The Influence of Horsetail (Equisetum arvense L.) Powder and Horsetail-Based Silica on the Crystallization Kinetics of Polylactide
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.1.1. Raw Materials
2.1.2. Filler Preparation
2.1.3. Preparation of Composites
2.2. Methods
2.2.1. Filler Characterization
2.2.2. Non-Isothermal Crystallization
2.2.3. Isothermal Crystallization
2.2.4. Isothermal Crystallization Kinetics Evaluation
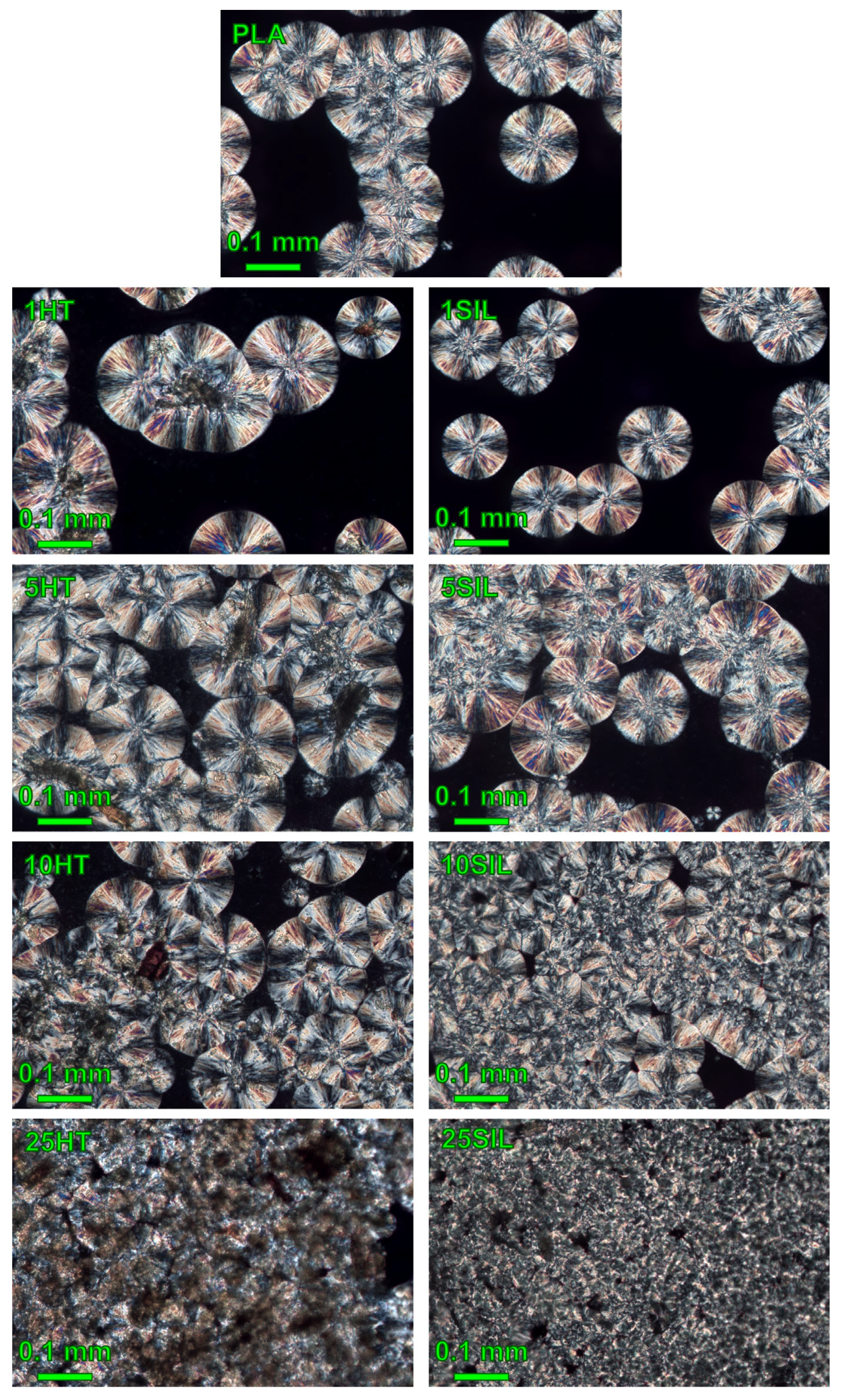
2.2.5. Microscopic Observations
3. Results
3.1. Filler Characterization
3.1.1. Particle Size Distribution
3.1.2. Surface Area and Porosity
3.1.3. Chemical Composition
3.1.4. Crystalline Structure
3.2. Crystallization of Composites
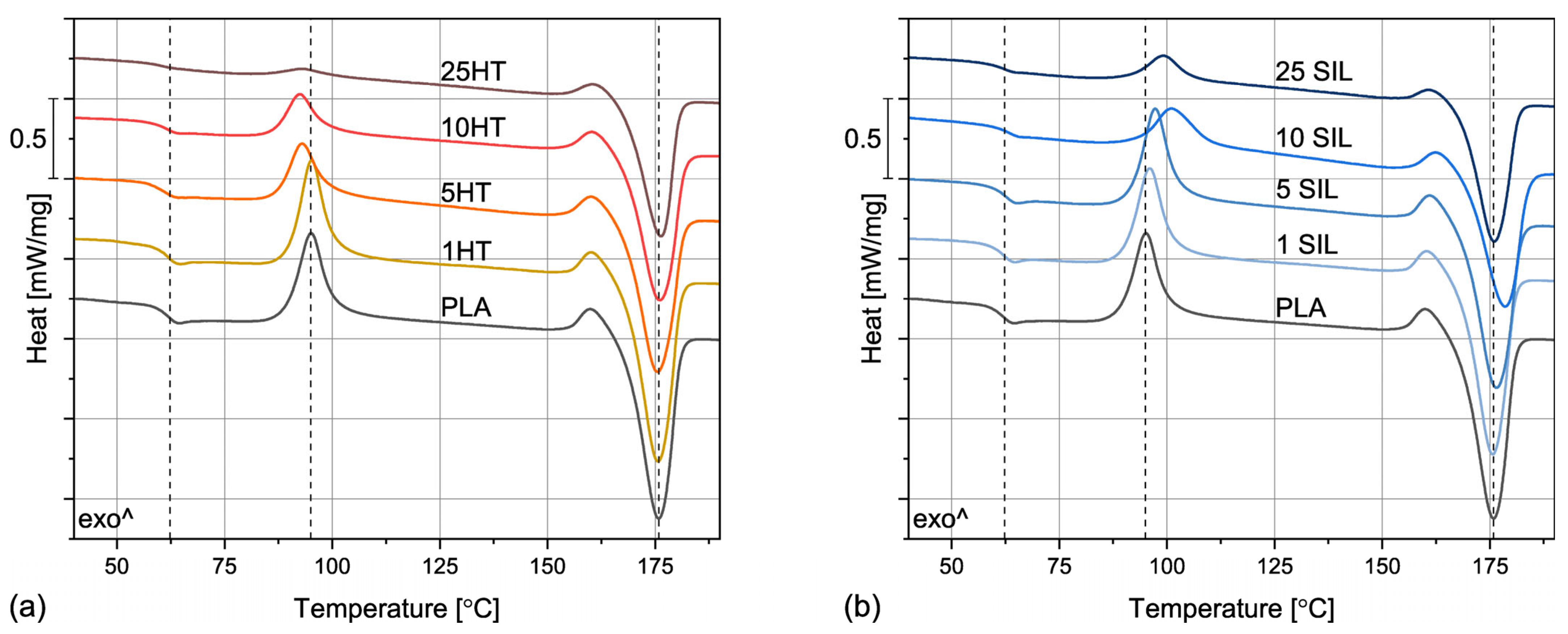
3.2.1. Non-Isothermal Crystallization
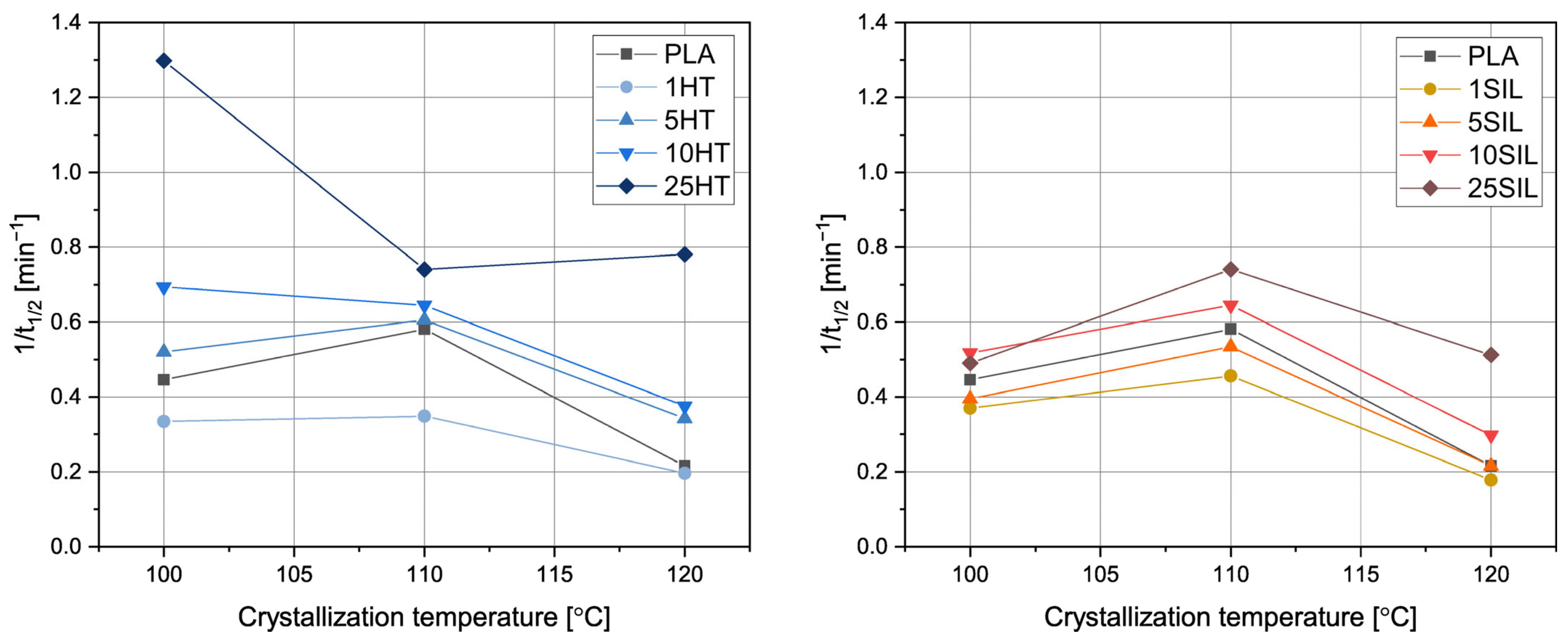
3.2.2. Isothermal Crystallization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, H.; Wang, F.; Zhang, C.; Shi, Y.; Jin, G.; Yuan, S. Preparation of Nano-Silica Materials: The Concept from Wheat Straw. J. Non-Cryst. Solids 2010, 356, 2781–2785. [Google Scholar] [CrossRef]
- Tonkha, O.; Butenko, A.; Bykova, O.; Kravchenko, Y.; Pikovska, O.; Kovalenko, V.; Evpak, I.; Masyk, I.; Zakharchenko, E. Spatial Heterogeneity of Soil Silicon in Ukrainian Phaozems and Chernozems. J. Ecol. Eng. 2020, 22, 111–119. [Google Scholar] [CrossRef] [PubMed]
- Cornelis, J.T.; Delvaux, B. Soil Processes Drive the Biological Silicon Feedback Loop. Funct. Ecol. 2016, 30, 1298–1310. [Google Scholar] [CrossRef]
- Schneider, D.; Wassersleben, S.; Weiß, M.; Denecke, R.; Stark, A.; Enke, D. A Generalized Procedure for the Production of High-Grade, Porous Biogenic Silica. Waste Biomass Valorization 2020, 11, 1–15. [Google Scholar] [CrossRef]
- Shen, Y. Rice Husk Silica Derived Nanomaterials for Sustainable Applications. Renew. Sustain. Energy Rev. 2017, 80, 453–466. [Google Scholar] [CrossRef]
- Dizaji, H.B.; Zeng, T.; Hartmann, I.; Enke, D.; Schliermann, T.; Lenz, V.; Bidabadi, M. Generation of High Quality Biogenic Silica by Combustion of Rice Husk and Rice Straw Combined with Pre- and Post-Treatment Strategies—A Review. Appl. Sci. 2019, 9, 1083. [Google Scholar] [CrossRef]
- Umeda, J.; Kondoh, K. High-Purification of Amorphous Silica Originated from Rice Husks by Combination of Polysaccharide Hydrolysis and Metallic Impurities Removal. Ind. Crops Prod. 2010, 32, 539–544. [Google Scholar] [CrossRef]
- Setiawan, W.K.; Chiang, K.Y. Crop Residues as Potential Sustainable Precursors for Developing Silica Materials: A Review. Waste Biomass Valorization 2021, 12, 2207–2236. [Google Scholar] [CrossRef]
- Soltani, N.; Bahrami, A.; Pech-Canul, M.I.; González, L.A. Review on the Physicochemical Treatments of Rice Husk for Production of Advanced Materials. Chem. Eng. J. 2015, 264, 899–935. [Google Scholar] [CrossRef]
- Zulfiqar, U.; Subhani, T.; Wilayat Husain, S. Towards Tunable Size of Silica Particles from Rice Husk. J. Non-Cryst. Solids 2015, 429, 61–69. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Delekta, M.; Czylkowska, A.; Strzelec, K. Natural Rubber Biocomposites Filled with Phyto-Ashes Rich in Biogenic Silica Obtained from Wheat Straw and Field Horsetail. Polymers 2021, 13, 1177. [Google Scholar] [CrossRef] [PubMed]
- Chun, J.; Lee, J.H. Recent Progress on the Development of Engineered Silica Particles Derived from Rice Husk. Sustainability 2020, 12, 10683. [Google Scholar] [CrossRef]
- Fuad, M.Y.A.; Jamaludin, M.; Ishak, Z.A.M.; Omar, Z.A.M. Rice Husk Ash as Fillers in Polypropylene: A Preliminary Study. Int. J. Polym. Mater. Polym. Biomater. 1993, 19, 75–92. [Google Scholar] [CrossRef]
- Kawai, F.; Nakadai, K.; Nishioka, E.; Nakajima, H.; Ohara, H.; Masaki, K.; Iefuji, H. Different Enantioselectivity of Two Types of Poly(Lactic Acid) Depolymerases toward Poly(l-Lactic Acid) and Poly(d-Lactic Acid). Polym. Degrad. Stab. 2011, 96, 1342–1348. [Google Scholar] [CrossRef]
- Castro-Aguirre, E.; Iñiguez-Franco, F.; Samsudin, H.; Fang, X.; Auras, R. Poly(Lactic Acid)—Mass Production, Processing, Industrial Applications, and End of Life. Adv. Drug Deliv. Rev. 2016, 107, 333–366. [Google Scholar] [CrossRef]
- Lim, L.-T.; Auras, R.; Rubino, M. Processing Technologies for Poly(Lactic Acid). Prog. Polym. Sci. 2008, 33, 820–852. [Google Scholar] [CrossRef]
- Auras, R.; Harte, B.; Selke, S. An Overview of Polylactides as Packaging Materials. Macromol. Biosci. 2004, 4, 835–864. [Google Scholar] [CrossRef]
- Nagarajan, V.; Zhang, K.; Misra, M.; Mohanty, A.K. Overcoming the Fundamental Challenges in Improving the Impact Strength and Crystallinity of PLA Biocomposites: Influence of Nucleating Agent and Mold Temperature. ACS Appl. Mater. Interfaces 2015, 7, 11203–11214. [Google Scholar] [CrossRef]
- Battegazzore, D.; Bocchini, S.; Alongi, J.; Frache, A. Rice Husk as Bio-Source of Silica: Preparation and Characterization of PLA-Silica Bio-Composites. RSC Adv. 2014, 4, 54703–54712. [Google Scholar] [CrossRef]
- Tipachan, C.; Gupta, R.K.; Kajorncheappunngam, S. Water Vapor Barrier Property of PLA Nanocomposites Using Rice Husk Ash and Layered Double Hydroxides as Fillers. Eng. Appl. Sci. Res. 2019, 46, 285–291. [Google Scholar] [CrossRef]
- Masnar, A.; Coorey, R. Application of Sago Pith Waste and Nanosilica from Rice Husk Ash as Hybrid Bio-Nanofiller Composite for Food Plastic Packaging. Ukr. Food J. 2017, 6, 618–631. [Google Scholar] [CrossRef]
- Tipachan, C.; Gupta, R.K.; Agarwal, S.; Kajorncheappunngam, S. Flame Retardant Properties and Thermal Stability of Polylactic Acid Filled with Layered Double Hydroxide and Rice Husk Ash Silica. J. Polym. Environ. 2020, 28, 948–961. [Google Scholar] [CrossRef]
- Carneiro, D.M.; Jardim, T.V.; Araújo, Y.C.L.; Arantes, A.C.; de Sousa, A.C.; Barroso, W.K.S.; Sousa, A.L.L.; da Cunha, L.C.; Cirilo, H.N.C.; Bara, M.T.F.; et al. Equisetum arvense: New Evidences Supports Medical Use in Daily Clinic. Pharmacogn. Rev. 2021, 13, 50–58. [Google Scholar] [CrossRef]
- Holzhüter, G.; Narayanan, K.; Gerber, T. Structure of Silica in Equisetum arvense. Anal. Bioanal. Chem. 2003, 376, 512–517. [Google Scholar] [CrossRef] [PubMed]
- Mimica-Dukic, N.; Simin, N.; Cvejic, J.; Jovin, E.; Orcic, D.; Bozin, B. Phenolic Compounds in Field Horsetail (Equisetum arvense L.) as Natural Antioxidants. Molecules 2008, 13, 1455–1464. [Google Scholar] [CrossRef]
- Masłowski, M.; Miedzianowska, J.; Czylkowska, A.; Strzelec, K. Horsetail (Equisetum arvense) as a Functional Filler for Natural Rubber Biocomposites. Materials 2020, 13, 2526. [Google Scholar] [CrossRef]
- Miedzianowska, J.; Masłowski, M.; Strzelec, K. Improving Performance of Natural Rubber Composites by the Application of Functional Biofiller: Horsetail Modified with Silane Coupling Agents. Cellulose 2023, 30, 10175–10198. [Google Scholar] [CrossRef]
- Szatkowski, P.; Szatkowska, M.; Gralewski, J.; Czechowski, L.; Kedziora, S. Biocomposites with Epoxy Resin Matrix Modified with Ingredients of Natural Origin. Materials 2022, 15, 7167. [Google Scholar] [CrossRef]
- Mysiukiewicz, O.; Barczewski, M.; Skórczewska, K.; Matykiewicz, D. Correlation between Processing Parameters and Degradation of Different Polylactide Grades during Twin-Screw Extrusion. Polymers 2020, 12, 1333. [Google Scholar] [CrossRef]
- Fischer, E.W.; Sterzel, H.J.; Wegner, G. Investigation of the Structure of Solution Grown Crystals of Lactide Copolymers by Means of Chemical Reactions. Colloid Polym. Sci. 1973, 251, 980–990. [Google Scholar]
- Mandelkern, L. Crystallization of Polymers. Volume 2: Kinetics and Mechanisms; Cambridge University Press: New York, NY, USA, 2004; ISBN 978-0-521-81682-3. [Google Scholar]
- Lorenzo, A.T.; Arnal, M.L.; Albuerne, J.; Müller, A.J. DSC Isothermal Polymer Crystallization Kinetics Measurements and the Use of the Avrami Equation to Fit the Data: Guidelines to Avoid Common Problems. Polym. Test. 2007, 26, 222–231. [Google Scholar] [CrossRef]
- Hosseini Mohtasham, N.; Gholizadeh, M. Horsetail Plant (Equisetum arvense) and Horsetail Plant Ash: Application and Comparison of Their Catalytic Activities as Novel and Natural Porous Lewis Acid Catalysts for the One-Pot Green Synthesis of 2-Amino-4H-Chromene Derivatives under Solvent-Free Conditions. J. Iran. Chem. Soc. 2020, 17, 397–409. [Google Scholar] [CrossRef]
- Tan, Y.; Wang, K.; Yan, Q.; Zhang, S.; Li, J.; Ji, Y. Synthesis of Amino-Functionalized Waste Wood Flour Adsorbent for High-Capacity Pb(II) Adsorption. ACS Omega 2019, 4, 10475–10484. [Google Scholar] [CrossRef] [PubMed]
- Allen, T. Particle Size Measurement; Chapman and Hall: London, UK, 1990; ISBN 041235070X. [Google Scholar]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S.W. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Alothman, Z.A. A Review: Fundamental Aspects of Silicate Mesoporous Materials. Materials 2012, 5, 2874–2902. [Google Scholar] [CrossRef]
- Santana Costa, J.A.; Paranhos, C.M. Systematic Evaluation of Amorphous Silica Production from Rice Husk Ashes. J. Clean. Prod. 2018, 192, 688–697. [Google Scholar] [CrossRef]
- Yuvakkumar, R.; Elango, V.; Rajendran, V.; Kannan, N. High-Purity Nano Silica Powder from Rice Husk Using a Simple Chemical Method. J. Exp. Nanosci. 2014, 9, 272–281. [Google Scholar] [CrossRef]
- Sola-Rabada, A.; Rinck, J.; Belton, D.J.; Powell, A.K.; Perry, C.C. Isolation of a Wide Range of Minerals from a Thermally Treated Plant: Equisetum arvense, a Mare’s Tale Topical Issue in Honor of R.J.P. Williams. J. Biol. Inorg. Chem. 2016, 21, 101–112. [Google Scholar] [CrossRef]
- Darie-Niță, R.N.; Irimia, A.; Grigoraș, V.C.; Mustață, F.; Tudorachi, N.; Râpă, M.; Ludwiczak, J.; Iwanczuk, A. Evaluation of Natural and Modified Castor Oil Incorporation on the Melt Processing and Physico-Chemical Properties of Polylactic Acid. Polymers 2022, 14, 3608. [Google Scholar] [CrossRef]
- Bouti, M.; Irinislimane, R.; Belhaneche-Bensemra, N. Properties Investigation of Epoxidized Sunflower Oil as Bioplasticizer for Poly (Lactic Acid). J. Polym. Environ. 2022, 30, 232–245. [Google Scholar] [CrossRef]
- Nair, D.G.; Jagadish, K.S.; Fraaij, A. Reactive Pozzolanas from Rice Husk Ash: An Alternative to Cement for Rural Housing. Cem. Concr. Res. 2006, 36, 1062–1071. [Google Scholar] [CrossRef]
- Venezia, A.M.; la Parola, V.; Longo, A.; Martorana, A. Effect of Alkali Ions on the Amorphous to Crystalline Phase Transition of Silica. J. Solid State Chem. 2001, 161, 373–378. [Google Scholar] [CrossRef]
- Mysiukiewicz, O.; Barczewski, M. Crystallization of Polylactide-Based Green Composites Filled with Oil-Rich Waste Fillers. J. Polym. Res. 2020, 27, 374. [Google Scholar] [CrossRef]
- Atanase, L.I.; Glaied, O.; Riess, G. Crystallization Kinetics of PCL Tagged with Well-Defined Positional Triazole Defects Generated by Click Chemistry. Polymer 2011, 52, 3074–3081. [Google Scholar] [CrossRef]
- Li, H.; Huneault, M.A. Crystallization of PLA/Thermoplastic Starch Blends. Int. Polym. Process. 2008, 23, 412–418. [Google Scholar] [CrossRef]
- Blázquez-Blázquez, E.; Barranco-García, R.; Díez-Rodríguez, T.M.; Cerrada, M.L.; Pérez, E. Role of the Plasticizers on the Crystallization of PLA and Its Composites with Mesoporous MCM-41. J. Mater. Sci. 2024, 59, 6305–6321. [Google Scholar] [CrossRef]
- Hakim, R.H.; Cailloux, J.; Santana, O.O.; Bou, J.; Sánchez-Soto, M.; Odent, J.; Raquez, J.M.; Dubois, P.; Carrasco, F.; Maspoch, M.L. PLA/SiO2 Composites: Influence of the Filler Modifications on the Morphology, Crystallization Behavior, and Mechanical Properties. J. Appl. Polym. Sci. 2017, 134, 45367. [Google Scholar] [CrossRef]
- Morales, J.; Michell, R.M.; Sommer-Márquez, A.; Rodrigue, D. Effect of Biobased SiO2 on the Morphological, Thermal, Mechanical, Rheological, and Permeability Properties of PLLA/PEG/SiO2 Biocomposites. J. Compos. Sci. 2023, 7, 150. [Google Scholar] [CrossRef]
- Lorenzo, A.T.; Müller, A.J. Estimation of the Nucleation and Crystal Growth Contributions to the Overall Crystallization Energy Barrier. J. Polym. Sci. B Polym. Phys. 2008, 46, 1478–1487. [Google Scholar] [CrossRef]
- Wu, D.; Cheng, Y.; Feng, S.; Yao, Z.; Zhang, M. Crystallization Behavior of Polylactide/Graphene Composites. Ind. Eng. Chem. Res. 2013, 52, 6731–6739. [Google Scholar] [CrossRef]
- Yang, B.; Wan, X. Trace Sorbitol-Modified Nano-Silica: Towards Nano-Nucleation for Poly(L-Lactic Acid). Int. J. Biol. Macromol. 2024, 274, 133236. [Google Scholar] [CrossRef] [PubMed]
- Papadopoulos, L.; Klonos, P.A.; Terzopoulou, Z.; Psochia, E.; Sanusi, O.M.; Hocine, N.A.; Benelfellah, A.; Giliopoulos, D.; Triantafyllidis, K.; Kyritsis, A.; et al. Comparative Study of Crystallization, Semicrystalline Morphology, and Molecular Mobility in Nanocomposites Based on Polylactide and Various Inclusions at Low Filler Loadings. Polymer 2021, 217, 123457. [Google Scholar] [CrossRef]
- Klonos, P.; Pissis, P. Effects of Interfacial Interactions and of Crystallization on Rigid Amorphous Fraction and Molecular Dynamics in Polylactide/Silica Nanocomposites: A Methodological Approach. Polymer 2017, 112, 228–243. [Google Scholar] [CrossRef]
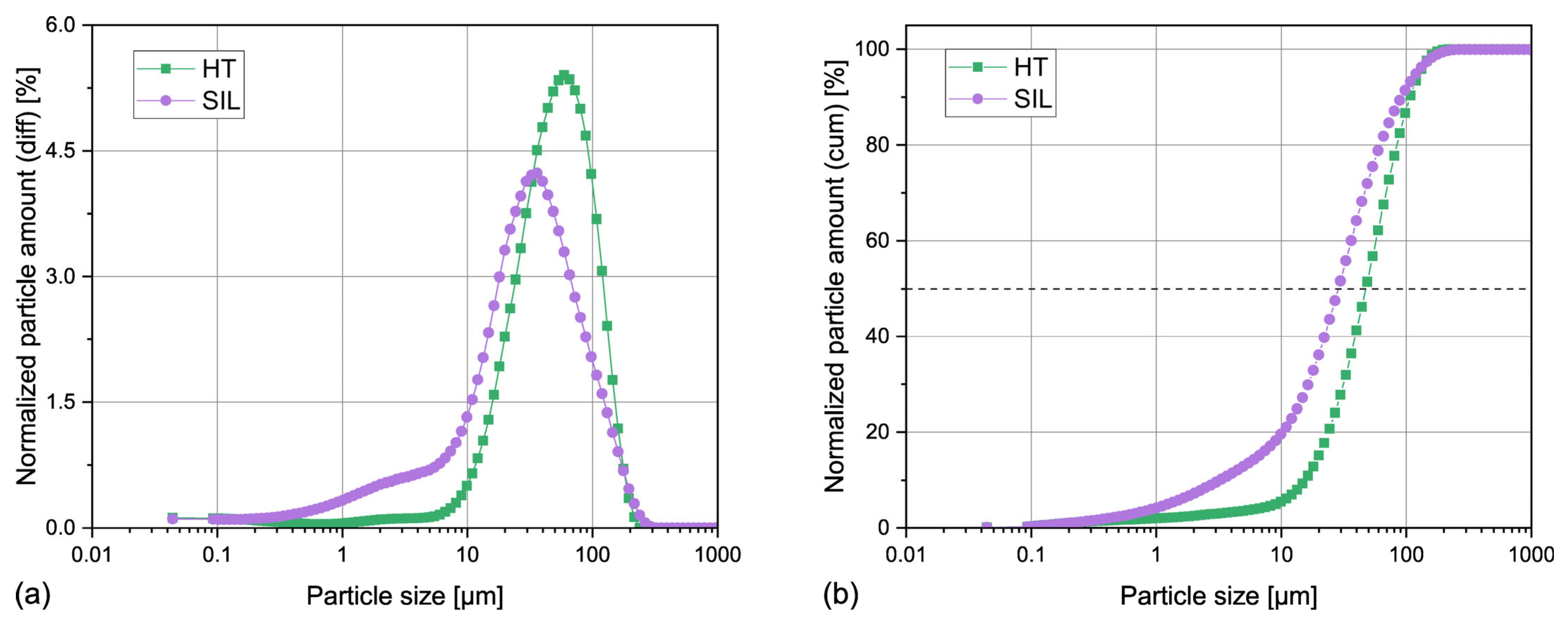
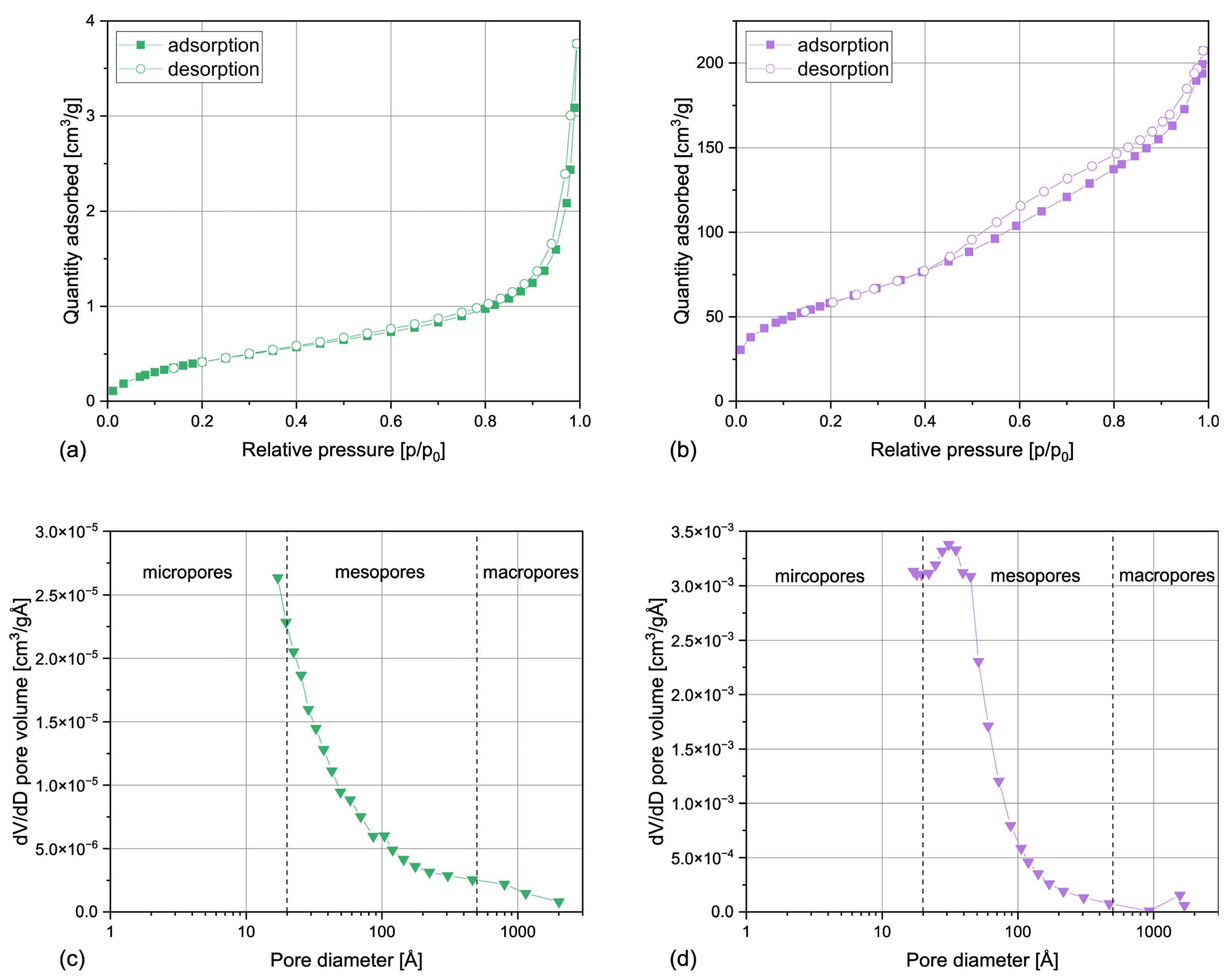
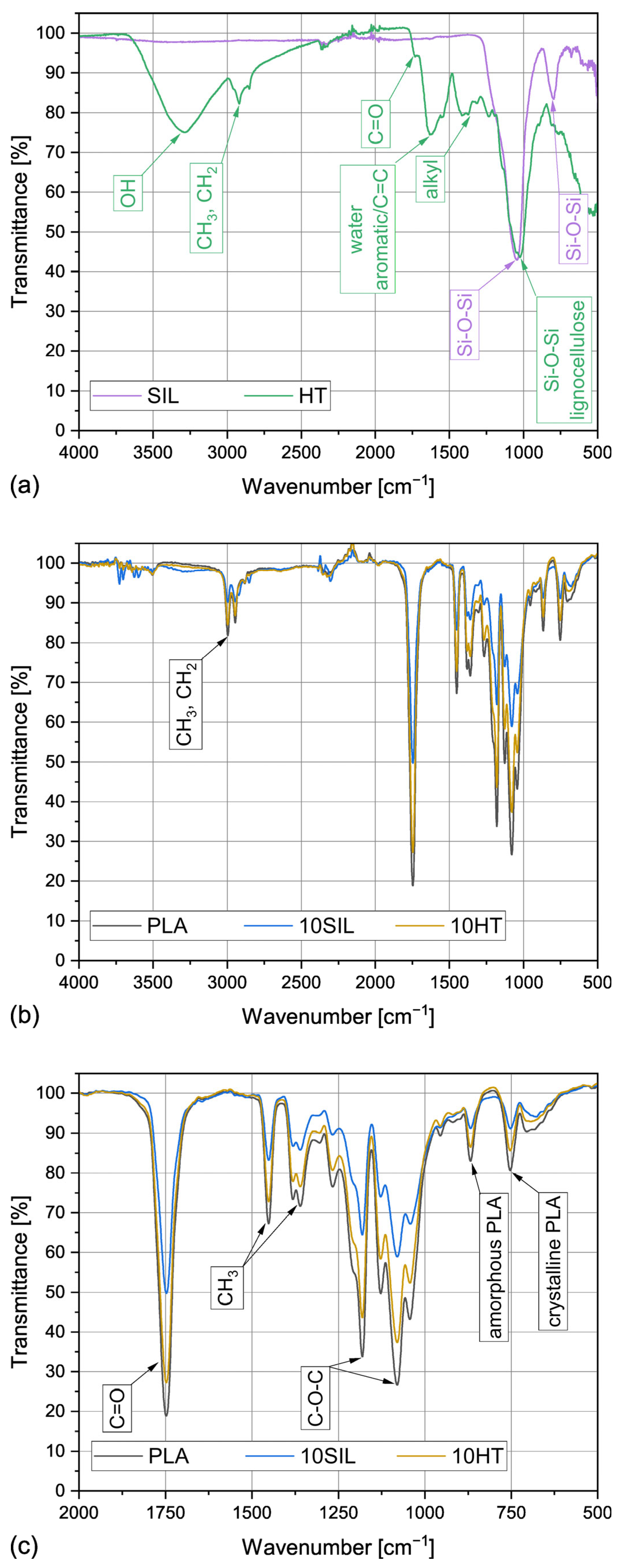
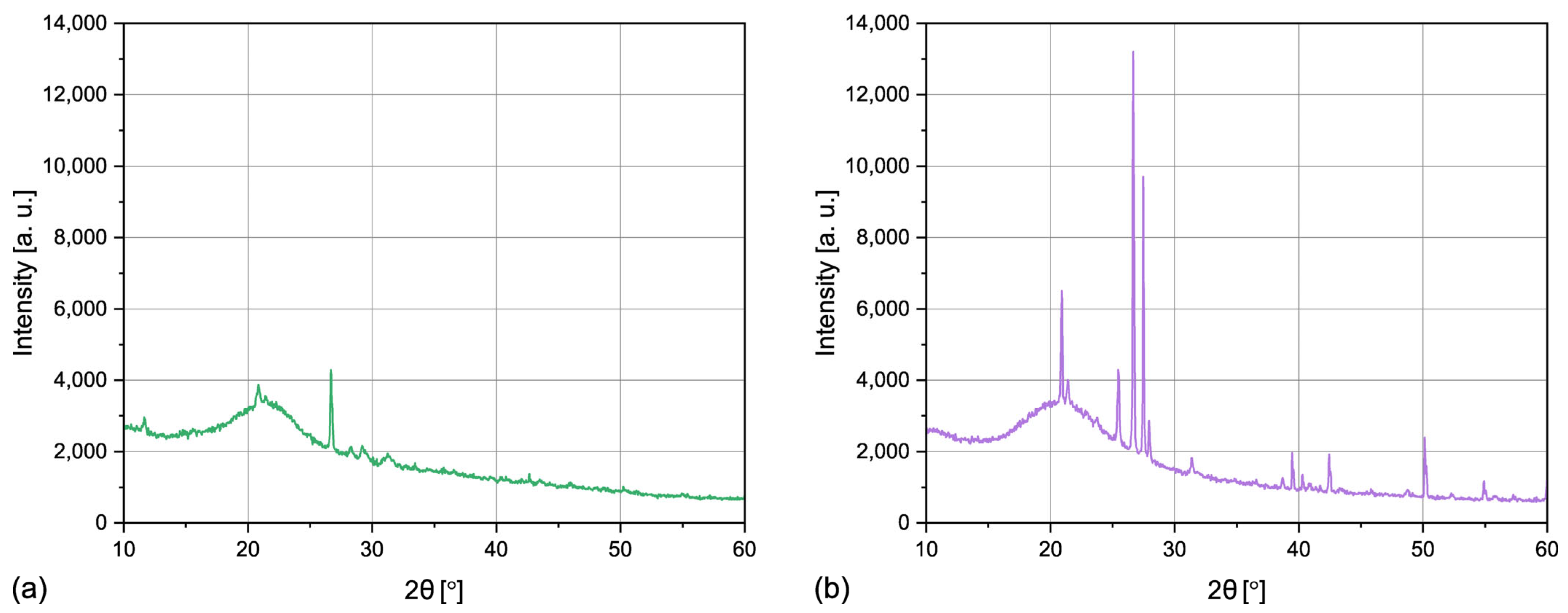

| Sample | SBET | SP | 4V/A |
|---|---|---|---|
| m2/g | m2/g | Å | |
| HT | 2 | 2 | 120 |
| SIL | 214 | 241 | 56 |
| Sample | TG [°C] | TCC [°C] | TM [°C] | TCR [°C] | XCR [%] |
|---|---|---|---|---|---|
| PLA | 62.3 | 95.0 | 175.8 | - | 25.5 |
| 1HT | 61.8 | 95.2 | 175.7 | - | 22.9 |
| 5HT | 61.3 | 93.0 | 175.6 | 99.1 | 34.9 |
| 10HT | 61.4 | 92.6 | 176.2 | 97.6 | 38.2 |
| 25HT | 62.4 | 92.9 | 176.3 | 99.4 | 52.5 |
| 1SIL | 62.4 | 95.9 | 175.7 | - | 22.7 |
| 5SIL | 63.1 | 97.2 | 176.4 | - | 25.8 |
| 10SIL | 63.4 | 101.1 | 178.4 | 99.2 | 41.2 |
| 25SIL | 63.1 | 99.1 | 175.9 | 103.2 | 51.1 |
| Temperature | Sample | t1/2 [min] | t1/2* [min] | n [-] | k [min−n] | R2 |
|---|---|---|---|---|---|---|
| 100 °C | PLA | 2.24 | 2.31 | 2.48 | 0.087 | 0.99997 |
| 1HT | 2.99 | 3.08 | 2.28 | 0.053 | 0.99999 | |
| 5HT | 1.92 | 1.92 | 2.55 | 0.131 | 0.99998 | |
| 10HT | 1.44 | 1.45 | 2.67 | 0.258 | 0.99999 | |
| 25HT | 0.77 | 0.77 | 2.86 | 1.462 | 0.99999 | |
| 1SIL | 2.70 | 2.79 | 2.38 | 0.060 | 0.99997 | |
| 5SIL | 2.53 | 2.63 | 2.40 | 0.068 | 0.99994 | |
| 10SIL | 1.93 | 1.97 | 2.57 | 0.122 | 0.99998 | |
| 25SIL | 2.04 | 1.97 | 2.40 | 0.137 | 0.99988 | |
| 110 °C | PLA | 1.72 | 1.74 | 2.55 | 0.169 | 0.99999 |
| 1HT | 2.87 | 2.95 | 2.26 | 0.060 | 0.99999 | |
| 5HT | 1.65 | 1.61 | 2.42 | 0.219 | 0.99991 | |
| 10HT | 1.55 | 1.55 | 2.73 | 0.209 | 0.99999 | |
| 25HT | 0.79 | 0.76 | 2.79 | 1.503 | 0.99993 | |
| 1SIL | 2.19 | 2.22 | 2.51 | 0.094 | 0.99999 | |
| 5SIL | 1.87 | 1.87 | 2.59 | 0.134 | 0.99999 | |
| 10SIL | 1.55 | 1.55 | 2.73 | 0.209 | 0.99999 | |
| 25SIL | 1.35 | 1.28 | 2.53 | 0.369 | 0.99987 | |
| 120 °C | PLA | 4.64 | 4.41 | 1.90 | 0.041 | 0.99879 |
| 1HT | 5.09 | 5.09 | 1.81 | 0.036 | 0.99946 | |
| 5HT | 2.92 | 2.85 | 2.27 | 0.064 | 0.99992 | |
| 10HT | 2.66 | 2.65 | 2.43 | 0.065 | 0.99998 | |
| 25HT | 1.28 | 1.25 | 2.63 | 0.389 | 0.99997 | |
| 1SIL | 5.61 | 5.99 | 1.77 | 0.029 | 0.99958 | |
| 5SIL | 4.64 | 4.96 | 1.94 | 0.031 | 0.99997 | |
| 10SIL | 3.36 | 3.42 | 2.26 | 0.043 | 0.99999 | |
| 25SIL | 1.95 | 1.86 | 2.39 | 0.157 | 0.99989 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mysiukiewicz, O.; Szulc, J.; Miklaszewski, A. The Influence of Horsetail (Equisetum arvense L.) Powder and Horsetail-Based Silica on the Crystallization Kinetics of Polylactide. Materials 2024, 17, 5697. https://doi.org/10.3390/ma17235697
Mysiukiewicz O, Szulc J, Miklaszewski A. The Influence of Horsetail (Equisetum arvense L.) Powder and Horsetail-Based Silica on the Crystallization Kinetics of Polylactide. Materials. 2024; 17(23):5697. https://doi.org/10.3390/ma17235697
Chicago/Turabian StyleMysiukiewicz, Olga, Joanna Szulc, and Andrzej Miklaszewski. 2024. "The Influence of Horsetail (Equisetum arvense L.) Powder and Horsetail-Based Silica on the Crystallization Kinetics of Polylactide" Materials 17, no. 23: 5697. https://doi.org/10.3390/ma17235697
APA StyleMysiukiewicz, O., Szulc, J., & Miklaszewski, A. (2024). The Influence of Horsetail (Equisetum arvense L.) Powder and Horsetail-Based Silica on the Crystallization Kinetics of Polylactide. Materials, 17(23), 5697. https://doi.org/10.3390/ma17235697









