Composites Based on Natural Zeolites and Green Materials for the Immobilization of Toxic Elements in Contaminated Soils: A Review
Abstract
1. Introduction
2. Amendments for Immobilization of Toxic Elements in Contaminated Soils
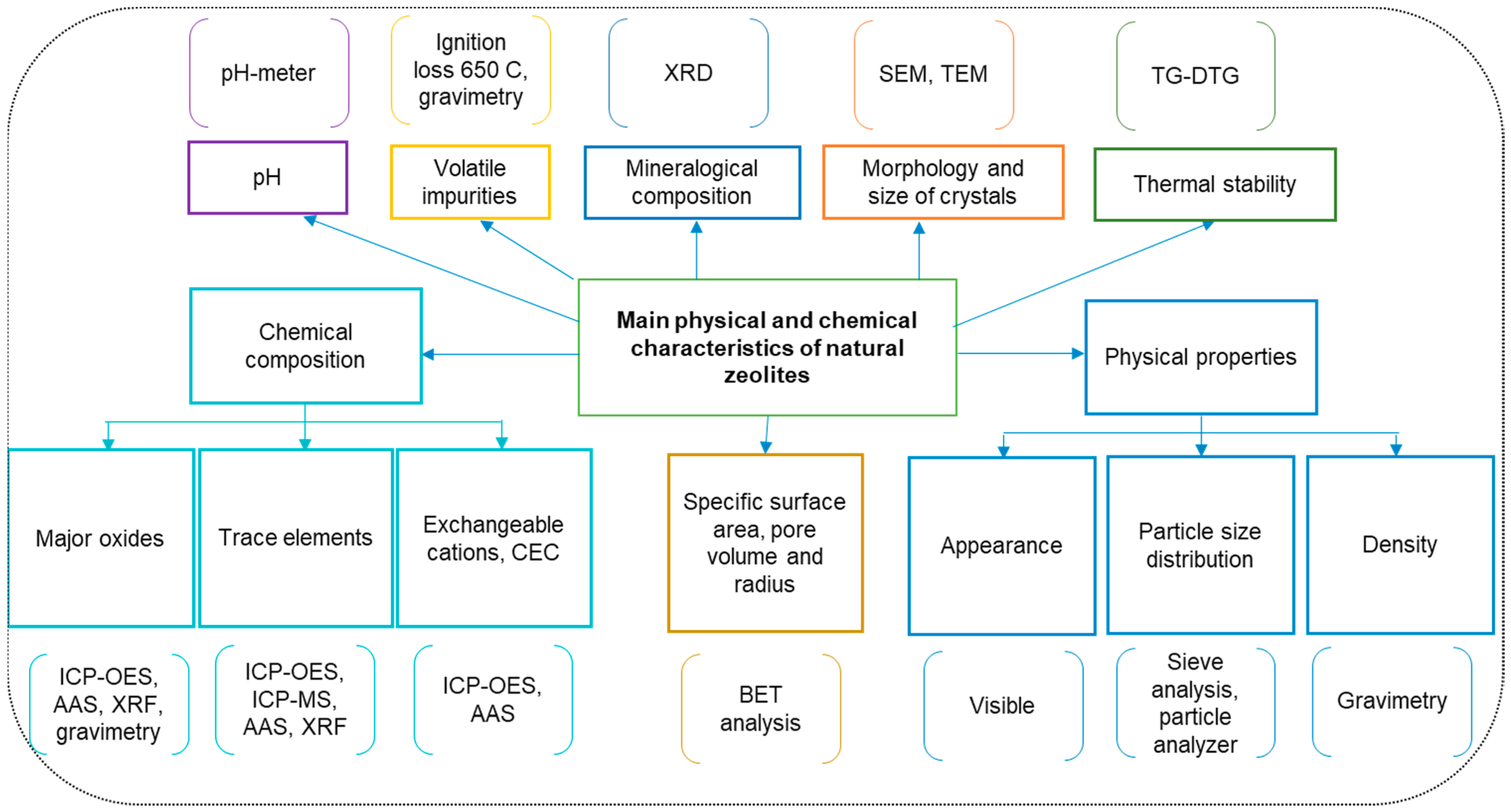
3. Type of Natural Zeolites and Their Main Properties
4. Application of Composites Based on Natural Zeolites and Green Materials in the Immobilization of Toxic Elements in Contaminated Soil
4.1. Biochar
4.2. Chitosan
4.3. Natural Minerals
4.4. Various Materials
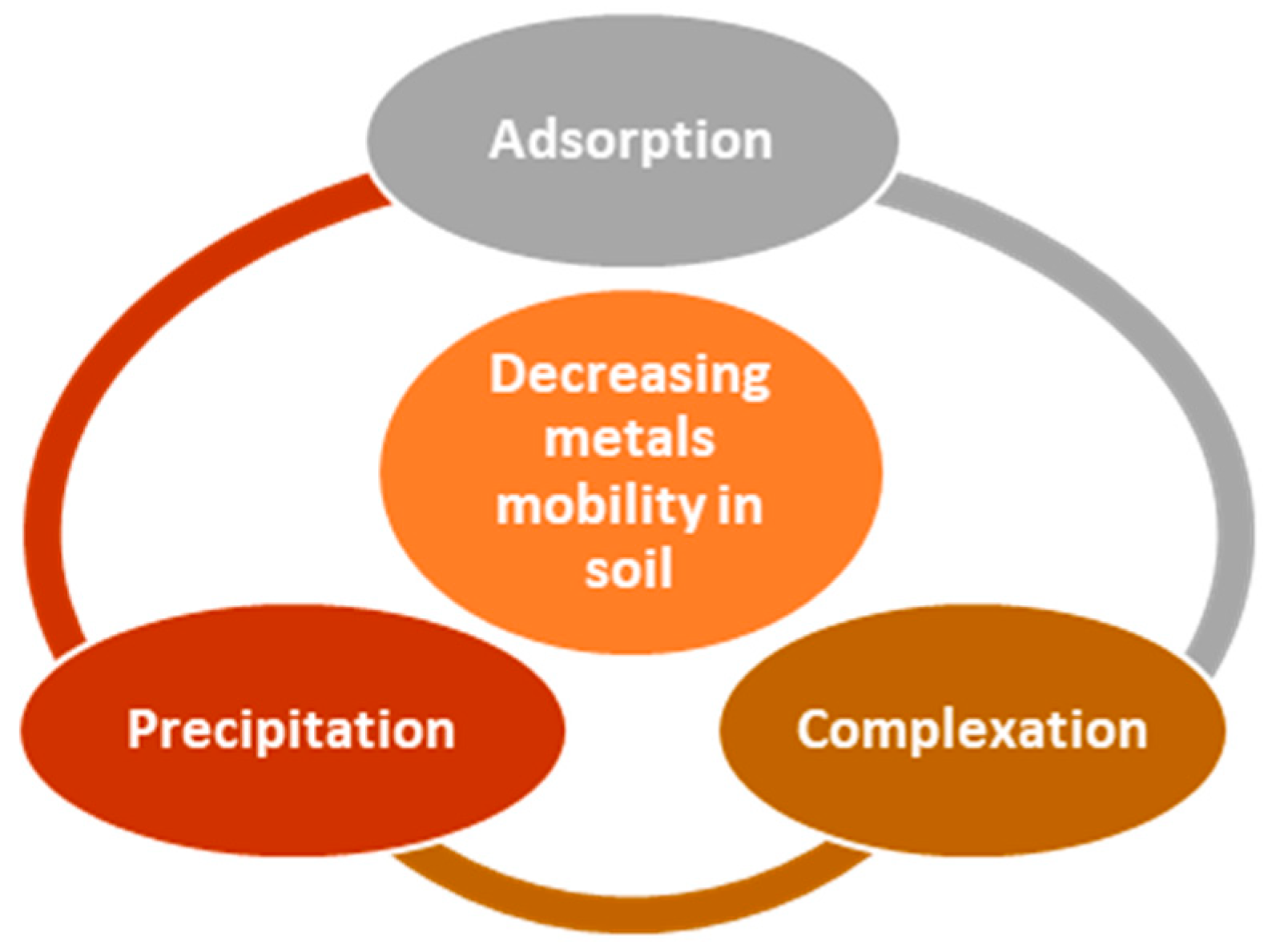
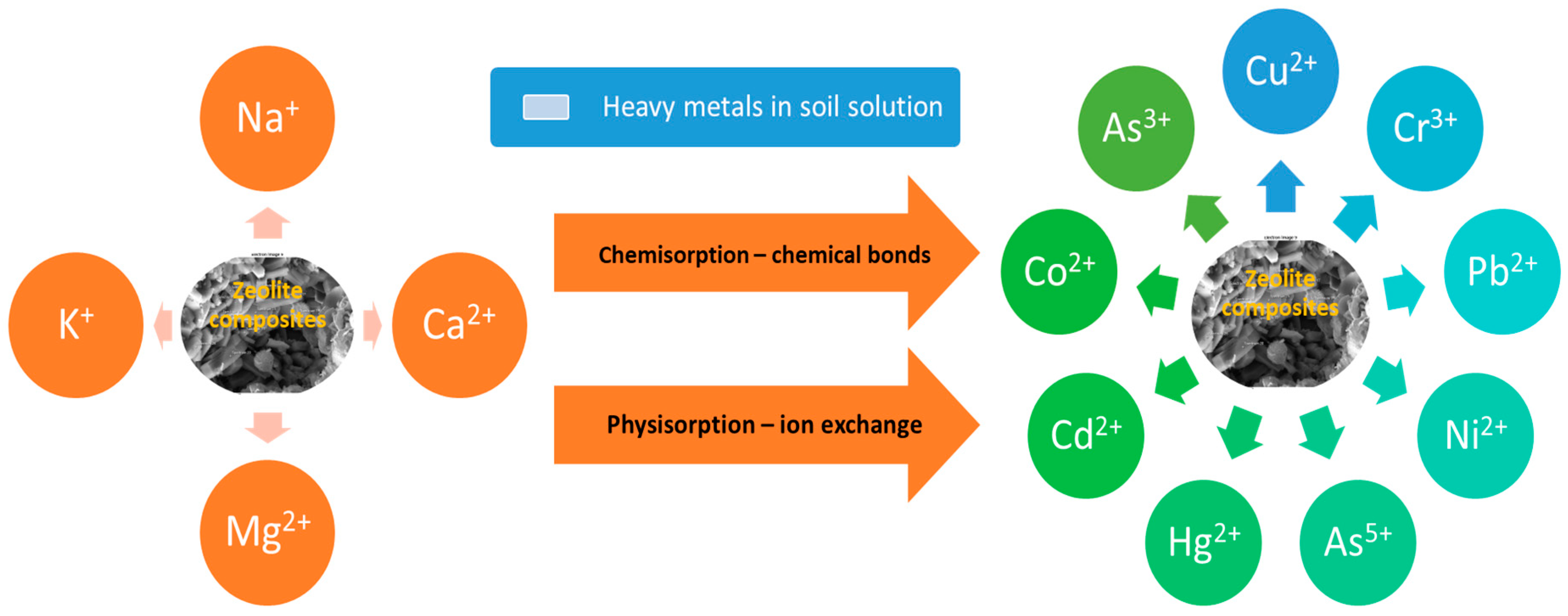
5. Mechanism of Toxic Elements Immobilization by Composites Based on Natural Zeolites and Green Materials
6. Effects of Composites Based on Natural Zeolites and Green Materials on Toxic Elements in Soil
7. Influences of Soil Characteristics and Soil Types on Metal Immobilization
8. Limitations
9. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Priya, A.K.; Muruganandam, M.; Ali, S.S.; Kornaros, M. Clean-Up of Heavy Metals from Contaminated Soil by Phytoremediation: A Multidisciplinary and Eco-Friendly Approach. Toxics 2023, 11, 422. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Castro, I.; Molina, L.; Prieto-Fernández, M.-Á.; Segura, A. Past, Present and Future Trends in the Remediation of Heavy-Metal Contaminated Soil—Remediation Techniques Applied in Real Soil-Contamination Events. Heliyon 2023, 9, e16692. [Google Scholar] [CrossRef] [PubMed]
- Mocek-Płóciniak, A.; Mencel, J.; Zakrzewski, W.; Roszkowski, S. Phytoremediation as an Effective Remedy for Removing Trace Elements from Ecosystems. Plants 2023, 12, 1653. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.J.; Irshad, M.K.; Kang, M.W.; Roh, H.-S.; Jeon, Y.; Lee, S.S. In-Situ Physical and Chemical Remediation of Cd and Pb Contaminated Mine Soils Cultivated with Chinese Cabbage: A Three-Year Field Study. J. Hazard. Mater. 2023, 459, 132091. [Google Scholar] [CrossRef] [PubMed]
- Radziemska, M.; Gusiatin, Z.M.; Bilgin, A. Potential of Using Immobilizing Agents in Aided Phytostabilization on Simulated Contamination of Soil with Lead. Ecol. Eng. 2017, 102, 490–500. [Google Scholar] [CrossRef]
- Bansal, O.P. The Influence of Potentially Toxic Elements on Soil Biological and Chemical Properties. In Metals in Soil-Contamination and Remediation; IntechOpen: London, UK, 2019. [Google Scholar] [CrossRef]
- Gholizadeh, M.; Hu, X. Removal of Heavy Metals from Soil with Biochar Composite: A Critical Review of the Mechanism. J. Environ. Chem. Eng. 2021, 9, 105830. [Google Scholar] [CrossRef]
- Yi, N.; Wu, Y.; Fan, L.; Hu, S. Remediating Cd-Contaminated Soils Using Natural and Chitosan-Introduced Zeolite, Bentonite, and Activated Carbon. Pol. J. Environ. Stud. 2019, 28, 1461–1468. [Google Scholar] [CrossRef]
- Chibuike, G.U.; Obiora, S.C. Heavy Metal Polluted Soils: Effect on Plants and Bioremediation Methods. Appl. Environ. Soil Sci. 2014, 2014, 752708. [Google Scholar] [CrossRef]
- Thalassinos, G.; Petropoulos, S.A.; Grammenou, A.; Antoniadis, V. Potentially Toxic Elements: A Review on Their Soil Behavior and Plant Attenuation Mechanisms against Their Toxicity. Agriculture 2023, 13, 1684. [Google Scholar] [CrossRef]
- Cao, L.; Lin, C.; Gao, Y.; Sun, C.; Xu, L.; Zheng, L.; Zhang, Z. Health Risk Assessment of Trace Elements Exposure through the Soil-Plant (Maize)-Human Contamination Pathway near a Petrochemical Industry Complex, Northeast China. Environ. Pollut. 2020, 263, 114414. [Google Scholar] [CrossRef]
- Antoniadis, V.; Levizou, E.; Shaheen, S.M.; Ok, Y.S.; Sebastian, A.; Baum, C.; Prasad, M.N.V.; Wenzel, W.W.; Rinklebe, J. Trace Elements in the Soil-Plant Interface: Phytoavailability, Translocation, and Phytoremediation—A Review. Earth-Sci. Rev. 2017, 171, 621–645. [Google Scholar] [CrossRef]
- Haidar, Z.; Fatema, K.; Shoily, S.S.; Sajib, A.A. Disease-Associated Metabolic Pathways Affected by Heavy Metals and Metalloid. Toxicol. Rep. 2023, 10, 554–570. [Google Scholar] [CrossRef]
- Mitra, S.; Chakraborty, A.J.; Tareq, A.M.; Emran, T.B.; Nainu, F.; Khusro, A.; Idris, A.M.; Khandaker, M.U.; Osman, H.; Alhumaydhi, F.A.; et al. Impact of Heavy Metals on the Environment and Human Health: Novel Therapeutic Insights to Counter the Toxicity. J. King Saud Univ.-Sci. 2022, 34, 101865. [Google Scholar] [CrossRef]
- Gazwi, H.S.S.; Yassien, E.E.; Hassan, H.M. Mitigation of Lead Neurotoxicity by the Ethanolic Extract of Laurus Leaf in Rats. Ecotoxicol. Environ. Saf. 2020, 192, 110297. [Google Scholar] [CrossRef]
- Vrînceanu, N.O.; Motelică, D.M.; Dumitru, M.; Calciu, I.; Tănase, V.; Preda, M. Assessment of Using Bentonite, Dolomite, Natural Zeolite and Manure for the Immobilization of Heavy Metals in a Contaminated Soil: The Copșa Mică Case Study (Romania). Catena 2019, 176, 336–342. [Google Scholar] [CrossRef]
- Kushwaha, J.; Singh, R. Cellulose Hydrogel and Its Derivatives: A Review of Application in Heavy Metal Adsorption. Inorg. Chem. Commun. 2023, 152, 110721. [Google Scholar] [CrossRef]
- Palansooriya, K.N.; Shaheen, S.M.; Chen, S.S.; Tsang, D.C.W.; Hashimoto, Y.; Hou, D.; Bolan, N.S.; Rinklebe, J.; Ok, Y.S. Soil Amendments for Immobilization of Potentially Toxic Elements in Contaminated Soils: A Critical Review. Environ. Int. 2020, 134, 105046. [Google Scholar] [CrossRef] [PubMed]
- Mourinha, C.; Palma, P.; Alexandre, C.; Cruz, N.; Rodrigues, S.M.; Alvarenga, P. Potentially Toxic Elements’ Contamination of Soils Affected by Mining Activities in the Portuguese Sector of the Iberian Pyrite Belt and Optional Remediation Actions: A Review. Environments 2022, 9, 11. [Google Scholar] [CrossRef]
- Rashid, A.; Schutte, B.J.; Ulery, A.; Deyholos, M.K.; Sanogo, S.; Lehnhoff, E.A.; Beck, L. Heavy Metal Contamination in Agricultural Soil: Environmental Pollutants Affecting Crop Health. Agronomy 2023, 13, 1521. [Google Scholar] [CrossRef]
- Li, J.; Yang, D.; Zou, W.; Feng, X.; Wang, R.; Zheng, R.; Luo, S.; Chu, Z.; Chen, H. Mechanistic insights into the synergetic remediation and amendment effects of zeolite/biochar composite on heavy metal-polluted red soil. Front. Environ. Sci. Eng. 2024, 18, 114. [Google Scholar] [CrossRef]
- Głąb, T.; Gondek, K.; Mierzwa–Hersztek, M. Biological Effects of Biochar and Zeolite Used for Remediation of Soil Contaminated with Toxic Heavy Metals. Sci. Rep. 2021, 11, 6998. [Google Scholar] [CrossRef] [PubMed]
- Zheng, R.; Feng, X.; Zou, W.; Wang, R.; Yang, D.; Wei, W.; Li, S.; Chen, H. Converting Loess into Zeolite for Heavy Metal Polluted Soil Remediation Based on “Soil for Soil-Remediation” Strategy. J. Hazard. Mater. 2021, 412, 125199. [Google Scholar] [CrossRef] [PubMed]
- Ugwu, E.I.; Othmani, A.; Nnaji, C.C. A Review on Zeolites as Cost-Effective Adsorbents for Removal of Heavy Metals from Aqueous Environment. Int. J. Environ. Sci. Technol. 2021, 19, 8061–8084. [Google Scholar] [CrossRef]
- Badora, A. The Influence of Zeolites on Quality Indicators of Soil-Plant Connection and Food Safety. In Zeolites-Useful Minerals; IntechOpen: London, UK, 2016. [Google Scholar]
- Senila, M.; Cadar, O. Modification of natural zeolites and their applications for heavy metal removal from polluted environments: Challenges, recent advances, and perspectives. Heliyon 2024, 10, e25303. [Google Scholar] [CrossRef]
- Morante-Carballo, F.; Montalván-Burbano, N.; Carrión-Mero, P.; Espinoza-Santos, N. Cation Exchange of Natural Zeolites: Worldwide Research. Sustainability 2021, 13, 7751. [Google Scholar] [CrossRef]
- Velarde, L.; Nabavi, M.S.; Escalera, E.; Antti, M.-L.; Akhtar, F. Adsorption of Heavy Metals on Natural Zeolites: A Review. Chemosphere 2023, 328, 138508. [Google Scholar] [CrossRef]
- Afzal, S.; Alghanem, S.M.S.I.; Alsudays, M.; Malik, Z.; Abbasi, G.H.; Ali, A.; Noreen, S.; Ali, M.; Irfan, M.; Rizwan, M. Effect of biochar, zeolite and bentonite on physiological and biochemical parameters and lead and zinc uptake by maize (Zea mays L.) plants grown in contaminated soil. J. Hazard. Mater. 2024, 469, 133927. [Google Scholar] [CrossRef]
- Belviso, C. Zeolite for Potential Toxic Metal Uptake from Contaminated Soil: A Brief Review. Processes 2020, 8, 820. [Google Scholar] [CrossRef]
- Kukowska, S.; Szewczuk-Karpisz, K. Biochar and Zeolite Uses in Improving Immobilization of Nutrients and Pollutants in Soils. Sep. Purif. Rev. 2024, 1–24. [Google Scholar] [CrossRef]
- Feng, S.; Zhang, P.; Hu, Y.; Jin, F.; Liu, Y.; Cai, S.; Song, Z.; Zhang, X.; Nadezhda, T.; Guo, Z.; et al. Combined Application of Biochar and Nano-Zeolite Enhanced Cadmium Immobilization and Promote the Growth of Pak Choi in Cadmium Contaminated Soil. NanoImpact 2022, 28, 100421. [Google Scholar] [CrossRef]
- Zheng, X.J.; Chen, M.; Wang, J.F.; Liu, Y.; Liao, Y.Q.; Liu, Y.C. Assessment of zeolite, biochar, and their combination for stabilization of multimetal-contaminated soil. ACS Omega 2020, 5, 27374–27382. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Ma, D.; Ye, Y. Adsorption, separation and recovery properties of blocky zeolite-biochar composites for remediation of cadmium contaminated soil. Chin. J. Chem. Eng. 2023, 54, 272–279. [Google Scholar] [CrossRef]
- Hong, Y.-K.; Kim, J.-W.; Lee, S.-P.; Yang, J.-E.; Kim, S.-C. Effect of Combined Soil Amendment on Immobilization of Bioavailable As and Pb in Paddy Soil. Toxics 2022, 10, 90. [Google Scholar] [CrossRef]
- Kumar, V.; Rout, C.; Singh, J.; Saharan, Y.; Goyat, R.; Umar, A.; Akbar, S.; Baskoutas, S. A Review on the Clean-up Technologies for Heavy Metal Ions Contaminated Soil Samples. Heliyon 2023, 9, e15472. [Google Scholar] [CrossRef]
- Shen, Z.; Jin, F.; O’Connor, D.; Hou, D. Solidification/Stabilization for Soil Remediation: An Old Technology with New Vitality. Environ. Sci. Technol. 2019, 53, 11615–11617. [Google Scholar] [CrossRef]
- Rađenović, D.; Kerkez, Đ.; Pilipović, D.T.; Dubovina, M.; Grba, N.; Krčmar, D.; Dalmacija, B. Long-Term Application of Stabilization/Solidification Technique on Highly Contaminated Sediments with Environment Risk Assessment. Sci. Total Environ. 2019, 684, 186–195. [Google Scholar] [CrossRef]
- Cheng, S.F.; Hseu, Z.Y. In-Situ Immobilization of Cadmium and Lead by Different Amendments in Two Contaminated Soils. Water Air Soil Pollut. 2002, 140, 73–84. [Google Scholar] [CrossRef]
- Liao, X.; Li, Y.; Miranda-Avilés, R.; Zha, X.; Anguiano, J.H.H.; Sánchez, C.D.M.; Puy-Alquiza, M.J.; González, V.P.; Garzon, L.F.R. In Situ Remediation and Ex Situ Treatment Practices of Arsenic-Contaminated Soil: An Overview on Recent Advances. J. Hazard. Mater. Adv. 2022, 8, 100157. [Google Scholar] [CrossRef]
- Sun, Z.; Zhao, M.; Chen, L.; Gong, Z.; Hu, J.; Ma, D. Electrokinetic Remediation for the Removal of Heavy Metals in Soil: Limitations, Solutions and Prospection. Sci. Total Environ. 2023, 903, 165970. [Google Scholar] [CrossRef]
- Feng, N.; Bitton, G.; Yeager, P.; Bonzongo, J.; Boularbah, A. Heavy Metal Removal from Soils Using Magnetic Separation: 1. Laboratory Experiments. CLEAN-Soil Air Water 2007, 35, 362–369. [Google Scholar] [CrossRef]
- Cameselle, C.; Gouveia, S.; Urréjola, S. Benefits of Phytoremediation Amended with DC Electric Field. Application to Soils Contaminated with Heavy Metals. Chemosphere 2019, 229, 481–488. [Google Scholar] [CrossRef] [PubMed]
- Raffa, C.M.; Chiampo, F.; Shanthakumar, S. Remediation of Metal/Metalloid-Polluted Soils: A Short Review. Appl. Sci. 2021, 11, 4134. [Google Scholar] [CrossRef]
- Wu, J.; Wan, T. Innovative Practice of Heavy Metal Soil Remediation Technology under the Background of Rural Revitalization by Integrating Agriculture, Culture and Tourism. Pol. J. Environ. Stud. 2024, 33, 4407–4419. [Google Scholar] [CrossRef]
- Aransiola, S.A.; Ijah, U.J.J.; Abioye, O.P.; Bala, J.D. Microbial-Aided Phytoremediation of Heavy Metals Contaminated Soil: A Review. Zenodo (CERN Eur. Organ. Nucl. Res.) 2019, 9, 104. [Google Scholar] [CrossRef]
- Contin, M.; Miho, L.; Pellegrini, E.; Gjoka, F.; Shkurta, E. Effects of Natural Zeolites on Ryegrass Growth and Bioavailability of Cd, Ni, Pb, and Zn in an Albanian Contaminated Soil. J. Soils Sediments 2019, 19, 4052–4062. [Google Scholar] [CrossRef]
- Bashir, S.; Salam, A.; Rehman, M.; Khan, S.; Gulshan, A.B.; Iqbal, J.; Shaaban, M.; Mehmood, S.; Zahra, A.; Hu, H. Effective Role of Biochar, Zeolite and Steel Slag on Leaching Behavior of CD and Its Fractionations in Soil Column Study. Bull. Environ. Contam. Toxicol. 2019, 102, 567–572. [Google Scholar] [CrossRef]
- Padhye, L.P.; Srivastava, P.; Jasemizad, T.; Bolan, S.; Hou, D.; Shaheen, S.M.; Rinklebe, J.; O’Connor, D.; Lamb, D.; Wang, H.; et al. Contaminant Containment for Sustainable Remediation of Persistent Contaminants in Soil and Groundwater. J. Hazard. Mater. 2023, 455, 131575. [Google Scholar] [CrossRef]
- Nejad, Z.D.; Jung, M.C.; Kim, K.-H. Remediation of Soils Contaminated with Heavy Metals with an Emphasis on Immobilization Technology. Environ. Geochem. Health 2017, 40, 927–953. [Google Scholar] [CrossRef]
- Sharma, I. Bioremediation Techniques for Polluted Environment: Concept, Advantages, Limitations, and Prospects. In Trace Metals in the Environment-New Approaches and Recent Advances; IntechOpen: London, UK, 2020. [Google Scholar]
- Radziemska, M. Study of Applying Naturally Occurring Mineral Sorbents of Poland (Dolomite Halloysite, Chalcedonite) for Aided Phytostabilization of Soil Polluted with Heavy Metals. CATENA 2017, 163, 123–129. [Google Scholar] [CrossRef]
- Katoh, M.; Lu, W.; Sato, T. Potential for Lead Release from Lead-Immobilized Animal Manure Compost in Rhizosphere Soil of Shooting Range. Appl. Environ. Soil Sci. 2016, 2016, 7410186. [Google Scholar] [CrossRef]
- Manzoor, M.Z.; Sarwar, G.; Alamery, S.; Ibrahim, M.; Sami, A.; Ahmed, B.; Ahsan, F.; Gul, S.; Attia, K.A.; Fiaz, S.; et al. Efficacy of Various Amendments for Immobilization of Potentially Toxic Elements in Wastewater Contaminated Soils. Sci. Rep. 2024, 14, 17350. [Google Scholar] [CrossRef] [PubMed]
- Shichalin, O.O.; Papynov, E.K.; Ivanov, N.P.; Balanov, M.I.; Dran’kov, A.N.; Shkuratov, A.L.; Zarubina, N.V.; Fedorets, A.N.; Mayorov, V.Y.; Lembikov, A.O.; et al. Study of Adsorption and Immobilization of Cs+, Sr2+, Co2+, Pb2+, La3+ Ions on Na-Faujasite Zeolite Transformed in Solid State Matrices. Sep. Purif. Technol. 2023, 332, 125662. [Google Scholar] [CrossRef]
- Hay, R.L.; Sheppard, R.A. Occurrence of Zeolites in Sedimentary Rocks: An Overview. Rev. Mineral. Geochem. 2001, 45, 217–234. [Google Scholar] [CrossRef]
- Wang, S.; Peng, Y. Natural Zeolites as Effective Adsorbents in Water and Wastewater Treatment. Chem. Eng. J. 2010, 156, 11–24. [Google Scholar] [CrossRef]
- Cappelletti, P.; Colella, A.; Langella, A.; Mercurio, M.; Catalanotti, L.; Monetti, V.; De Gennaro, B. Use of Surface Modified Natural Zeolite (SMNZ) in Pharmaceutical Preparations Part 1. Mineralogical and Technological Characterization of Some Industrial Zeolite-Rich Rocks. Microporous Mesoporous Mater. 2017, 250, 232–244. [Google Scholar] [CrossRef]
- Colella, C.; Wise, W.S. The IZA Handbook of Natural Zeolites: A Tool of Knowledge on the Most Important Family of Porous Minerals. Microporous Mesoporous Mater. 2014, 189, 4–10. [Google Scholar] [CrossRef]
- Mamytbekov, G.K.; Zheltov, D.A.; Milts, O.S.; Nurtazin, Y.R. Polymer–Zeolite Composites: Synthesis, Characterization and Application. Colloids Interfaces 2024, 8, 8. [Google Scholar] [CrossRef]
- Tasharrofi, S.; Rouzitalab, Z.; Maklavany, D.M.; Esmaeili, A.; Rabieezadeh, M.; Askarieh, M.; Rashidi, A.; Taghdisian, H. Adsorption of Cadmium Using Modified Zeolite-Supported Nanoscale Zero-Valent Iron Composites as a Reactive Material for PRBs. Sci. Total Environ. 2020, 736, 139570. [Google Scholar] [CrossRef]
- Structure Commission of the International Zeolite Association IZA-SC. Database of Zeolite Structures. 2020. Available online: https://europe.iza-structure.org/IZA-SC/ftc_table.php (accessed on 25 November 2024).
- Hudcová, B.; Osacký, M.; Vítková, M.; Mitzia, A.; Komárek, M. Investigation of Zinc Binding Properties onto Natural and Synthetic Zeolites: Implications for Soil Remediation. Microporous Mesoporous Mater. 2021, 317, 111022. [Google Scholar] [CrossRef]
- Oggiano, G.; Pokimica, B.; Popović, T.; Takić, M. Beneficial Properties of Zeolite. Ital. J. Food Sci. 2023, 35, 72–78. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Papynov, E.K.; Belov, A.A.; Ivanov, N.P.; Buravlev, I.Y.; Lembikov, A.O.; Dvornik, M.I.; Chigrin, P.G.; Vlasova, N.M.; Mirovoy, Y.A.; et al. Immobilization of 137Cs in NaY Type Zeolite Matrices Using Various Heat Treatment Methods. Solid State Sci. 2024, 154, 107619. [Google Scholar] [CrossRef]
- Shichalin, O.O.; Papynov, E.K.; Nepomnyushchaya, V.A.; Ivanets, A.I.; Belov, A.A.; Dran’kov, A.N.; Yarusova, S.B.; Buravlev, I.Y.; Tarabanova, A.E.; Fedorets, A.N.; et al. Hydrothermal Synthesis and Spark Plasma Sintering of NaY Zeolite as Solid-State Matrices for Cesium-137 Immobilization. J. Eur. Ceram. Soc. 2022, 42, 3004–3014. [Google Scholar] [CrossRef]
- Pérez-Botella, E.; Valencia, S.; Rey, F. Zeolites in Adsorption Processes: State of the Art and Future Prospects. Chem. Rev. 2022, 122, 17647–17695. [Google Scholar] [CrossRef] [PubMed]
- Krstić, V. Role of Zeolite Adsorbent in Water Treatment. In Handbook of Nanomaterials for Wastewater Treatment; Elsevier: Amsterdam, The Netherlands, 2021; pp. 417–481. [Google Scholar]
- Cadar, O.; Vagner, I.; Miu, I.; Scurtu, D.; Senila, M. Preparation, characterization, and performance of natural zeolites as alternative materials for beer filtration. Materials 2023, 16, 1914. [Google Scholar] [CrossRef]
- Senila, M.; Neag, E.; Tanaselia, C.; Senila, L. Removal of Cesium and Strontium ions from aqueous solutions by thermally treated natural zeolite. Materials 2023, 16, 2965. [Google Scholar] [CrossRef]
- Senila, M.; Neag, E.; Cadar, O.; Kovacs, E.D.; Aschilean, I.; Kovacs, M.H. Simultaneous Removal of Heavy Metals (Cu, Cd, Cr, Ni, Zn and Pb) from Aqueous Solutions Using Thermally Treated Romanian Zeolitic Volcanic Tuff. Molecules 2022, 27, 3938. [Google Scholar] [CrossRef]
- Nuić, I.; Gosar, M.; Ugrina, M.; Trgo, M. Assessment of Natural Zeolite Clinoptilolite for Remediation of Mercury-Contaminated Environment. Processes 2022, 10, 639. [Google Scholar] [CrossRef]
- Ma, Y.; Cheng, L.; Zhang, D.; Zhang, F.; Zhou, S.; Ma, Y.; Guo, J.; Zhang, Y.; Xing, B. Stabilization of Pb, Cd, and Zn in Soil by Modified-Zeolite: Mechanisms and Evaluation of Effectiveness. Sci. Total Environ. 2022, 814, 152746. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Wang, M.; Zhao, Z.; Li, X.; Han, Y.; Chen, S. Alleviation of Cadmium Phytotoxicity to Wheat Is Associated with Cd Re-Distribution in Soil Aggregates as Affected by Amendments. RSC Adv. 2018, 8, 17426–17434. [Google Scholar] [CrossRef]
- Mai, X.; Tang, J.; Tang, J.; Zhu, X.; Yang, Z.; Liu, X.; Zhuang, X.; Feng, G.; Tang, L. Research Progress on the Environmental Risk Assessment and Remediation Technologies of Heavy Metal Pollution in Agricultural Soil. J. Environ. Sci. 2024, 149, 1–20. [Google Scholar] [CrossRef]
- Jarosz, R.; Szerement, J.; Gondek, K.; Mierzwa-Hersztek, M. The Use of Zeolites as an Addition to Fertilisers—A Review. Catena 2022, 213, 106125. [Google Scholar] [CrossRef]
- Al-Shaeli, M.; Al-Juboori, R.A.; Al Aani, S.; Ladewig, B.P.; Hilal, N. Natural and recycled materials for sustainable membrane modification: Recent trends and prospects. Sci. Total Environ. 2022, 838, 156014. [Google Scholar] [CrossRef] [PubMed]
- Safie, N.N.; Zahrim, A.Y. Recovery of nutrients from sewage using zeolite-chitosan-biochar adsorbent: Current practices and perspectives. J. Water Process. Eng. 2021, 40, 101845. [Google Scholar] [CrossRef]
- Kim, H.S.; Kim, K.R.; Kim, H.J.; Yoon, J.H.; Yang, J.E.; Ok, Y.S.; Owens, G.; Kim, K.H. Effect of biochar on heavy metal immobilization and uptake by lettuce (Lactuca sativa L.) in agricultural soil. Environ. Earth Sci. 2015, 74, 1249–1259. [Google Scholar] [CrossRef]
- Mansoor, S.; Kour, N.; Manhas, S.; Zahid, S.; Wani, O.A.; Sharma, V.; Wijaya, L.; Alyemeni, M.N.; Alsahli, A.A.; El-Serehy, H.A.; et al. Biochar as a Tool for Effective Management of Drought and Heavy Metal Toxicity. Chemosphere 2020, 271, 129458. [Google Scholar] [CrossRef]
- Boostani, H.R.; Hardie, A.G.; Najafi-Ghiri, M.; Khalili, D. Investigation of cadmium immobilization in a contaminated calcareous soil as influenced by biochars and natural zeolite application. Int. J. Environ. Sci. Technol. 2018, 15, 2433–2446. [Google Scholar] [CrossRef]
- Liu, W.; Huo, R.; Xu, J.; Liang, S.; Li, J.; Zhao, T.; Wang, S. Effects of biochar on nitrogen transformation and heavy metals in sludge composting. Bioresour. Technol. 2017, 235, 43–49. [Google Scholar] [CrossRef]
- Manu, M.K.; Wang, C.; Li, D.; Varjani, S.; Wong, J.W.C. Impact of zeolite amendment on composting of food waste digestate. J. Clean. Prod. 2022, 371, 133408. [Google Scholar] [CrossRef]
- Wang, Q.; Awasthi, M.K.; Ren, X.; Zhao, J.; Li, R.; Wang, Z.; Chen, H.; Wang, M.; Zhang, Z. Comparison of biochar, zeolite and their mixture amendment for aiding organic matter transformation and nitrogen conservation during pig manure composting. Bioresour. Technol. 2017, 245, 300–308. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, H.; He, L.; Lu, K.; Sarmah, A.; Li, J.; Bolan, N.S.; Pei, J.; Huang, H. Using Biochar for Remediation of Soils Contaminated with Heavy Metals and Organic Pollutants. Environ. Sci. Pollut. Res. 2013, 20, 8472–8483. [Google Scholar] [CrossRef]
- Rahimi, M.; Bertalan-Balázs, B.; Adelinia, A.; Ebrahimi, E.; Ojani, M. Impact Assessment of Zeolite, Ca-Bentonite and Biochar Amendments on Cd Bioavailability and Fractions in Polluted Calcareous Soils. Environ. Earth Sci. 2024, 83, 506. [Google Scholar] [CrossRef]
- Deng, Z.; Gu, S.; Cheng, H.; Xing, D.; Twagirayezu, G.; Wang, X.; Ning, W.; Mao, M. Removal of phosphate from aqueous solution by zeolite-biochar composite: Adsorption performance and regulation mechanism. Appl. Sci. 2022, 12, 5334. [Google Scholar] [CrossRef]
- Awasthi, M.K.; Wang, M.; Pandey, A.; Chen, H.; Awasthi, S.K.; Wang, Q.; Ren, X.; Lahori, A.H.; Li, D.-S.; Li, R.; et al. Heterogeneity of Zeolite Combined with Biochar Properties as a Function of Sewage Sludge Composting and Production of Nutrient-Rich Compost. Waste Manag. 2017, 68, 760–773. [Google Scholar] [CrossRef]
- Esmaeili, V.; Zhang, S.; Hu, X.; Gholizadeh, M. The fate of char in controlling the rate of heavy metal transfer from soil to potato. Chem. Pap. 2022, 76, 1171–1183. [Google Scholar] [CrossRef]
- Zhang, J.; Li, C.; Li, G.; He, Y.; Yang, J.; Zhang, J. Effects of biochar on heavy metal bioavailability and uptake by tobacco (Nicotiana tabacum) in two soils. Agric. Ecosyst. Environ. 2021, 317, 107453. [Google Scholar] [CrossRef]
- Guo, X.; Cui, X.; Li, H.; Xiong, B. Purifying effect of biochar-zeolite constructed wetlands on arsenic-containing biogas slurry in large-scale pig farms. J. Clean. Prod. 2021, 279, 123579. [Google Scholar] [CrossRef]
- Kocaturk-Schumacher, N.P.; Zwart, K.; Bruun, S.; Jensen, L.S.; Sørensen, H.; Brussaard, L. Recovery of nutrients from the liquid fraction of digestate: Use of enriched zeolite and biochar as nitrogen fertilizers. J. Plant Nutr. Soil Sci. 2019, 182, 187–195. [Google Scholar] [CrossRef]
- Wu, X.; Ren, L.; Luo, L.; Zhang, J.; Zhang, L.; Huang, H. Bacterial and fungal community dynamics and shaping factors during agricultural waste composting with zeolite and biochar addition. Sustainability 2020, 12, 7082. [Google Scholar] [CrossRef]
- Waqas, M.; Nizami, A.S.; Aburiazaiza, A.S.; Barakat, M.A.; Asam, Z.Z.; Khattak, B.; Rashid, M.I. Untapped potential of zeolites in optimization of food waste composting. J. Environ. Manag. 2019, 241, 99–112. [Google Scholar] [CrossRef]
- Mosa, A.; El-Ghamry, A.; Tolba, M. Biochar-supported natural zeolite composite for recovery and reuse of aqueous phosphate and humate: Batch sorption–desorption and bioassay investigations. Environ. Technol. Innov. 2020, 19, 100807. [Google Scholar] [CrossRef]
- Yang, X.; Arias, M.E.; Ergas, S.J. Hybrid constructed wetlands amended with zeolite/biochar for enhanced landfill leachate treatment. Ecol. Eng. 2023, 192, 106990. [Google Scholar] [CrossRef]
- Amalina, F.; Razak, A.S.A.; Krishnan, S.; Sulaiman, H.; Zularisam, A.W.; Nasrullah, M. Biochar Production Techniques Utilizing Biomass Waste-Derived Materials and Environmental Applications—A Review. J. Hazard. Mater. Adv. 2022, 7, 100134. [Google Scholar] [CrossRef]
- Santos, V.P.; Marques, N.S.S.; Maia, P.C.S.V.; Lima, M.A.B.d.; Franco, L.d.O.; Campos-Takaki, G.M.d. Seafood Waste as Attractive Source of Chitin and Chitosan Production and Their Applications. Int. J. Mol. Sci. 2020, 21, 4290. [Google Scholar] [CrossRef] [PubMed]
- Tripathi, N.; Choppala, G.; Singh, R.S. Evaluation of Modified Chitosan for Remediation of Zinc Contaminated Soils. J. Geochem. Explor. 2016, 182, 180–184. [Google Scholar] [CrossRef]
- Mahmodi, G.; Zarrintaj, P.; Taghizadeh, A.; Taghizadeh, M.; Manouchehri, S.; Dangwal, S.; Ronte, A.; Ganjali, M.R.; Ramsey, J.D.; Kim, S.-J.; et al. From Microporous to Mesoporous Mineral Frameworks: An Alliance between Zeolite and Chitosan. Carbohydr. Res. 2020, 489, 107930. [Google Scholar] [CrossRef]
- Wen, J.; Zeng, G. Chemical and Biological Assessment of Cd-Polluted Sediment for Land Use: The Effect of Stabilization Using Chitosan-Coated Zeolite. J. Environ. Manag. 2018, 212, 46–53. [Google Scholar] [CrossRef]
- Şenol, Z.M.; Elma, E.; Messaoudi, N.E.; Mehmeti, V. Performance of Cross-Linked Chitosan-Zeolite Composite Adsorbent for Removal of Pb2+ Ions from Aqueous Solutions: Experimental and Monte Carlo Simulations Studies. J. Mol. Liq. 2023, 391, 123310. [Google Scholar] [CrossRef]
- Gu, S.; Kang, X.; Wang, L.; Lichtfouse, E.; Wang, C. Clay Mineral Adsorbents for Heavy Metal Removal from Wastewater: A Review. Environ. Chem. Lett. 2018, 17, 629–654. [Google Scholar] [CrossRef]
- Huang, Z.; Li, Y.; Chen, W.; Shi, J.; Zhang, N.; Wang, X.; Li, Z.; Gao, L.; Zhang, Y. Modified Bentonite Adsorption of Organic Pollutants of Dye Wastewater. Mater. Chem. Phys. 2017, 202, 266–276. [Google Scholar] [CrossRef]
- Wan, Y.; Liu, J.; Zhuang, Z.; Wang, Q.; Li, H. Heavy Metals in Agricultural Soils: Sources, Influencing Factors, and Remediation Strategies. Toxics 2024, 12, 63. [Google Scholar] [CrossRef]
- Wang, Y.; Joseph, S.; Chen, C.; Qi, X.; Mitchell, D.R.G.; Si, H.; Shang, J. Goethite-Enriched Biochar Mitigates Soil Emissions of CO2 during Arsenic Passivation: Effect and Mechanisms. Chem. Eng. J. 2023, 476, 146542. [Google Scholar] [CrossRef]
- Argiri, A.; Ioannou, Z.; Dimirkou, A. Impact of New Soil Amendments on the Uptake of Lead by Crops. Commun. Soil Sci. Plant Anal. 2012, 44, 566–573. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, X.; Xue, H.; Gong, B.; Li, Q.; Guo, W.; Meng, X. Effective Immobilization and Biosafety Assessment of Antimony in Soil with Zeolite-Supported Nanoscale Zero-Valent Iron. Environ. Pollut. 2024, 352, 124082. [Google Scholar] [CrossRef]
- Li, Z.; Wang, L.; Meng, J.; Liu, X.; Xu, J.; Wang, F.; Brookes, P. Zeolite-Supported Nanoscale Zero-Valent Iron: New Findings on Simultaneous Adsorption of Cd(II), Pb(II), and As(III) in Aqueous Solution and Soil. J. Hazard. Mater. 2017, 344, 1–11. [Google Scholar] [CrossRef]
- Shi, W.-Y.; Shao, H.-B.; Li, H.; Shao, M.-A.; Du, S. Progress in the Remediation of Hazardous Heavy Metal-Polluted Soils by Natural Zeolite. J. Hazard. Mater. 2009, 170, 1–6. [Google Scholar] [CrossRef]
- Chen, Y.L.; Hua, L.C.; Tsai, M.H.; Chien, T.Y.; Huang, C. Chemisorption of fluoride onto manganese-oxide-coated activated alumina in aqueous solution. J. Hazard. Mater. Adv. 2022, 6, 100095. [Google Scholar] [CrossRef]
- Wang, H.; Feng, Q.; Liu, K.; Li, Z.; Tang, X.; Li, G. Highly efficient fluoride adsorption from aqueous solution by nepheline prepared from kaolinite through alkali-hydrothermal process. J. Environ. Manag. 2017, 196, 72–79. [Google Scholar] [CrossRef]
- Erdem, E.; Karapinar, N.; Donat, R. The Removal of Heavy Metal Cations by Natural Zeolites. J. Colloid Interface Sci. 2004, 280, 309–314. [Google Scholar] [CrossRef]
- Kragović, M.; Daković, A.; Sekulić, Ž.; Trgo, M.; Ugrina, M.; Perić, J.; Gatta, G.D. Removal of Lead from Aqueous Solutions by Using the Natural and Fe(III)-Modified Zeolite. Appl. Surf. Sci. 2011, 258, 3667–3673. [Google Scholar] [CrossRef]
- Min, F.; Wang, X.; Li, L.; Xin, Z.; Li, X.; Zhang, T.; Sun, X.; You, H. Effects of Silicate Stabilizers on Cadmium Reduction and the Quality of Rice Grains in Acidic Paddy Soil. Sci. Rep. 2024, 14, 20551. [Google Scholar] [CrossRef]
- Lahori, A.H.; Zhang, Z.; Shaheen, S.M.; Rinklebe, J.; Guo, Z.; Li, R.; Mahar, A.; Wang, Z.; Ren, C.; Mi, S.; et al. Mono-and Co-Applications of Ca-Bentonite with Zeolite, Ca-Hydroxide, and Tobacco Biochar Affect Phytoavailability and Uptake of Copper and Lead in a Gold Mine-Polluted Soil. J. Hazard. Mater. 2019, 374, 401–411. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, P.; Santona, L.; Enzo, S.; Melis, P. Sorption Processes and XRD Analysis of a Natural Zeolite Exchanged with Pb2+, Cd2+ and Zn2+ Cations. J. Hazard. Mater. 2008, 156, 428–434. [Google Scholar] [CrossRef]
- Farooqi, Z.U.R.; Ahmad, I.; Qadir, A.A.; Murtaza, G.; Rafiq, S.; Jamal, A.; Zeeshan, N.; Murtaza, B.; Javed, W.; Radicetti, E.; et al. Zeolite-Assisted Immobilization and Health Risks of Potentially Toxic Elements in Wastewater-Irrigated Soil under Brinjal (Solanum melongena) Cultivation. Agronomy 2022, 12, 2433. [Google Scholar] [CrossRef]
- Król, M. Natural vs. Synthetic Zeolites. Crystals 2020, 10, 622. [Google Scholar] [CrossRef]
- Sushkevich, V.L.; Safonova, O.V.; Palagin, D.; Newton, M.A.; van Bokhoven, J.A. Structure of copper sites in zeolites examined by Fourier and wavelet transform analysis of EXAFS. Chem. Sci. 2020, 11, 5299–5312. [Google Scholar] [CrossRef] [PubMed]
- Pankin, I.A.; Martini, A.; Lomachenko, K.A.; Soldatov, A.V.; Bordiga, S.; Borfecchia, E. Identifying Cu-oxo species in Cu-zeolites by XAS: A theoretical survey by DFT-assisted XANES simulation and EXAFS wavelet transform. Catal. Today 2020, 345, 125–135. [Google Scholar] [CrossRef]
- Chen, Z.; Wei, D.; Li, Q.; Wang, X.; Yu, S.; Liu, L.; Liu, B.; Xie, S.; Wang, J.; Chen, D.; et al. Macroscopic and microscopic investigation of Cr(VI) immobilization by nanoscaled zero-valent iron supported zeolite MCM-41 via batch, visual, XPS and EXAFS techniques. J. Clean. Prod. 2018, 181, 745–752. [Google Scholar] [CrossRef]
- Kunene, S.C.; Lin, K.S.; Mdlovu, N.V.; Lin, Y.S.; Mdlovu, N.B. Speciation and fate of toxic cadmium in contaminated paddy soils and rice using XANES/EXAFS spectroscopy. J. Hazard. Mater. 2021, 407, 124879. [Google Scholar] [CrossRef]
- Senila, M.; Levei, E.; Miclean, M.; Senila, L.; Stefanescu, L.; Marginean, S.; Ozunu, A.; Roman, C. Influence of pollution level on heavy metals mobility in soil from NW Romania. Environ. Eng. Manag. J. 2011, 10, 59–64. [Google Scholar] [CrossRef]
- Ibrahim, E.A.; El-Sherbini, M.a.A.; Selim, E.-M.M. Effects of Biochar, Zeolite and Mycorrhiza Inoculation on Soil Properties, Heavy Metal Availability and Cowpea Growth in a Multi-Contaminated Soil. Sci. Rep. 2023, 13, 6621. [Google Scholar] [CrossRef]
- Senila, M.; Cadar, O.; Senila, L.; Angyus, B.S. Simulated Bioavailability of Heavy Metals (Cd, Cr, Cu, Pb, Zn) in Contaminated Soil Amended with Natural Zeolite Using Diffusive Gradients in Thin-Films (DGT) Technique. Agriculture 2022, 12, 321. [Google Scholar] [CrossRef]
- Shi, J.; Yang, Z.; Dai, H.; Lu, X.; Peng, L.; Tan, X.; Shi, L.; Fahim, R. Preparation and application of modified zeolites as adsorbents in wastewater treatment. Water Sci. Technol. 2018, 2017, 621–635. [Google Scholar] [CrossRef] [PubMed]
- Amirahmadi, E.; Ghorbani, M.; Moudrý, J. Effects of zeolite on aggregation, nutrient availability, and growth characteristics of corn (Zea mays L.) in cadmium-contaminated soils. Water Air Soil Pollut 2022, 233, 436. [Google Scholar] [CrossRef]
- Demir, Y. The effects of the applications of zeolite and biochar to the soils irrigated with treated wastewater on the heavy metal concentrations of the soils and leaching waters from the soils. Carpath. J. Earth Environ. 2021, 16, 223–236. [Google Scholar] [CrossRef]
- Szewczuk-Karpisz, K.; Tomczyk, A.; Kercheva, M.; Paparkova, T.; Grygorczuk-Płaneta, K.; Siryk, O.; Kukowska, S.; Panek, R. Reclamation of Degraded Soils: Analysis of Selected Parameters after Organic/Inorganic Modifications. J. Soils Sediments 2024, 24, 1704–1723. [Google Scholar] [CrossRef]
- Guan, D.X.; He, S.X.; Li, G.; Teng, H.H.; Ma, L.Q. Application of diffusive gradients in thin-films technique for speciation, bioavailability, modeling and mapping of nutrients and contaminants in soils. Crit. Rev. Environ. Sci. Technol. 2022, 52, 3035–3079. [Google Scholar] [CrossRef]
- Senila, M.; Kovacs, E. Use of diffusive gradients in thin-film technique to predict the mobility and transfer of nutrients and toxic elements from agricultural soil to crops—An overview of recent studies. Environ. Sci. Pollut. Res. 2024, 31, 34817–34838. [Google Scholar] [CrossRef]
- Miclean, M.; Levei, E.A.; Tanaselia, C.; Cadar, O. Rare Earth Elements Transfer from Soil to Vegetables and Health Risks Associated with Vegetable Consumption in a Former Mining Area. Agronomy 2023, 13, 1399. [Google Scholar] [CrossRef]
- Lee, H.; Sam, K.; Coulon, F.; De Gisi, S.; Notarnicola, M.; Labianca, C. Recent Developments and Prospects of Sustainable Remediation Treatments for Major Contaminants in Soil: A Review. Sci. Total Environ. 2023, 912, 168769. [Google Scholar] [CrossRef]
- Zhu, J.; Fu, Q.; Qiu, G.; Liu, Y.; Hu, H.; Huang, Q.; Violante, A. Influence of low molecular weight anionic ligands on the sorption of heavy metals by soil constituents: A review. Environ. Chem. Lett. 2019, 17, 1271–1280. [Google Scholar] [CrossRef]
- Honma, T.; Ohba, H.; Kaneko-Kadokura, A.; Makino, T.; Nakamura, K.; Katou, H. Optimal soil Eh, pH, and water management for simultaneously minimizing arsenic and cadmium concentrations in rice grains. Environ. Sci. Technol. 2016, 50, 4178–4185. [Google Scholar] [CrossRef] [PubMed]
- Zhong, X.; Chen, Z.; Li, Y.; Ding, K.; Liu, W.; Liu, Y.; Yuan, Y.; Zhang, M.; Baker, A.J.M.; Yang, W.; et al. Factors influencing heavy metal availability and risk assessment of soils at typical metal mines in Eastern China. J. Hazard. Mater. 2020, 400, 123289. [Google Scholar] [CrossRef] [PubMed]
- Wuana, R.A.; Okieimen, F.E. Heavy Metals in Contaminated Soils: A Review of Sources, Chemistry, Risks and Best Available Strategies for Remediation. ISRN Ecol. 2011, 2011, 402647. [Google Scholar] [CrossRef]
- Zamzow, M.J.; Eichbaum, B.R.; Sandgren, K.R.; Shanks, D.E. Removal of Heavy Metals and Other Cations from Wastewater Using Zeolites. Sep. Sci. Technol. 1990, 25, 1555–1569. [Google Scholar] [CrossRef]
- Belova, T.P. Adsorption of Heavy Metal Ions (Cu2+, Ni2+, Co2+ and Fe2+) from Aqueous Solutions by Natural Zeolite. Heliyon 2019, 5, e02320. [Google Scholar] [CrossRef]
- Asare, M.O.; Pellegrini, E.; Száková, J.; Blöcher, J.R.; Najmanová, J.; Tlustoš, P.; Contin, M. Organic Amendments to Short Rotation Coppice (SRC) Plantation Affect Species Richness and Metal Accumulation of Spontaneously Growing Herbaceous Plants. J. Soil Sci. Plant Nutr. 2024, 24, 1474–1488. [Google Scholar] [CrossRef]
- Cadar, O.; Stupar, Z.; Senila, M.; Levei, L.; Moldovan, A.; Becze, A.; Ozunu, A.; Levei, E.A. Zeolites Reduce the Transfer of Potentially Toxic Elements from Soil to Leafy Vegetables. Materials 2022, 15, 5657. [Google Scholar] [CrossRef]
- Kordala, N.; Wyszkowski, M. Zeolite Properties, Methods of Synthesis, and Selected Applications. Molecules 2024, 29, 1069. [Google Scholar] [CrossRef]
- Misaelides, P. Application of Natural Zeolites in Environmental Remediation: A Short Review. Microporous Mesoporous Mater. 2011, 144, 15–18. [Google Scholar] [CrossRef]
- Belviso, C.; Cavalcante, F.; Di Gennaro, S.; Palma, A.; Ragone, P.; Fiore, S. Mobility of Trace Elements in Fly Ash and in Zeolitised Coal Fly Ash. Fuel 2015, 144, 369–379. [Google Scholar] [CrossRef]
- Legese, W.; Taddesse, A.M.; Kibret, K.; Wogi, L. Assessing the Influence of Natural Zeolite on Toxic Heavy Metals Immobilization and Their Transfer into Zea mays L. Bull. Chem. Soc. Ethiop. 2023, 37, 1351–1368. [Google Scholar] [CrossRef]
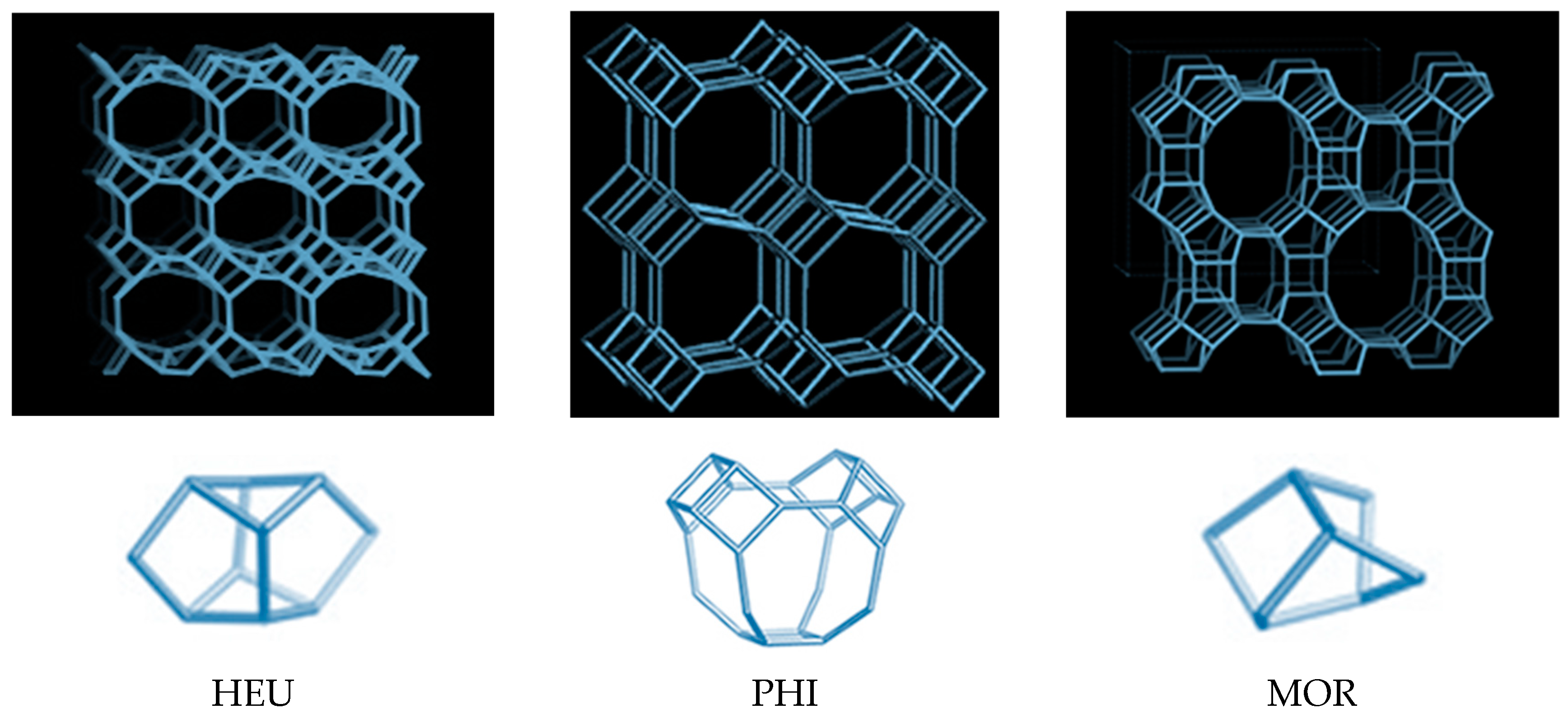
| Zeolite Type | Si/(Al + Fe3+) Ratio | Water Molecules | Main Exchangeable Cations |
|---|---|---|---|
| Clinoptilolite | 4.0–5.6 | 3.5–4.0 | Na+, Ca2+, K+ |
| Heulandite | 2.7–4.0 | 2.5–3.1 | Ca2+, K+, Na+, Sr2+ |
| Phillipsite | 1.08–3.35 | 1.7–3.3 | Ca, K+, Na+ |
| Laumontite | 1.9–2.4 | 2.0 | Ca2+ |
| Analcime | 1.5–2.9 | 1.0–1.3 | Na+ |
| Modernite | 4.1–5.7 | 3.0–3.5 | Ca2+, Na+, K+ |
| Chabazite | 1.4–4.1 | 2.7–4.1 | Ca2+, Na+ |
| Natrolite | 1.5 | 1.0 | Na+ |
| Erionite | 2.6–3.8 | 3.0–3.5 | Na+, K+, Ca2+ |
| Stilbite | 2.6–3.5 | 2.8–3.5 | Na+, Ca2+ |
| Wairakite | 2.0 | 1.0 | Ca2+ |
| Type of Zeolites and Their Modification | Combination of Zeolites | Mode of Application on Soil | Effect | References |
|---|---|---|---|---|
| Analcime zeolite obtained from red soil | A composite material containing zeolite and biochar from rice straw. | Composite materials of zeolite and biochar added to contaminated soil at amounts of 10, 20, and 30 g/kg. | Zeolite and biochar composite showed synergetic effect on soil remediation. The contents of TN, TOC, available P and available K in soil increased by 41.2%, 42%, 172.0%, and 878.0%. Pb and Cd in the plant stems diminished by 92.8% and 92.9%. | [21] |
| Zeolite | Application of organo-mineral amendments: biochar bentonite and zeolite alone and in combination (20% biochar, 80% bentonite or zeolite; 20% biochar, 40% zeolite, 40% bentonite). | Control soil and soil artificially spiked with Pb and Zn, and then kept in the dark for 1 month. Soil was used for growing maize in greenhouse pot experiments. | The amendments reduced the mobile fractions of metals in soil and reduced the transfer of Pb and Zn in maize roots by 24–59% and 42–68% and leaves by 19–60% and 43–75%. | [29] |
| Natural zeolite from Iran with grain size < 0.5 mm | Natural zeolite added to soil with biochar produced from corn straw, wheat straw, rice husk, licorice root pulp, and sheep manure. | Natural zeolite applied at 0, 3 and 6% (w/w) combined with biochar at 3% (w/w). | Combination of sheep manure biochar with zeolite provided the best results to decrease Cd mobility in soil. EDTA-extractable Cd decreased with 54.2%. | [81] |
| Natural zeolite | Natural zeolite, biochar and their mixture used as amendments for soil. | Ninety-day incubation in pot experiments for an application rate of 5% amendment to soil. | Bioavailability of As, Cd, Pb, and W decreased by 57.4, 62.7, 56.4, and 22.5%, respectively, following amendments application to soil. | [33] |
| Natural zeolite powder | Zeolite–biochar composite obtained from co-pyrolysis of 50% zeolite with 50% feedstock, then activated by NaOH. | Composites mixed with soil at 5% mass, batch experiments. | Bioavailability of Cd in the soil decreased by 59.70% and 68.54%, respectively, following zeolite–biochar composite application to soil. | [97] |
| Natural zeolite anzimit type | Natural zeolite alone. | Zeolite application at increasing amounts of 0, 5, 10, and 15 g kg−1 of soil contaminated with Cd. Soil was used to grow corn. | Cd availability was significantly reduced in all soils amended by zeolites, until 86.84%. Growth characteristics of corn improved and N, P, and K in leaf increased by 71.20%, 47.01%, and 20.19% | [128] |
| Clinoptilolite type natural zeolite from Turkey | Zeolites and biochar separately added for comparison. | Zeolite at 5%, 10%, and 20% and biochar at 1%, 2%, and 4% were added to soils irrigated with wastewater. | Biochar and zeolite addition to the soils reduced the metals mobility. The concentrations of heavy metals in the leaching waters decreased. | [129] |
| Natural zeolite from Bulgaria | Zeolite, zeolite–biochar composite, and vermicompost added to degraded soil. | One percent of soil amendments were mixed with soils: Eutric Cambisol (Poland) and Epicalcic Cher nozem (Bulgaria). | Zeolitic and vermicompost materials enhanced Cd adsorption. The amounts of Cd adsorbed were 1.04 mg/g and 2.97 mg/g in the two soils from a solution with an initial Cd concentration of 50 mg/L. | [130] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senila, M.; Cadar, O. Composites Based on Natural Zeolites and Green Materials for the Immobilization of Toxic Elements in Contaminated Soils: A Review. Materials 2024, 17, 5977. https://doi.org/10.3390/ma17235977
Senila M, Cadar O. Composites Based on Natural Zeolites and Green Materials for the Immobilization of Toxic Elements in Contaminated Soils: A Review. Materials. 2024; 17(23):5977. https://doi.org/10.3390/ma17235977
Chicago/Turabian StyleSenila, Marin, and Oana Cadar. 2024. "Composites Based on Natural Zeolites and Green Materials for the Immobilization of Toxic Elements in Contaminated Soils: A Review" Materials 17, no. 23: 5977. https://doi.org/10.3390/ma17235977
APA StyleSenila, M., & Cadar, O. (2024). Composites Based on Natural Zeolites and Green Materials for the Immobilization of Toxic Elements in Contaminated Soils: A Review. Materials, 17(23), 5977. https://doi.org/10.3390/ma17235977







