Oxidation Behavior of Nanocrystalline Alloys
Abstract
1. Introduction
- 1
- An initial and transient stage wherein scales of the most thermodynamically stable oxide under given conditions are established;
- 2
- The steady-state growth stage is wherein the established oxide scale grows in the normal direction to the alloy surface;
- 3
- Breakaway oxidation, where the reservoir of the primarily oxidating element facilitating the TGO is depleted.
- 1
- Adsorption of oxygen gas to the alloy surface;
- 2
- Nucleation of numerous individual protective oxide islands through rapid selective oxidation of the favored element;
- 3
- Lateral growth of protective oxide nuclei to form a coalesced, continuous layer;
- 4
- Thickening of a growing oxide layer and subsequent steady-state growth stage;
- 5
- Spalling and exfoliation of the TGO layer amid breakaway oxidation or thermally cyclic effects accelerating depletion of the primary oxidating element.
2. Influence of Nanocrystallization on Oxidation Behavior
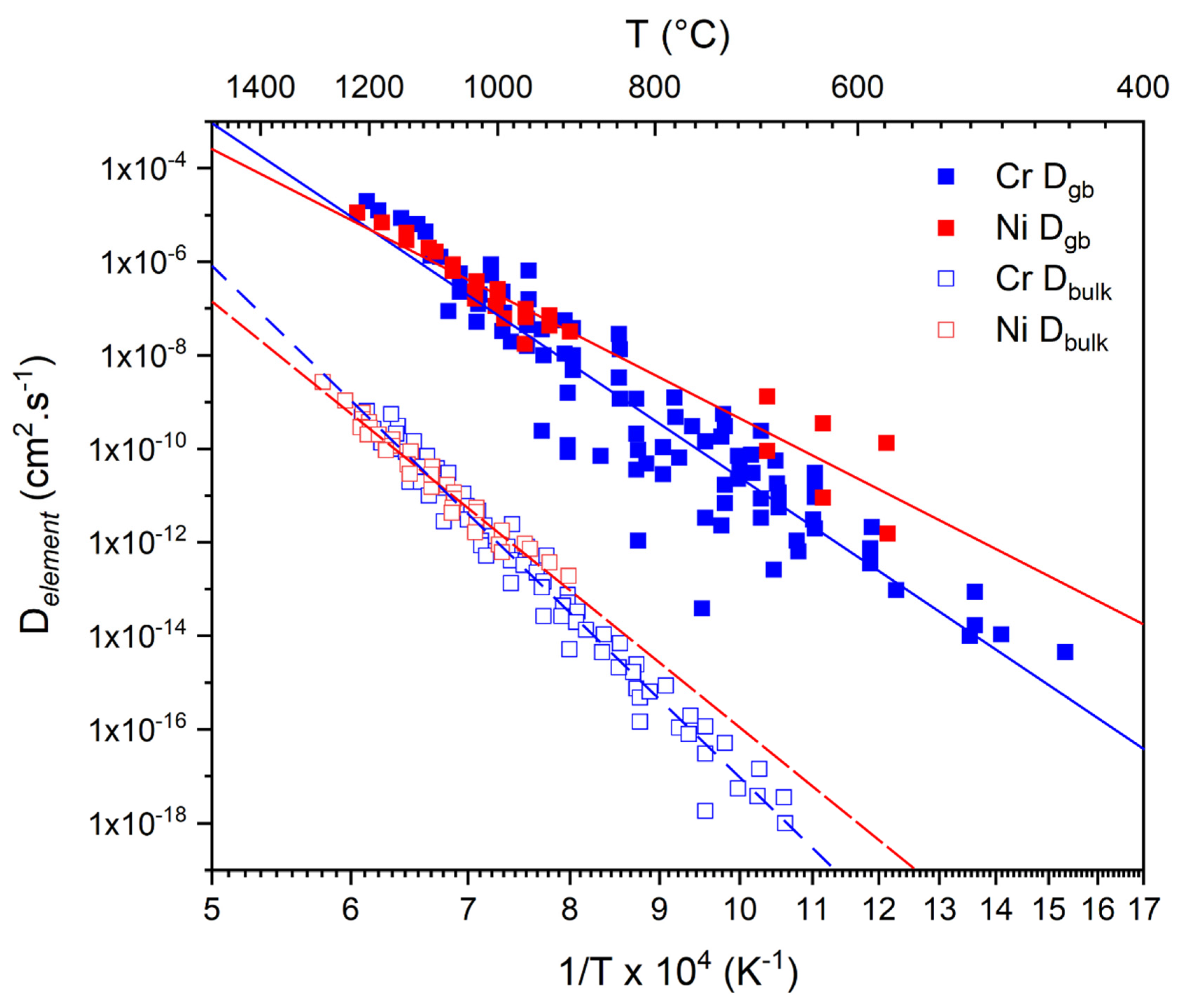
2.1. Bulk vs. GB Diffusion in NC Alloys
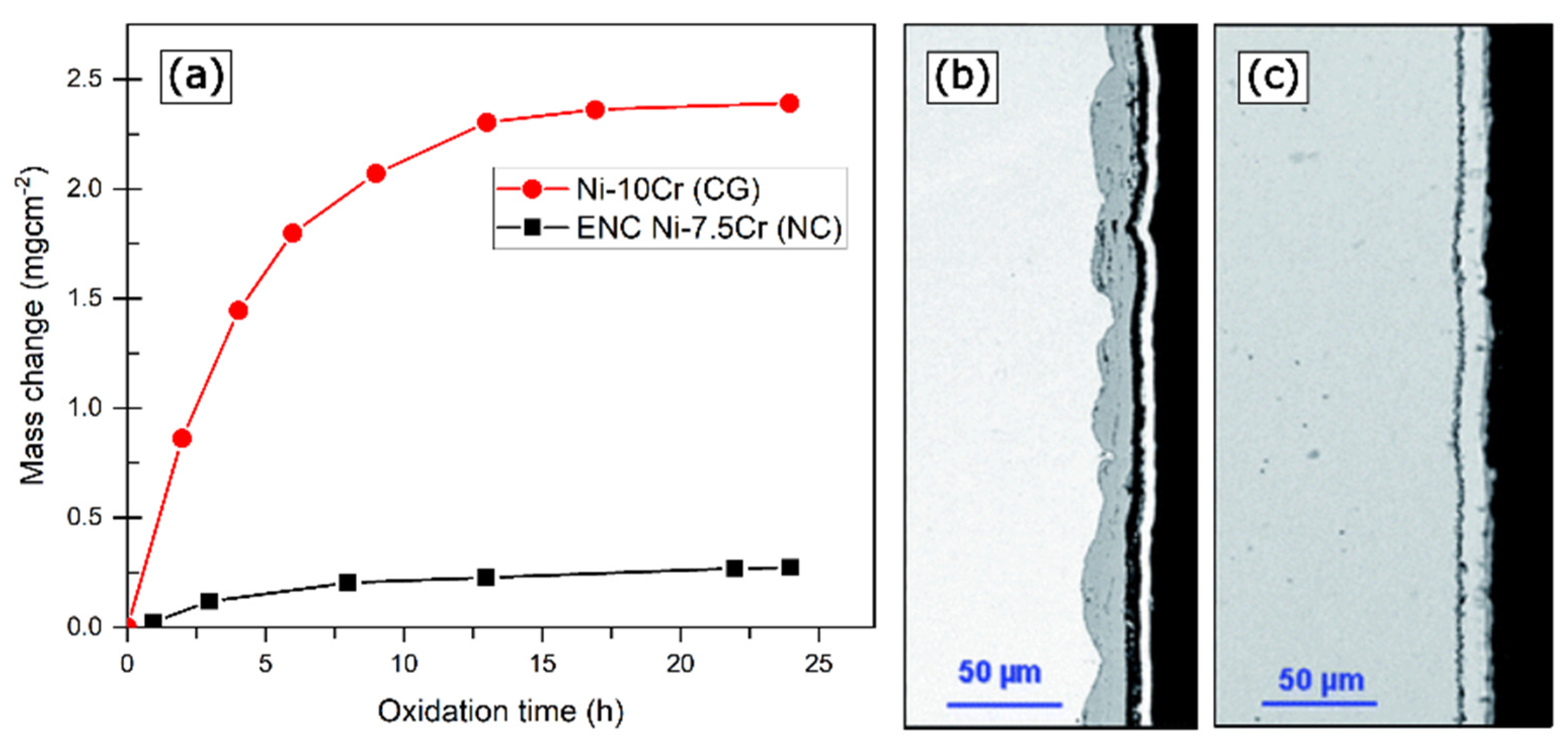
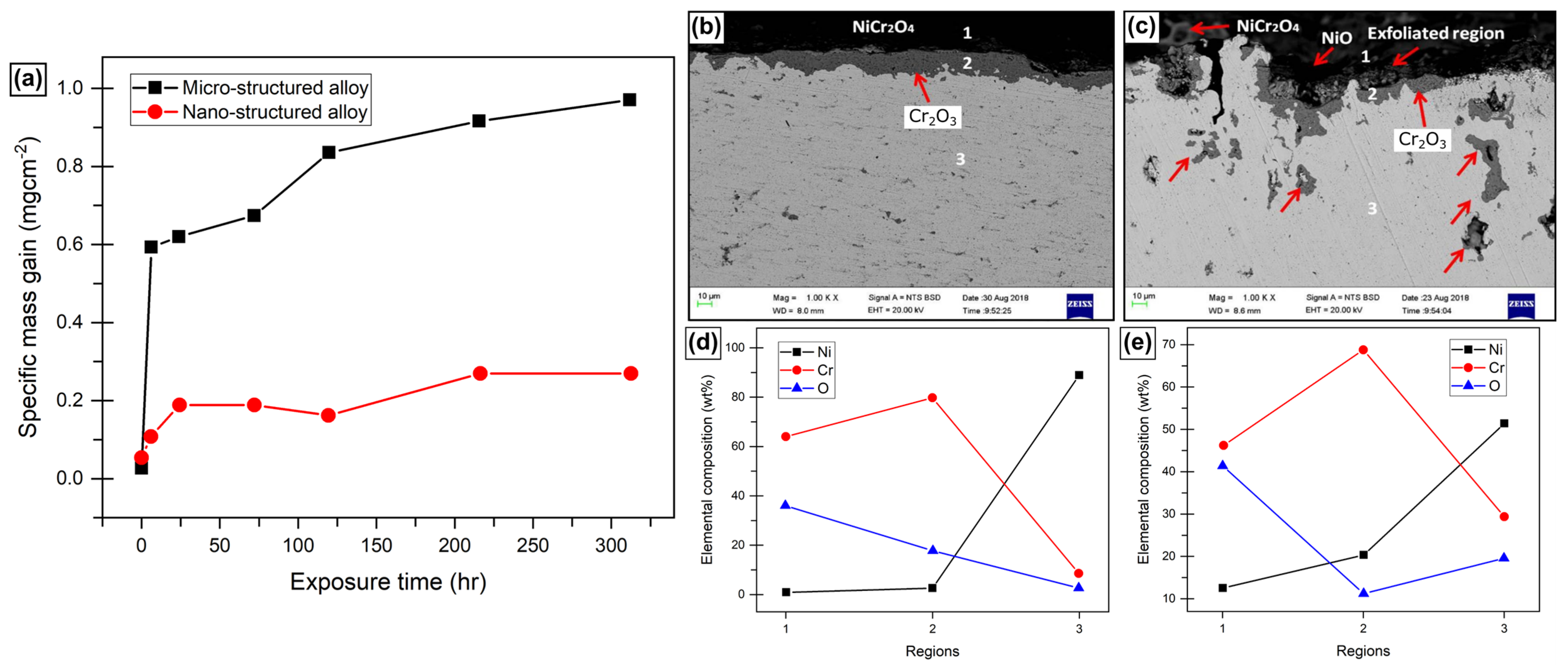
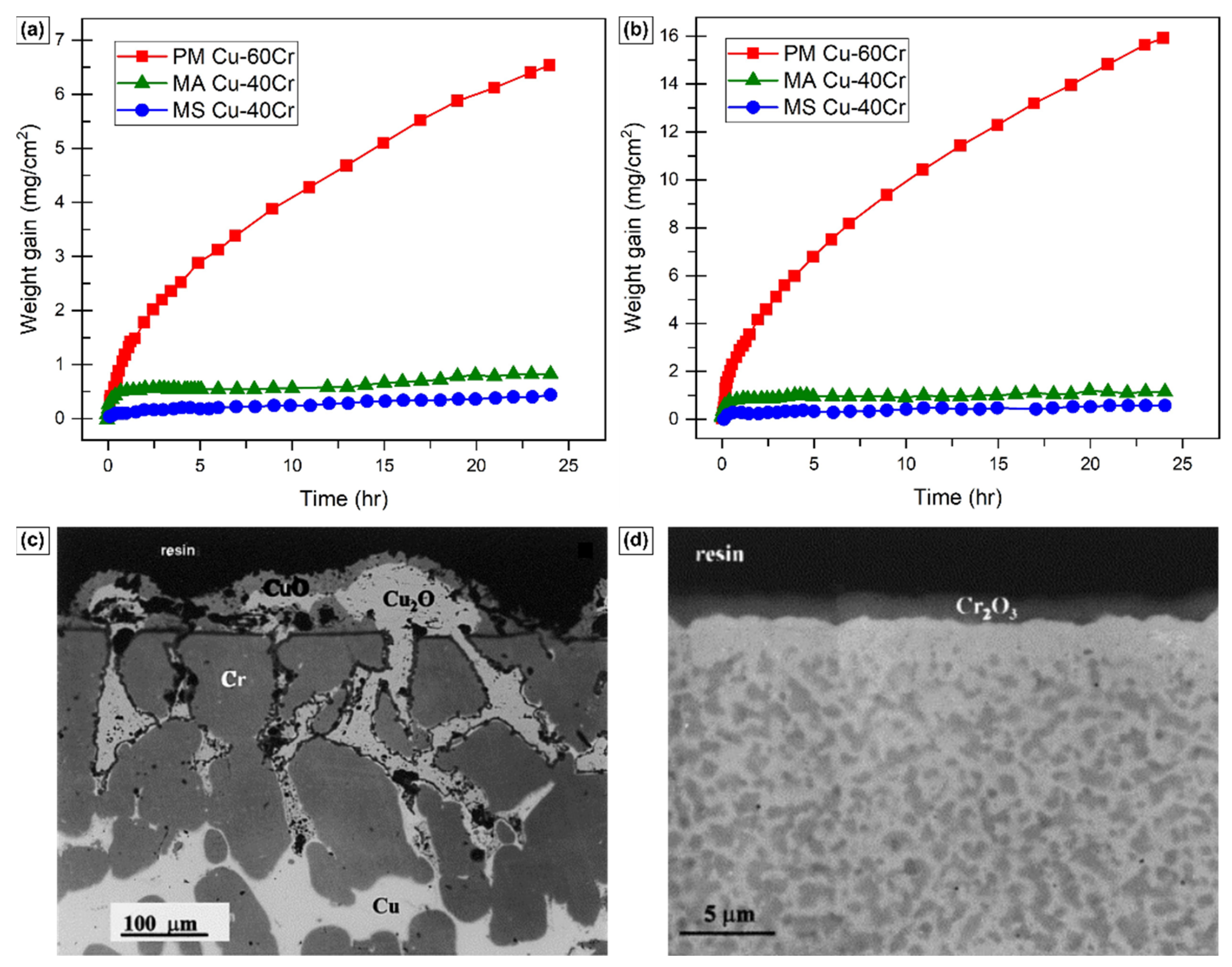
2.2. Effect on Oxidative Kinetics
3. Influence of Alloy Chemistry on Oxidation Behavior
3.1. Third Element Effect
3.2. Reactive Element Effect
3.3. Effect of Growth Stresses
4. Perspective
4.1. Micro-Structurally Stable Bulk NC Alloys
4.2. Surface Mechanical Attrition Treatment (SMAT)
5. Summary
Funding
Conflicts of Interest
References
- Birringer, R.; Gleiter, H.; Klein, H.-P.; Marquardt, P. Nanocrystalline Materials an Approach to a Novel Solid Structure with Gas-like Disorder? Phys. Lett. A 1984, 102, 365–369. [Google Scholar] [CrossRef]
- Mütschele, T.; Kirchheim, R. Hydrogen as a Probe for the Average Thickness of a Grain Boundary. Scr. Metall. 1987, 21, 1101–1104. [Google Scholar] [CrossRef]
- Rupert, T.J. The Role of Complexions in Metallic Nano-Grain Stability and Deformation. Curr. Opin. Solid State Mater. Sci. 2016, 20, 257–267. [Google Scholar] [CrossRef]
- Grigorian, C.M.; Rupert, T.J. Thick Amorphous Complexion Formation and Extreme Thermal Stability in Ternary Nanocrystalline Cu-Zr-Hf Alloys. Acta Mater. 2019, 179, 172–182. [Google Scholar] [CrossRef]
- Schuh, C.A.; Lu, K. Stability of Nanocrystalline Metals: The Role of Grain-Boundary Chemistry and Structure. MRS Bull. 2021, 46, 225–235. [Google Scholar] [CrossRef]
- Mishin, Y.; Asta, M.; Li, J. Atomistic Modeling of Interfaces and Their Impact on Microstructure and Properties. Acta Mater. 2010, 58, 1117–1151. [Google Scholar] [CrossRef]
- Armstrong, R.W. 60 Years of Hall-Petch: Past to Present Nano-Scale Connections. Mater. Trans. 2014, 55, 2–12. [Google Scholar] [CrossRef]
- Kale, C.; Turnage, S.; Garg, P.; Adlakha, I.; Srinivasan, S.; Hornbuckle, B.C.; Darling, K.; Solanki, K.N. Thermo-Mechanical Strengthening Mechanisms in a Stable Nanocrystalline Binary Alloy—A Combined Experimental and Modeling Study. Mater. Des. 2019, 163, 107551. [Google Scholar] [CrossRef]
- Kale, C.; Srinivasan, S.; Hornbuckle, B.C.; Koju, R.K.; Darling, K.; Mishin, Y.; Solanki, K.N. An Experimental and Modeling Investigation of Tensile Creep Resistance of a Stable Nanocrystalline Alloy. Acta Mater. 2020, 199, 141–154. [Google Scholar] [CrossRef]
- Kale, C.; Srinivasan, S.; Sharma, S.; Hornbuckle, B.C.; Koju, R.K.; Grendahl, S.; Darling, K.; Mishin, Y.; Solanki, K. Exceptional Fatigue Strength of a Microstructurally Stable Bulk Nanocrystalline Alloy. Acta Mater. 2023, 255, 119049. [Google Scholar] [CrossRef]
- Chookajorn, T.; Murdoch, H.A.; Schuh, C.A. Design of Stable Nanocrystalline Alloys. Science 2012, 337, 951–954. [Google Scholar] [CrossRef]
- Van Swygenhoven, H.; Weertman, J.R. Deformation in Nanocrystalline Metals. Mater. Today 2006, 9, 24–31. [Google Scholar] [CrossRef]
- Darling, K.A.; Rajagopalan, M.; Komarasamy, M.; Bhatia, M.A.; Hornbuckle, B.C.; Mishra, R.S.; Solanki, K.N. Extreme Creep Resistance in a Microstructurally Stable Nanocrystalline Alloy. Nature 2016, 537, 378–381. [Google Scholar] [CrossRef]
- Koch, C.C. Structural Nanocrystalline Materials: An Overview. J. Mater. Sci. 2007, 42, 1403–1414. [Google Scholar] [CrossRef]
- Turnage, S.A.; Rajagopalan, M.; Darling, K.A.; Garg, P.; Kale, C.; Bazehhour, B.G.; Adlakha, I.; Hornbuckle, B.C.; Williams, C.L.; Peralta, P.; et al. Anomalous Mechanical Behavior of Nanocrystalline Binary Alloys under Extreme Conditions. Nat. Commun. 2018, 9, 2699. [Google Scholar] [CrossRef]
- Rajagopalan, M.; Darling, K.A.; Kale, C.; Turnage, S.A.; Koju, R.K.; Hornbuckle, B.C.; Mishin, Y.; Solanki, K.N. Nanotechnology Enabled Design of a Structural Material with Extreme Strength as Well as Thermal and Electrical Properties. Mater. Today 2019, 31, 10–20. [Google Scholar] [CrossRef]
- Srinivasan, S.; Sharma, S.; Turnage, S.; Hornbuckle, B.C.; Kale, C.; Darling, K.A.; Solanki, K. Role of Tantalum Concentration, Processing Temperature, and Strain-Rate on the Mechanical Behavior of Copper-Tantalum Alloys. Acta Mater. 2021, 208, 116706. [Google Scholar] [CrossRef]
- Lu, L.; Wang, L.B.; Ding, B.Z.; Lu, K. High-Tensile Ductility in Nanocrystalline Copper. J. Mater. Res. 2000, 15, 270–273. [Google Scholar] [CrossRef]
- Lu, L.; Sui, M.L.; Lu, K. Superplastic Extensibility of Nanocrystalline Copper at Room Temperature. Science 2000, 287, 1463–1466. [Google Scholar] [CrossRef]
- Gialanella, S.; Marino, F. Effect of Microstructure on Thermal Expansion Behaviour of Nanocrystalline Metallic Materials. J. Mater. Sci. 2010, 45, 824–830. [Google Scholar] [CrossRef]
- Sui, M.L.; Lu, K. Thermal Expansion Behavior of Nanocrystalline Ni P Alloys of Different Grain Sizes. Nanostruct. Mater. 1995, 6, 651–654. [Google Scholar] [CrossRef]
- Trelewicz, J.R.; Schuh, C.A. Grain Boundary Segregation and Thermodynamically Stable Binary Nanocrystalline Alloys. Phys. Rev. B 2009, 79, 094112. [Google Scholar] [CrossRef]
- Kalidindi, A.R.; Schuh, C.A. Stability Criteria for Nanocrystalline Alloys. Acta Mater. 2017, 132, 128–137. [Google Scholar] [CrossRef]
- Saber, M.; Kotan, H.; Koch, C.C.; Scattergood, R.O. Thermodynamic Stabilization of Nanocrystalline Binary Alloys. J. Appl. Phys. 2013, 113, 063515. [Google Scholar] [CrossRef]
- Chookajorn, T.; Schuh, C.A. Thermodynamics of Stable Nanocrystalline Alloys: A Monte Carlo Analysis. Phys. Rev. B 2014, 89, 064102. [Google Scholar] [CrossRef]
- Würschum, R.; Herth, S.; Brossmann, U. Diffusion in Nanocrystalline Metals and Alloys—A Status Report. Adv. Eng. Mater. 2003, 5, 365–372. [Google Scholar] [CrossRef]
- Chattopadhyay, P.P.; Pabi, S.K.; Manna, I. On the Enhancement of Diffusion Kinetics in Nanocrystalline Materials. Mater. Chem. Phys. 2001, 68, 80–84. [Google Scholar] [CrossRef]
- Kaszkur, Z.; Juszczyk, W.; Łomot, D. Self-Diffusion in Nanocrystalline Alloys. Phys. Chem. Chem. Phys. 2015, 17, 28250–28255. [Google Scholar] [CrossRef]
- Gil Sevillano, J.; Aldazabal, J. Ductilization of Nanocrystalline Materials for Structural Applications. Scr. Mater. 2004, 51, 795–800. [Google Scholar] [CrossRef]
- Weston, D.P.; Harris, S.J.; Capel, H.; Ahmed, N.; Shipway, P.H.; Yellup, J.M. Nanostructured Co–W Coatings Produced by Electrodeposition to Replace Hard Cr on Aerospace Components. Trans. IMF 2010, 88, 47–56. [Google Scholar] [CrossRef]
- Schuh, C.A.; Nieh, T.G.; Yamasaki, T. Hall–Petch Breakdown Manifested in Abrasive Wear Resistance of Nanocrystalline Nickel. Scr. Mater. 2002, 46, 735–740. [Google Scholar] [CrossRef]
- Inoue, A. Bulk Amorphous and Nanocrystalline Alloys with High Functional Properties. Mater. Sci. Eng. A 2001, 304–306, 1–10. [Google Scholar] [CrossRef]
- Wei, Y.; Zheng, S.; Pu, J.; Zhou, D.; Wang, L.; Guo, W.; He, G. Effect of Aluminum Concentration on Microstructure and Evolution Behavior of Oxide Layer on NiAlSiY Coating at 500 °C. Corros. Sci. 2020, 165, 108400. [Google Scholar] [CrossRef]
- Anghel, E.M.; Marcu, M.; Banu, A.; Atkinson, I.; Paraschiv, A.; Petrescu, S. Microstructure and Oxidation Resistance of a NiCrAlY/Al2O3-Sprayed Coating on Ti-19Al-10Nb-V Alloy. Ceram. Int. 2016, 42, 12148–12155. [Google Scholar] [CrossRef]
- Reuban, A.; Povstugar, I.; Rasiński, M.; Vayyala, A.; Litnovsky, A.; Coenen, J.W.; Linsmeier, C.; Guillon, O.; Gonzalez-Julian, J. Unveiling the Diffusion Pathways under High-Temperature Oxidation of Cr2AlC MAX Phase via Nanoscale Analysis. Corros. Sci. 2024, 235, 112179. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, Z.; Lin, J.; Zhou, Z. A Study on High Temperature Oxidation Behavior of High-Velocity Arc Sprayed Fe-Based Coatings. Surf. Coat. Technol. 2015, 283, 255–261. [Google Scholar] [CrossRef]
- Gupta, M.; Markocsan, N.; Li, X.-H.; Kjellman, B. Development of Bondcoats for High Lifetime Suspension Plasma Sprayed Thermal Barrier Coatings. Surf. Coat. Technol. 2019, 371, 366–377. [Google Scholar] [CrossRef]
- Hejrani, E.; Sebold, D.; Nowak, W.J.; Mauer, G.; Naumenko, D.; Vaßen, R.; Quadakkers, W.J. Isothermal and Cyclic Oxidation Behavior of Free Standing MCrAlY Coatings Manufactured by High-Velocity Atmospheric Plasma Spraying. Surf. Coat. Technol. 2017, 313, 191–201. [Google Scholar] [CrossRef]
- Muñoz Saldaña, J.; Schulz, U.; Mondragón Rodríguez, G.C.; Caceres-Diaz, L.A.; Lau, H. Microstructure and Lifetime of Hf or Zr Doped Sputtered NiAlCr Bond Coat/7YSZ EB-PVD TBC Systems. Surf. Coat. Technol. 2018, 335, 41–51. [Google Scholar] [CrossRef]
- Gao, J.; Tang, Z.; Wang, C.; Guo, M.; Cui, Y. Microstructure, Mechanical and Oxidation Characteristics of Detonation Gun and HVOF Sprayed MCrAlYX Coatings. Trans. Nonferrous Met. Soc. China 2015, 25, 817–823. [Google Scholar] [CrossRef]
- Stephenson, G.B. Deformation during Interdiffusion. Acta Metall. 1988, 36, 2663–2683. [Google Scholar] [CrossRef]
- El Kadiri, H.; Horstemeyer, M.F.; Bammann, D.J. A Theory for Stress-Driven Interfacial Damage upon Cationic-Selective Oxidation of Alloys. J. Mech. Phys. Solids 2008, 56, 3392–3415. [Google Scholar] [CrossRef]
- Suo, Z.; Kubair, D.V.; Evans, A.G.; Clarke, D.; Tolpygo, V.K. Stresses Induced in Alloys by Selective Oxidation. Acta Mater. 2003, 51, 959–974. [Google Scholar] [CrossRef]
- Suzuki, A.; Mishin, Y. Diffusion Mechanisms in Grain Boundaries. J. Metastable Nanocryst. Mater. 2004, 19, 1–24. [Google Scholar] [CrossRef]
- Huang, X.; Martinelli, L.; Bosonnet, S.; Fossati, P.C.M.; Latu-Romain, L.; Wouters, Y. Chromium Depletion in a Ni-30Cr Alloy During High-Temperature Oxidation. High Temp. Corros. Mater. 2023, 100, 745–773. [Google Scholar] [CrossRef]
- Peng, X. Nanoscale Assembly of High-Temperature Oxidation-Resistant Nanocomposites. Nanoscale 2010, 2, 262–268. [Google Scholar] [CrossRef]
- Babalola, B.J.; Shongwe, M.B.; Maledi, N.; Jeje, S.O.; Ayodele, O.O.; Lawan, A.R.; Olubambi, P.A. Effect of Nanocrystalline Nickel Powder and Co, Mo, Ta, and Al Additions on Isothermal Oxidation Behavior of Ni–17Cr Alloy. Metallogr. Microstruct. Anal. 2020, 9, 75–85. [Google Scholar] [CrossRef]
- Viskari, L.; Hörnqvist, M.; Moore, K.L.; Cao, Y.; Stiller, K. Intergranular Crack Tip Oxidation in a Ni-Base Superalloy. Acta Mater. 2013, 61, 3630–3639. [Google Scholar] [CrossRef]
- Viskari, L.; Johansson, S.; Stiller, K. Oxygen Influenced Intergranular Crack Propagation: Analysing Microstructure and Chemistry in the Crack Tip Region. Mater. High Temp. 2011, 28, 336–341. [Google Scholar] [CrossRef]
- Ramsay, J.D.; Evans, H.E.; Child, D.J.; Taylor, M.P.; Hardy, M.C. The Influence of Stress on the Oxidation of a Ni-Based Superalloy. Corros. Sci. 2019, 154, 277–285. [Google Scholar] [CrossRef]
- Fu, G.; Niu, Y.; Gesmundo, F. Microstructural Effects on the High Temperature Oxidation of Two-Phase Cu–Cr Alloys in 1 Atm O2. Corros. Sci. 2003, 45, 559–574. [Google Scholar] [CrossRef]
- Pan, T.J.; Chen, J.; He, Y.X.; Wei, W.; Hu, J. Influence of Grain Refinement on Oxidation Behavior of Two-Phase Cu–Cr Alloys at 973–1,073 K in Air. High Temp. Mater. Process. 2016, 35, 1005–1011. [Google Scholar] [CrossRef]
- Peng, X.; Clarke, D.R.; Wang, F. Transient-Alumina Transformations during the Oxidation of Magnetron-Sputtered CoCrAl Nanocrystalline Coatings. Oxid. Met. 2003, 60, 225–240. [Google Scholar] [CrossRef]
- Kumar, R.; Singh Raman, R.K.; Bakshi, S.R.; Raja, V.S.; Parida, S. Effect of Nanocrystalline Structure on the Oxidation Behavior of Fe–20Cr–3Al Alloy at High Temperatures. Oxid. Met. 2022, 97, 307–321. [Google Scholar] [CrossRef]
- Yang, X.; Peng, X.; Xu, C.; Wang, F. Electrochemical Assembly of Ni – XCr – YAl Nanocomposites with Excellent High-Temperature Oxidation Resistance. J. Electrochem. Soc. 2009, 156, C167. [Google Scholar] [CrossRef]
- Niu, Y.; Cao, Z.Q.; Gesmundo, F.; Farnè, G.; Randi, G.; Wang, C.L. Grain Size Effects on the Oxidation of Two Ternary Cu–Ni–20wt.% Cr Alloys at 700–800 °C in 1 Atm O2. Corros. Sci. 2003, 45, 1125–1142. [Google Scholar] [CrossRef]
- Huang, Z.; Peng, X.; Xu, C.; Wang, F. On the Exclusive Growth of External Chromia Scale on the Novel Electrodeposited Cu–Ni–Cr Nanocomposites. J. Mater. Res. 2007, 22, 3166–3177. [Google Scholar] [CrossRef]
- Hamadi, S.; Bacos, M.-P.; Poulain, M.; Seyeux, A.; Maurice, V.; Marcus, P. Oxidation Resistance of a Zr-Doped NiAl Coating Thermochemically Deposited on a Nickel-Based Superalloy. Surf. Coat. Technol. 2009, 204, 756–760. [Google Scholar] [CrossRef]
- Jedlinski, J.; Godlewski, K.; Mrowec, S. The Influence of Implanted Yttrium and Cerium on the Protective Properties of a β-NiAl Coating on a Nickel-Base Superalloy. Mater. Sci. Eng. A 1989, 120–121, 539–543. [Google Scholar] [CrossRef]
- Guo, H.; Li, D.; Zheng, L.; Gong, S.; Xu, H. Effect of Co-Doping of Two Reactive Elements on Alumina Scale Growth of β-NiAl at 1200 °C. Corros. Sci. 2014, 88, 197–208. [Google Scholar] [CrossRef]
- Boll, T.; Unocic, K.A.; Pint, B.A.; Mårtensson, A.; Stiller, K. Grain Boundary Chemistry and Transport Through Alumina Scales on NiAl Alloys. Oxid. Met. 2017, 88, 469–479. [Google Scholar] [CrossRef]
- Calvarin-Amiri, G.; Molins, R.; Huntz, A.M. Effect of the Application of a Mechanical Load on the Oxide-Layer Microstructure and on the Oxidation Mechanism of Ni–20Cr Foils. Oxid. Met. 2000, 53, 399–426. [Google Scholar] [CrossRef]
- Moulin, G.; Arevalo, P.; Salleo, A. Influence of External Mechanical Loadings (Creep, Fatigue) on Oxygen Diffusion during Nickel Oxidation. Oxid. Met. 1996, 45, 153–181. [Google Scholar] [CrossRef]
- Osgerby, S.; Berriche-Bouhanek, K.; Evans, H.E. Tensile Cracking of a Chromia Layer on a Stainless Steel during Thermal Cycling with Hold Periods. Mater. Sci. Eng. A 2005, 412, 182–190. [Google Scholar] [CrossRef]
- Evans, H.E. Stress Effects in High Temperature Oxidation of Metals. Int. Mater. Rev. 1995, 40, 1–40. [Google Scholar] [CrossRef]
- Hibbard, G.; McCrea, J.; Palumbo, G.; Aust, K.; Erb, U. An Initial Analysis of Mechanisms Leading to Late Stage Abnormal Grain Growth in Nanocrystalline Ni. Scr. Mater. 2002, 47, 83–87. [Google Scholar] [CrossRef]
- Ganapathi, S.K.; Owen, D.M.; Chokshi, A.H. The Kinetics of Grain Growth in Nanocrystalline Copper. Scr. Metall. Mater. 1991, 25, 2699–2704. [Google Scholar] [CrossRef]
- Weissmüller, J. Alloy Thermodynamics in Nanostructures. J. Mater. Res. 1994, 9, 4–7. [Google Scholar] [CrossRef]
- Kirchheim, R. Grain Coarsening Inhibited by Solute Segregation. Acta Mater. 2002, 50, 413–419. [Google Scholar] [CrossRef]
- Michels, A.; Krill, C.E.; Ehrhardt, H.; Birringer, R.; Wu, D.T. Modelling the Influence of Grain-Size-Dependent Solute Drag on the Kinetics of Grain Growth in Nanocrystalline Materials. Acta Mater. 1999, 47, 2143–2152. [Google Scholar] [CrossRef]
- Darling, K.A.; Chan, R.N.; Wong, P.Z.; Semones, J.E.; Scattergood, R.O.; Koch, C.C. Grain-Size Stabilization in Nanocrystalline FeZr Alloys. Scr. Mater. 2008, 59, 530–533. [Google Scholar] [CrossRef]
- Srinivasan, S.; Kale, C.; Hornbuckle, B.C.; Darling, K.A.; Peralta, P.; Solanki, K.N. Thermomechanical Response of an Ultrafine-Grained Nickel-Yttrium Alloy. Scr. Mater. 2020, 187, 434–438. [Google Scholar] [CrossRef]
- Darling, K.A.; Hornbuckle, B.C.; Marvel, C.J.; Hammond, V.H.; Solanki, K. Effect of Constrained Inter-Granular Regions on the Inverse Hall-Petch Phenomena. Mater. Sci. Eng. A 2023, 875, 145125. [Google Scholar] [CrossRef]
- Darling, K.A.; Kale, C.; Turnage, S.; Hornbuckle, B.C.; Luckenbaugh, T.L.; Grendahl, S.; Solanki, K.N. Nanocrystalline Material with Anomalous Modulus of Resilience and Springback Effect. Scr. Mater. 2017, 141, 36–40. [Google Scholar] [CrossRef]
- Darling, K.A.; Srinivasan, S.; Koju, R.K.; Hornbuckle, B.C.; Smeltzer, J.; Mishin, Y.; Solanki, K.N. Stress-Driven Grain Refinement in a Microstructurally Stable Nanocrystalline Binary Alloy. Scr. Mater. 2021, 191, 185–190. [Google Scholar] [CrossRef]
- Hornbuckle, B.C.; Dean, S.W.; Zhou, X.; Giri, A.K.; Williams, C.L.; Solanki, K.N.; Thompson, G.B.; Darling, K.A. Laser Shocking of Nanocrystalline Materials: Revealing the Extreme Pressure Effects on the Microstructural Stability and Deformation Response. Appl. Phys. Lett. 2020, 116, 231901. [Google Scholar] [CrossRef]
- Hornbuckle, B.C.; Williams, C.L.; Dean, S.W.; Zhou, X.; Kale, C.; Turnage, S.A.; Clayton, J.D.; Thompson, G.B.; Giri, A.K.; Solanki, K.N. Stable Microstructure in a Nanocrystalline Copper–tantalum Alloy during Shock Loading. Commun. Mater. 2020, 1, 22. [Google Scholar] [CrossRef]
- Hornbuckle, B.C.; Kale, C.; Srinivasan, S.; Luckenbaugh, T.L.; Solanki, K.N.; Darling, K.A. Revealing Cryogenic Mechanical Behavior and Mechanisms in a Microstructurally-Stable, Immiscible Nanocrystalline Alloy. Scr. Mater. 2019, 160, 33–38. [Google Scholar] [CrossRef]
- Hornbuckle, B.C.; Luckenbaugh, T.L.; Fudger, S.J.; Roberts, A.J.; Jannotti, P.; Byun, T.S.; Hoelzer, D.T.; Solanki, K.; Darling, K.A. Role of Geometric Dynamic Recrystallization in Nanocrystalline Alloys. Materialia 2023, 30, 101807. [Google Scholar] [CrossRef]
- Srinivasan, S.; Hornbuckle, B.C.; Chancey, M.R.; Darling, K.A.; Wang, Y.; Solanki, K. Role of Tantalum Concentration on the High Dose Self-Ion Irradiation Behavior of Nanocrystalline Binary Alloys. Scr. Mater. 2023, 223, 115100. [Google Scholar] [CrossRef]
- Srinivasan, S.; Hornbuckle, B.C.; Darling, K.A.; Kim, H.; Wang, Y.Q.; Solanki, K. Helium Partitioning to the Core-Shelled Ta Nanoclusters in Nanocrystalline Cu-Ta Alloy. Scr. Mater. 2022, 208, 114344. [Google Scholar] [CrossRef]
- Srinivasan, S.; Kale, C.; Hornbuckle, B.C.; Darling, K.A.; Chancey, M.R.; Hernández-Rivera, E.; Chen, Y.; Koenig, T.R.; Wang, Y.Q.; Thompson, G.B.; et al. Radiation Tolerance and Microstructural Changes of Nanocrystalline Cu-Ta Alloy to High Dose Self-Ion Irradiation. Acta Mater. 2020, 195, 621–630. [Google Scholar] [CrossRef]
- Srinivasan, S.; Lang, E.; Burns, K.; Hattar, K.; Hornbuckle, B.C.; Darling, K.A.; Solanki, K. In-Situ TEM Bubble to Cavity Evolution Due to Annealing Post Helium and Dual Ion Irradiation in Cu-10Ta and Cu-3Ta. Mater. Charact. 2023, 202, 113038. [Google Scholar] [CrossRef]
- Bhatia, M.; Rajagopalan, M.; Darling, K.; Tschopp, M.; Solanki, K. The Role of Ta on Twinnability in Nanocrystalline Cu–Ta Alloys. Mater. Res. Lett. 2017, 5, 48–54. [Google Scholar] [CrossRef]
- Lu, K.; Lu, J. Nanostructured Surface Layer on Metallic Materials Induced by Surface Mechanical Attrition Treatment. Mater. Sci. Eng. A 2004, 375–377, 38–45. [Google Scholar] [CrossRef]
- Beura, V.K.; Karanth, Y.; Darling, K.; Solanki, K. Role of Gradient Nanograined Surface Layer on Corrosion Behavior of Aluminum 7075 Alloy. Npj Mater. Degrad. 2022, 6, 62. [Google Scholar] [CrossRef]
- Beura, V.; Zhang, D.; Overman, N.; Darsell, J.; Herling, D.R.; Solanki, K.; Joshi, V.V. Enhanced Mechanical Behavior and Corrosion Resistance of AZ31 Magnesium Alloy through a Novel Solid-Phase Processing. Corros. Sci. 2022, 197, 110074. [Google Scholar] [CrossRef]
- Olugbade, T.O.; Lu, J. Literature Review on the Mechanical Properties of Materials after Surface Mechanical Attrition Treatment (SMAT). Nano Mater. Sci. 2020, 2, 3–31. [Google Scholar] [CrossRef]
- Xia, Z.X.; Zhang, C.; Huang, X.F.; Liu, W.B.; Yang, Z.G. Improve Oxidation Resistance at High Temperature by Nanocrystalline Surface Layer. Sci. Rep. 2015, 5, 13027. [Google Scholar] [CrossRef]
- Singh, D.; Cemin, F.; Jimenez, M.J.M.; Antunes, V.; Alvarez, F.; Orlov, D.; Figueroa, C.A.; Hosmani, S.S. High-Temperature Oxidation Behaviour of Nanostructure Surface Layered Austenitic Stainless Steel. Appl. Surf. Sci. 2022, 581, 152437. [Google Scholar] [CrossRef]
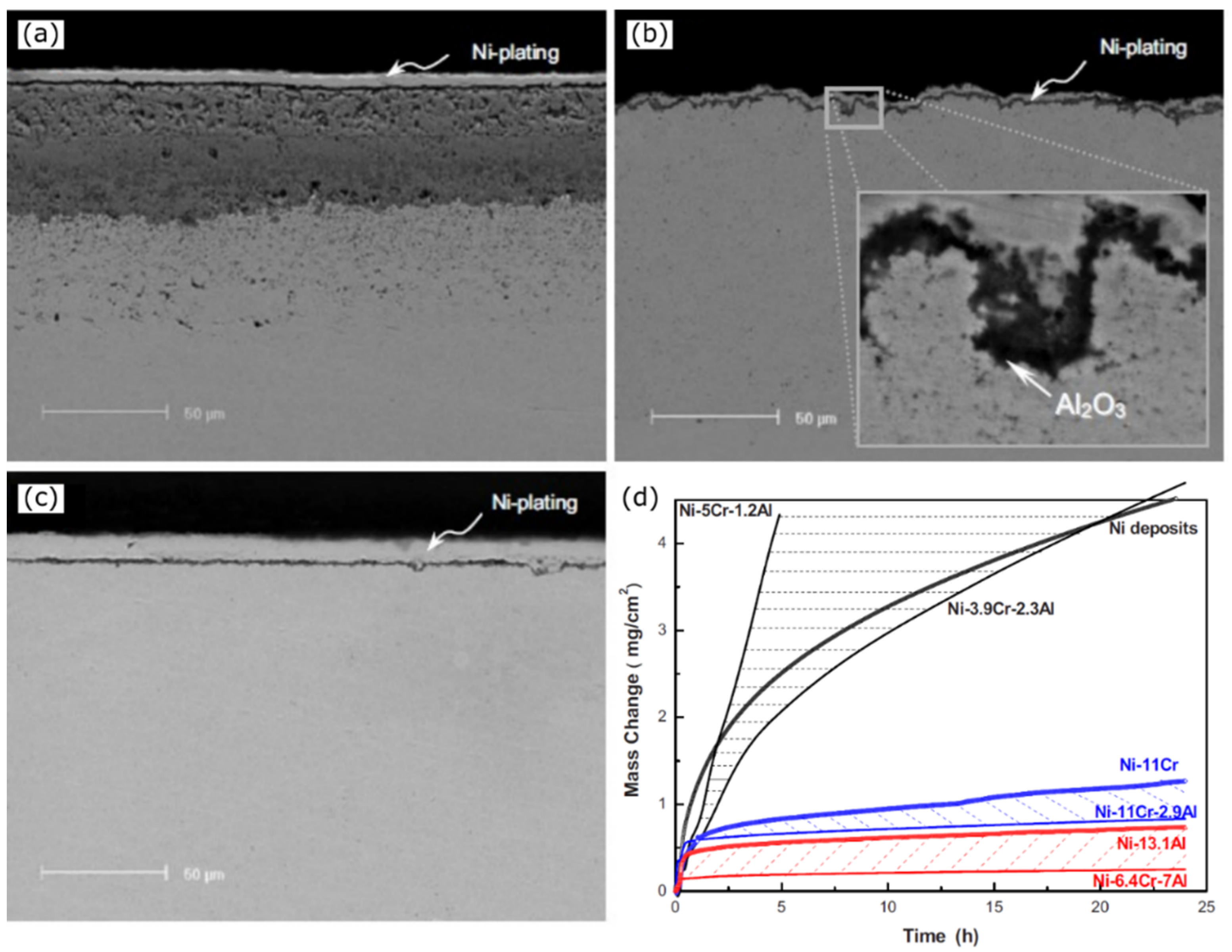

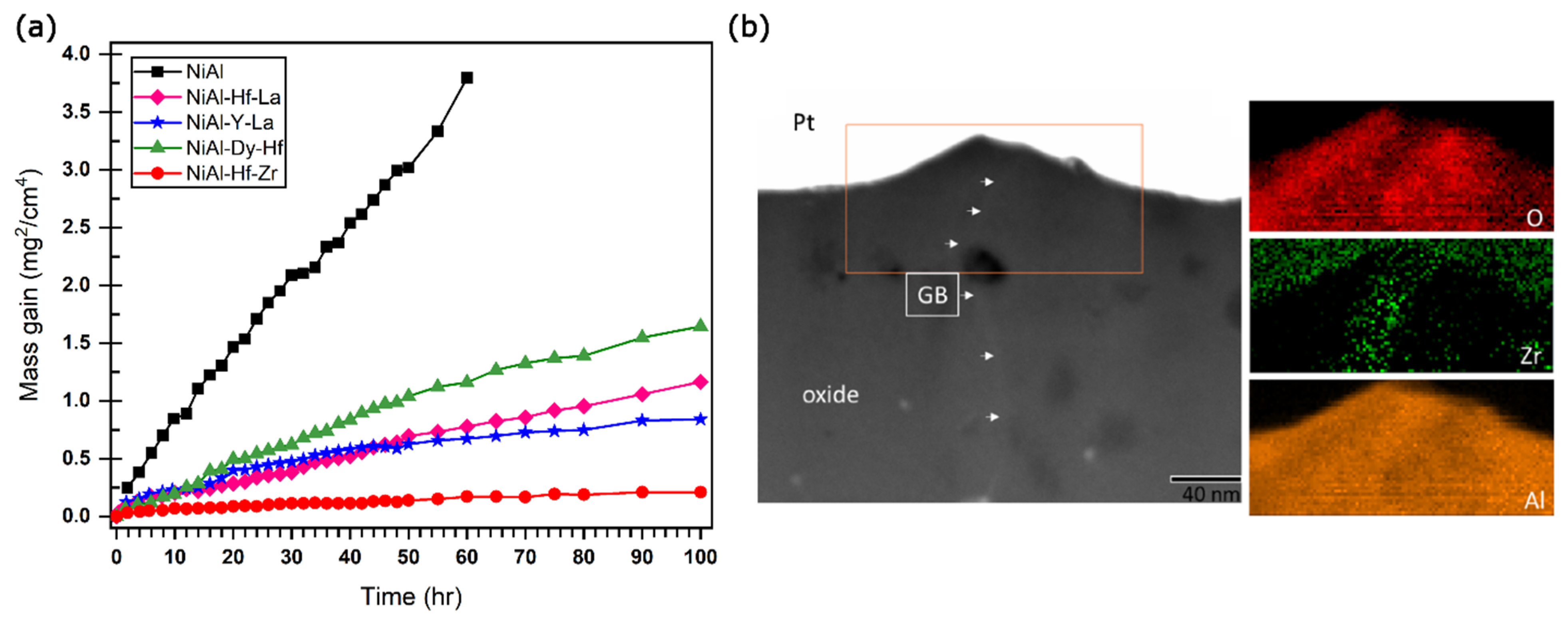
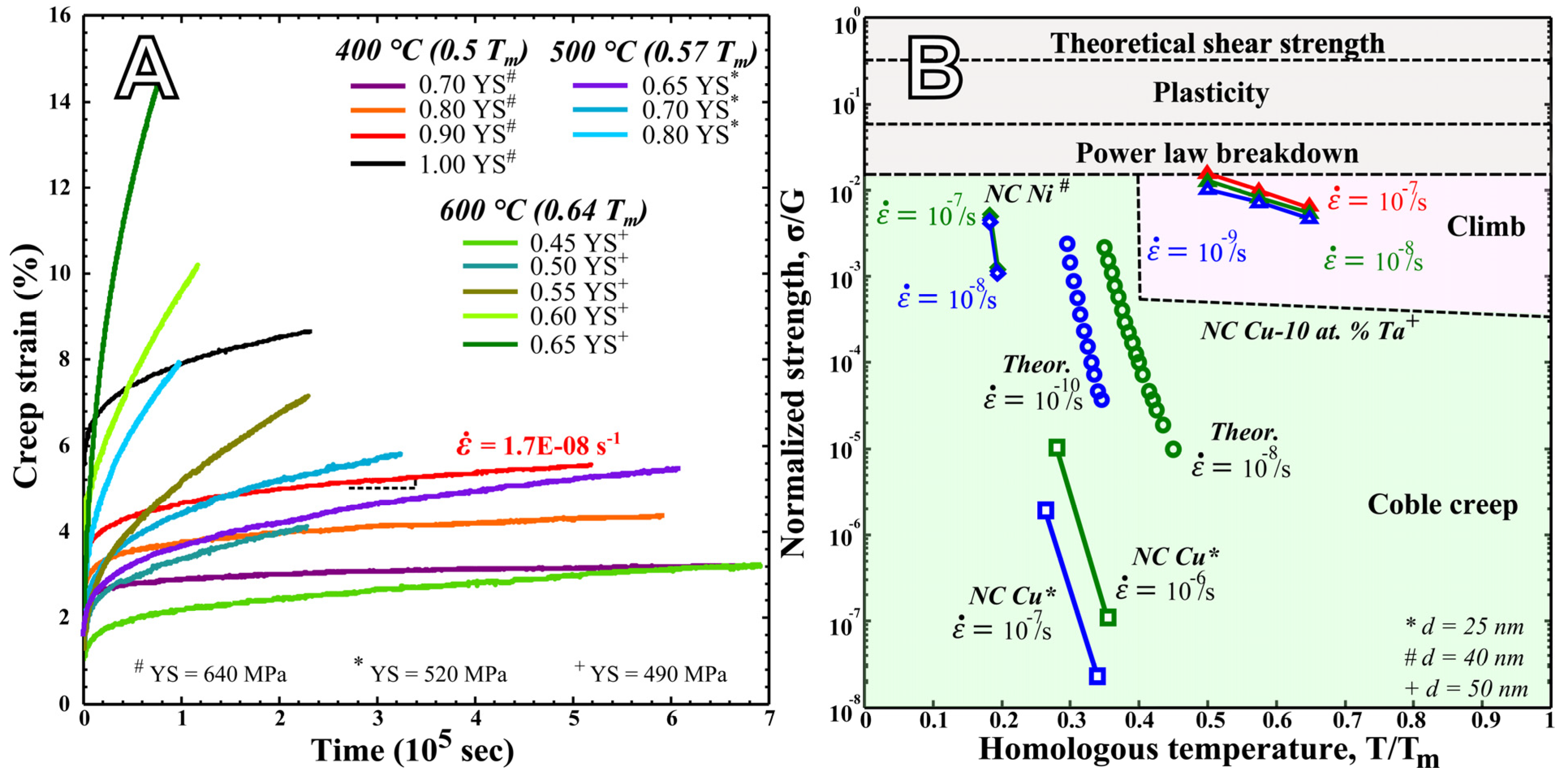
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Karanth, Y.; Sharma, S.; Darling, K.; El Kadiri, H.; Solanki, K. Oxidation Behavior of Nanocrystalline Alloys. Materials 2024, 17, 5842. https://doi.org/10.3390/ma17235842
Karanth Y, Sharma S, Darling K, El Kadiri H, Solanki K. Oxidation Behavior of Nanocrystalline Alloys. Materials. 2024; 17(23):5842. https://doi.org/10.3390/ma17235842
Chicago/Turabian StyleKaranth, Yashaswini, Saurabh Sharma, Kris Darling, Haitham El Kadiri, and Kiran Solanki. 2024. "Oxidation Behavior of Nanocrystalline Alloys" Materials 17, no. 23: 5842. https://doi.org/10.3390/ma17235842
APA StyleKaranth, Y., Sharma, S., Darling, K., El Kadiri, H., & Solanki, K. (2024). Oxidation Behavior of Nanocrystalline Alloys. Materials, 17(23), 5842. https://doi.org/10.3390/ma17235842






