Electrode Conductivity of Carbon Nanotube@Mesoporous Carbon Fibers for High Performance Aluminum–Selenium Batteries. Small 2019, 15, 1904310. [Google Scholar] [CrossRef]
- Song, X.; Ning, G.; Ma, X.; Yu, Z.; Wang, G. N-Doped Carbon Nanotube-Reinforced N-Doped Mesoporous Carbon for Flue Gas Desulfurization. Ind. Eng. Chem. Res. 2018, 57, 4245–4252. [Google Scholar] [CrossRef]
- Nazari, M.; Rahmanifar, M.S.; Noori, A.; Li, W.; Zhang, C.; Mousavi, M.F. The Ordered Mesoporous Carbon Nitride-Graphene Aerogel Nanocomposite for High-Performance Supercapacitors. J. Power Sources 2021, 494, 229741. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Joseph, S.; Ilbeygi, H.; Park, D.-H.; Sarkar, S.; Chandra, G.; Umapathy, S.; Srinivasan, S.; Talapaneni, S.N.; Vinu, A. Highly Crystalline Mesoporous C60 with Ordered Pores: A Class of Nanomaterials for Energy Applications. Angew. Chem. Int. Ed. 2018, 57, 569–573. [Google Scholar] [CrossRef]
- Benzigar, M.R.; Joseph, S.; Baskar, A.V.; Park, D.-H.; Chandra, G.; Umapathy, S.; Talapaneni, S.N.; Vinu, A. Ordered Mesoporous C70 with Highly Crystalline Pore Walls for Energy Applications. Adv. Funct. Mater. 2018, 28, 1803701. [Google Scholar] [CrossRef]
- Baskar, A.V.; Ruban, A.M.; Davidraj, J.M.; Singh, G.; Al-Muhtaseb, A.H.; Lee, J.M.; Yi, J.; Vinu, A. Single-Step Synthesis of 2D Mesoporous C60/Carbon Hybrids for Supercapacitor and Li-Ion Battery Applications. Bull. Chem. Soc. Jpn. 2021, 94, 133–140. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.; Song, H.; Noonan, O.; Liang, C.; Huang, X.; Yu, C. Single-Layered Mesoporous Carbon Sandwiched Graphene Nanosheets for High Performance Ionic Liquid Supercapacitors. J. Phys. Chem. C 2017, 121, 23947–23954. [Google Scholar] [CrossRef]
- Lu, S.; Guo, K.; Xie, Y.; Ning, J. Ordered Mesoporous Carbons Loading on Sulfonated Graphene by Multi-Components Co-Assembly for Supercapacitor Applications. Energy Technol. 2018, 6, 1975–1985. [Google Scholar] [CrossRef]
- Song, Y.; Yang, J.; Wang, K.; Haller, S.; Wang, Y.; Wang, C.; Xia, Y. In-Situ Synthesis of Graphene/Nitrogen-Doped Ordered Mesoporous Carbon Nanosheet for Supercapacitor Application. Carbon 2016, 96, 955–964. [Google Scholar] [CrossRef]
- Sui, L.; Wang, Y.; Ji, W.; Kang, H.; Dong, L.; Yu, L. N-Doped Ordered Mesoporous Carbon/Graphene Composites with Supercapacitor Performances Fabricated by Evaporation Induced Self-Assembly. Int. J. Hydrogen Energy 2017, 42, 29820–29829. [Google Scholar] [CrossRef]
- Zhu, J.; Feng, T.; Du, X.; Wang, J.; Hu, J.; Wei, L. High Performance Asymmetric Supercapacitor Based on Polypyrrole/Graphene Composite and Its Derived Nitrogen-Doped Carbon Nano-Sheets. J. Power Sources 2017, 346, 120–127. [Google Scholar] [CrossRef]
- Zhen, Q.; Ma, H.; Jin, Z.; Zhu, D.; Liu, X.; Sun, Y.; Zhang, C.; Pang, H. Electrochemical Sensor for Rutin Detection Based on N-Doped Mesoporous Carbon Nanospheres and Graphene. New J. Chem. 2021, 45, 4986–4993. [Google Scholar] [CrossRef]
- Shen, Z.; Du, J.; Mo, Y.; Chen, A. Nanocomposites of Reduced Graphene Oxide Modified with Mesoporous Carbon Layers Anchored by Hollow Carbon Spheres for Energy Storage. Carbon 2021, 173, 22–30. [Google Scholar] [CrossRef]
- Lei, Z.; Christov, N.; Zhao, X.S. Intercalation of Mesoporous Carbon Spheres between Reduced Graphene Oxide Sheets for Preparing High-Rate Supercapacitor Electrodes. Energy Environ. Sci. 2011, 4, 1866–1873. [Google Scholar] [CrossRef]
- Song, Y.; Li, Z.; Guo, K.; Shao, T. Hierarchically Ordered Mesoporous Carbon/Graphene Composites as Supercapacitor Electrode Materials. Nanoscale 2016, 8, 15671–15680. [Google Scholar] [CrossRef]
- Chen, P.; Lu, S.; Yang, C.; He, Z.; Chen, X.; Guo, K. Ordered Mesoporous Carbons Loading on Graphene after Different Molten Salt Activations for Supercapacitor Applications. Energy Technol. 2018, 6, 2273–2281. [Google Scholar] [CrossRef]
- Yang, S.; Feng, X.; Wang, L.; Tang, K.; Maier, J.; Müllen, K. Graphene-Based Nanosheets with a Sandwich Structure. Angew. Chem. Int. Ed. 2010, 49, 4795–4799. [Google Scholar] [CrossRef]
- Ma, T.-Y.; Liu, L.; Yuan, Z.-Y. Direct Synthesis of Ordered Mesoporous Carbons. Chem. Soc. Rev. 2013, 42, 3977–4003. [Google Scholar] [CrossRef] [PubMed]
- Qiu, J.; Wang, Y.; Wu, F.; Liu, W.; Zhang, S. Graphene-Containing Ordered Mesoporous Carbons Synthesized by One-Pot Aqueous Route and Its Electrochemical Performance. Polym. Compos. 2017, 38, 1438–1446. [Google Scholar] [CrossRef]
- Chen, P.; Yang, C.; He, Z.; Guo, K. One-Pot Facile Route to Fabricate the Precursor of Sulfonated Graphene/N-Doped Mesoporous Carbons Composites for Supercapacitors. J. Mater. Sci. 2019, 54, 4180–4191. [Google Scholar] [CrossRef]
- Liu, J.; Bo, X.; Zhou, M.; Guo, L. A Nanocomposite Prepared from Metal-Free Mesoporous Carbon Nanospheres and Graphene Oxide for Voltammetric Determination of Doxorubicin. Microchim. Acta 2019, 186, 639. [Google Scholar] [CrossRef]
- Zhu, X.; Xia, Y.; Zhang, X.; Al-Khalaf, A.A.; Zhao, T.; Xu, J.; Peng, L.; Hozzein, W.N.; Li, W.; Zhao, D. Synthesis of Carbon Nanotubes@mesoporous Carbon Core–Shell Structured Electrocatalysts via a Molecule-Mediated Interfacial Co-Assembly Strategy. J. Mater. Chem. A 2019, 7, 8975–8983. [Google Scholar] [CrossRef]
- Wang, L.; Sun, L.; Tian, C.; Tan, T.; Mu, G.; Zhang, H.; Fu, H. A Novel Soft Template Strategy to Fabricate Mesoporous Carbon/Graphene Composites as High-Performance Supercapacitor Electrodes. RSC Adv. 2012, 2, 8359–8367. [Google Scholar] [CrossRef]
- Li, M.; Ober, C.K. Block Copolymer Patterns and Templates. Mater. Today 2006, 9, 30–39. [Google Scholar] [CrossRef]
- Naumann, S. Strategies for Pore-Diameter Control in Mesoporous Carbons Derived from Organic Self-Assembly Processes. Org. Mater. 2021, 03, 283–294. [Google Scholar] [CrossRef]
- Zhao, D.; Huo, Q.; Feng, J.; Chmelka, B.F.; Stucky, G.D. Nonionic Triblock and Star Diblock Copolymer and Oligomeric Surfactant Syntheses of Highly Ordered, Hydrothermally Stable, Mesoporous Silica Structures. J. Am. Chem. Soc. 1998, 120, 6024–6036. [Google Scholar] [CrossRef]
- Zhai, C.; Li, S.; Wang, J.; Liu, Y. Nitrogen-Doped Porous Carbon Sphere Supported Pt Nanoparticles for Methanol and Ethanol Electro-Oxidation in Alkaline Media. RSC Adv. 2018, 8, 36353–36359. [Google Scholar] [CrossRef]
- Wang, Z.; Stein, A. Morphology Control of Carbon, Silica, and Carbon/Silica Nanocomposites: From 3D Ordered Macro-/Mesoporous Monoliths to Shaped Mesoporous Particles. Chem. Mater. 2008, 20, 1029–1040. [Google Scholar] [CrossRef]
- Libbrecht, W.; Verberckmoes, A.; Thybaut, J.W.; Van Der Voort, P.; De Clercq, J. Tunable Large Pore Mesoporous Carbons for the Enhanced Adsorption of Humic Acid. Langmuir 2017, 33, 6769–6777. [Google Scholar] [CrossRef] [PubMed]
- Meng, Y.; Gu, D.; Zhang, F.; Shi, Y.; Yang, H.; Li, Z.; Yu, C.; Tu, B.; Zhao, D. Ordered Mesoporous Polymers and Homologous Carbon Frameworks: Amphiphilic Surfactant Templating and Direct Transformation. Angew. Chem. Int. Ed. 2005, 44, 7053–7059. [Google Scholar] [CrossRef] [PubMed]
- Xing, L.; Song, C.; Kong, A. N–S-Codoped Mesoporous Carbons from Melamine-2-Thenaldehyde Polymers on Carbon Nanotubes for Oxygen Reduction and Zn-Air Batteries. J. Solid State Chem. 2020, 287, 121348. [Google Scholar] [CrossRef]
- Chang, Y.; Ren, Y.; Zhu, L.; Li, Y.; Li, T.; Ren, B. Preparation of Macadamia Nut Shell Porous Carbon and Its Electrochemical Performance as Cathode Material for Lithium–Sulfur Batteries. Electrochimica Acta 2022, 420, 140454. [Google Scholar] [CrossRef]
- Gao, Y.; Wang, Q.; Ji, G.; Li, A.; Niu, J. Doping Strategy, Properties and Application of Heteroatom-Doped Ordered Mesoporous Carbon. RSC Adv. 2021, 11, 5361–5383. [Google Scholar] [CrossRef]
- Wang, X.; Lee, J.S.; Zhu, Q.; Liu, J.; Wang, Y.; Dai, S. Ammonia-Treated Ordered Mesoporous Carbons as Catalytic Materials for Oxygen Reduction Reaction. Chem. Mater. 2010, 22, 2178–2180. [Google Scholar] [CrossRef]
- Khan, S.; Ul-Islam, M.; Sajjad, M.; Hussain, I.; Idrees, M.; Saeed, M.; Imran, M.; Javed, M.S. Nitrogen and Sulfur Co-Doped Two-Dimensional Highly Porous Carbon Nanosheets for High-Performance Lithium–Sulfur Batteries. Energy Fuels 2022, 36, 2220–2227. [Google Scholar] [CrossRef]
- Ke, Q.; Wang, J. Graphene-Based Materials for Supercapacitor Electrodes—A Review. J. Mater. 2016, 2, 37–54. [Google Scholar] [CrossRef]
- Li, M.; Ding, J.; Xue, J. Mesoporous Carbon Decorated Graphene as an Efficient Electrode Material for Supercapacitors. J. Mater. Chem. A 2013, 1, 7469. [Google Scholar] [CrossRef]
- Chandrasekaran, N.; Premkumar, V.; Senthil Kumar, S.M.; Ram, R. Single-Step Rapid Synthesis of Monolithic Mesoporous Carbon/Graphene Aerogels with Improved Double Layer Capacitance. New J. Chem. 2018, 42, 7371–7376. [Google Scholar] [CrossRef]
- Baruah, K.; Sarmah, D.; Kumar, A. Ternary Hybrid Nanocomposites of Polypyrrole Nanotubes with 2D Self-Assembled Heterostructures of Protonated g-C3N4-rGO as Supercapacitor Electrodes. Ionics 2021, 27, 3153–3168. [Google Scholar] [CrossRef]
- Zhang, L.; Wang, T.; Gao, T.-N.; Xiong, H.; Zhang, R.; Liu, Z.; Song, S.; Dai, S.; Qiao, Z.-A. Multistage Self-Assembly Strategy: Designed Synthesis of N-Doped Mesoporous Carbon with High and Controllable Pyridine N Content for Ultrahigh Surface-Area-Normalized Capacitance. CCS Chem. 2021, 3, 870–881. [Google Scholar] [CrossRef]
- Sridevi, G.; Narmatha, M.; Sathish, M. N-Containing Carbon/Graphene Nanocomposites for Electrochemical Supercapacitor Applications. J. Nanosci. Nanotechnol. 2017, 17, 1267–1274. [Google Scholar] [CrossRef]
- Liu, K.-K.; Jin, B.; Meng, L.-Y. Glucose/Graphene-Based Aerogels for Gas Adsorption and Electric Double Layer Capacitors. Polymers 2018, 11, 40. [Google Scholar] [CrossRef]
- Zheng, L.; Xia, K.; Han, B.; Zhou, C.; Gao, Q.; Wang, H.; Pu, S.; Wu, J. N/P Codoped Porous Carbon-Coated Graphene Nanohybrid as a High-Performance Electrode for Supercapacitors. ACS Appl. Nano Mater. 2018, 1, 6742–6751. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, Y.; Zhang, Q.; Fang, C.; Wu, L.; Bai, M.; Yao, Y. Facile Synthesis of Mesoporous Carbon Microspheres/Graphene Composites in Situ for Application in Supercapacitors. RSC Adv. 2019, 9, 32258–32269. [Google Scholar] [CrossRef]
- Kong, L.; Ma, Q.; Xu, Z.; Shen, X.; Wang, J.; Zhu, J. Three-Dimensional Graphene Network Deposited with Mesoporous Nitrogen-Doped Carbon from Non-Solvent Induced Phase Inversion for High-Performance Supercapacitors. J. Colloid Interface Sci. 2020, 558, 21–31. [Google Scholar] [CrossRef]
- Liu, Z.-A.; Tao, Y.; Song, X.-Z.; Bao, M.; Tan, Z. A Three Dimensional N-Doped Graphene/CNTs/AC Hybrid Material for High-Performance Supercapacitors. RSC Adv. 2017, 7, 6664–6670. [Google Scholar] [CrossRef]
- Siemiaszko, G.; Breczko, J.; Hryniewicka, A.; Ilnicka, A.; Markiewicz, K.H.; Terzyk, A.P.; Plonska-Brzezinska, M.E. Composites Containing Resins and Carbon Nano-Onions as Efficient Porous Carbon Materials for Supercapacitors. Sci. Rep. 2023, 13, 6606. [Google Scholar] [CrossRef]
- Krestinin, A.V.; Knerel’man, E.I.; Dremova, N.N.; Golodkov, O.N. Carbon Nanopaper Produced from Carbon Nanotubes/Resorcinol–Formaldehyde Xerogel Nanocomposite for Electrochemical Supercapasitors. Russ. J. Electrochem. 2023, 59, 666–677. [Google Scholar] [CrossRef]
- Krestinin, A.V.; Tarasenko, A.B.; Kochanova, S.A.; Kislenko, S.A. Characteristics of Power Supercapacitor with Electrodes Made of Composite Carbon Nanopaper Based on Carbon Nanotubes and Resorcinol–Formaldehyde Xerogel. Russ. J. Electrochem. 2024, 60, 513–525. [Google Scholar] [CrossRef]
- Chen, X.; Wang, S.; Wang, Y.; Yang, Z.; Liu, S.; Gao, J.; Su, Z.; Zhu, P.; Zhao, X.; Wang, G. Nitrogen-Doped Mesoporous Carbon Layer with Embedded Co/CoOx Nanoparticles Coated on CNTs for Oxygen Reduction Reaction in Zn–Air Battery. Electrocatalysis 2019, 10, 277–286. [Google Scholar] [CrossRef]
- Wang, Z.; Cheng, J.; Song, H.; Xue, D.; Zhong, X.; Wang, J. Polar and Nonpolar Matrix Consisting of Twined Multiwalled Carbon Nanotube and High Nitrogen-Doped Porous Carbon Derived from Ionic Liquid for Stable Li-S Battery. Energy Technol. 2019, 7, 1900470. [Google Scholar] [CrossRef]
- Zhe, R.; Zhu, T.; Wei, X.; Ren, Y.; Qing, C.; Li, N.; Wang, H.-E. Graphene Oxide Wrapped Hollow Mesoporous Carbon Spheres as a Dynamically Bipolar Host for Lithium–Sulfur Batteries. J. Mater. Chem. A 2022, 10, 24422–24433. [Google Scholar] [CrossRef]
- Wu, X.; Li, S.; Wang, B.; Liu, J.; Yu, M. Free-Standing 3D Network-like Cathode Based on Biomass-Derived N-Doped Carbon/Graphene/g-C3N4 Hybrid Ultrathin Sheets as Sulfur Host for High-Rate Li-S Battery. Renew. Energy 2020, 158, 509–519. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Zhao, Y.-S.; Yang, X.-X.; Ren, M.-X.; Lei, B.-Y.; Meng, W.-J.; Zhao, D.-L. Graphene Nanosheet@spherical Ordered Mesoporous Carbon/Sulfur Nanocomposites as Cathode Material for High-Performance Lithium-Sulfur Batteries. Int. J. Hydrogen Energy 2020, 45, 32654–32663. [Google Scholar] [CrossRef]
- Xi, J.; Liu, J.; Gong, Z.; Wang, S.; Li, X.; Yuan, J.; Mei, X.; Luo, Z. Graphene Coated and Nitrogen-Rich Porous Carbon Composites with Sulfur Encapusulation as Cathode for Lithium-Sulfur Batteries. Mater. Lett. 2022, 316, 132050. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhao, K.; Ji, G.; Guo, X.; Han, M.; Wen, J.; Ren, Z.; Zhao, S.; Gao, Z.; Wang, R.; et al. High Sulfur Loading, rGO-Linked and Polymer Binder-Free Cathodes Based on rGO Wrapped N,P-Codoped Mesoporous Carbon as Sulfur Host for Li-S Batteries. Chem. Eng. J. 2019, 361, 1043–1052. [Google Scholar] [CrossRef]
- Kim, Y.; Yun, J.; Shin, H.-S.; Jung, K.-N.; Lee, J.-W. Synergistic Nanoarchitecture of Mesoporous Carbon and Carbon Nanotubes for Lithium–Oxygen Batteries. Nano Converg. 2021, 8, 17. [Google Scholar] [CrossRef]
- Shu, C.; Lin, Y.; Su, D. N-Doped Onion-like Carbon as an Efficient Oxygen Electrode for Long-Life Li–O2 Battery. J. Mater. Chem. A 2016, 4, 2128–2136. [Google Scholar] [CrossRef]
- Ren, M.-X.; He, C.-J.; Duan, Y.-J.; Wang, Y.-Q.; Meng, W.-J.; Hou, Y.-L.; Zhao, D.-L. Mesoporous Silicon Nanocubes Coated by Nitrogen-Doped Carbon Shell and Wrapped by Graphene for High Performance Lithium-Ion Battery Anodes. Ceram. Int. 2022, 48, 4812–4820. [Google Scholar] [CrossRef]
- Jeong, Y.; Lee, K.; Kim, K.; Kim, S. Pore-Structure-Optimized CNT-Carbon Nanofibers from Starch for Rechargeable Lithium Batteries. Materials 2016, 9, 995. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Huang, X.; Qu, D.; Zheng, D.; Wang, G.; Harris, J.; Si, J.; Ding, T.; Chen, J.; Qu, D. Confined Phosphorus in Carbon Nanotube-Backboned Mesoporous Carbon as Superior Anode Material for Sodium/Potassium-Ion Batteries. Nano Energy 2018, 52, 1–10. [Google Scholar] [CrossRef]
- Xi, Y.; Yang, D.; Liu, W.; Qin, Y.; Qiu, X. Preparation of Porous Lignin-Derived Carbon/Carbon Nanotube Composites by Hydrophobic Self-Assembly and Carbonization to Enhance Lithium Storage Capacity. Electrochim. Acta 2019, 303, 1–8. [Google Scholar] [CrossRef]
- Wang, Y.; Fugetsu, B.; Wang, Z.; Gong, W.; Sakata, I.; Morimoto, S.; Hashimoto, Y.; Endo, M.; Dresselhaus, M.; Terrones, M. Nitrogen-Doped Porous Carbon Monoliths from Polyacrylonitrile (PAN) and Carbon Nanotubes as Electrodes for Supercapacitors. Sci. Rep. 2017, 7, 40259. [Google Scholar] [CrossRef]
- Hoffmann, F.; Fröba, M. Vitalising Porous Inorganic Silica Networks with Organic Functions—PMOs and Related Hybrid Materials. Chem. Soc. Rev. 2011, 40, 608–620. [Google Scholar] [CrossRef]
- Peng, C.; Li, Z.; Zhang, X.; Zhou, S.; Zhang, W.; Liu, X.; Zhao, P. Simultaneous Determination of Hydroquinone, Catechol and Resorcinol with High Selectivity Based on Hollow Nitrogen-Doped Mesoporous Carbon Spheres Decorated Graphene. J. Electrochem. Soc. 2018, 165, B212–B219. [Google Scholar] [CrossRef]
- Zhu, D.; Chu, M.; Xin, J.; Wang, X.; O’Halloran, K.P.; Ma, H.; Pang, H.; Tan, L.; Yang, G. Hierarchical and Hollow Boron/Nitrogen Co-Doped Yolk-Shell Mesoporous Carbon Nanospheres Attached to Reduced Graphene Oxide with High Sensing Performance for the Simultaneous Detection of Xanthine and Guanosine. Sens. Actuators B Chem. 2021, 343, 130068. [Google Scholar] [CrossRef]
- Zhou, M.; Guo, J.; Guo, L.; Bai, J. Electrochemical Sensing Platform Based on the Highly Ordered Mesoporous Carbon−Fullerene System. Anal. Chem. 2008, 80, 4642–4650. [Google Scholar] [CrossRef]
- Castelo-Quibén, J.; Bailón-García, E.; Pérez-Fernández, F.J.; Carrasco-Marín, F.; Pérez-Cadenas, A.F. Mesoporous Carbon Nanospheres with Improved Conductivity for Electro-Catalytic Reduction of O2 and CO2. Carbon. 2019, 155, 88–99. [Google Scholar] [CrossRef]
- Kim, C.; Talapaneni, S.N.; Dai, L. Porous Carbon Materials for CO2 Capture, Storage and Electrochemical Conversion. Mater. Rep. Energy 2023, 3, 100199. [Google Scholar] [CrossRef]
- Steele, B.C.H.; Heinzel, A. Materials for Fuel-Cell Technologies. Nature 2001, 414, 345–352. [Google Scholar] [CrossRef]
- Han, S.; Wu, D.; Li, S.; Zhang, F.; Feng, X. Porous Graphene Materials for Advanced Electrochemical Energy Storage and Conversion Devices. Adv. Mater. 2014, 26, 849–864. [Google Scholar] [CrossRef]
- Zhou, Z.; Pang, S.; Liu, Z.; Xu, H.; Cui, G. Interface Engineering for High-Performance Perovskite Hybrid Solar Cells. J. Mater. Chem. A 2015, 3, 19205–19217. [Google Scholar] [CrossRef]
- Kim, I.Y.; Kim, S.; Jin, X.; Premkumar, S.; Chandra, G.; Lee, N.-S.; Mane, G.P.; Hwang, S.-J.; Umapathy, S.; Vinu, A. Ordered Mesoporous C3N5 with a Combined Triazole and Triazine Framework and Its Graphene Hybrids for the Oxygen Reduction Reaction (ORR). Angew. Chem. Int. Ed. 2018, 57, 17135–17140. [Google Scholar] [CrossRef]
- Qu, K.; Zheng, Y.; Dai, S.; Qiao, S.Z. Graphene Oxide-Polydopamine Derived N, S-Codoped Carbon Nanosheets as Superior Bifunctional Electrocatalysts for Oxygen Reduction and Evolution. Nano Energy 2016, 19, 373–381. [Google Scholar] [CrossRef]
- Niu, W.; Li, L.; Liu, J.; Wang, N.; Li, W.; Tang, Z.; Zhou, W.; Chen, S. Graphene-Supported Mesoporous Carbons Prepared with Thermally Removable Templates as Efficient Catalysts for Oxygen Electroreduction. Small 2016, 12, 1900–1908. [Google Scholar] [CrossRef]
- Kim, I.Y.; Kim, S.; Premkumar, S.; Yang, J.; Umapathy, S.; Vinu, A. Thermodynamically Stable Mesoporous C3N7 and C3N6 with Ordered Structure and Their Excellent Performance for Oxygen Reduction Reaction. Small 2020, 16, 1903572. [Google Scholar] [CrossRef]
- Wang, N.; Tian, H.; Zhu, S.-Y.; Yan, D.-Y.; Mai, Y.-Y. Two-Dimensional Nitrogen-Doped Mesoporous Carbon/Graphene Nanocomposites from the Self-Assembly of Block Copolymer Micelles in Solution. Chin. J. Polym. Sci. 2018, 36, 266–272. [Google Scholar] [CrossRef]
- Hou, D.; Zhang, J.; Tian, H.; Li, Q.; Li, C.; Mai, Y. Pore Engineering of 2D Mesoporous Nitrogen-Doped Carbon on Graphene through Block Copolymer Self-Assembly. Adv. Mater. Interfaces 2019, 6, 1901476. [Google Scholar] [CrossRef]
- Xu, Z.; Li, L.; Chen, X.; Xiao, G. N,S-Codoped Mesoporous Carbons Derived from Polymer Micelle-Based Assemblies for the Oxygen Reduction Reaction. ACS Appl. Energy Mater. 2021, 4, 1954–1961. [Google Scholar] [CrossRef]
- Li, X.; Fang, Y.; Zhao, S.; Wu, J.; Li, F.; Tian, M.; Long, X.; Jin, J.; Ma, J. Nitrogen-Doped Mesoporous Carbon Nanosheet/Carbon Nanotube Hybrids as Metal-Free Bi-Functional Electrocatalysts for Water Oxidation and Oxygen Reduction. J. Mater. Chem. A 2016, 4, 13133–13141. [Google Scholar] [CrossRef]
- Xu, C.; Gu, Q.; Li, S.; Ma, J.; Zhou, Y.; Zhang, X.; Jiang, C.; Pham-Huu, C.; Liu, Y. Heteroatom-Doped Monolithic Carbocatalysts with Improved Sulfur Selectivity and Impurity Tolerance for H2S Selective Oxidation. ACS Catal. 2021, 11, 8591–8604. [Google Scholar] [CrossRef]
- Ba, H.; Liu, Y.; Truong-Phuoc, L.; Duong-Viet, C.; Nhut, J.-M.; Nguyen, D.L.; Ersen, O.; Tuci, G.; Giambastiani, G.; Pham-Huu, C. N-Doped Food-Grade-Derived 3D Mesoporous Foams as Metal-Free Systems for Catalysis. ACS Catal. 2016, 6, 1408–1419. [Google Scholar] [CrossRef]
- Long, D.; Chen, W.; Rao, X.; Zheng, S.; Zhang, Y. Synergetic Effect of C60/g-C3N4 Nanowire Composites for Enhanced Photocatalytic H2 Evolution under Visible Light Irradiation. ChemCatChem 2020, 12, 2022–2031. [Google Scholar] [CrossRef]
- Mi, M.; Liu, X.; Kong, W.; Ge, Y.; Dang, W.; Hu, J. Hierarchical Composite of N-Doped Carbon Sphere and Holey Graphene Hydrogel for High-Performance Capacitive Deionization. Desalination 2019, 464, 18–24. [Google Scholar] [CrossRef]
- Feng, B.; Khan, Z.U.; Khan, W.U. 3D Graphene-Supported N-Doped Hierarchically Porous Carbon for Capacitive Deionization of Saline Water. Environ. Sci. Nano 2023, 10, 1163–1176. [Google Scholar] [CrossRef]
- Liu, K.; Chen, B.; Feng, A.; Wu, J.; Hu, X.; Zhou, J.; Yu, Y. Bio-Composite Nanoarchitectonics for Graphene Tofu as Useful Source Material for Capacitive Deionization. Desalination 2022, 526, 115461. [Google Scholar] [CrossRef]
- Li, Y.; Qi, J.; Zhang, W.; Zhang, M.; Li, J. Fabrication of Polyvinylidene Fluoride-Derived Porous Carbon Heterostructure with Inserted Carbon Nanotube via Phase-Inversion Coupled with Annealing for Capacitive Deionization Application. J. Colloid Interface Sci. 2019, 554, 353–361. [Google Scholar] [CrossRef]
- Mondal, S.K. Synthesis of Mesoporous Fullerene and Its Platinum Composite: A Catalyst for PEMFc. J. Electrochem. Soc. 2012, 159, K156–K160. [Google Scholar] [CrossRef]
- Mondal, S.K. Synthesis of Novel Mesoporous Fullerene and Its Application to Electro-Oxidation of Methanol. arXiv 2011, arXiv:1004.4046. [Google Scholar]
- Song, J.; Murata, T.; Tsai, K.; Jia, X.; Sciortino, F.; Ma, R.; Yamauchi, Y.; Hill, J.P.; Shrestha, L.K.; Ariga, K. Fullerphene Nanosheets: A Bottom-Up 2D Material for Single-Carbon-Atom-Level Molecular Discrimination. Adv. Mater. Interfaces 2022, 9, 2102241. [Google Scholar] [CrossRef]
- Ren, W.; Tang, D.; Lu, X.; Sun, J.; Li, M.; Qiu, S.; Fan, D. Novel Multilayer ACF@rGO@OMC Cathode Composite with Enhanced Activity for Electro-Fenton Degradation of Phthalic Acid Esters. Ind. Eng. Chem. Res. 2016, 55, 11085–11096. [Google Scholar] [CrossRef]










| Hard-Template Method | Soft-Template Method | |
|---|---|---|
| Advantages |
|
|
| Disadvantages |
|
|
| Carbon Nanostructure | Precursor of Mesoporous Carbon | Template | Pore-Forming Agent | T (°C) | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | Hetero-Atom (%) | Capacity (mAh g−1) | Applications | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|---|
| Graphene | n-Butanol, S | Hard | P123, TEOS | 850 | 381 | 0.27 | 3.0–4.2 | S | 1159 | Li-S batteries | [90] |
| Graphene | SiO2/MF, S | Hard | SiO2 | 800 | 879 | 2 | - | N (18.6) S (70 wt%) | 880.8 | [91] | |
| CNTs | Emim-dca, S | - | 800 | - | - | <8 | N | 1558.6 | [87] | ||
| Graphene | Chitin, urea, g-C3N4, S | - | - | 800 | 342 | - | 2–5 | N S (78 wt%) | 1130 | [89] | |
| Graphene | Aniline, phytic acid | - | - | 800 | 683 | 0.26 | - | N (4.57) P (5.07) | 1469 | [92] | |
| CNTs | Macadamia nut shells, S | - | - | 900 | - | - | - | S | 1253 | [68] | |
| Graphene | m-Aminobenzene sulfonic acid | - | - | 900 | 185 | - | 3 | N (4.07) S (1.28) | 1355 | [71] | |
| Graphene | Resorcinol, HCHO, ammonia, S | Hard | TPOS | 800 | 973 | [88] | |||||
| CNTs | Resorcinol, HCHO, ethylenediamine | Hard | TEOS | 700 | 749 | 0.90 | ~2.7 | N | conductivity 1.54 S cm−1 | Al-Se batteries | [37] |
| CNTs | Dicyandiamide | - | - | 800 | 1685 | - | - | N | 1840 | Li-O2 batteries | [93] |
| CNOs | Nitric acid | - | - | 550 | 406 | - | 20.0 | N | 12,181 | [94] | |
| Fullerene C60 | Sucrose | Hard | SBA-15 | 900 | 808 | 1.5 | 3.6 | - | 1299 | Li-ion batteries | [42] |
| Graphene | 3-Aminopropyl triethoxysilane | Hard | TEOS | 750 | 224 | - | 3.8 | N | 1368 | [95] | |
| CNTs | Polyvinyl alcohol, starch | - | - | 700 | 982 | 0.48 | 2.0 | - | 743 | [96] | |
| CNTs | Dopamine hydrochloride | - | - | 800 | 321 | - | ~3.7 | N (1.6) | - | Zn–air batteries | [86] |
| CNTs | Resorcinol, HCHO | Soft | F-127 | 800 | 146 | - | ~3 | - | 203.6 | Na-ion batteries | [23] |
| CNTs | Resorcinol, HCHO | Hard | TEOS | 800 | 1045 | 1.09 | 8.3 | - | 1000 | Na/K-ion batteries | [97] |
| CNTs | Aniline, (NH4)2S2O8 | Hard | SiO2 | 900 | 786 | 1.66 | 7–22 | N (7.3) | 700 | Flexible sulfur electrodes | [35] |
| CNTs | Lignin | - | - | 900 | 1050 | 1.55 | 39 | - | 905 | Lithium storage | [98] |
| Graphene | Resorcinol, HCHO | Hard | TEOS, IL | 800 | 740 | 0.87 | 2.7 | N (5.5) | 347.3 | Energy storage systems | [49] |
| Fullerene C70 | 1-Chloronaphtalene | Hard | SBA-15 | 900 | 586 | 0.79 | 10.1 | - | Cs = 172 F g−1 | [41] | |
| CNTs | Dopamine, TMB | Soft | F-127 | 800 | 768 | 0.57 | 6.9 | N (6.9) | - | [58] | |
| CNTs | Phenol, HCHO | Soft | F-127 | 600 | - | - | - | - | Cs = 20 F g−1 | [99] | |
| CNOs | Resorcinol, HCHO | Sof | F-127 | 850 | 700 | 1.14 | 13.7 | - | - | [33] | |
| Fullerene C60 | 1-Chloronaphtalene | Hard | SBA-15 | 900 | 680 | 0.85 | 10.6 | - | Cs = 116 F g−1 | fuel cell | [40] |
| Carbon Nanostructure | Precursor of Mesoporous Carbon | Template | Pore-Forming Agent | T (°C) | Surface Area (m2 g−1) | Pore Size (nm) | Heteroatom | Linear Range | Limit of Detection | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Graphene | Polydopamine | Hard | TEOS | 800 | - | - | N | 0.5–400 mM for HQ) 1–300 mM (for CC) 3–200 mM (for RC) | 0.15 mM 0.3 mM 1.0 mM | [101] |
| Graphene | Resorcinol, HCHO, boric acid, CTAB | Hard | TEOS | 700 | 1239 | 6 | N B | 0.0915–103 μM (for X) 0.0822–128 μM (for G) | 0.0503 μM 0.0462 μM | [102] |
| Graphene | Resorcinol, HCHO, CTAB | Hard | TEOS | 800 | - | - | N | 0.5–189 mM (for rutin) | 0.05 mM | [48] |
| Fullerene C60 | Sucrose | Hard | SBA-15 | 900 | 1302 | 3.21 | - | - | - | [103] |
| Graphene | Phenol, HCHO | Soft | F127 | 700 | 514 | 3 | - | 10 nM–10 μM (for doxorubicine) | 1.50 nM | [57] |
| Carbon Nanostructure | Precursor of Mesoporous Carbon | Template | Pore-Forming AGENT | T (°C) | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | Heteroatom (%) | Feature | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Application in the ORR | ||||||||||
| Graphene | Dopamine, mercapto-ethanol | - | - | 800 | 273 | 0.33 | 3.6 | N (4.1) S (6.1) | Double-layer capacitance 11.1 mF cm−2 | [110] |
| Graphene | 2-Fluoroaniline | Hard | FeOOH | 800 | 821 | 0.66 | - | - | Current density 6.1 mA cm−2 | [111] |
| Graphene | 5-Amino-1H-tetrazole | Hard | P123 TEOS | 540 | 301 | - | 3.4 | N | Current density 11.1 mA cm−2 | [109] |
| Graphene | 5-Amino-1H-tetrazole | Hard | P123 TEOS | 250 300 | 114 167 | - | 4.4 3.3 | N | Current density 8.2 mA cm−2 7.5 mA cm−2 | [112] |
| Graphene | m-Phenylene-diamine | Soft | PS-b-PEO | 900 | 812 | 0.69 | 19 | N (2.2) | Current density 5.2 mA cm−2 | [113] |
| Graphene | m-Phenylene-diamine | Soft | P123 or F127 | 800 | 420 | - | 8 | N (2.6) | Current density 4.8 mA cm−2 | [114] |
| Graphene | m-Amino-thiophenol | Soft | PS-b-PEO | 900 | 799 | 0.94 | 9.4 | N (3.4) S (0.4) | Current density 5.66 mA cm–2 | [115] |
| CNTs | Melamine, 2-thenaldehyde | - | - | 900 | 407 | - | 5.44 | N (1.9) S (0.23) | - | [67] |
| CNTs | Urea, glucose | - | - | 800 | 594.1 | 0.58 | 2–50 | N (8.5) | ORR and OER activity with a low onset potential | [116] |
| Application in selective H2S oxidation | ||||||||||
| CNTs | D-glucose, citric acid, (NH4)2CO3 | - | - | 900 | 537 | 0.73 | 6.1 | N (4.0–9.1) | - | [32] |
| CNTs | D-glucose, citric acid, (NH4)2CO3 | - | - | 800 | 330 | - | - | N (4.0–5.4) P (0.5–3.3) | - | [117] |
| Application in catalysis and photocatalysis | ||||||||||
| CNTs | D-glucose,citric acid, (NH4)2CO3 | - | - | 900 | 516 | - | ~4 | N (19.5) | - | [118] |
| Fullerene C60 | Urea | - | - | 600 | 117.47 | - | 2.0–6.0 | N | - | [119] |
| Carbon Nanostructure | Precursor of Mesoporous Carbon | Template | Pore-Forming Agent | T (°C) | Surface Area (m2 g−1) | Pore Volume (cm3 g−1) | Pore Size (nm) | Heteroatom (%) | Feature | Ref. |
|---|---|---|---|---|---|---|---|---|---|---|
| Application in capacitive deionization | ||||||||||
| Graphene | Dopamine | Hard | TEOS | 800 | 1270 | 1.6 | 8–10 | N (4.2) | Cs = 125.7 F g−1 | [4] |
| Graphene | Bean protein | - | - | 850 | 1286 | 1.15 | 3.58 | N (1.93) | Cs = 370 F g−1 adsorption capacity 38.5 mg g−1 | [122] |
| Graphene | Resorcinol, HCHO | Hard | TPOS | 700 | 338 | 0.62 | - | N (3.11) | Cs = 226.5 F g−1 electrosorption capacity 17.8 mg g−1 in 500 mg L−1 NaCl | [120] |
| CNTs | Poly(vinylidene fluoride) | - | - | 800 | 905 | 0.48 | 2–10 | - | Electrosorption capacity 15.1 mg g−1 in 500 mg L−1 NaCl | [123] |
| Graphene | Polyacrylonitrile, polystyrene | Soft | PS spheres | 700 | 650 | 0.16 | 10.3 | N | Electrosorption capacity 25.5 mg g−1 in 500 mg L−1 NaCl | [121] |
| Application in proton-exchange membrane fuel cells | ||||||||||
| Fullerene C60 | 1,2,4-Trinitrobenzen | Hard | KIT-6 SBA15 | 900 | 310.5 100.0 | - | 2.5 3.5 | - | - | [124,125] |
| Application in single-carbon-atom-level molecular discrimination | ||||||||||
| Fullerene C60 | Ethylenediamine | - | - | 700 | 655.2 | 0.659 | 3.66 | N (1.2) | - | [126] |
| Fullerene C70 | Ethylenediamine | - | - | 700 | 114.1 | 0.257 | 3.88 | N (1.7) | - | [126] |
| Application in flue gas desulfurization | ||||||||||
| CNTs | Melamine, phenolic resin | Hard | MgO | 700 | 223 | - | 20 | N (6.1) | SO2 capacity 21.2 mg g−1 | [38] |
| Application in the electro-Fenton process | ||||||||||
| Graphene | Active carbon fiber, resol | Soft | F127 | 800 | 533 | 0.45 | 3.8 | - | Electroactive surface area 486 cm2 g–1 Electron transfer resistance 8.60 Ω | [127] |
| Electrical applications | ||||||||||
| CNTs | Resorcinol, HCHO | Soft | NaDBS | 1050 | 1507.5 | 0.99 | 3.26 | - | - | [31] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hryniewicka, A.; Siemiaszko, G.; Plonska-Brzezinska, M.E. Mesoporous Carbon Composites Containing Carbon Nanostructures: Recent Advances in Synthesis and Applications in Electrochemistry. Materials 2024, 17, 6195. https://doi.org/10.3390/ma17246195
Hryniewicka A, Siemiaszko G, Plonska-Brzezinska ME. Mesoporous Carbon Composites Containing Carbon Nanostructures: Recent Advances in Synthesis and Applications in Electrochemistry. Materials. 2024; 17(24):6195. https://doi.org/10.3390/ma17246195
Chicago/Turabian StyleHryniewicka, Agnieszka, Gabriela Siemiaszko, and Marta E. Plonska-Brzezinska. 2024. "Mesoporous Carbon Composites Containing Carbon Nanostructures: Recent Advances in Synthesis and Applications in Electrochemistry" Materials 17, no. 24: 6195. https://doi.org/10.3390/ma17246195
APA StyleHryniewicka, A., Siemiaszko, G., & Plonska-Brzezinska, M. E. (2024). Mesoporous Carbon Composites Containing Carbon Nanostructures: Recent Advances in Synthesis and Applications in Electrochemistry. Materials, 17(24), 6195. https://doi.org/10.3390/ma17246195








