Anion-Doped Perovskite Oxygen-Permeable Membranes Fabricated via an Improved One-Step Thermal Processing Approach
Abstract
1. Introduction
2. Experimental Section
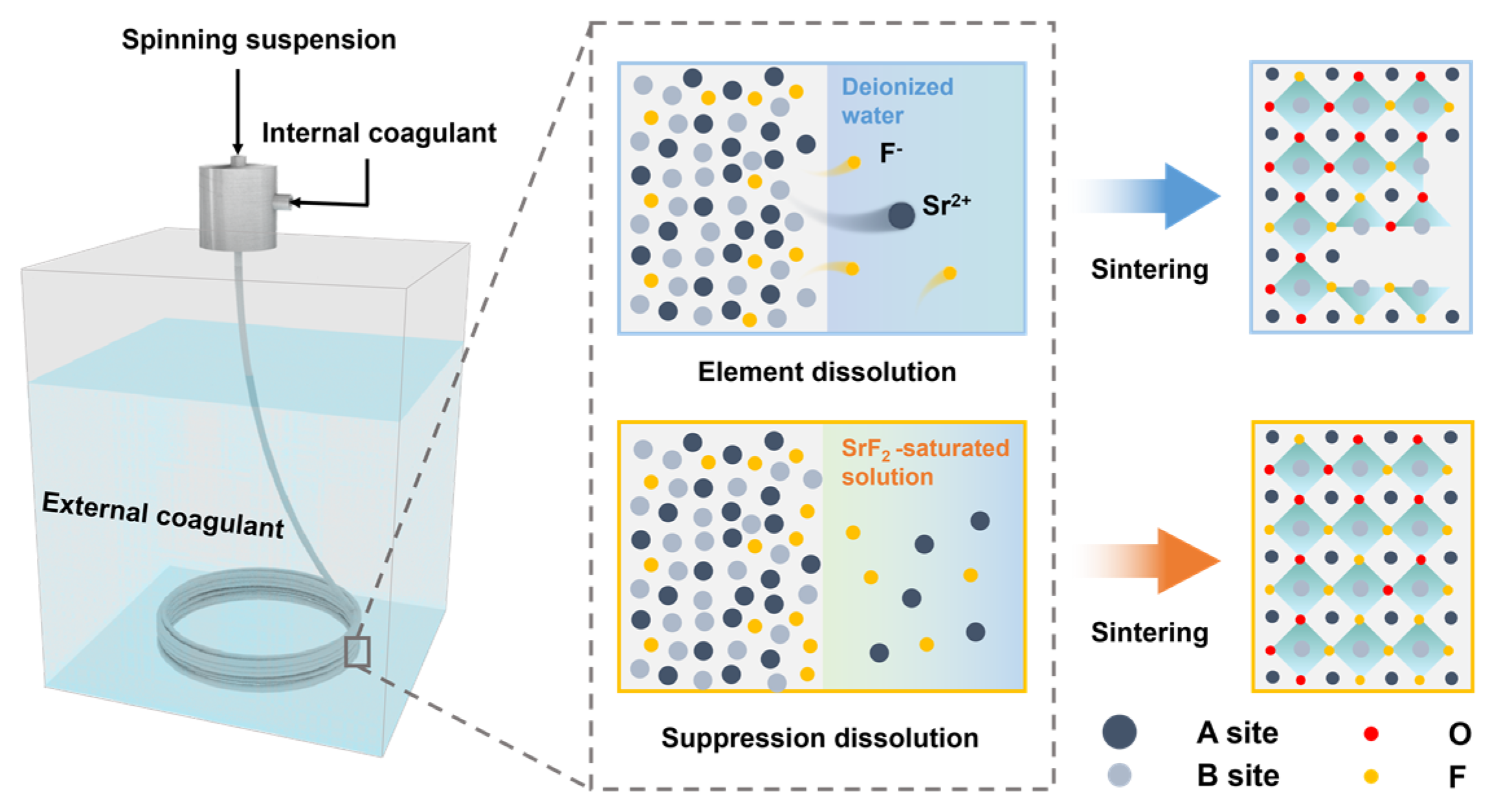
2.1. Preparation of HF Membranes
2.2. Characterizations
2.3. Oxygen Permeation Measurement
3. Results and Discussion
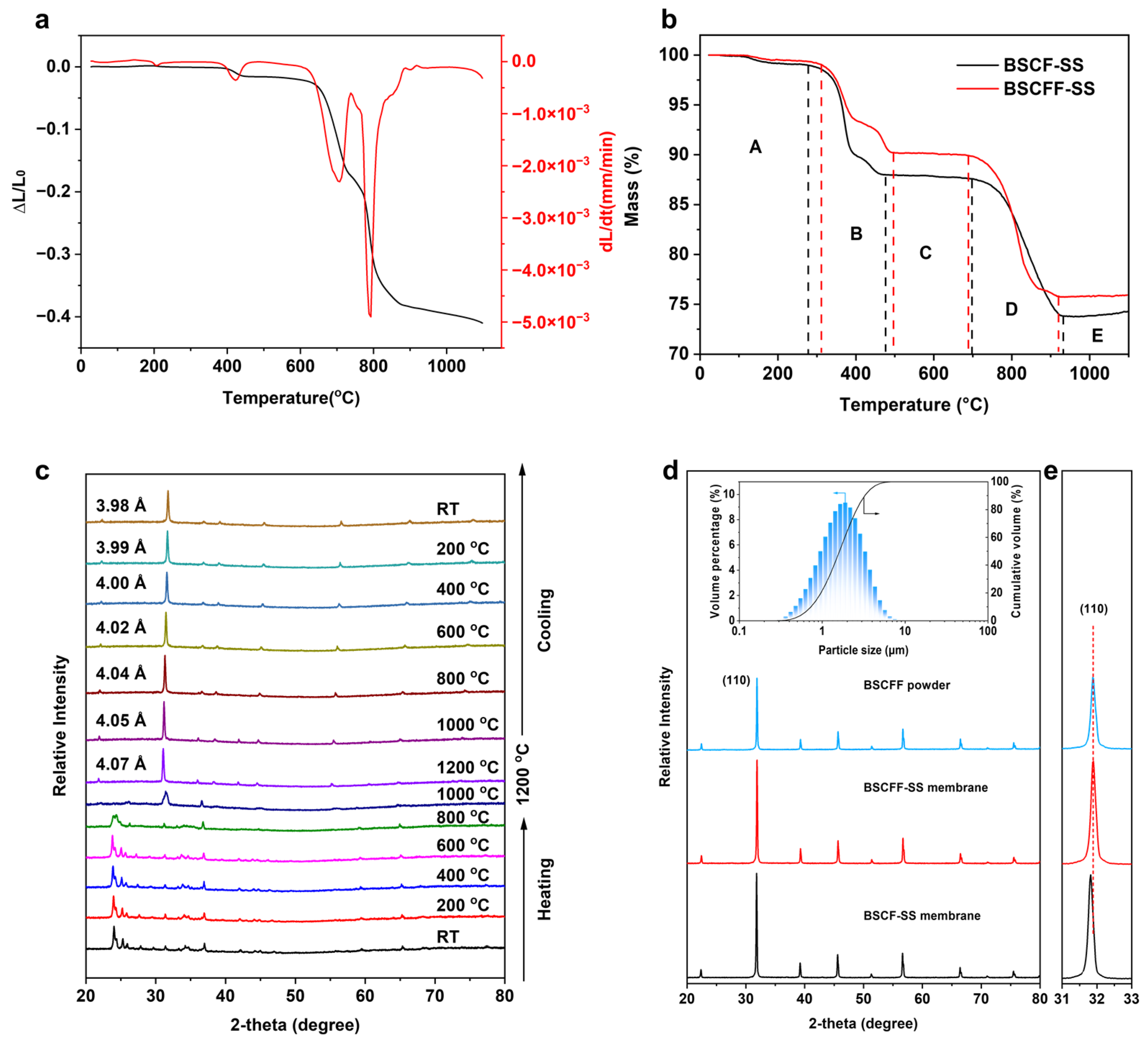
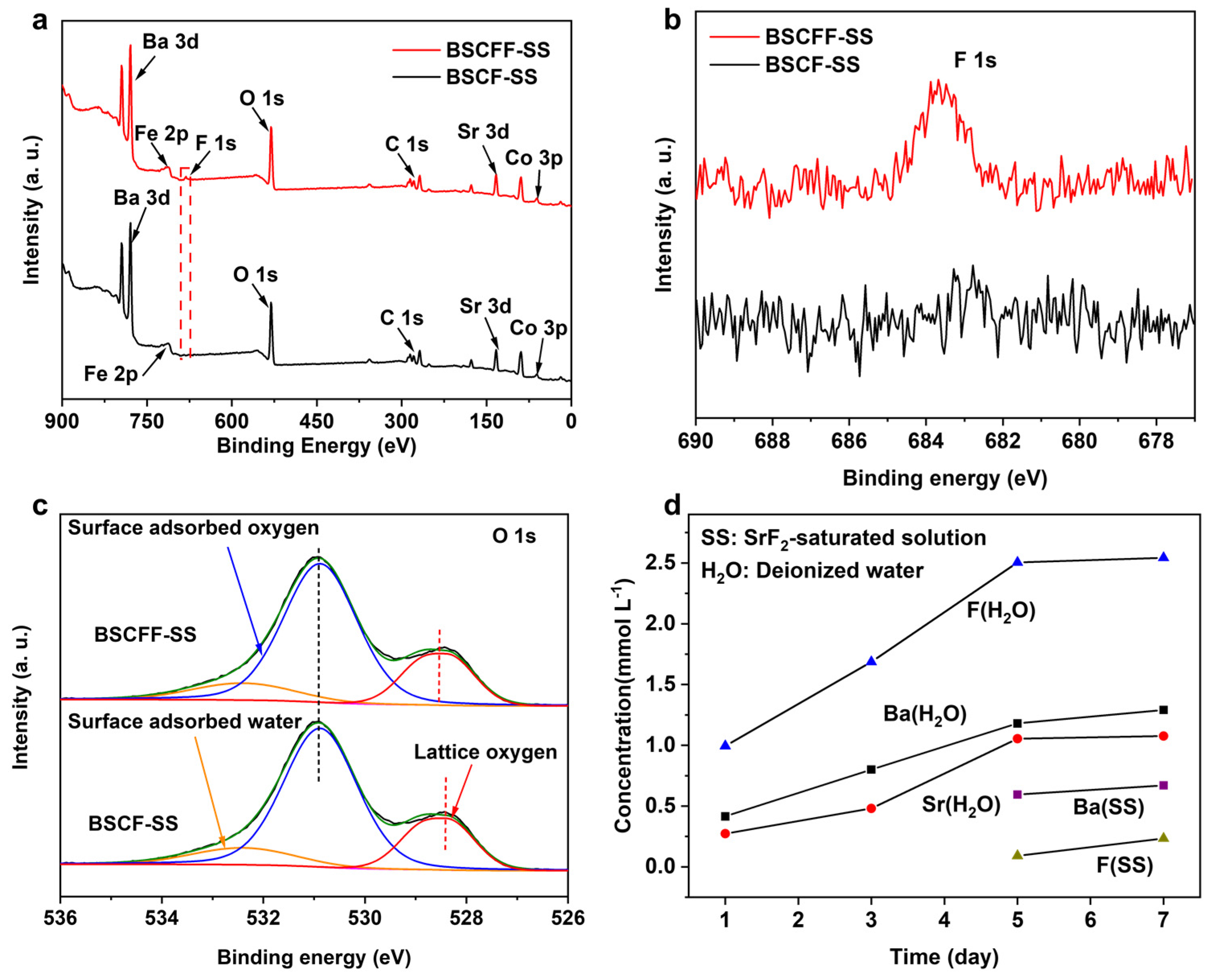
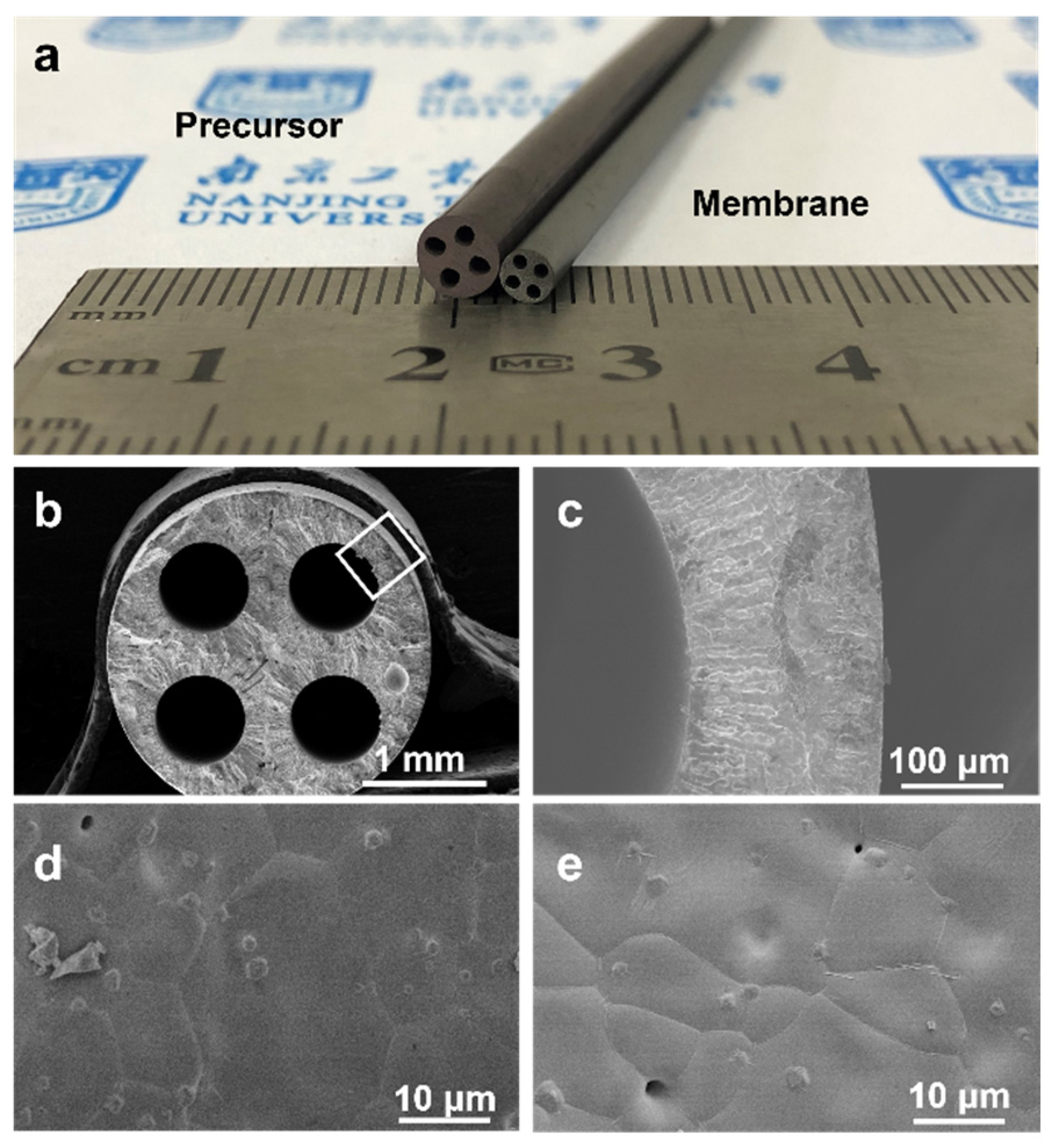
3.1. Fabrication of BSCFF HF Membrane
3.2. Oxygen Permeation
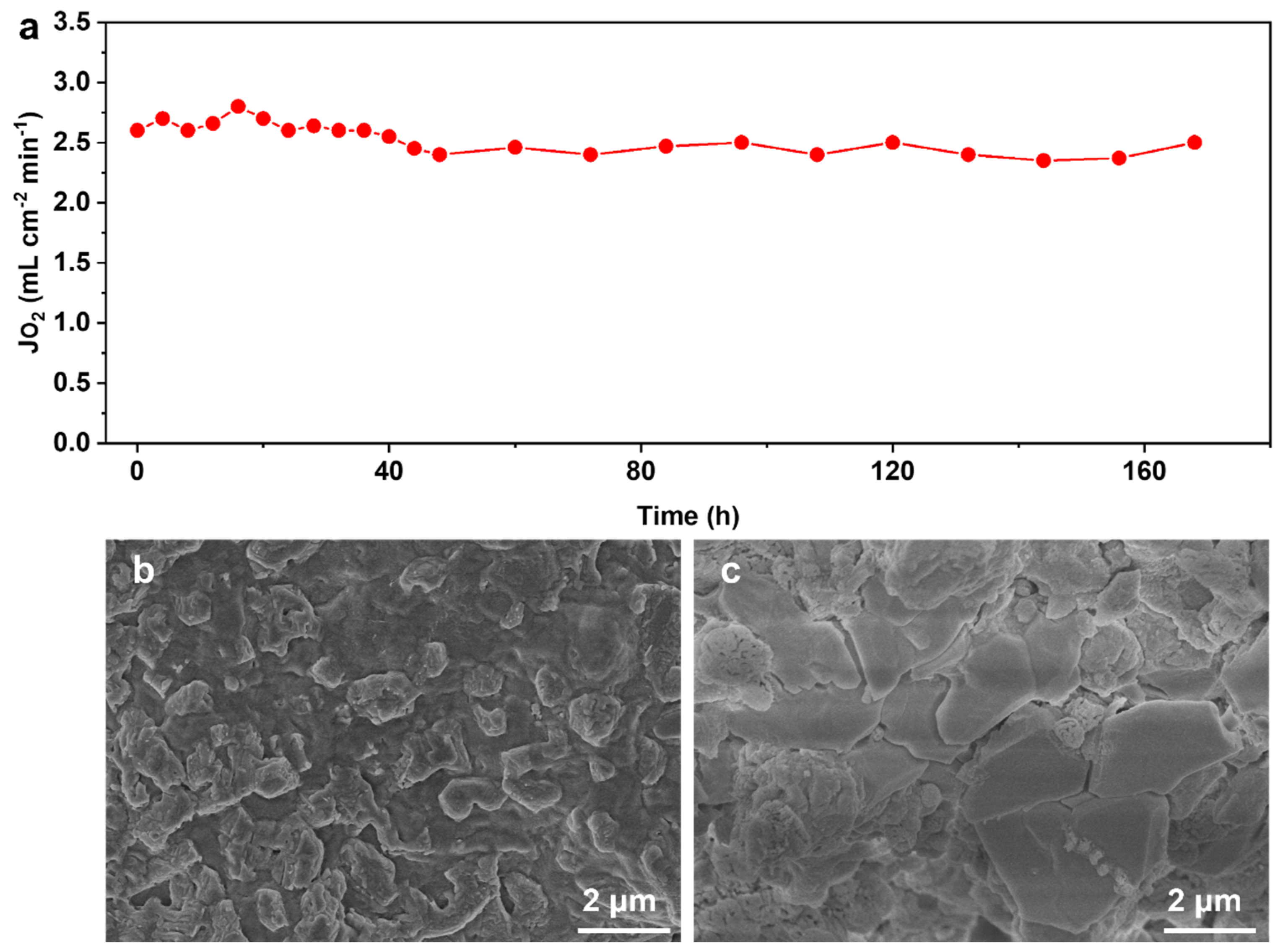
3.3. Long-Term Stability
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Z.; Zhou, W.; Jin, W.; Xu, N. Effect of pH on synthesis and properties of perovskite oxide via a citrate process. AIChE J. 2005, 52, 769–776. [Google Scholar] [CrossRef]
- Miller, C.F.; Chen, J.; Carolan, M.F.; Foster, E.P. Advances in ion transport membrane technology for Syngas production. Catal. Today 2014, 228, 152–157. [Google Scholar] [CrossRef]
- Sunarso, J.; Baumann, S.; Serra, J.M.; Meulenberg, W.A.; Liu, S.; Lin, Y.S.; Diniz da Costa, J.C. Mixed ionic–electronic conducting (MIEC) ceramic-based membranes for oxygen separation. J. Membr. Sci. 2008, 320, 13–41. [Google Scholar] [CrossRef]
- Zhu, X.; Yang, W. Microstructural and Interfacial Designs of Oxygen-Permeable Membranes for Oxygen Separation and Reaction–Separation Coupling. Adv. Mater. 2019, 31, 1902547. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Lin, J.Y.S. Fixed-bed performance for production of oxygen-enriched carbon dioxide stream by perovskite-type ceramic sorbent. Sep. Purif. Technol. 2006, 49, 27–35. [Google Scholar] [CrossRef]
- Madsen, B.D.; Kobsiriphat, W.; Wang, Y.; Marks, L.D.; Barnett, S.A. Nucleation of nanometer-scale electrocatalyst particles in solid oxide fuel cell anodes. J. Power Sources 2007, 166, 64–67. [Google Scholar] [CrossRef]
- Wei, Y.; Yang, W.; Caro, J.; Wang, H. Dense ceramic oxygen permeable membranes and catalytic membrane reactors. Chem. Eng. J. 2013, 220, 185–203. [Google Scholar] [CrossRef]
- Kruidhof, H.; Bouwmeester, H.J.; Doorn, R.V.; Burggraaf, A.J. Influence of order-disorder transitions on oxygen permeability through selected nonstoichiometric perovskite-type oxides. Solid State Ion. 1993, 63–65, 816–822. [Google Scholar] [CrossRef]
- Liang, F.; Jiang, H.; Luo, H.; Caro, J.; Feldhoff, A. Phase Stability and Permeation Behavior of a Dead-End Ba0.5Sr0.5Co0.8Fe0.2O3−δ Tube Membrane in High-Purity Oxygen Production. Chem. Mater. 2011, 23, 4765–4772. [Google Scholar] [CrossRef]
- Shao, Z.; Yang, W.; Cong, Y.; Dong, H.; Tong, J.; Xiong, G. Investigation of the permeation behavior and stability of a Ba0.5Sr0.5Co0.8Fe0.2O3−δ oxygen membrane. J. Membr. Sci. 2000, 172, 177–188. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, W.; Xu, X.; Marcel Veder, J.-P.; Shao, Z. Recent advances in anion-doped metal oxides for catalytic applications. J. Mater. Chem. A 2019, 7, 7280–7300. [Google Scholar] [CrossRef]
- Cook, R.L.; Sammells, A.F. On the systematic selection of perovskite solid electrolytes for intermediate temperature fuel cells. Solid State Ion. 1991, 45, 311–321. [Google Scholar] [CrossRef]
- Zhu, J.; Liu, G.; Liu, Z.; Chu, Z.; Jin, W.; Xu, N. Unprecedented Perovskite Oxyfluoride Membranes with High-Efficiency Oxygen Ion Transport Paths for Low-Temperature Oxygen Permeation. Adv. Mater. 2016, 28, 3511–3515. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Liu, L.; Xue, J.; Zhuang, L.; Wang, H. Tailoring hydrogen separation performance through the ceramic lanthanum tungstate membranes by chlorine doping. J. Membr. Sci. 2019, 573, 117–125. [Google Scholar] [CrossRef]
- Chen, L.; Zhuang, L.; Xue, J.; Wei, Y.; Wang, H. Tuning the separation performance of hydrogen permeable membranes using an anion doping strategy. J. Mater. Chem. A 2017, 5, 20482–20490. [Google Scholar] [CrossRef]
- Xue, J.; Li, J.; Zhuang, L.; Chen, L.; Feldhoff, A.; Wang, H. Anion doping CO2-stable oxygen permeable membranes for syngas production. Chem. Eng. J. 2018, 347, 84–90. [Google Scholar] [CrossRef]
- Liu, Y.; Meng, X.; Yu, F.; Yin, M.; Yang, N.; Meng, B.; Sofianos, M.V.; Liu, S. Enhancing oxygen reduction reaction activity of perovskite oxides cathode for solid oxide fuel cells using a novel anion doping strategy. Int. J. Hydrogen Energy 2018, 43, 12328–12336. [Google Scholar] [CrossRef]
- Jiansheng, L.; Lianjun, W.; Yanxia, H.; Xiaodong, L.; Xiuyun, S. Preparation and characterization of Al2O3 hollow fiber membranes. J. Membr. Sci. 2005, 256, 1–6. [Google Scholar] [CrossRef]
- Zhu, J.; Zhang, G.; Liu, G.; Liu, Z.; Jin, W.; Xu, N. Perovskite Hollow Fibers with Precisely Controlled Cation Stoichiometry via One-Step Thermal Processing. Adv. Mater. 2017, 29, 1606377. [Google Scholar] [CrossRef]
- Zhu, J.; Dong, Z.; Liu, Z.; Zhang, K.; Zhang, G.; Jin, W. Multichannel mixed-conducting hollow fiber membranes for oxygen separation. AIChE J. 2014, 60, 1969–1976. [Google Scholar] [CrossRef]
- Feng, P.; Cao, P.; Ren, S.; Ren, J.; Dong, Y.; Wu, G.; Tang, R. The mechanical and hydrochemical properties of cemented calcareous soil under long-term soaking. Sci. Rep. 2024, 14, 24532. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jin, W.; Huang, P.; Xu, N.; Shi, J.; Hu, M.Z.C.; Payzant, E.A.; Ma, Y.H. Perovskite-related ZrO2-doped SrCo0.4Fe0.6O3-δ membrane for oxygen permeation. AIChE J. 2004, 45, 276–284. [Google Scholar] [CrossRef]
- Zhou, W.; Fan, Z.; Jiang, H.; Wu, Z.; Liu, Z.; Zhang, G.; Jin, W. Cold sintering of perovskite-based mixed conducting membrane for oxygen separation. AIChE J. 2024, 70, e18378. [Google Scholar] [CrossRef]
- Zhu, Y.; Lei, J.; Liu, J.; Tan, J.; Zhang, G.; Liu, Z.; Jin, W. Fabrication of CO2-tolerant SrFe0.8Nb0.2O3-δ/SrCo0.9Nb0.1O3-δ dual-layer 7-channel hollow fiber membrane by co-spinning and one-step thermal process. J. Membr. Sci. 2023, 670, 121346. [Google Scholar] [CrossRef]
- Baumann, S.; Schulze-Küppers, F.; Roitsch, S.; Betz, M.; Zwick, M.; Pfaff, E.M.; Meulenberg, W.A.; Mayer, J.; Stöver, D. Influence of sintering conditions on microstructure and oxygen permeation of Ba0.5Sr0.5Co0.8Fe0.2O3−δ (BSCF) oxygen transport membranes. J. Membr. Sci. 2010, 359, 102–109. [Google Scholar] [CrossRef]
- Wu, D.; Shen, C.; Zhou, W.; Tan, J.; Niu, Y.; Liu, Z.; Zhang, G.; Jin, W. Exploring the potential of decade-air exposed perovskite membranes through sustainable recycling approaches. Chem. Eng. J. 2024, 489, 151406. [Google Scholar] [CrossRef]
- Zhao, T.; Tan, J.; Zhou, W.; Gu, Z.; Niu, Y.; Liu, Z.; Zhang, G.; Jin, W. Engineering robust porous/dense composite hollow fiber membranes for highly efficient hydrogen separation. J. Membr. Sci. 2024, 705, 122872. [Google Scholar] [CrossRef]
- Mao, H.K.; Hemley, R.J.; Fei, Y.; Shu, J.F.; Chen, L.C.; Jephcoat, A.P.; Wu, Y.; Bassett, W.A. Effect of pressure, temperature, and composition on lattice parameters and density of (Fe,Mg)SiO3-perovskites to 30 GPa. J. Geophys. Res. Solid Earth 2012, 96, 8069–8079. [Google Scholar] [CrossRef]
- Tan, X.; Liu, S.; Li, K. Preparation and characterization of inorganic hollow fiber membranes. J. Membr. Sci. 2001, 188, 87–95. [Google Scholar] [CrossRef]
- Ullah, S.; Wang, S.; Ahmad, M.S.; Sharif, H.M.A.; Liu, Q.; Kida, T.; Shafique, A.; Rehman, M.U.; Wang, G.; Qiu, J. Investigating the role of oxygen vacancies in metal oxide for enhanced electrochemical reduction of NO3− to NH3: Mechanistic insights. Inorg. Chem. Front. 2023, 10, 6440–6488. [Google Scholar] [CrossRef]
- Qiu, K.; Liu, Y.; Tan, J.; Wang, T.; Zhang, G.; Liu, Z.; Jin, W. Fluorine-doped barium cobaltite perovskite membrane for oxygen separation and syngas production. Ceram. Int. 2020, 46, 27469–27475. [Google Scholar] [CrossRef]
- Wu, X.; Miao, H.; Hu, R.; Chen, B.; Yin, M.; Zhang, H.; Xia, L.; Zhang, C.; Yuan, J. A-site deficient perovskite nanofibers boost oxygen evolution reaction for zinc-air batteries. Appl. Surf. Sci. 2021, 536, 147806. [Google Scholar] [CrossRef]
- Moon, E.J.; Xie, Y.; Laird, E.D.; Keavney, D.J.; Li, C.Y.; May, S.J. Fluorination of Epitaxial Oxides: Synthesis of Perovskite Oxyfluoride Thin Films. J. Am. Chem. Soc. 2014, 136, 2224–2227. [Google Scholar] [CrossRef] [PubMed]
- Yang, L.; Cao, Z.; Zhu, Y.; Wang, J.; Pang, B.; Zhang, W.; Zhang, L.; Hu, S.; Zhu, X.; Yang, W. Single- and dual-phase capillary membranes prepared through plastic extrusion method for oxygen permeation. Ceram. Int. 2021, 47, 18510–18516. [Google Scholar] [CrossRef]
- Steele, B. Ceramic ion conducting membranes and their technological applications. C.R. Acad. Sci. Ser. IIC Chem. 1998, 1, 533–543. [Google Scholar] [CrossRef]
- Wu, Z.; Dong, X.; Jin, W.; Fan, Y.; Xu, N. A dense oxygen separation membrane deriving from nanosized mixed conducting oxide. J. Membr. Sci. 2007, 291, 172–179. [Google Scholar] [CrossRef]
- Wang, B.; Zydorczak, B.; Wu, Z.-T.; Li, K. Stabilities of La0.6Sr0.4Co0.2Fe0.8O3−δ oxygen separation membranes—Effects of kinetic demixing/decomposition and impurity segregation. J. Membr. Sci. 2009, 344, 101–106. [Google Scholar] [CrossRef]

| Fabrication Parameters | Value |
|---|---|
| Spinning temperature | 20 °C |
| Injection rate of internal coagulant | 10 mL min−1 |
| Injection rate of suspension | 4 mL min−1 |
| Air gap | 0 cm |
| Element | EDX/Atomic% | XPS/Atomic% | Target/Atomic% |
|---|---|---|---|
| Ba | 13.69 | 13.97 | 10 |
| Sr | 8.05 | 8.47 | 10 |
| Co | 11.05 | 7.07 | 16 |
| Fe | 3.72 | 2.22 | 4 |
| O | 62.27 | 67.05 | 59 |
| F | 1.21 | 1.23 | 1 |
| Temperature (°C) | Character (mm) | Fm (N) | Gas-Tightness |
|---|---|---|---|
| 1050 | OD = 2.76 ID = 0.75 | 37.12 ± 0.83 | Gas leaking |
| 1060 | OD = 3.24 ID = 0.75 | 45.25 ± 1.08 | |
| 1070 | OD = 2.79 ID = 0.75 | 39.56 ± 2.53 | |
| 1080 | OD = 3.15 ID = 0.75 | 38.37 ± 0.73 | |
| 1090 | OD = 3.24 ID = 0.75 | 39.65 ± 1.61 | |
| 1100 | OD = 3.09 ID = 0.75 | 45.97 ± 1.30 | |
| 1110 | OD = 3.03 ID = 0.75 | 38.39 ± 0.96 | |
| 1120 | OD = 2.97 ID = 0.75 | 39.62 ± 1.28 | Gas-tight |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Niu, Y.; Zhou, W.; Ni, S.; Liu, Z.; Zhang, G.; Jin, W. Anion-Doped Perovskite Oxygen-Permeable Membranes Fabricated via an Improved One-Step Thermal Processing Approach. Materials 2024, 17, 5929. https://doi.org/10.3390/ma17235929
Niu Y, Zhou W, Ni S, Liu Z, Zhang G, Jin W. Anion-Doped Perovskite Oxygen-Permeable Membranes Fabricated via an Improved One-Step Thermal Processing Approach. Materials. 2024; 17(23):5929. https://doi.org/10.3390/ma17235929
Chicago/Turabian StyleNiu, Yongqiang, Wanglin Zhou, Shuyang Ni, Zhengkun Liu, Guangru Zhang, and Wanqin Jin. 2024. "Anion-Doped Perovskite Oxygen-Permeable Membranes Fabricated via an Improved One-Step Thermal Processing Approach" Materials 17, no. 23: 5929. https://doi.org/10.3390/ma17235929
APA StyleNiu, Y., Zhou, W., Ni, S., Liu, Z., Zhang, G., & Jin, W. (2024). Anion-Doped Perovskite Oxygen-Permeable Membranes Fabricated via an Improved One-Step Thermal Processing Approach. Materials, 17(23), 5929. https://doi.org/10.3390/ma17235929







