Experimental Validation of Hydrogen Affinity as a Design Criterion for Alloys
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Design
Hydrogen Affinity
2.2. Material Preparation
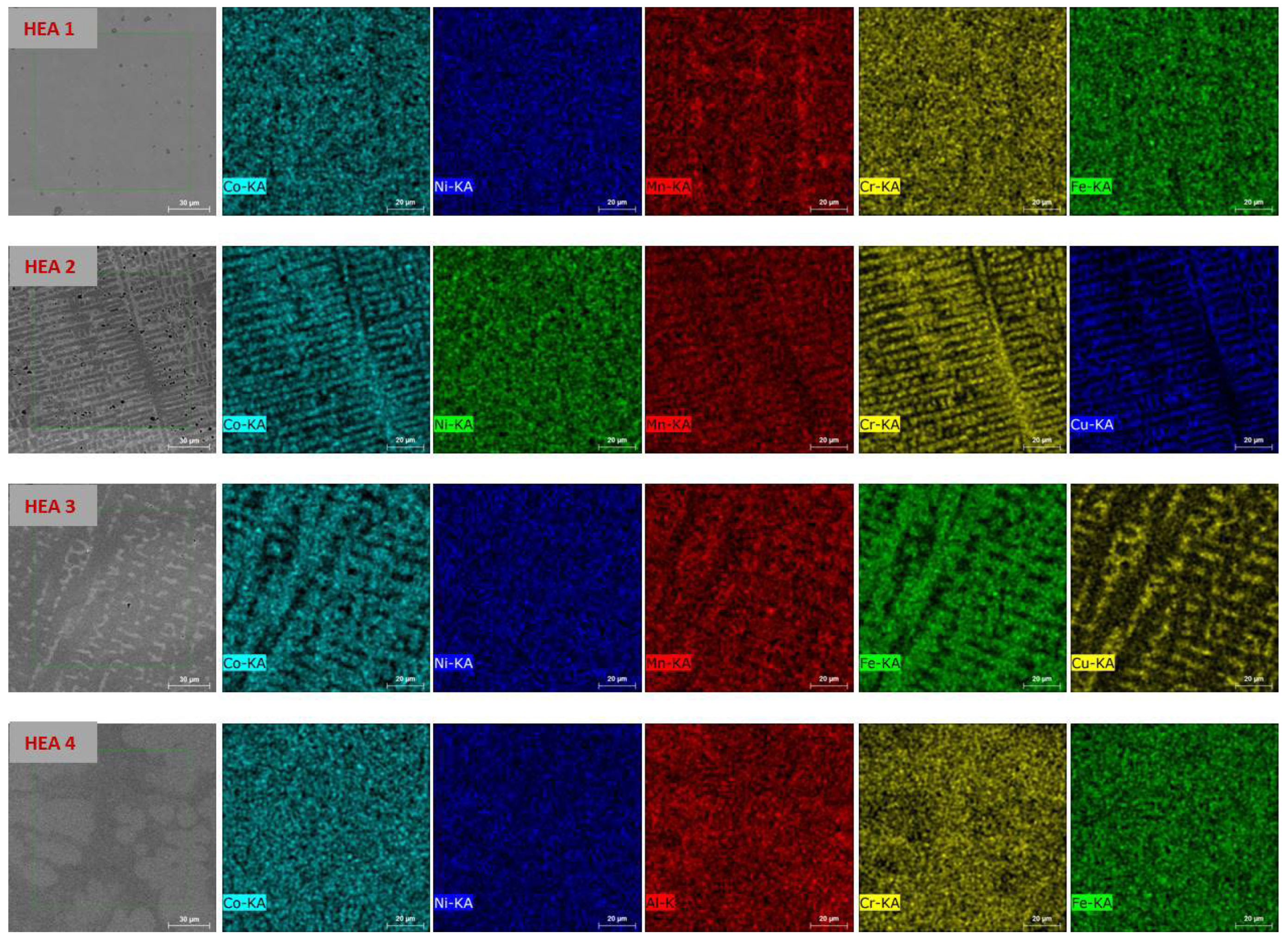
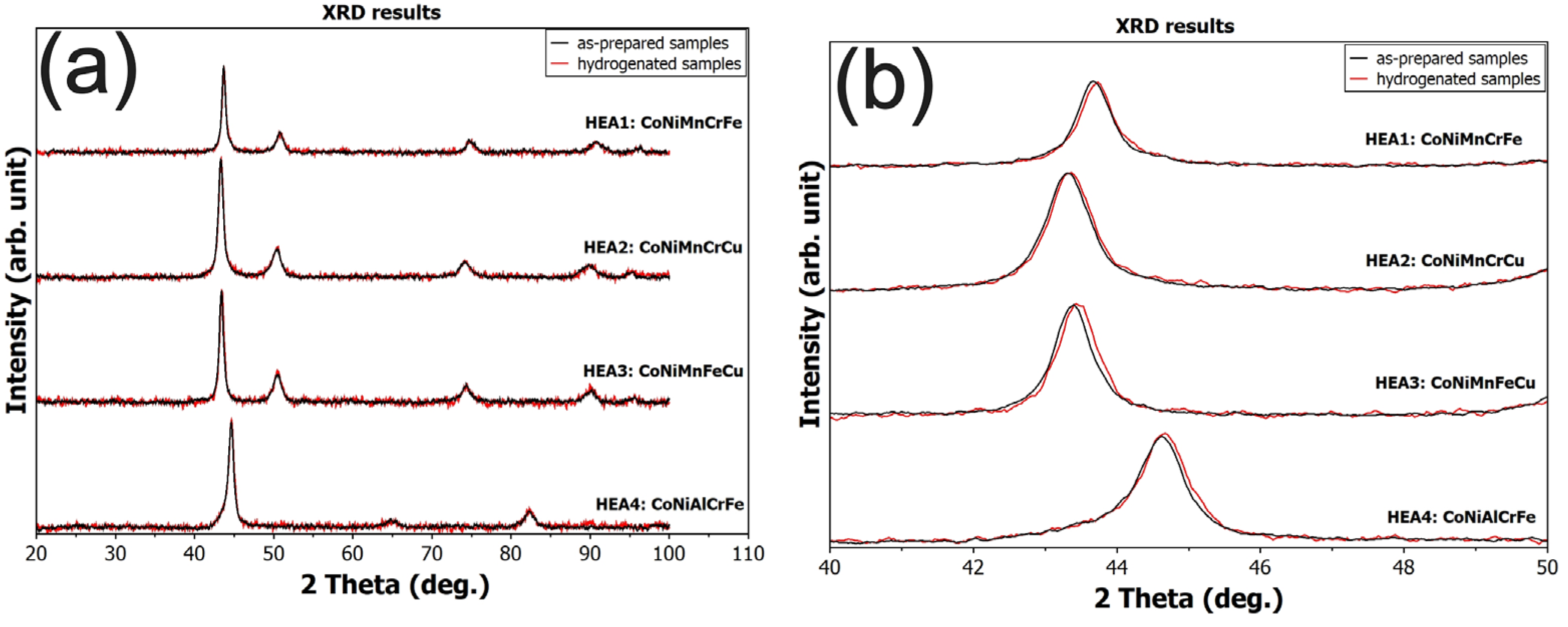
2.3. Material Characterization
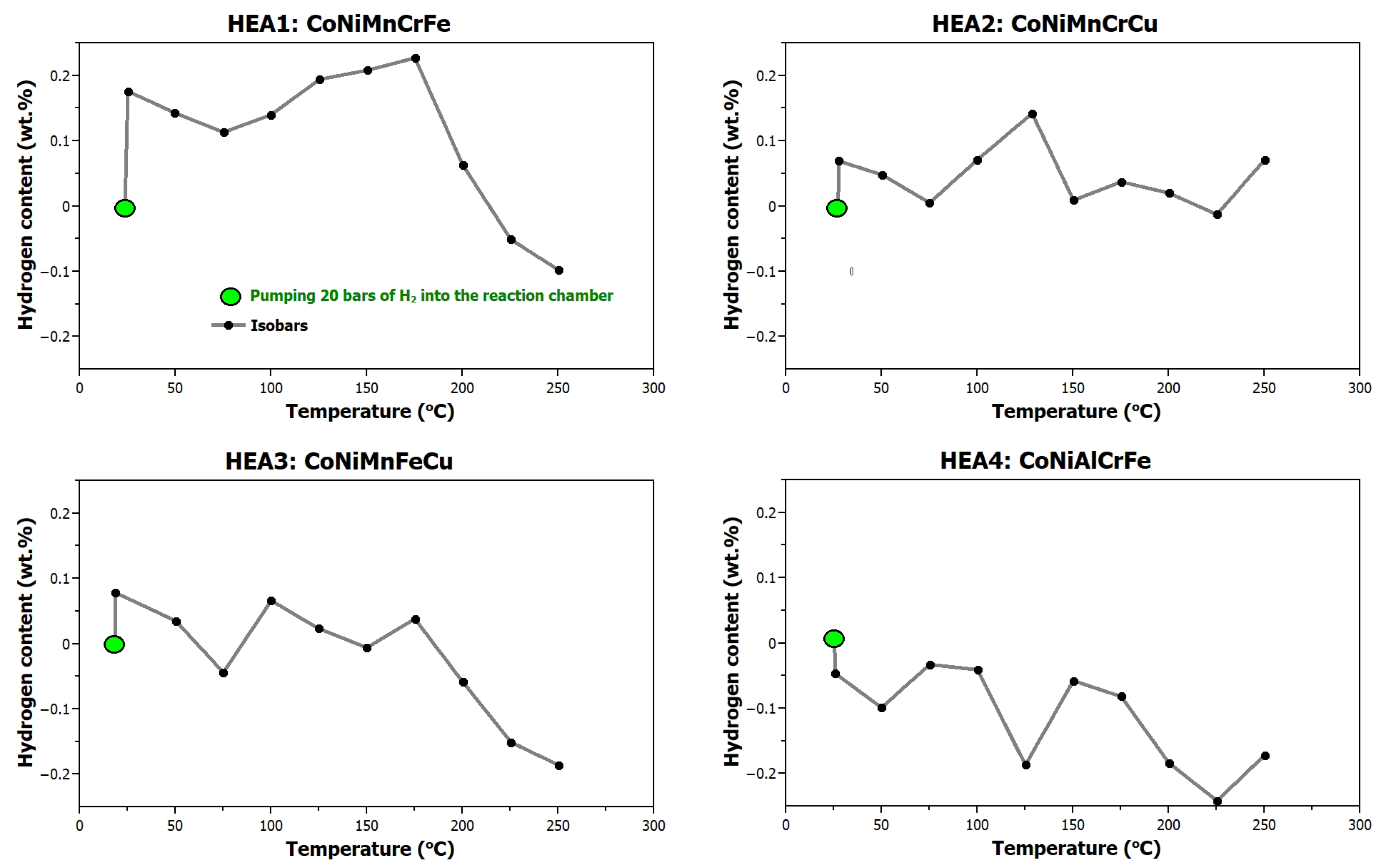
2.4. Hydrogen Absorption Experiments
- Approximately 1 g of the powder alloy was placed in the reaction chamber of the magnetic suspension balance. The system was sealed and evacuated to a vacuum. The first hydrogen-absorption measurement began by filling the reaction chamber with hydrogen to a pressure of 2 MPa, followed by increasing the temperature from RT to 250 °C in 25 °C increments, with each step being held for 30 min. The sample mass was monitored by the magnetic suspension balance. Following this measurement, the sample was cooled to room temperature under a hydrogen pressure of 2 MPa.
- Next, the chamber was evacuated and heated to 400 °C for 3 h to desorb hydrogen from the sample and then cooled to RT.
- The reaction chamber was heated to 250 °C. After reaching the required temperature, the system was filled with hydrogen to a pressure of 2 MPa. The measurement was held for 30 min.
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jena, P. Materials for Hydrogen Storage: Past, Present, and Future. J. Phys. Chem. Lett. 2011, 2, 206–211. [Google Scholar] [CrossRef]
- Sakintuna, B.; Lamari-Darkrim, F.; Hirscher, M. Metal hydride materials for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2007, 32, 1121–1140. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Ahmed, S.; Haq, M.; Alraih, A.M.; Hidouri, T.; Kamyab, H.; Vo, D.-V.N.; Ibrahim, H. Advancements in sorption-based materials for hydrogen storage and utilization: A comprehensive review. Energy 2024, 309, 132855. [Google Scholar] [CrossRef]
- Trajkovska Petkoska, A.; Samakoski, B.; Samardjioska Azmanoska, B.; Velkovska, V. Towpreg—An Advanced Composite Material with a Potential for Pressurized Hydrogen Storage Vessels. J. Compos. Sci. 2024, 8, 374. [Google Scholar] [CrossRef]
- Qureshi, F.; Yusuf, M.; Kamyab, H.; Zaidi, S.; Khalil, M.J.; Khan, M.A.; Alam, M.A.; Masood, F.; Bazli, L.; Chelliapan, S.; et al. Current trends in hydrogen production, storage and applications in India: A review. Sustain. Energy Technol. Assess. 2022, 53, 102677. [Google Scholar] [CrossRef]
- Prachi, R.P.; Mahesh, M.W.; Aneesh, C.G. A Review on Solid State Hydrogen Storage Material. Adv. Energy Power 2016, 4, 11–22. [Google Scholar] [CrossRef]
- Zhang, Y.H.; Jia, Z.C.; Yuan, Z.M.; Yang, T.; Qi, Y.; Zhao, D.L. Development and Application of Hydrogen Storage. J. Iron Steel Res. Int. 2015, 22, 757–770. [Google Scholar] [CrossRef]
- Nartita, R.; Ionita, D.; Demetrescu, I. A Modern Approach to HEAs: From Structure to Properties and Potential Applications. Crystals 2024, 14, 451. [Google Scholar] [CrossRef]
- Varcholová, D.; Kušnírová, K.; Oroszová, L.; Möllmer, J.; Lange, M.; Gáborová, K.; Buľko, B.; Demeter, P.; Saksl, K. New-Generation Materials for Hydrogen Storage in Medium-Entropy Alloys. Materials 2024, 17, 2897. [Google Scholar] [CrossRef]
- Qureshi, T.; Khan, M.M.; Pali, H.S. The future of hydrogen economy: Role of high entropy alloys in hydrogen storage. J. Alloys Compd. 2024, 1004, 175668. [Google Scholar] [CrossRef]
- Sharma, P.; Dwivedi, V.K.; Dwivedi, S.P. Development of high entropy alloys: A review. Mater. Today Proc. 2021, 43, 502–509. [Google Scholar] [CrossRef]
- Bolar, S.; Ito, Y.; Fujita, T. Future prospects of high-entropy alloys as next-generation industrial electrode materials. Chem. Sci. 2024, 15, 8664–8722. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Chen, L.; Li, L.; Liu, S.; Li, Y.; Li, C.; Li, L.; Cui, J.; Li, Y. High-entropy alloys for solid hydrogen storage: A review. Int. J. Hydrogen Energy 2024, 50, 406–430. [Google Scholar] [CrossRef]
- Tsai, M.H.; Yeh, J.W. High-Entropy Alloys: A Critical Review. Mater. Res. Lett. 2014, 2, 107–123. [Google Scholar] [CrossRef]
- Zhang, Y.; Zuo, T.T.; Tang, Z.; Gao, M.C.; Dahmen, K.A.; Liaw, P.K.; Lu, Z.P. Microstructures and properties of high-entropy alloys. Prog. Mater. Sci. 2014, 61, 1–93. [Google Scholar] [CrossRef]
- Keith, A.; Zlotea, C.; Szilagyi, P.A. Perspective of interstitial hydrides of high-entropy alloys for vehicular hydrogen storage. Int. J. Hydrogen Energy 2024, 52, 531–546. [Google Scholar] [CrossRef]
- Griessen, R.; Riesterer, T. Heat of formation models. In Hydrogen in Intermetallic Compounds I; Topics in Applied Physics; Schlapbach, L., Ed.; Springer: Berlin/Heidelberg, Germany, 1988; Volume 63. [Google Scholar]
- Guo, S. Phase selection rules for cast high entropy alloys: An overview. Mater. Sci. Technol. 2015, 31, 1223–1230. [Google Scholar] [CrossRef]
- Miracle, D.B.; Senkov, O.N. A critical review of high entropy alloys and related concepts. Acta Mater. 2017, 122, 448–511. [Google Scholar] [CrossRef]
- Takeuchi, A.; Inoue, A. Classification of Bulk Metallic Glasses by Atomic Size Difference, Heat of Mixing and Period of Constituent Elements and Its Application to Characterization of the Main Alloying Element. Mater. Trans. 2005, 46, 2817–2829. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhou, Y.J.; Lin, J.P.; Chen, G.L.; Liaw, P.K. Solid-solution phase formation rules for multi-component alloys. Adv. Eng. Mater. 2008, 10, 534–538. [Google Scholar] [CrossRef]
- Moellmer, J.; Moeller, A.; Dreisbach, F.; Glaeser, R.; Staudt, R. High pressure adsorption of hydrogen, nitrogen, carbon dioxide and methane on the metal–organic framework HKUST-1. Microporous Mesoporous Mater. 2011, 138, 140–148. [Google Scholar] [CrossRef]

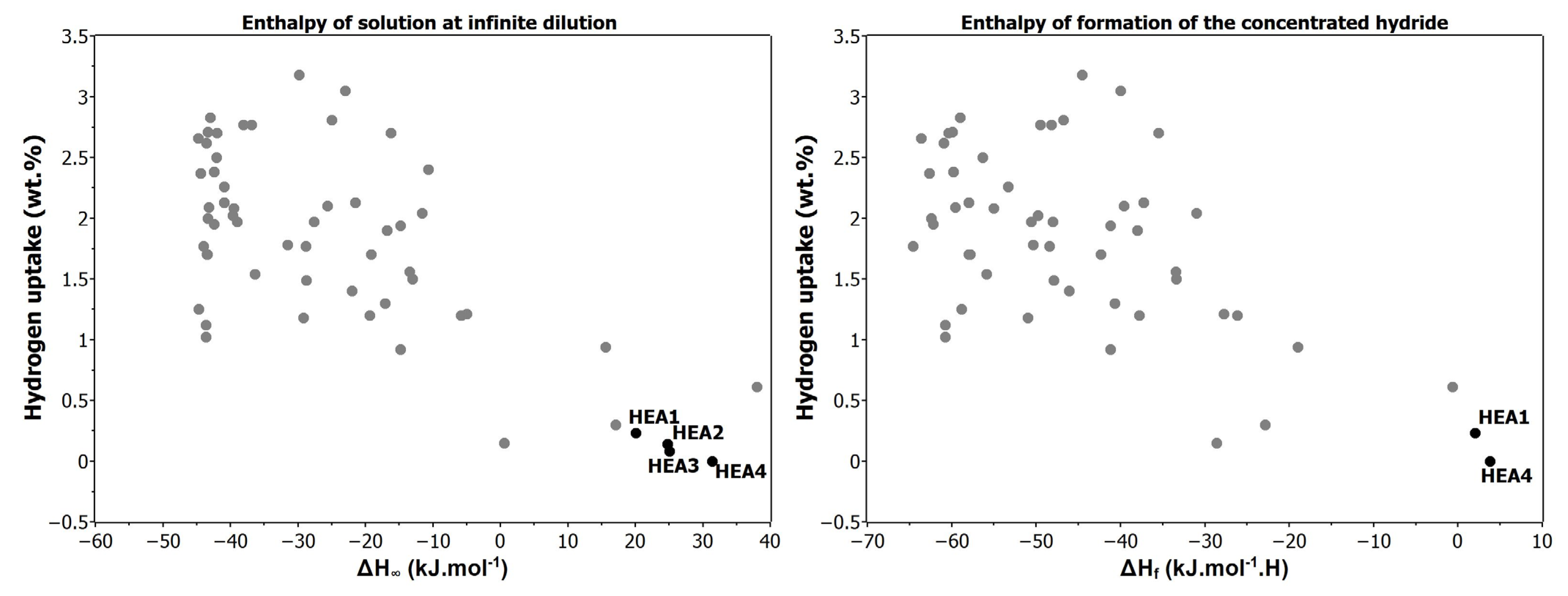
| Alloy | δ [%] | ΔHmix [kJ∙mol−1] | ΔH∞ [kJ∙mol−1] | ΔHf [kJ∙mol−1] | Ω | ΔSmix [J∙K−1∙mol−1] | Tm [K] | VEC |
|---|---|---|---|---|---|---|---|---|
| HEA1: CoNiMnCrFe | 3.01 | −4.08 | 20.08 | 2.05 | 5.90 | 1.60 R | 1808 | 8.01 |
| HEA2: CoNiMnCrCu | 2.98 | +0.94 | 24.79 | - | 24.21 | 1.61 R | 1701 | 8.66 |
| HEA3: CoNiMnFeCu | 3.02 | +2.02 | 25.03 | - | 10.85 | 1.61 R | 1643 | 9.06 |
| HEA4: CoNiAlCrFe | 5.78 | −12.32 | 31.40 | 3.8 | 1.82 | 1.61 R | 1673 | 7.20 |
| Alloy EDX Composition [at.%] | Density [g∙cm−3] | Hardness HV0.3 | XRD | Temperature of max. H2 Absorption [°C] | Maximum H2 Capacity [wt.%] (H/M) |
|---|---|---|---|---|---|
| HEA1 CoNiMnCrFe Co21Ni20Mn16Cr22Fe21 | 7.99 | 130 ± 5 | FCC | 175 | 0.23 (0.13) |
| HEA2 CoNiMnCrCu Co21Ni20Mn18Cr20Cu21 | 7.81 | 220 ± 4 | FCC | 125 | 0.14 (0.08) |
| HEA3 CoNiMnFeCu Co21Ni21Mn17Fe21Cu20 | 7.99 | 170 ± 5 | FCC | RT | 0.08 (0.04) |
| HEA4 CoNiAlCrFe Co20Ni20Al20Cr20Fe20 | 7.06 | 492 ± 11 | BCC | RT | 0 (0) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nigutová, K.; Oroszová, L.; Molčanová, Z.; Csík, D.; Gáborová, K.; Möllmer, J.; Lange, M.; Saksl, K. Experimental Validation of Hydrogen Affinity as a Design Criterion for Alloys. Materials 2024, 17, 6106. https://doi.org/10.3390/ma17246106
Nigutová K, Oroszová L, Molčanová Z, Csík D, Gáborová K, Möllmer J, Lange M, Saksl K. Experimental Validation of Hydrogen Affinity as a Design Criterion for Alloys. Materials. 2024; 17(24):6106. https://doi.org/10.3390/ma17246106
Chicago/Turabian StyleNigutová, Katarína, Lenka Oroszová, Zuzana Molčanová, Dávid Csík, Katarína Gáborová, Jens Möllmer, Marcus Lange, and Karel Saksl. 2024. "Experimental Validation of Hydrogen Affinity as a Design Criterion for Alloys" Materials 17, no. 24: 6106. https://doi.org/10.3390/ma17246106
APA StyleNigutová, K., Oroszová, L., Molčanová, Z., Csík, D., Gáborová, K., Möllmer, J., Lange, M., & Saksl, K. (2024). Experimental Validation of Hydrogen Affinity as a Design Criterion for Alloys. Materials, 17(24), 6106. https://doi.org/10.3390/ma17246106






