Green Synthesis of CuO Nanoparticles—Structural, Morphological, and Dielectric Characterization
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
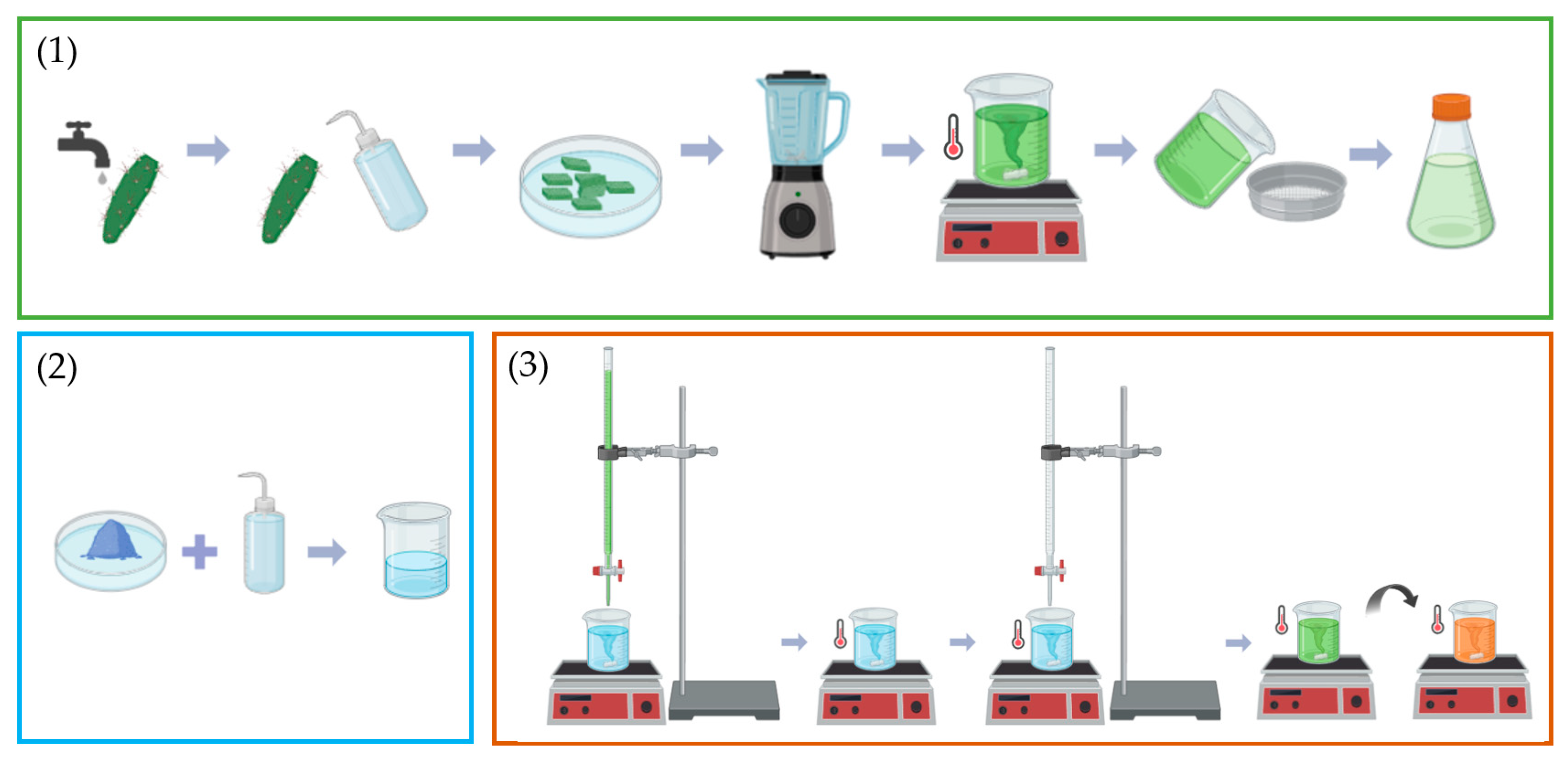
2.2. Preparation of the Opuntia ficus-indica Cladode Extract
2.3. Synthesis of the CuO Nanoparticles
2.4. Characterization Techniques
3. Results
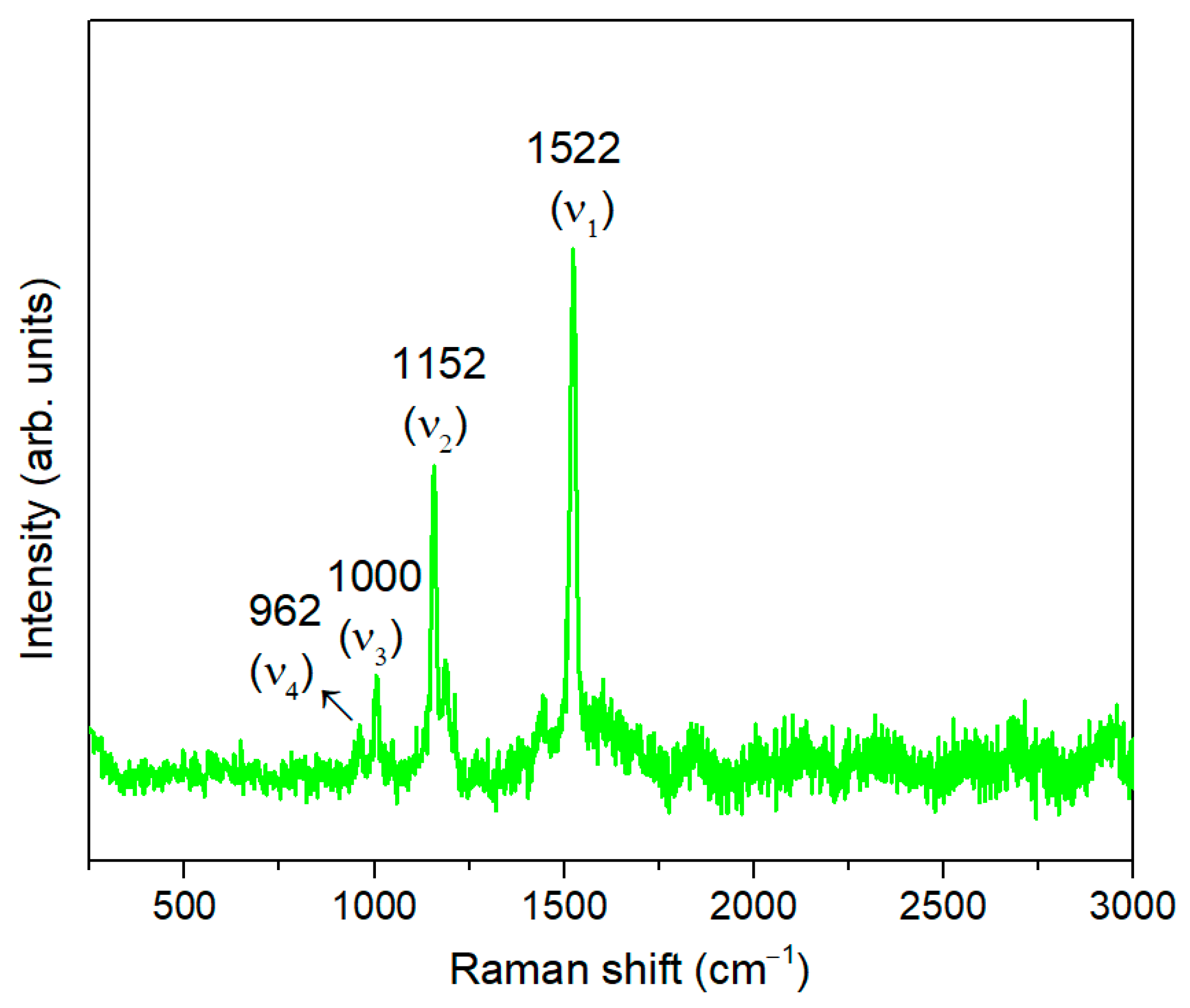
3.1. Opuntia ficus-indica
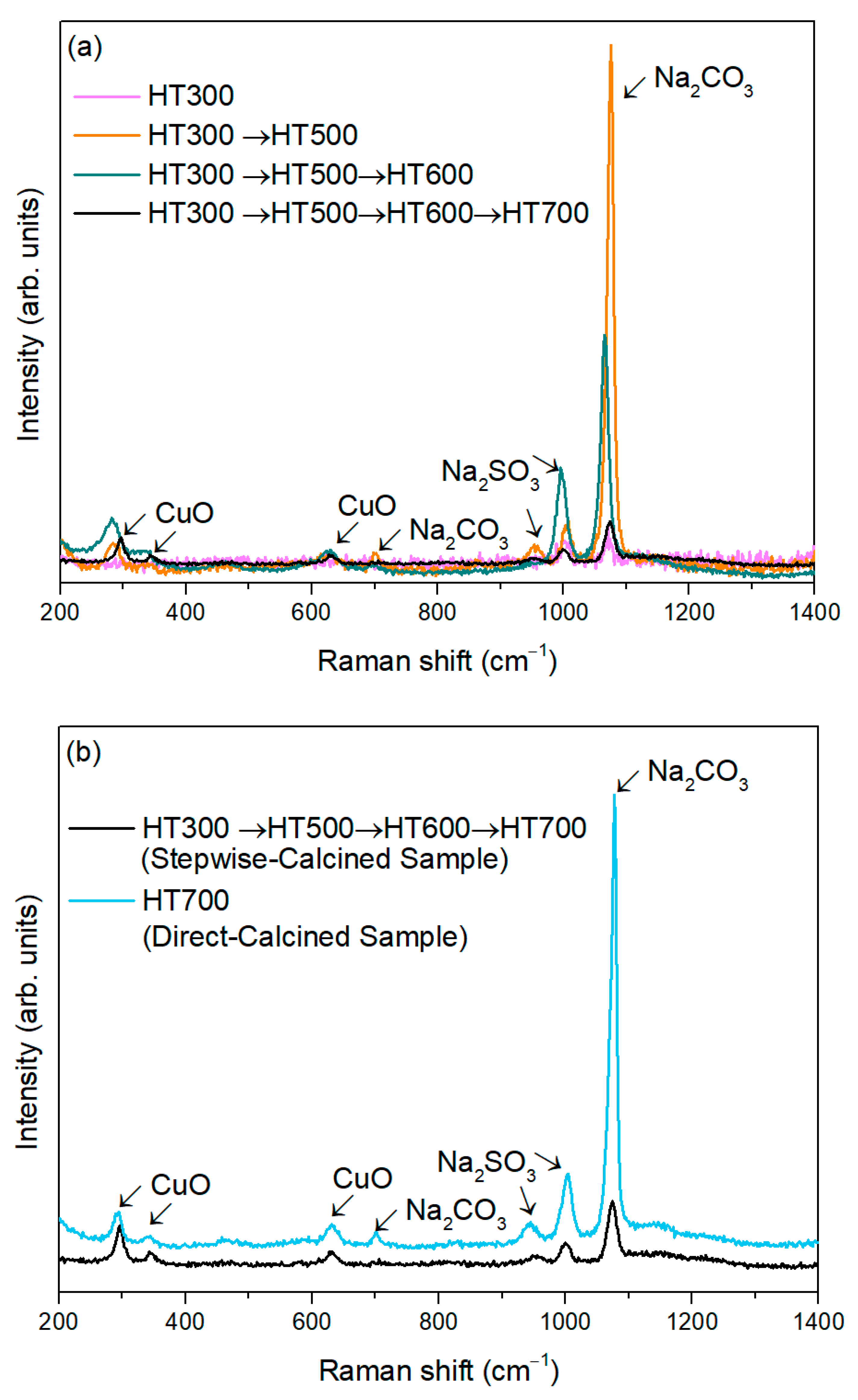
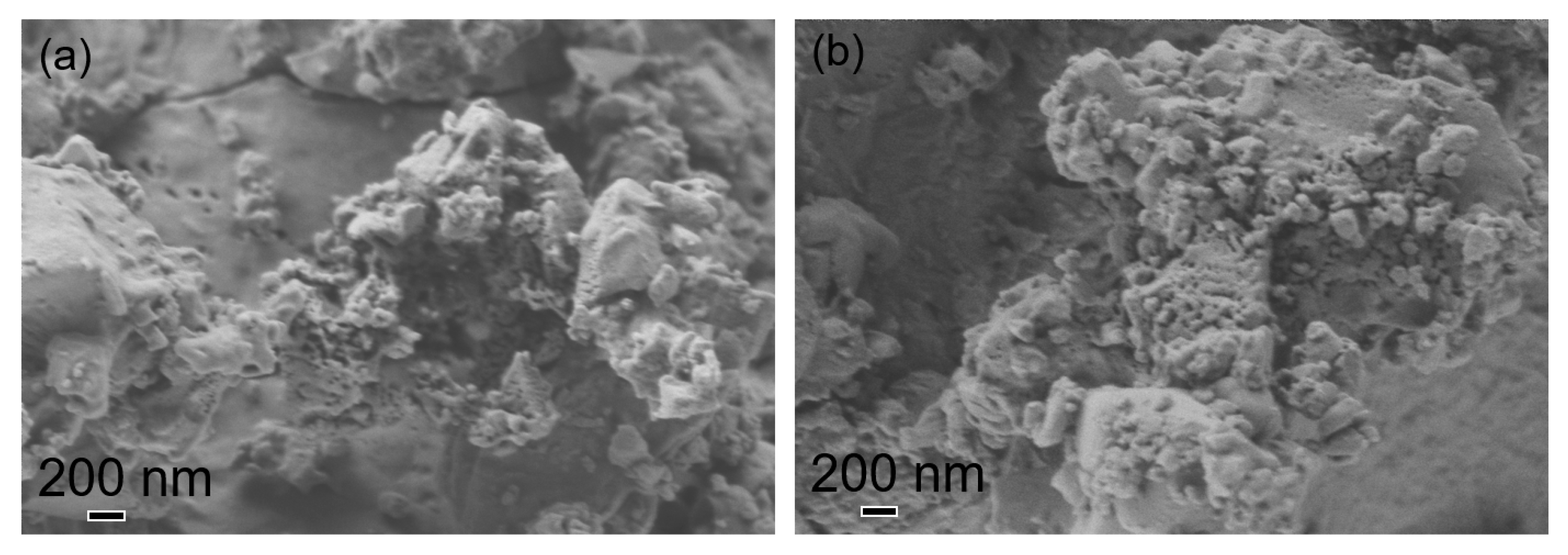
3.2. CuO Nanoparticles
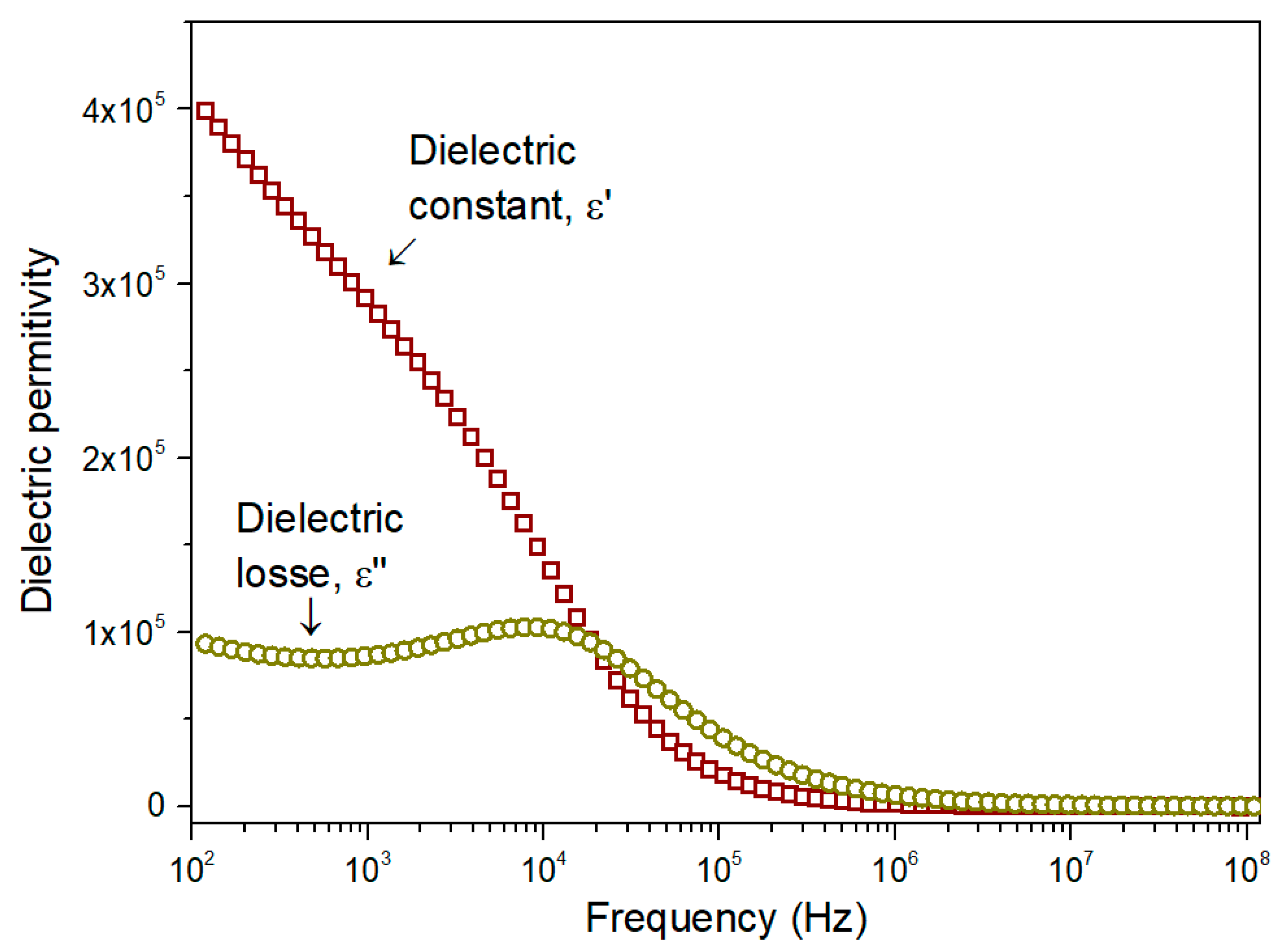
3.3. Dielectric Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Taghavi Fardood, S.; Ramazani, A.; Joo, S.W. Green Chemistry Approach for the Synthesis of Copper Oxide Nanoparticles Using Tragacanth Gel and Their Structural Characterization. J. Struct. Chem. 2018, 59, 482–486. [Google Scholar] [CrossRef]
- Auffan, M.; Rose, J.; Bottero, J.-Y.; Lowry, G.V.; Jolivet, J.-P.; Wiesner, M.R. Towards a Definition of Inorganic Nanoparticles from an Environmental, Health and Safety Perspective. Nat. Nanotechnol. 2009, 4, 634–641. [Google Scholar] [CrossRef] [PubMed]
- Borm, P.J.; Robbins, D.; Haubold, S.; Kuhlbusch, T.; Fissan, H.; Donaldson, K.; Schins, R.; Stone, V.; Kreyling, W.; Lademann, J.; et al. The Potential Risks of Nanomaterials: A Review Carried out for ECETOC. Part. Fibre Toxicol. 2006, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Joudeh, N.; Linke, D. Nanoparticle Classification, Physicochemical Properties, Characterization, and Applications: A Comprehensive Review for Biologists. J. Nanobiotechnology 2022, 20, 262. [Google Scholar] [CrossRef]
- Szczyglewska, P.; Feliczak-Guzik, A.; Nowak, I. Nanotechnology–General Aspects: A Chemical Reduction Approach to the Synthesis of Nanoparticles. Molecules 2023, 28, 4932. [Google Scholar] [CrossRef]
- Sajid, M.; Płotka-Wasylka, J. Nanoparticles: Synthesis, Characteristics, and Applications in Analytical and Other Sciences. Microchem. J. 2020, 154, 104623. [Google Scholar] [CrossRef]
- Khalil, M.; Jan, B.M.; Tong, C.W.; Berawi, M.A. Advanced Nanomaterials in Oil and Gas Industry: Design, Application and Challenges. Appl. Energy 2017, 191, 287–310. [Google Scholar] [CrossRef]
- Alhalili, Z. Green Synthesis of Copper Oxide Nanoparticles CuO NPs from Eucalyptus Globoulus Leaf Extract: Adsorption and Design of Experiments. Arab. J. Chem. 2022, 15, 103739. [Google Scholar] [CrossRef]
- Kumar, N.; Parui, S.S.; Limbu, S.; Mahato, D.K.; Tiwari, N.; Chauhan, R.N. Structural and Optical Properties of Sol–Gel Derived CuO and Cu2O Nanoparticles. Mater. Today Proc. 2021, 41, 237–241. [Google Scholar] [CrossRef]
- Yang, C.; Xiao, F.; Wang, J.; Su, X. Synthesis and Microwave Modification of CuO Nanoparticles: Crystallinity and Morphological Variations, Catalysis, and Gas Sensing. J. Colloid Interface Sci. 2014, 435, 34–42. [Google Scholar] [CrossRef]
- Chavali, M.S.; Nikolova, M.P. Metal Oxide Nanoparticles and Their Applications in Nanotechnology. SN Appl. Sci. 2019, 1, 607. [Google Scholar] [CrossRef]
- Oruç, Ç.; Altındal, A. Structural and Dielectric Properties of CuO Nanoparticles. Ceram. Int. 2017, 43, 10708–10714. [Google Scholar] [CrossRef]
- Chand, P.; Manisha; Kumar, P. Effect of Precursors Medium on Structural, Optical and Dielectric Properties of CuO Nanostructures. Optik 2018, 156, 743–753. [Google Scholar] [CrossRef]
- Ighalo, J.O.; Sagboye, P.A.; Umenweke, G.; Ajala, O.J.; Omoarukhe, F.O.; Adeyanju, C.A.; Ogunniyi, S.; Adeniyi, A.G. CuO Nanoparticles (CuO NPs) for Water Treatment: A Review of Recent Advances. Environ. Nanotechnol. Monit. Manag. 2021, 15, 100443. [Google Scholar] [CrossRef]
- Ijaz, I.; Gilani, E.; Nazir, A.; Bukhari, A. Detail Review on Chemical, Physical and Green Synthesis, Classification, Characterizations and Applications of Nanoparticles. Green Chem. Lett. Rev. 2020, 13, 223–245. [Google Scholar] [CrossRef]
- Khan, I.; Saeed, K.; Khan, I. Nanoparticles: Properties, Applications and Toxicities. Arab. J. Chem. 2019, 12, 908–931. [Google Scholar] [CrossRef]
- Alsaiari, N.S.; Alzahrani, F.M.; Amari, A.; Osman, H.; Harharah, H.N.; Elboughdiri, N.; Tahoon, M.A. Plant and Microbial Approaches as Green Methods for the Synthesis of Nanomaterials: Synthesis, Applications, and Future Perspectives. Molecules 2023, 28, 463. [Google Scholar] [CrossRef]
- Alhalili, Z. Metal Oxides Nanoparticles: General Structural Description, Chemical, Physical, and Biological Synthesis Methods, Role in Pesticides and Heavy Metal Removal through Wastewater Treatment. Molecules 2023, 28, 3086. [Google Scholar] [CrossRef]
- Bokov, D.; Turki Jalil, A.; Chupradit, S.; Suksatan, W.; Javed Ansari, M.; Shewael, I.H.; Valiev, G.H.; Kianfar, E. Nanomaterial by Sol-Gel Method: Synthesis and Application. Adv. Mater. Sci. Eng. 2021, 2021, 1–21. [Google Scholar] [CrossRef]
- Prabakaran, S.; Rajan, M. Biosynthesis of Nanoparticles and Their Roles in Numerous Areas. Compr. Anal. Chem. 2021, 94, 1–47. [Google Scholar]
- Raduwan, N.F.; Shaari, N.; Kamarudin, S.K.; Masdar, M.S.; Yunus, R.M. An Overview of Nanomaterials in Fuel Cells: Synthesis Method and Application. Int. J. Hydrogen Energy 2022, 47, 18468–18495. [Google Scholar] [CrossRef]
- Deena Raj, K.M.; Sujatha, S.; Chanthini, K.M.-P. Green Synthesis of CuO Nanoparticle Using Cyperus Rotundus and Evaluation of Their Anticancer Potentials against Lung Cancer Cells Invitro. Biocatal. Agric. Biotechnol. 2024, 57, 103119. [Google Scholar] [CrossRef]
- Kerour, A.; Boudjadar, S.; Bourzami, R.; Allouche, B. Eco-Friendly Synthesis of Cuprous Oxide (Cu2O) Nanoparticles and Improvement of Their Solar Photocatalytic Activities. J. Solid State Chem. 2018, 263, 79–83. [Google Scholar] [CrossRef]
- Govindasamy, R.; Govindarasu, M.; Alharthi, S.S.; Mani, P.; Bernaurdshaw, N.; Gomathi, T.; Ansari, M.A.; Alomary, M.N.; Atwah, B.; Malik, M.S.; et al. Sustainable Green Synthesis of Yttrium Oxide (Y2O3) Nanoparticles Using Lantana Camara Leaf Extracts: Physicochemical Characterization, Photocatalytic Degradation, Antibacterial, and Anticancer Potency. Nanomaterials 2022, 12, 2393. [Google Scholar] [CrossRef] [PubMed]
- Gu, H.; Chen, X.; Chen, F.; Zhou, X.; Parsaee, Z. Ultrasound-Assisted Biosynthesis of CuO-NPs Using Brown Alga Cystoseira Trinodis: Characterization, Photocatalytic AOP, DPPH Scavenging and Antibacterial Investigations. Ultrason. Sonochem. 2018, 41, 109–119. [Google Scholar] [CrossRef]
- Lv, Q.; Zhang, B.; Xing, X.; Zhao, Y.; Cai, R.; Wang, W.; Gu, Q. Biosynthesis of Copper Nanoparticles Using Shewanella Loihica PV-4 with Antibacterial Activity: Novel Approach and Mechanisms Investigation. J. Hazard. Mater. 2018, 347, 141–149. [Google Scholar] [CrossRef]
- Cunha, F.A.; Cunha, M.d.C.S.O.; da Frota, S.M.; Mallmann, E.J.J.; Freire, T.M.; Costa, L.S.; Paula, A.J.; Menezes, E.A.; Fechine, P.B.A. Biogenic Synthesis of Multifunctional Silver Nanoparticles from Rhodotorula Glutinis and Rhodotorula Mucilaginosa: Antifungal, Catalytic and Cytotoxicity Activities. World J. Microbiol. Biotechnol. 2018, 34, 127. [Google Scholar] [CrossRef]
- Jalal, M.; Ansari, M.A.; Alzohairy, M.A.; Ali, S.G.; Khan, H.M.; Almatroudi, A.; Raees, K. Biosynthesis of Silver Nanoparticles from Oropharyngeal Candida Glabrata Isolates and Their Antimicrobial Activity against Clinical Strains of Bacteria and Fungi. Nanomaterials 2018, 8, 586. [Google Scholar] [CrossRef]
- Love, A.J.; Makarov, V.; Yaminsky, I.; Kalinina, N.O.; Taliansky, M.E. The Use of Tobacco Mosaic Virus and Cowpea Mosaic Virus for the Production of Novel Metal Nanomaterials. Virology 2014, 449, 133–139. [Google Scholar] [CrossRef]
- Akintelu, S.A.; Folorunso, A.S.; Folorunso, F.A.; Oyebamiji, A.K. Green Synthesis of Copper Oxide Nanoparticles for Biomedical Application and Environmental Remediation. Heliyon 2020, 6, e04508. [Google Scholar] [CrossRef]
- Nasrollahzadeh, M.; Sajadi, S.M.; Issaabadi, Z.; Sajjadi, M. Biological Sources Used in Green Nanotechnology. Interface Sci. Technol. 2019, 28, 81–111. [Google Scholar] [CrossRef]
- Miu, B.A.; Dinischiotu, A. New Green Approaches in Nanoparticles Synthesis: An Overview. Molecules 2022, 27, 6472. [Google Scholar] [CrossRef] [PubMed]
- Aigbe, U.O.; Osibote, O.A. Green Synthesis of Metal Oxide Nanoparticles, and Their Various Applications. J. Hazard. Mater. Adv. 2024, 13, 100401. [Google Scholar] [CrossRef]
- Berra, D.; Laouini, S.E.; Benhaoua, B.; Ouahrani, M.R.; Berrani, D.; Rahal, A. Green Synthesis of Copper Oxide Nanoparticles by Pheonix Dactylifera L Leaves Extract. Dig. J. Nanomater. Biostruct. 2018, 13, 1231–1238. [Google Scholar]
- Bhatia, N.; Kumari, A.; Chauhan, N.; Thakur, N.; Sharma, R. Duchsnea Indica Plant Extract Mediated Synthesis of Copper Oxide Nanomaterials for Antimicrobial Activity and Free-Radical Scavenging Assay. Biocatal. Agric. Biotechnol. 2023, 47, 102574. [Google Scholar] [CrossRef]
- Saif, S.; Tahir, A.; Asim, T.; Chen, Y. Plant Mediated Green Synthesis of CuO Nanoparticles: Comparison of Toxicity of Engineered and Plant Mediated CuO Nanoparticles towards Daphnia Magna. Nanomaterials 2016, 6, 205. [Google Scholar] [CrossRef]
- Vishveshvar, K.; Aravind Krishnan, M.V.; Haribabu, K.; Vishnuprasad, S. Green Synthesis of Copper Oxide Nanoparticles Using Ixiro Coccinea Plant Leaves and Its Characterization. Bionanoscience 2018, 8, 554–558. [Google Scholar] [CrossRef]
- Aragona, M.; Lauriano, E.R.; Pergolizzi, S.; Faggio, C. Opuntia ficus-indica (L.) Miller as a Source of Bioactivity Compounds for Health and Nutrition. Nat. Prod. Res. 2018, 32, 2037–2049. [Google Scholar] [CrossRef]
- Silva, M.A.; Albuquerque, T.G.; Pereira, P.; Ramalho, R.; Vicente, F.; Oliveira, M.B.P.P.; Costa, H.S. Opuntia ficus-indica (L.) Mill.: A Multi-Benefit Potential to Be Exploited. Molecules 2021, 26, 951. [Google Scholar] [CrossRef]
- de Cortázar, V.G.; Nobel, P.S. Biomass and Fruit Production for the Prickly Pear Cactus, Opuntia ficus-indica. J. Am. Soc. Hortic. Sci. 1992, 117, 558–562. [Google Scholar] [CrossRef]
- Gebretinsae, H.G.; Tsegay, M.G.; Nuru, Z.Y. Biosynthesis of Nickel Oxide (NiO) Nanoparticles from Cactus Plant Extract. Mater. Today Proc. 2021, 36, 566–570. [Google Scholar] [CrossRef]
- Chiteva, R.; Wairagu, N. Chemical and Nutritional Content of Opuntia ficus-indica (L.). Afr. J. Biotechnol. 2013, 12, 3309–3312. [Google Scholar] [CrossRef]
- Shoukat, R.; Cappai, M.; Pia, G.; Pilia, L. An Updated Review: Opuntia Ficus Indica (OFI) Chemistry and Its Diverse Applications. Appl. Sci. 2023, 13, 7724. [Google Scholar] [CrossRef]
- Kim, J.-Y.; Lim, J.-C.; Kim, S.; Hwang, S.-M.; Shin, W.-H.; Yang, H.; Lee, K.H.; Kim, H.-S. Impact of Two-Step Calcination on Microstructure, Phase, Electronic, and Dielectric Properties of KCa2Nb3O10 Bulk Layered Perovskite. J. Asian Ceram. Soc. 2023, 11, 188–196. [Google Scholar] [CrossRef]
- Udensi, J.; Loughman, J.; Loskutova, E.; Byrne, H.J. Raman Spectroscopy of Carotenoid Compounds for Clinical Applications—A Review. Molecules 2022, 27, 9017. [Google Scholar] [CrossRef]
- Mehta, M.; Naffa, R.; Zhang, W.; Schreurs, N.M.; Waterland, M.; Cooper, S.; Holmes, G. Validity and Reliability of Raman Spectroscopy for Carotenoid Assessment in Cattle Skin. Biochem. Biophys. Rep. 2021, 27, 101036. [Google Scholar] [CrossRef]
- Udensi, J.; Loskutova, E.; Loughman, J.; Byrne, H.J. Quantitative Raman Analysis of Carotenoid Protein Complexes in Aqueous Solution. Molecules 2022, 27, 4724. [Google Scholar] [CrossRef]
- Figueirôa, J.A.; Menezes Novaes, G.U.; de Souza Gomes, H.; de Morais Silva, V.L.M.; de Moraes Lucena, D.; Lima, L.M.R.; de Souza, S.A.; Viana, L.G.F.C.; Rolim, L.A.; da Silva Almeida, J.R.G.; et al. Opuntia ficus-indica Is an Excellent Eco-Friendly Biosorbent for the Removal of Chromium in Leather Industry Effluents. Heliyon 2021, 7, e07292. [Google Scholar] [CrossRef]
- Lavado-Meza, C.; Fernandez-Pezua, M.C.; Gamarra-Gómez, F.; Sacari-Sacari, E.; Angeles-Suazo, J.; Dávalos-Prado, J.Z. Single and Binary Removals of Pb(II) and Cd(II) with Chemically Modified Opuntia Ficus Indica Cladodes. Molecules 2023, 28, 4451. [Google Scholar] [CrossRef]
- Perucini-Avendaño, M.; Nicolás-García, M.; Jiménez-Martínez, C.; Perea-Flores, M.d.J.; Gómez-Patiño, M.B.; Arrieta-Báez, D.; Dávila-Ortiz, G. Cladodes: Chemical and Structural Properties, Biological Activity, and Polyphenols Profile. Food Sci. Nutr. 2021, 9, 4007–4017. [Google Scholar] [CrossRef]
- Buzgar, N.; Apopei, A.I. The Raman Study of Certain Carbonates. Geol. Tomul L 2009, 2, 97–112. [Google Scholar]
- Tran, T.H.; Nguyen, V.T. Phase Transition of Cu2O to CuO Nanocrystals by Selective Laser Heating. Mater. Sci. Semicond. Process. 2016, 46, 6–9. [Google Scholar] [CrossRef]
- Debbichi, L.; Marco de Lucas, M.C.; Pierson, J.F.; Krüger, P. Vibrational Properties of CuO and Cu 4 O 3 from First-Principles Calculations, and Raman and Infrared Spectroscopy. J. Phys. Chem. C 2012, 116, 10232–10237. [Google Scholar] [CrossRef]
- Brajesh, K.; Ranjan, S.; Kumar, R.; Gupta, R.; Dixit, A.; Garg, A. Room-temperature Structural, Magnetic, and Dielectric Characteristics of La-doped CuO Bulk Multiferroic. J. Am. Ceram. Soc. 2023, 106, 6769–6777. [Google Scholar] [CrossRef]
- Xu, J.F.; Ji, W.; Shen, Z.X.; Tang, S.H.; Ye, X.R.; Jia, D.Z.; Xin, X.Q. Preparation and Characterization of CuO Nanocrystals. J. Solid State Chem. 1999, 147, 516–519. [Google Scholar] [CrossRef]
- Yang, J.-Q.; Yu, H.-H.; Jin, L.; Yang, F.-Z.; Wu, D.-Y.; Zhan, D.-P.; Tian, Z.-Q. Insights into the Effects of Chloride Ions on Cyanide-Free Gold Electrodeposition. J. Electrochem. Soc. 2020, 167, 102514. [Google Scholar] [CrossRef]
- Åsbrink, S.; Norrby, L.J. A Refinement of the Crystal Structure of Copper(II) Oxide with a Discussion of Some Exceptional e.s.d.’s. Acta Crystallogr. B 1970, 26, 8–15. [Google Scholar] [CrossRef]
- Dusek, M.; Chapuis, G.; Meyer, M.; Petricek, V. Sodium Carbonate Revisited. Acta Crystallogr. B 2003, 59, 337–352. [Google Scholar] [CrossRef]
- Doebelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Cryst. 2015, 48, 1573–1580. [Google Scholar] [CrossRef]
- Toby, B.H. R Factors in Rietveld Analysis: How Good Is Good Enough? Powder Diffr. 2006, 21, 67–70. [Google Scholar] [CrossRef]
- Sharma, S.; Yadav, D.K.; Chawla, K.; Lal, N.; Lal, C. Synthesis and Characterization of CuO Nanoparticles by Aloe Barbadensis Leaves. Quantum J. Eng. Sci. Technol. 2021, 2, 1–9. [Google Scholar]
- Chan, Y.; Selvanathan, V.; Tey, L.-H.; Akhtaruzzaman, M.; Anur, F.; Djearamane, S.; Watanabe, A.; Aminuzzaman, M. Effect of Calcination Temperature on Structural, Morphological and Optical Properties of Copper Oxide Nanostructures Derived from Garcinia Mangostana L. Leaf Extract. Nanomaterials 2022, 12, 3589. [Google Scholar] [CrossRef] [PubMed]
- Sajjad, A.; Bhatti, S.H.; Ali, Z.; Jaffari, G.H.; Khan, N.A.; Rizvi, Z.F.; Zia, M. Photoinduced Fabrication of Zinc Oxide Nanoparticles: Transformation of Morphological and Biological Response on Light Irradiance. ACS Omega 2021, 6, 11783–11793. [Google Scholar] [CrossRef] [PubMed]
- You, W.; Ahn, J.C.; Boopathi, V.; Arunkumar, L.; Rupa, E.J.; Akter, R.; Kong, B.M.; Lee, G.S.; Yang, D.C.; Kang, S.C.; et al. Enhanced Antiobesity Efficacy of Tryptophan Using the Nanoformulation of Dendropanax Morbifera Extract Mediated with ZnO Nanoparticle. Materials 2021, 14, 824. [Google Scholar] [CrossRef] [PubMed]
- Baharudin, K.B.; Abdullah, N.; Derawi, D. Effect of Calcination Temperature on the Physicochemical Properties of Zinc Oxide Nanoparticles Synthesized by Coprecipitation. Mater. Res. Express 2018, 5, 125018. [Google Scholar] [CrossRef]
- Devesa, S.; Gonçalves, F.; Graça, M. Influence of the Preparation Method on the Structural, Morphological and Dielectric Properties of FeNbO4 Ceramics. Materials 2023, 16, 3202. [Google Scholar] [CrossRef]
- Devesa, S.; Amorim, C.O.; Belo, J.H.; Araújo, J.P.; Teixeira, S.S.; Graça, M.P.F.; Costa, L.C. Comprehensive Characterization of Bi1.34Fe0.66Nb1.34O6.35 Ceramics: Structural, Morphological, Electrical, and Magnetic Properties. Magnetochemistry 2024, 10, 79. [Google Scholar] [CrossRef]
- Devesa, S.; Ferreira, N.M.; Peixoto, M.V.; Costa, F.M.; Graça, M.P.F. Growth Rate Effect on the Dielectric Properties of FeNbO4 Fibres Processed by the Laser Floating Zone Technique. Ceram. Int. 2024, 50, 34143–34152. [Google Scholar] [CrossRef]
- Gomes, E.d.S.; Lima, A.M.d.O.; Gavinho, S.R.; Graça, M.P.F.; Devesa, S.; Macêdo, A.A.M. Influence of Polymeric Blends on Bioceramics of Hydroxyapatite. Crystals 2023, 13, 1429. [Google Scholar] [CrossRef]
- Vindhya, P.S.; Jeyasingh, T.; Kavitha, V.T. Dielectric Properties of Copper Oxide Nanoparticles Using AnnonaMuricata Leaf. AIP Conf. Proc. 2019, 2162, 020021. [Google Scholar] [CrossRef]

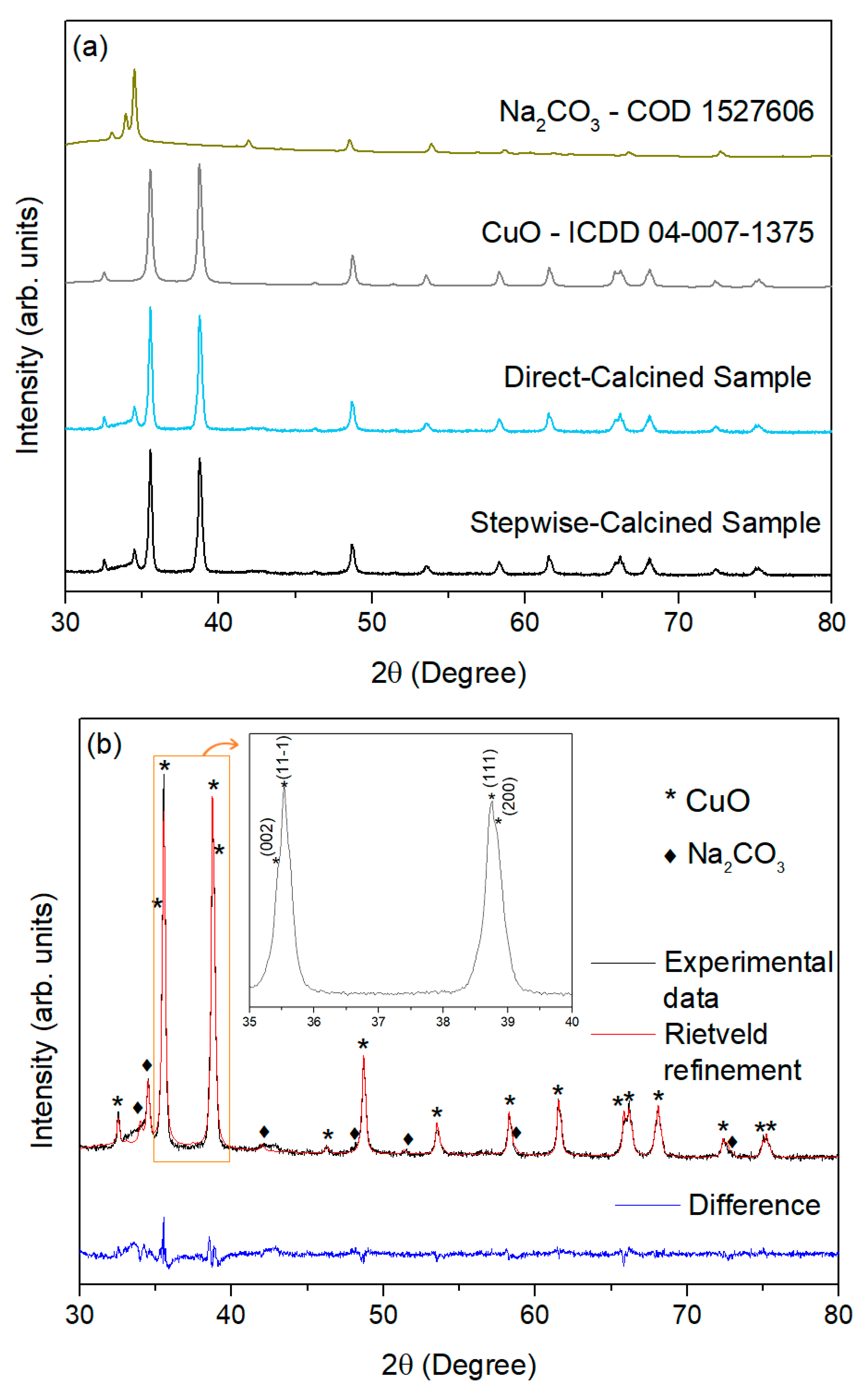
| Sample | Stepwise-Calcined Sample | Directly Calcined Sample | |||
|---|---|---|---|---|---|
| Crystalline phases | CuO | Na2CO3 | CuO | Na2CO3 | |
| Content (wt%) | 77.15 | 22.85 | 75.10 | 24.92 | |
| Rietveld fitting parameters | Rwp = 3.90 Rexp = 2.46 | GoF = 1.59 | Rwp = 3.65 Rexp = 1.76 | GoF = 2.07 | |
| Lattice parameters | a (nm) | b (nm) | c (nm) | β (°) | Crystallite size (nm) |
| CuO stepwise-calcined sample | 0.46925 | 0.34228 | 0.51342 | 99.54 | (111) 52.9 ± 0.7 |
| CuO Directly calcined sample | 0.46896 | 0.34226 | 0.51322 | 99.51 | (111) 43.0 ± 0.5 |
| CuO Reference 04-007-1375 [57] | 0.46837 | 0.34226 | 0.51288 | 99.54 | --------- |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neiva, J.; Benzarti, Z.; Carvalho, S.; Devesa, S. Green Synthesis of CuO Nanoparticles—Structural, Morphological, and Dielectric Characterization. Materials 2024, 17, 5709. https://doi.org/10.3390/ma17235709
Neiva J, Benzarti Z, Carvalho S, Devesa S. Green Synthesis of CuO Nanoparticles—Structural, Morphological, and Dielectric Characterization. Materials. 2024; 17(23):5709. https://doi.org/10.3390/ma17235709
Chicago/Turabian StyleNeiva, Joana, Zohra Benzarti, Sandra Carvalho, and Susana Devesa. 2024. "Green Synthesis of CuO Nanoparticles—Structural, Morphological, and Dielectric Characterization" Materials 17, no. 23: 5709. https://doi.org/10.3390/ma17235709
APA StyleNeiva, J., Benzarti, Z., Carvalho, S., & Devesa, S. (2024). Green Synthesis of CuO Nanoparticles—Structural, Morphological, and Dielectric Characterization. Materials, 17(23), 5709. https://doi.org/10.3390/ma17235709








