1. Introduction
High-Mn–low-Cr steel alloys with a face-centered cubic (FCC) structure at room temperature have attracted significant attention owing to their desirable combination of superior mechanical properties and long-term lifespan even in harsh chloride environments [
1,
2,
3]. Their intermediate products (e.g., casting slab), however, can sometimes fail, caused mainly by various types of surface and subsurface damage during subsequent manufacturing processes. Among these, the high temperature and severe plastic deformation provided by the surface grinding process can significantly modify the physical, mechanical, and electrochemical properties [
4,
5,
6]. This modification may involve phase transformation, crystallographic texture modifications, work hardening, thermal softening, and dynamic softening including recovery and recrystallization [
4,
7,
8]. According to Yao et al. [
9], when grinding Cr-bearing steel using a flat grinder, its outer surface can be exposed to high temperatures of more than 1000 °C, leading to phase transformations. Moreover, the sample surface undergoes severe plastic deformation due to shearing and extrusion by abrasive particles. This abrasive grinding process is often regarded as a superb machining strategy for structural steel alloys, achieving a high-quality mechanically strengthened layer owing to the mechanical–thermal coupling effect [
9,
10]. On the other hand, the microstructural modifications on the distorted surface generated by severe plastic deformation can increase electrochemical reactivity, leading to a higher susceptibility to anodic metal dissolution of the surface.
It is known that adding even small quantities of Cr to various steel alloys, including the high-Mn–low-Cr steel alloys investigated in this work, enhances corrosion resistance in aqueous media [
11,
12,
13,
14,
15,
16,
17,
18]. Specifically, alloying 3 wt.% Cr to 24 wt.% Mn-bearing steel alloys significantly improves corrosion resistance in brine environments by forming bi-layered corrosion products, consisting of an inner Cr-enriched oxide (or hydroxide) and an outer Fe(Mn)-based oxide (or hydroxide) [
12,
13]. However, the intermediate products of high-Mn–low-Cr steel, such as casting slabs, may have inferior corrosion resistance primarily due to the formation of coarse grains and (Cr,Mn)-bearing precipitates resulting from slow cooling rates during the casting process. Additionally, their corrosion behaviors, such as corrosion type and kinetics, can depend greatly on environmental conditions to which they are exposed, such as temperature and humidity levels. Despite the considerable body of literature analyzing the mechanical properties of steel alloys undergoing the abrasive grinding process [
4,
5,
6,
7,
8,
9,
10], the corrosion behavior of ground high-Mn–low-Cr cast steels in humid environments has not been mechanistically investigated.
The primary objective of the present study is to elucidate the relationship between abrasive grinding-induced microstructural changes and corrosion behaviors of high-Mn–low-Cr cast steel under humid environments. To accomplish this, a range of microstructural characterizations via field-emission scanning electron microscopy (FE-SEM) (Hitachi, Tokyo, Japan), electron backscatter diffraction (EBSD) (Symmetry, Oxford, UK), and transmission electron microscopy (TEM) (SELMI, Sumy, Ukraine) were conducted. Additionally, corrosion-induced surface degradation in humid environments was examined through periodic water spraying and simulated temperature–humidity exposure testing, followed by surface analysis using FE-SEM.
2. Experimental
2.1. Material Preparation
The material investigated in this study was high-Mn–low-Cr-based casting steel (POSCO technical research laboratories, Pohang, Republic of Korea), with a chemical composition of 0.3~0.4 wt.% C, 20~24 wt.% Mn, 0.2~0.3 wt.% Si, 2~3 wt.% Cr, 0.02~0.03 wt.% Ni, and balance Fe. After casting a square-shaped large-scale slab with dimensions of 700 mm in width, 4000 in length, and 225 mm in thickness, it was air-cooled to 200 °C. Subsequently, the surface of the cast slab was ground using a grinder (GRN-20, Shigiya, Schaumburg, IL, USA) with a ceramic bond white corundum grinding wheel (F46 of 300 mm in diameter and 25 mm in width). The grinding depth, feed rate, and grinding wheel speed were 0.1 mm, 20 mm/s, and 35,000 mm/s, respectively.
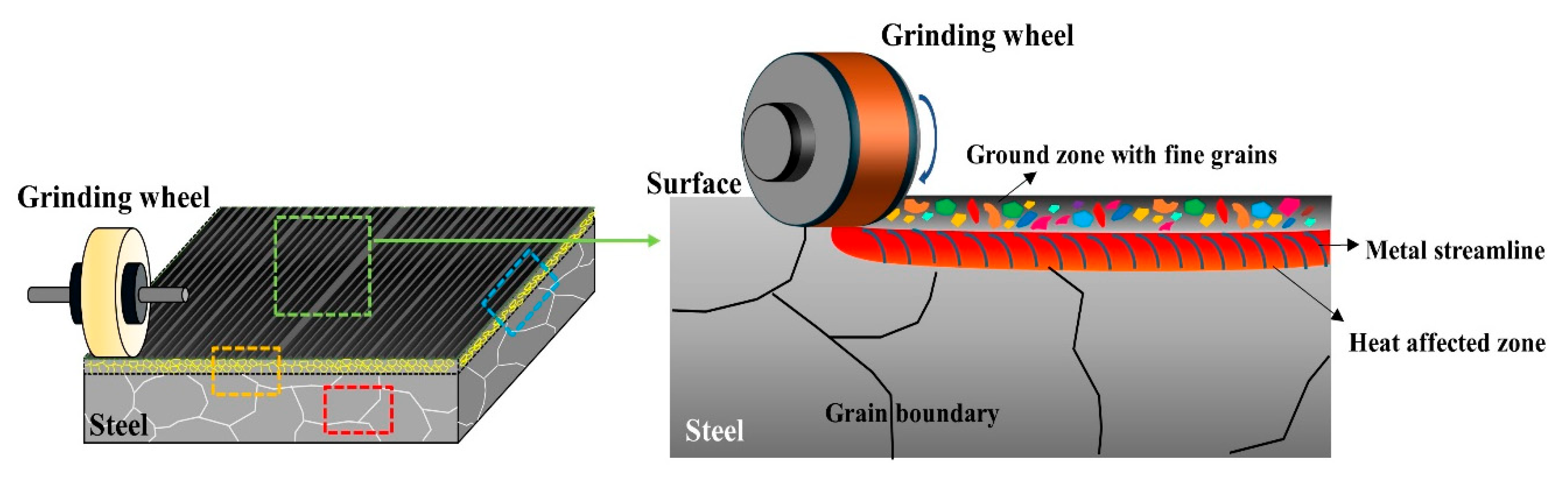
Figure 1 depicts the schematic diagram of the grinding process on the steel slab.
2.2. Microstructure Characterization
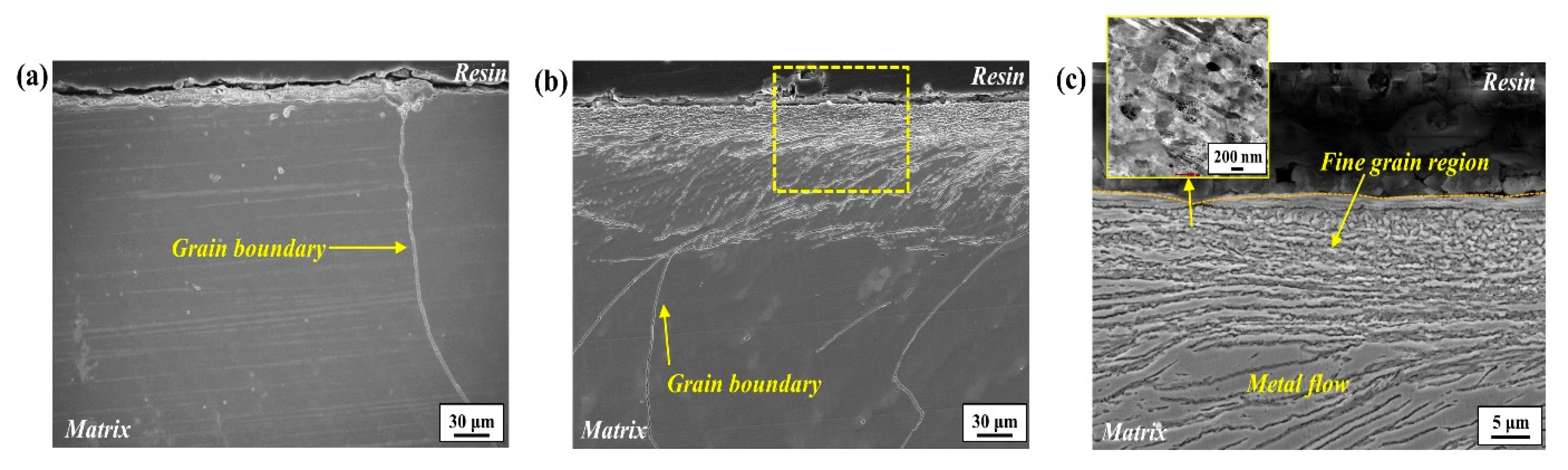
The surface part of the ground steel slab was cut using electrical discharge machining to obtain samples (40 × 50 × 5 mm
3) for microstructural examination employing FE-SEM (Hitachi, Tokyo, Japan), EBSD (Symmetry, Oxford, UK), and TEM (TECNAI 200, FEI, Amsterdam, The Netherlands). The cross-section morphology of the ground sample surface was examined in both the front view (grinding direction, GD) and side view (transverse direction, TD) of grinding, as indicated by the orange and blue dashed boxes in
Figure 1. Additionally, the cross-section morphology below the damaged surface region, as indicated by the red dashed box in
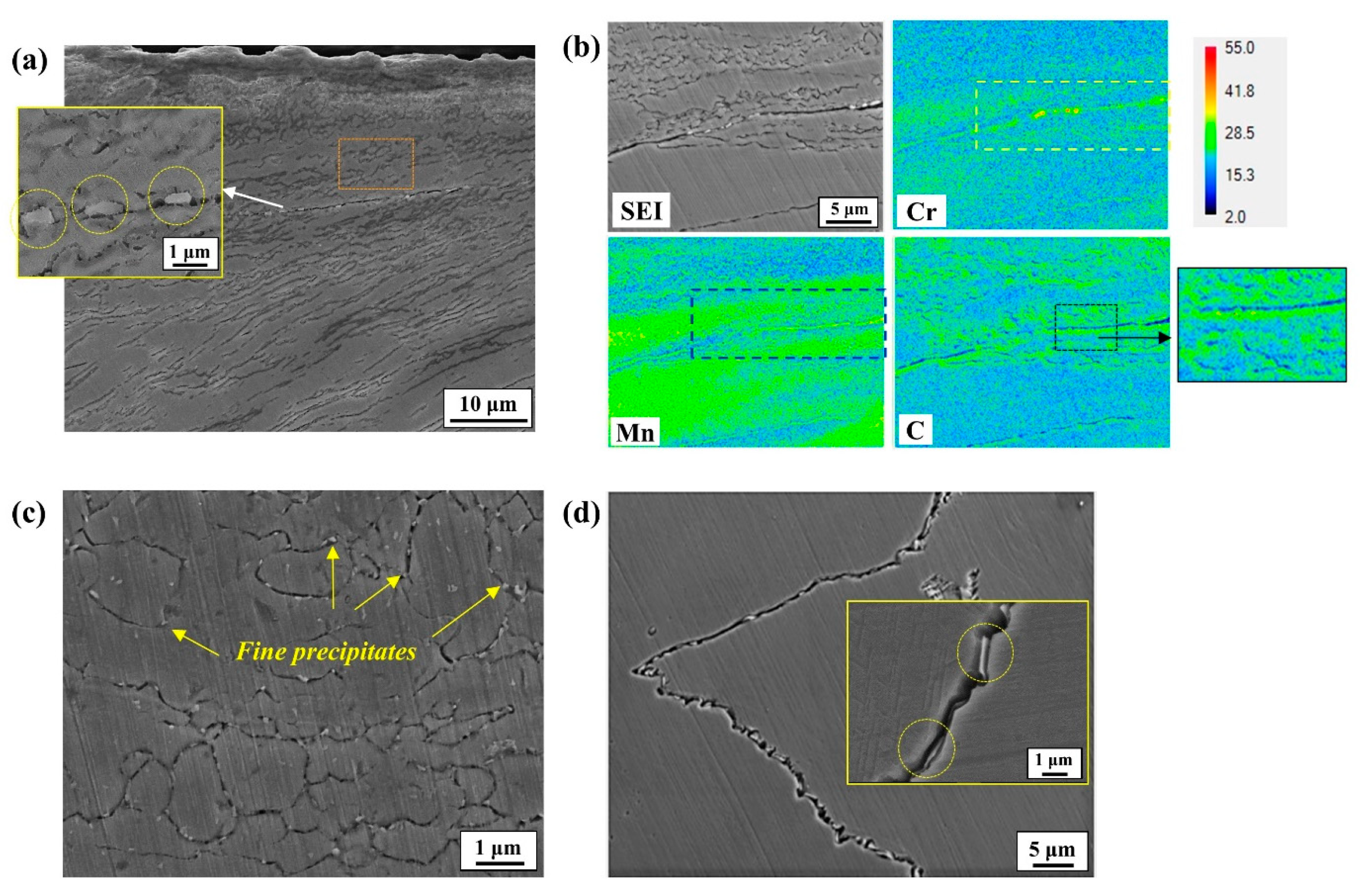
Figure 1, was also analyzed. Fine precipitates in the microstructure were characterized by the EBSD phase mapping and TEM diffraction pattern with a thin foil specimen. For EBSD analysis, the cross-section of the specimens was polished to 0.04 μm using a diamond suspension. The acceleration voltage, beam current, and step size were 20 kV, 1nA, and 40 nm, respectively.
2.3. Corrosion Testing and Analyses
To precisely comprehend the mechanism of corrosion-induced surface degradation in the samples, two types of corrosion tests were conducted: the periodic water spraying test and the simulated temperature–humidity exposure test.
In the periodic water spraying test, the surfaces of ground and unground samples were exposed to tap water (Ca: 19.7 mg/L, Mg: 4.5 mg/L, Na: 13.3 mg/L, K: 4.4 mg/L, Cl−: 13.3 mg/L, SO42−: 21 mg/L) by spraying in a perpendicular direction once every 12 h for seven days. This causes a portion of the tap water to remain on the sample surface. The rationale for selecting tap water as the test solution is that, unlike distilled water, it effectively simulates the presence of Cl−, Na+, Ca, and other elements that may be present in the moisture condensing on the surfaces of steel produced in manufacturing plants located near coastal areas. After drying in air, the cross-sections of the samples were examined on days one, three, and seven of the testing periods. The corrosion product formed on the surface after seven days of testing was additionally characterized through X-ray diffraction (XRD, Bruker type diffractometer using a Cu anode) (New D8-Advance, Bruker, Ettlingen, Germany) with a thin film mode.
In a separately conducted simulated temperature–humidity exposure test, the conditions under which the specimens were exposed were categorized into four groups: summer–dry season (SD), summer–rainy season (SR), winter–dry season (WD), and winter–rainy season (WR). Summer and winter corresponded to the periods from June to July and December to January, respectively. The dry season was defined as the midpoint of a seven-day period without rain, while the rainy season was set as the midpoint of a five-day period with rainfall. Detailed variations in temperature and humidity within each group were based on data from the province of Jeonnam provided by the Meteorological Administration in South Korea, as shown in
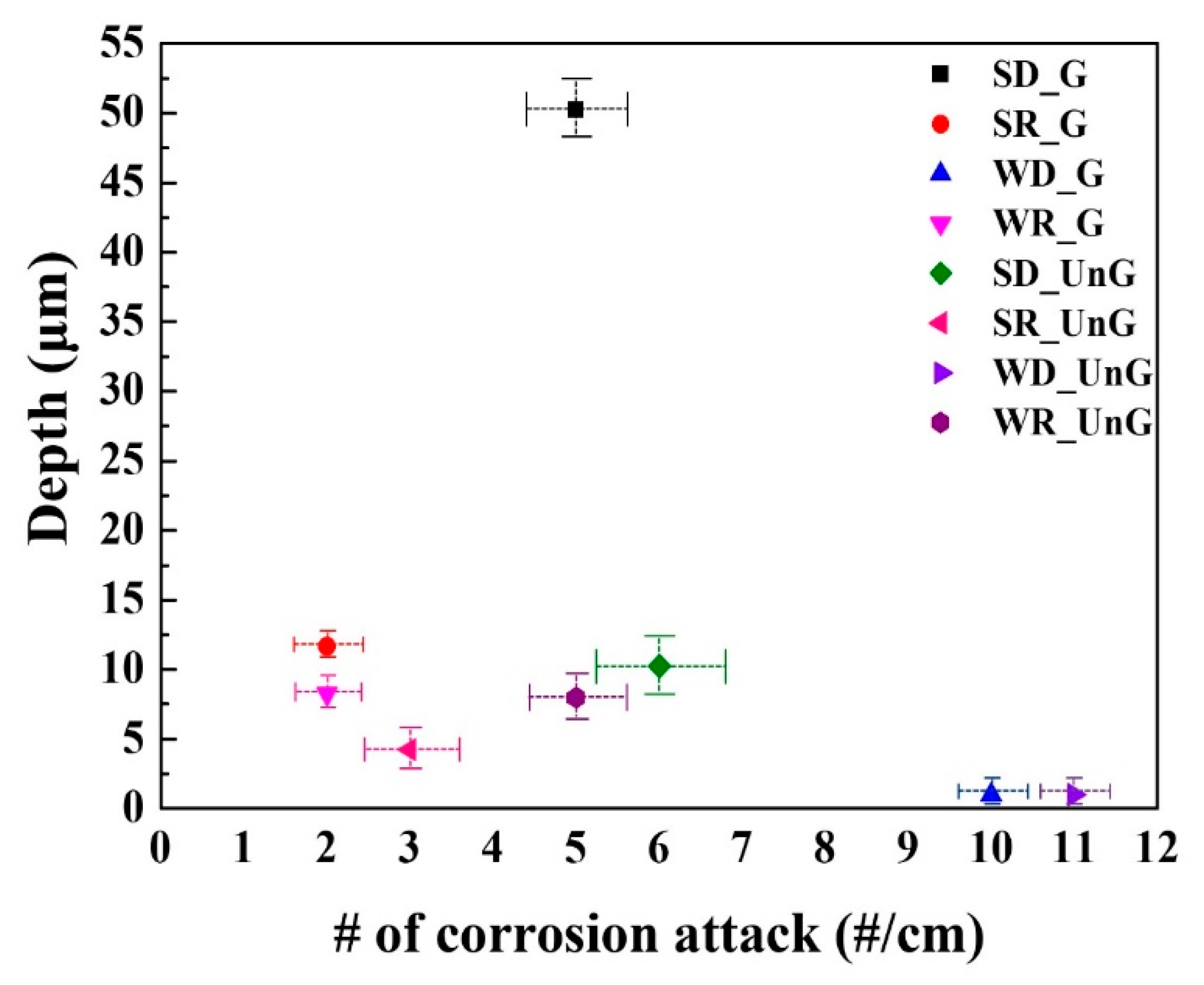
Supplementary Figure S1. These temperature–humidity exposures were conducted in a constant temperature and humidity chamber (TH-150U, Seoul, Republic of Korea). The number of corrosion-induced attacks per unit centimeter and the average depth of the attacks were determined by taking the average values from measurements at 10 different locations observed in the cross-sections of the corroded samples.
For a more detailed mechanistic understanding of the effect of fine precipitates (carbides) within the microstructure of the ground sample on corrosion behavior, additional annealing was conducted by heating to 1100 °C for 10 min using Gleeble simulator. This was followed by cooling with two different cooling rates (5 °C/s and 200 °C/s), resulting in two samples with different fractions of carbide precipitation. Typically, 200 °C/s corresponds to a sufficiently rapid cooling rate that can suppress the precipitation of carbides (e.g., when quenching the steel sample in water), while 5 °C/s represents a sufficiently slow cooling rate that allows for carbide precipitation (e.g., during natural cooling in air). Their corrosion behaviors were examined electrochemically using a potentiostat (Reference 600, Gamry, Warminster, PA, USA), and the corrosion parameters of corrosion current density (icorr) and polarization resistance (Rp) were determined through the linear polarization resistance (LPR) test and electrochemical impedance spectroscopy (EIS) test, respectively. For these electrochemical measurements, a three-electrode system consisting of working (WE), counter (CE), and reference (RE) electrodes was employed. A Pt grid and a saturated calomel electrode (SCE) served as the CE and RE, respectively. Prior to the experiments, the samples serving as the WE were polished with a 2000-grit sand paper and ultrasonically cleaned in ethanol. The LPR test was performed by polarizing the samples from −20 to 20 mV vs. OCP at a scan rate of 0.2 mV/s in a 3.5% NaCl solution for 3 d, and potential–current density plots in semi-log format were obtained. The EIS test was conducted over the frequency range from 100 kHz to 10 mHz, applying an amplitude of ±10 mV AC potential vs. the OCP, and Nyquist plots were obtained. To ensure the reproducibility and repeatability of the electrochemical measurements, each type of test was repeated three times.
4. Discussion
The grinding heat and severe plastic deformation, generated by the abrasive grinding process for this high-Mn–low-Cr based cast steel resulted in the formation of a surface deformed region composed of a grain refinement layer and multiple metal streamlines. This grain refining can be interpreted as a result of dynamic recrystallization occurring under thermal–mechanical coupling conditions. The size of recrystallized grains is dependent upon the nucleation rate, which can be described as a function of strain rate and temperature, according to nucleation model proposed by Ding et al. [
19]. This can be expressed as:
where
,
C,
m,
Qact,
R, and
T denote the nucleation rate, stain rate, rate constant [
20], exponent, activation energy, gas constant, and temperature, respectively.
Hence, the fact that the recrystallized grains become much finer when the grinding process is conducted on the sample right after it reaches a higher temperature (600 °C) during the cooling process after casting can be understood (refer to the EBSD analysis in
Supplementary Figure S3). It is considered that the fine-grain recrystallized layer contributes positively to other mechanical properties, such as strength, toughness, and fatigue resistance [
21,
22]. From an electrochemical perspective, however, fine grains with a high density of grain boundaries can have higher chemical reactivity and a higher local anodic dissolution rate than larger ones [
23,
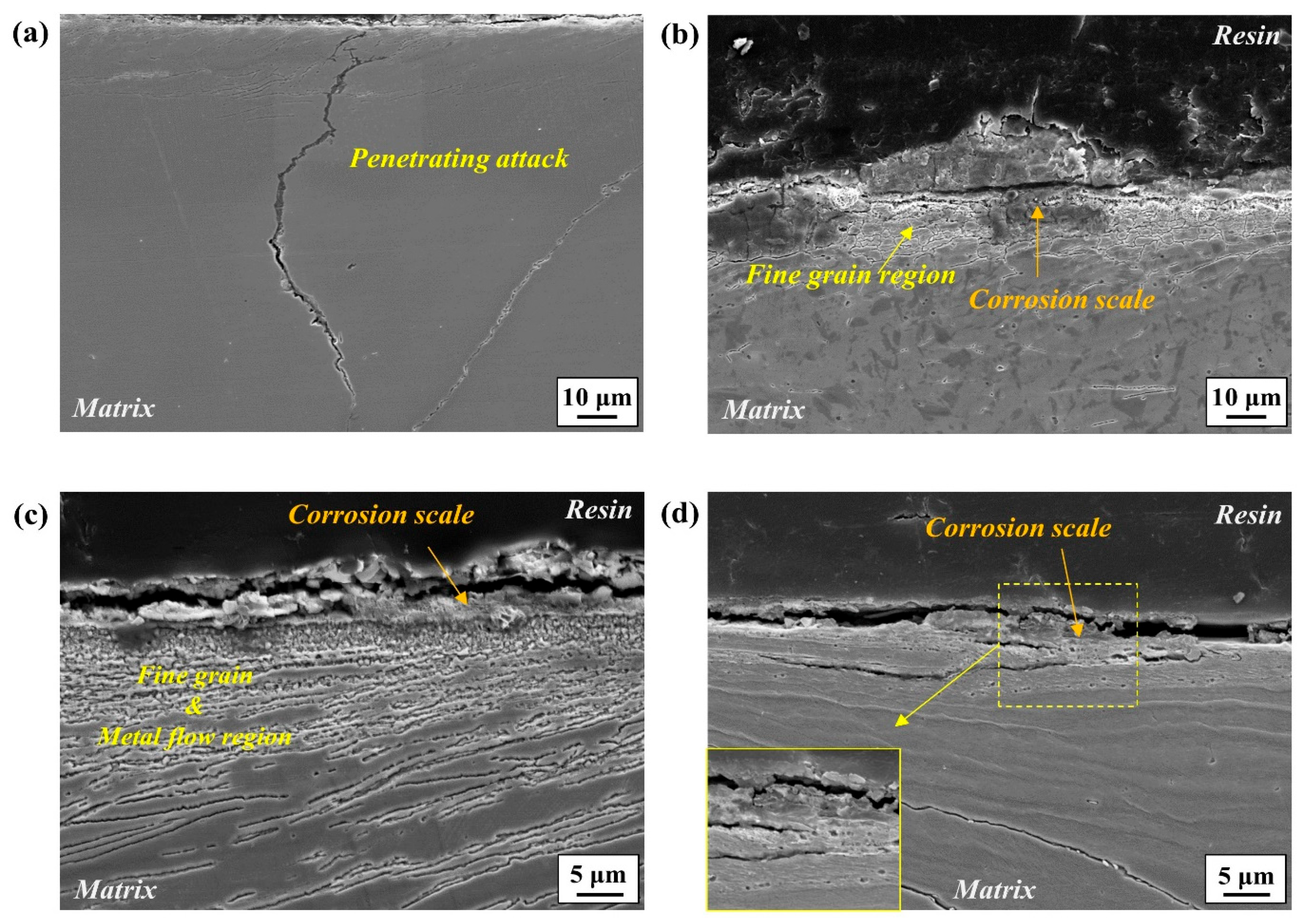
24]. In light of this, it is expected that the early stages of corrosion occur preferentially along the boundaries of recrystallized fine grains, as noticed in
Figure 7a and
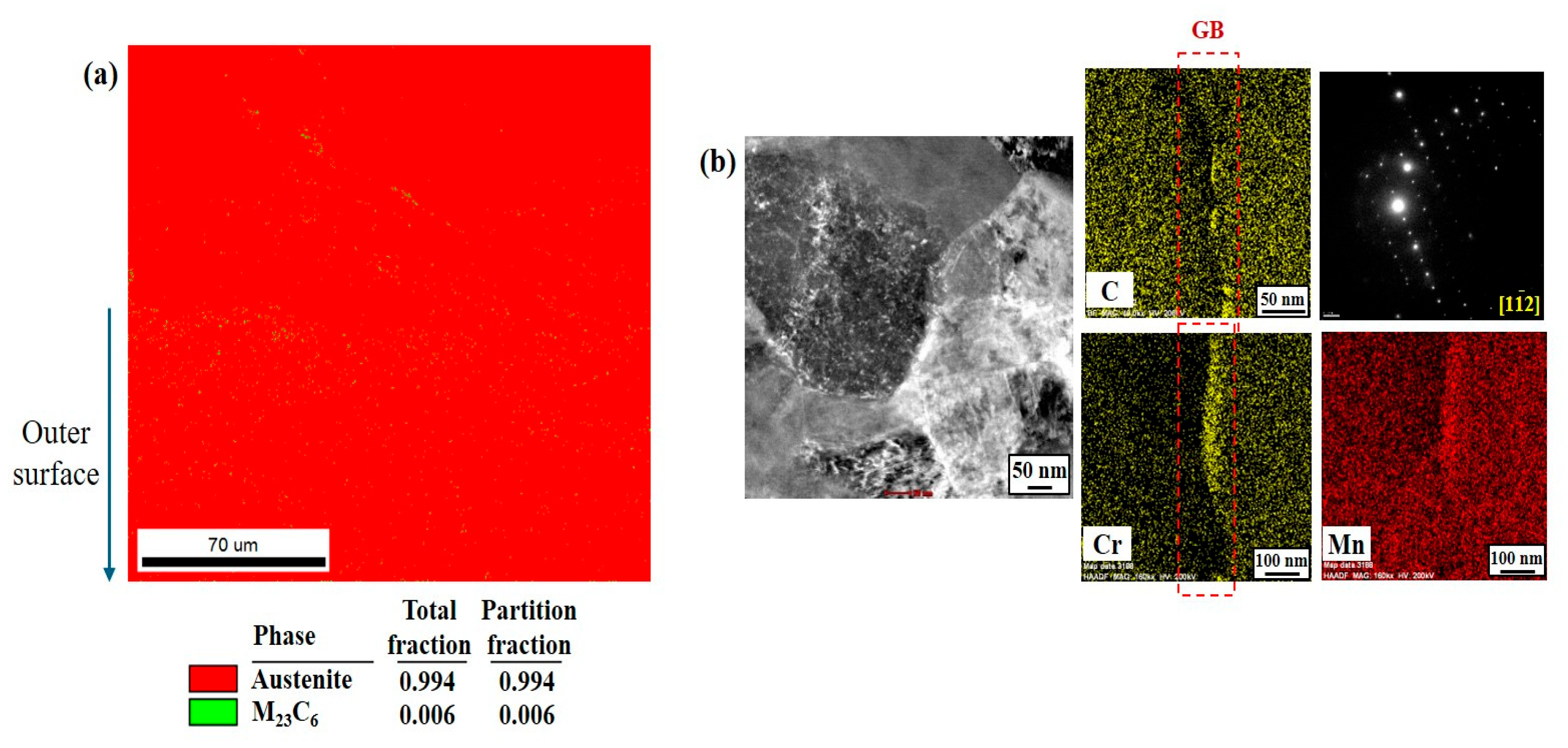
Figure 8a,d. However, the susceptibility of boundaries to corrosion may vary depending greatly on their physical and chemical characteristics. Among these characteristics, a major feature in this high-Mn–low-Cr cast steel alloy could be the precipitation of alloy carbides, mostly along the boundary areas. From a thermodynamic point of view, the addition of Cr to high-Mn steel favors the precipitation of M
23C
6 [
11]. In the present cast alloy system, the alloy carbides were characterized as Cr(Mn)
23C
6 precipitated along the boundaries. According to Xu et al. [
25], the M
23C
6 particle has a coherent interface and a cube–cube orientation relationship with the austenite grain from which it precipitated. This carbide can play a significant role in aggravating corrosion resistance from two aspects. Firstly, it serves as an electrochemical cathode when exposed to aqueous media, leading to higher anodic dissolution from the matrix due to microgalvanic action. Secondly, its precipitation causes the local depletion of Cr, resulting in corrosion propagation in a localized form, as observed clearly in
Figure 8f. The annealed and FC sample with a higher density of the carbide exhibited a higher
icorr, and lower
Ecorr and
Rp can also be understood in this aspect. Specifically, the
Ecorr values for the FC and WQ samples are −0.686 V
SCE and −0.652 V
SCE, respectively, indicating a slightly lower value for the FC sample. Although the difference in
Ecorr is minor, the
Rp values of 2550 Ω·cm
2 and 3120 Ω·cm
2 reveal a more noticeable contrast. The
icorr values, which are inversely related to
Rp and commonly indicative of corrosion kinetics, are 8.18 μA cm
−2 and 2.5 μA cm
−2, with the FC sample exhibiting a value over three times higher. This suggests that the carbides (Cr(Mn)
23C
6) precipitated within the microstructure of the FC sample play a significant role in reducing corrosion resistance. This is further supported by the
Rct values, which reflect the resistance to charge transfer reactions at the interface, derived from the EIS experiments (2150 Ω·cm
2 for FC and 3820 Ω·cm
2 for WQ), demonstrating an inverse relationship with
icorr. Additional parameters obtained from the electrochemical tests can be found in
Table 1 and
Table 2. Although this electrochemical behavior is not directly indicative of the actual corrosion process, the fact that a higher precipitation of Cr(Mn)
23C
6 negatively affected the corrosion resistance of the steel sample can at least be accepted.
The metal streamlines formed beneath the recrystallized grains can also affect the corrosion behavior: they serve as a propagation path of corrosion attacks (
Figure 7c and
Figure 8c), due mainly to the high strain energy leading to a high density of dislocations. This can be supported in part by the higher kernel average misorientation (KAM) in the metal streamline areas, as shown in
Supplementary Figure S3c. In general, highly deformed areas with high strain energy and metallurgical defects in metallic materials have higher exchange current densities for anodic and cathodic reactions by lowering the free energy [
26,
27,
28], which is expressed as:
where
,
A, and
are exchange current density, Arrhenius pre-exponential factor, and energy barrier (activation energy), respectively.
Nevertheless, there may be uncertainty regarding why only localized areas are preferentially attacked in this alloy without forming a passive layer. For a precise understanding, the corrosion initiation site on the outer surface was observed, revealing that corrosion was initiated in the valley region where the surface was highly ground, as shown in
Figure 12.
This valley could also be the region where a small amount of moisture can persist on the surface for longer times. Here, the hydrolysis reaction of Fe(Mn) can be facilitated, making it conducive to the reduction in local pH during the corrosion process [
29,
30], as described below:
Moreover, the formation of corrosion scale (Fe-based hydroxides) could be facilitated within a thin water film due to the extremely low solubility (solubility product ~ 10
−38) [
31]. Considering the higher volumetric expansion ratio of Fe-based oxyhydroxides in comparison with steel [
32], the expansive force can create spaces on the steel surface, causing capillary action that facilitates the easy penetration of corrosive agents, including oxygen and moisture. Hence, the local propagating form of corrosion attacks with the rapid formation of corrosion scale in an initial stage can be understood. This was mechanistically represented in
Supplementary Figure S4. To the best of our knowledge, this is the first experimental study to report the corrosion-assisted penetrating attack on ground high-Mn–low-Cr casting steel in humid environments, in contrast to the uniform corrosion behaviors typically exhibited by conventional low-alloyed steels.
Although this type of corrosion attack could occur more severely and frequently during the summer season, relative humidity is the most critical factor. It is worth noting that the highest severity occurs under relative humidity levels between 60% and 80%, rather than 100%. This can be interpreted in association with variations in the ease of oxygen reduction reactions (Equation (4)) on the steel surface depending on relative humidity. In contrast to conditions where relative humidity levels are around 100%, forming a bulk water layer on the steel surface, a relative humidity of approximately 60~70% allows a thin water film to form. Under these conditions, oxygen access to the steel surface becomes much easier [
33], leading to more reduction reactions and overall corrosion attacks. This promotes the formation of corrosion products with a high volumetric expansion ratio, while capillary action further facilitates the penetration of corrosive agents, as discussed above. In spite of the formation of a thin water film under such humidity levels, the corrosion attacks can be limited under winter conditions of low temperature due to the lower diffusion kinetics of oxygen to the steel surface.
Based on the results, several technical strategies are recommended to mitigate corrosion-induced penetrating attacks on ground high-Mn–low-Cr casting steel slabs. These strategies include maintaining uniform surface roughness during the grinding process, controlling temperature and humidity (specifically, keeping relative humidity from 60% to 80% rather than 100%), and applying effective surface treatments. From this perspective, these findings offer valuable insights into the industrial applicability of high-Mn–low-Cr steels by significantly reducing surface damage (e.g., corrosion-induced penetrating attack).
5. Conclusions
This study investigated the metallurgical and environmental factors that cause corrosion-induced penetrating attacks on high-Mn–low-Cr casting steel subjected to surface grinding. Through various experimental and analytical tests, the underlying mechanism of this type of corrosion damage was interpreted. The main findings are summarized as follows.
The abrasive grinding process for this high-Mn–low-Cr based cast steel resulted in the formation of a surface deformed region composed of a grain refinement layer and multiple metal streamlines. Moreover, Cr(Mn)23C6 particles were densely precipitated at the boundary areas (recrystallized fine grain boundaries, metal streamlines, and coarse grain boundaries).
The early stages of corrosion occur preferentially along the boundaries of recrystallized fine grains, and the corrosion scale, characterized as Fe-based oxyhydroxides, was formed. With prolonged exposure to a corrosive environment, the fine-grain areas were gradually detached, and additional corrosion damage occurred along the metal streamlines located beneath them. The primary propagation path of further corrosion attacks was the boundary where Cr(Mn)23C6 particles were precipitated.
Macroscopically, the corrosion was initiated in the valley region where the surface was highly ground, facilitating the formation of corrosion scale (Fe-based oxyhydroxides) within a remaining thin water film. The expansive force caused by the higher volumetric expansion ratio of the corrosion scale detaches the surface deformed regions, enabling the easy penetration of corrosive media.
The local propagating form of corrosion attacks occurs more severely and frequently during the summer–dry (SD) season (i.e., relative humidity levels around 60% to 80%, rather than 100%) when a thin water film can form on the steel surface. The higher reduction rate based on oxygen accessibility to the surface during the SD season makes the steel highly susceptible to corrosion damage in humid environments.
It is recommended that technical strategies—including maintaining uniform surface roughness during the grinding process, controlling temperature and humidity (specifically, keeping relative humidity from 60% to 80% rather than 100%), and applying effective surface treatments—be implemented to mitigate corrosion-induced penetrating attacks on high-Mn–low-Cr casting steel slabs.