Synthesis of Polyphosphate Flame Retardant Bisphenol AP Bis(Diphenyl Phosphate) and Its Application in Polycarbonate/Acrylonitrile-Butadiene-Styrene
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
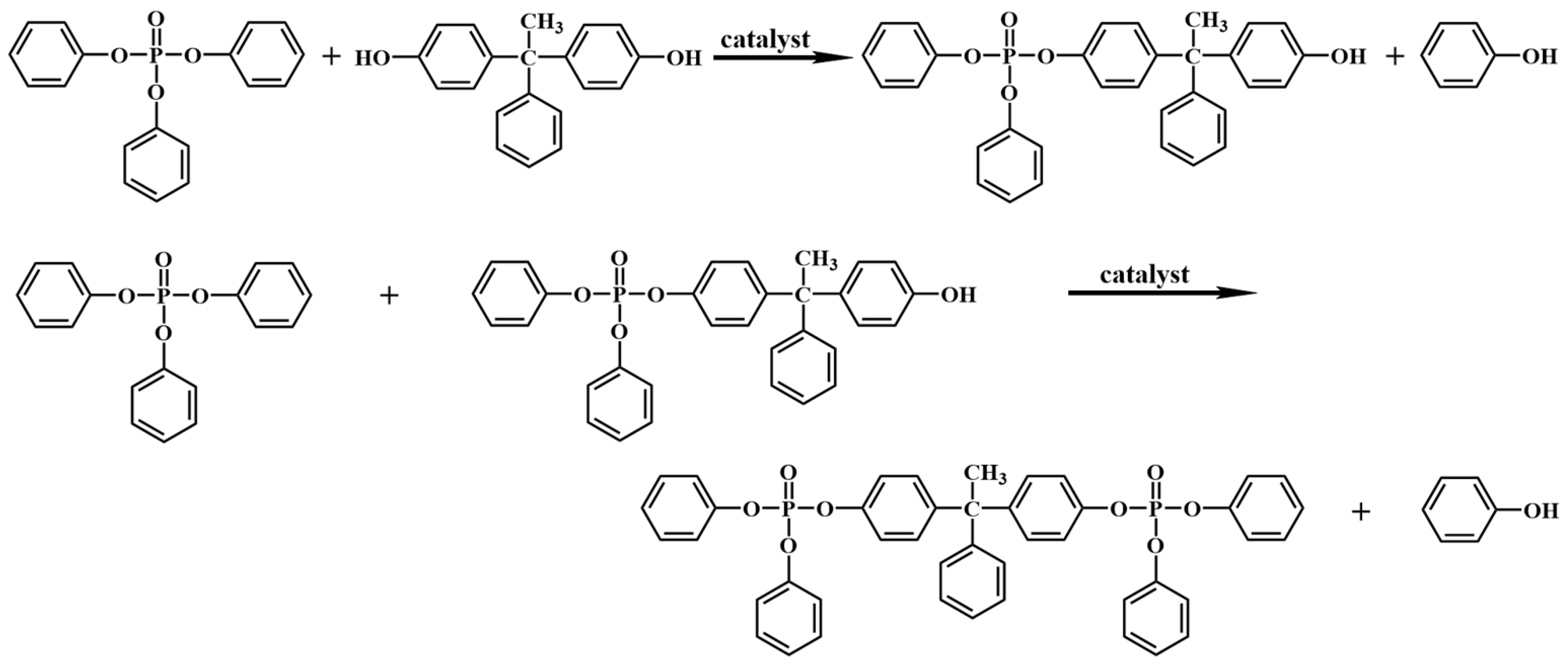
2.2. Synthesis of BAPDP
2.3. Preparation of PC/ABS/BAPDP Composites
2.4. Characterization
3. Results and Discussion
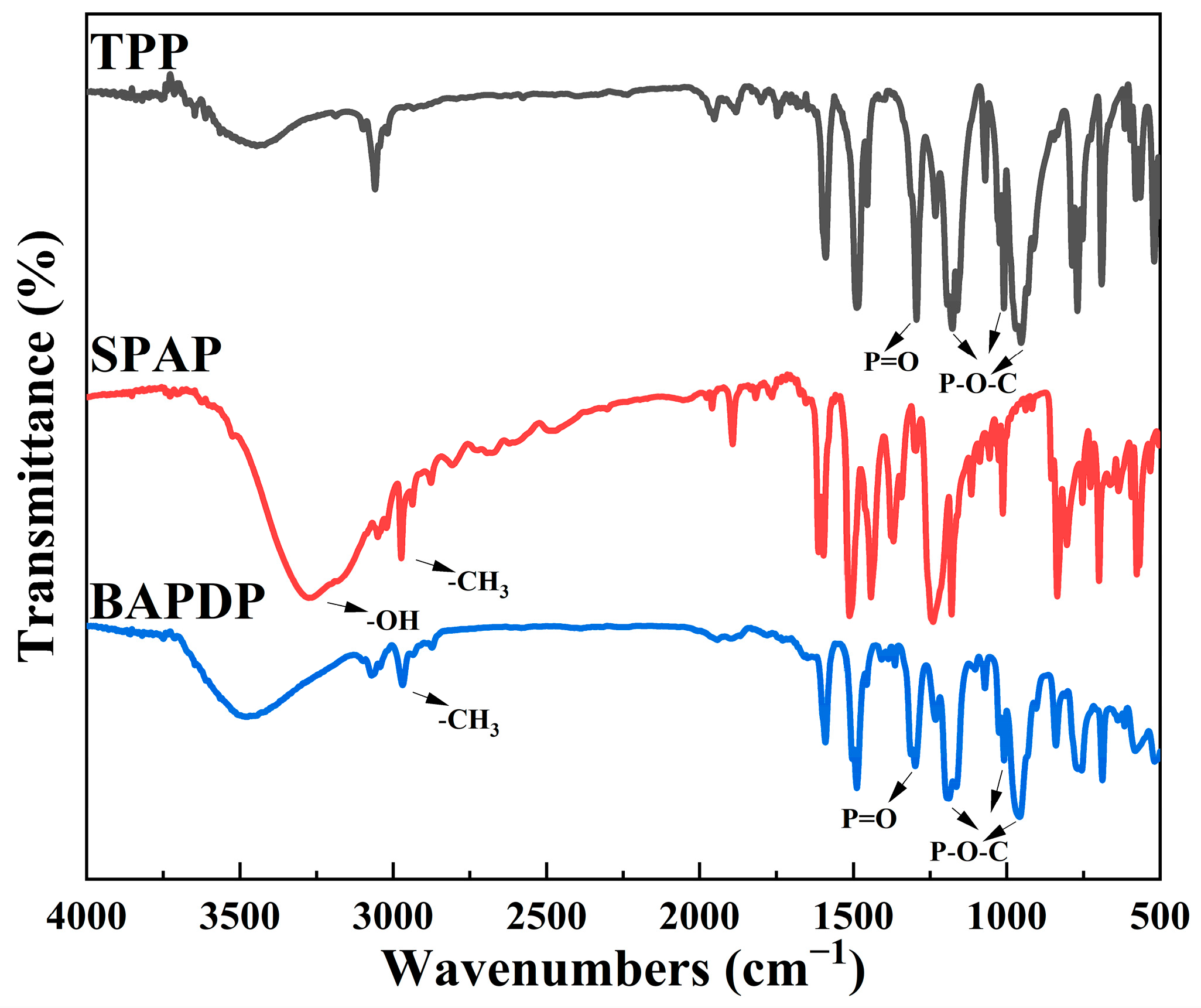
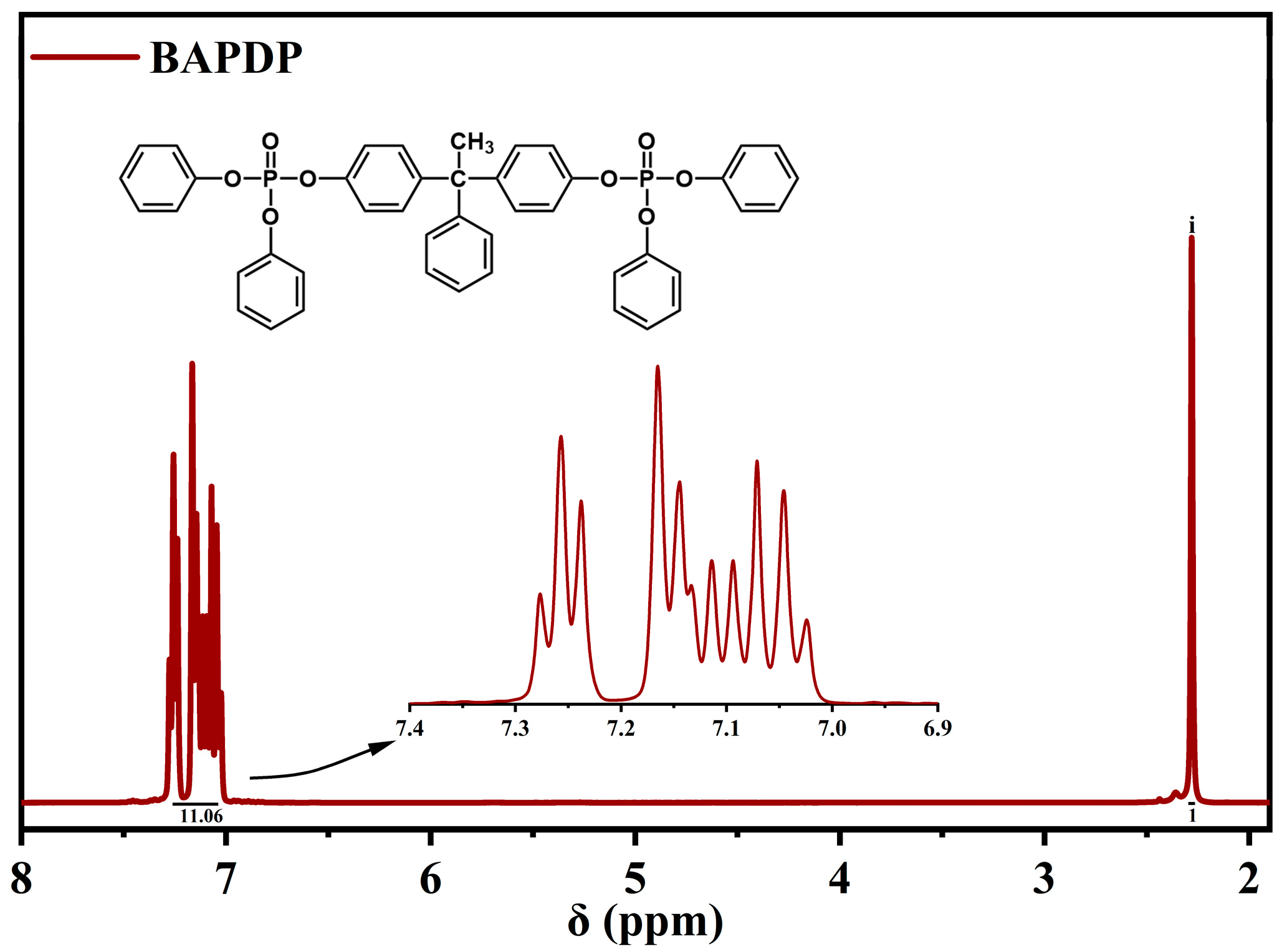
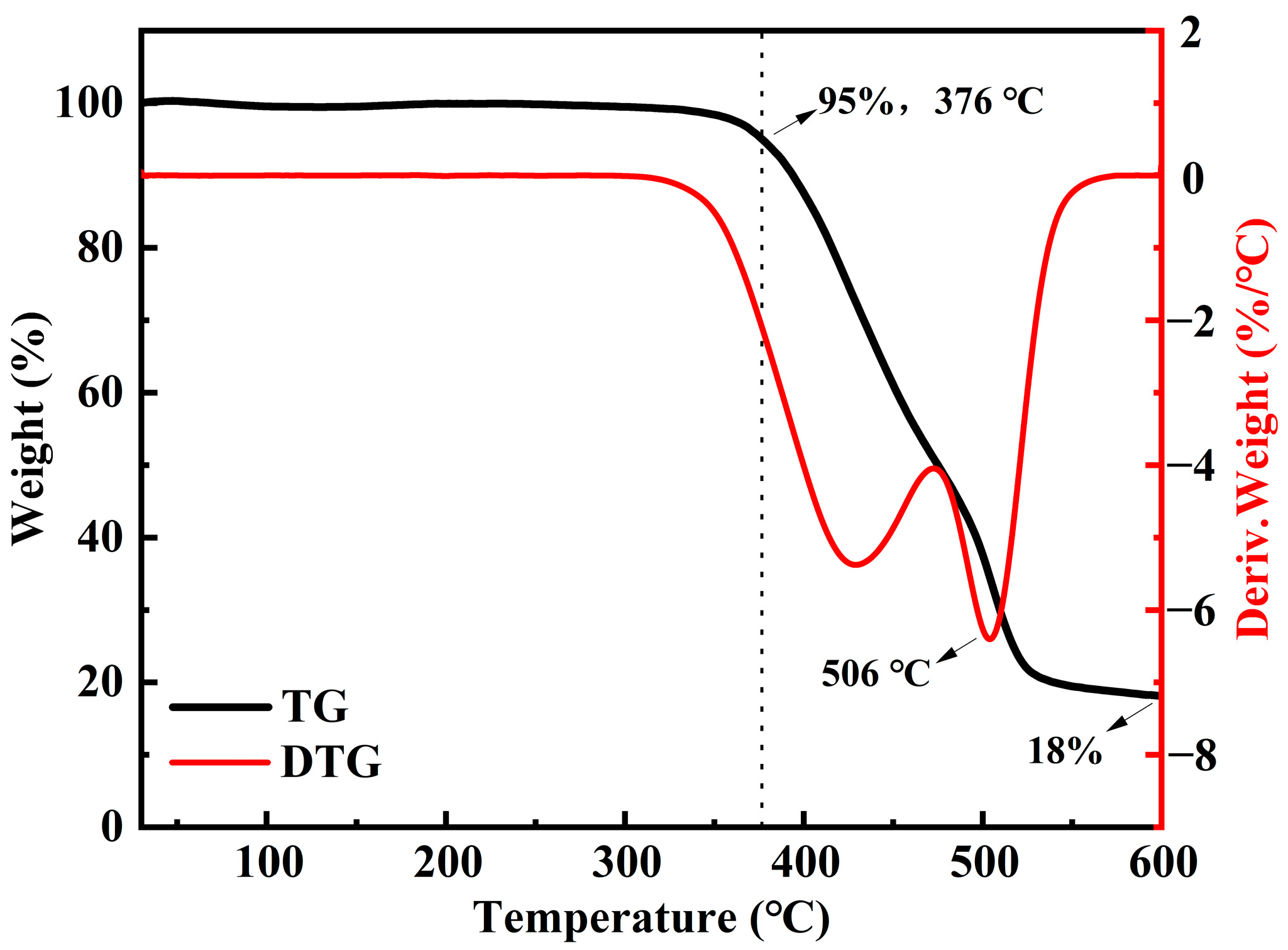
3.1. Characterizations of BAPDP
3.2. Effect of BAPDP on Flame Retardancy of PC/ABS
3.3. Effects of BAPDP on Mechanical Properties of PC/ABS
3.4. Effect of BAPDP on Thermal Deformation Temperature of PC/ABS
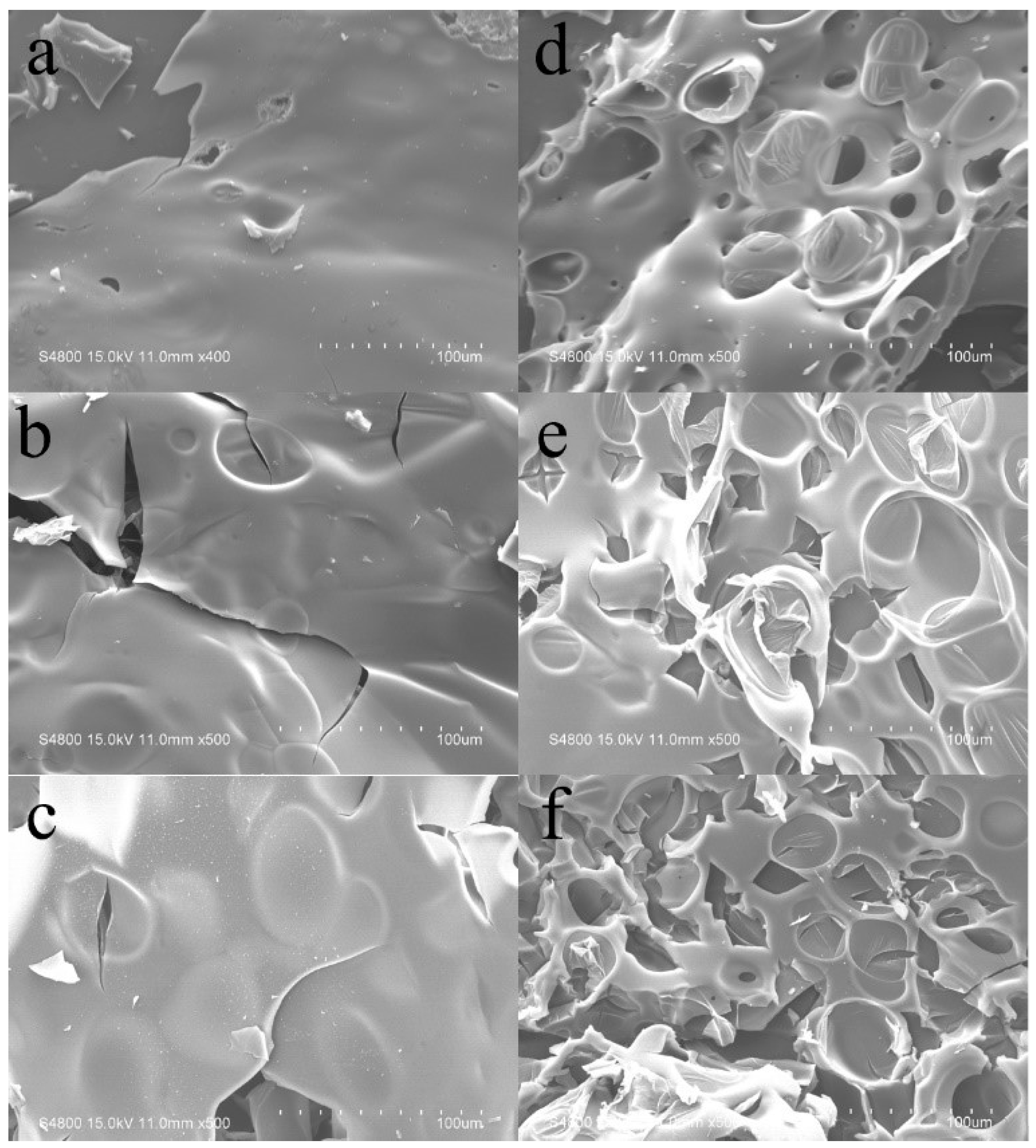
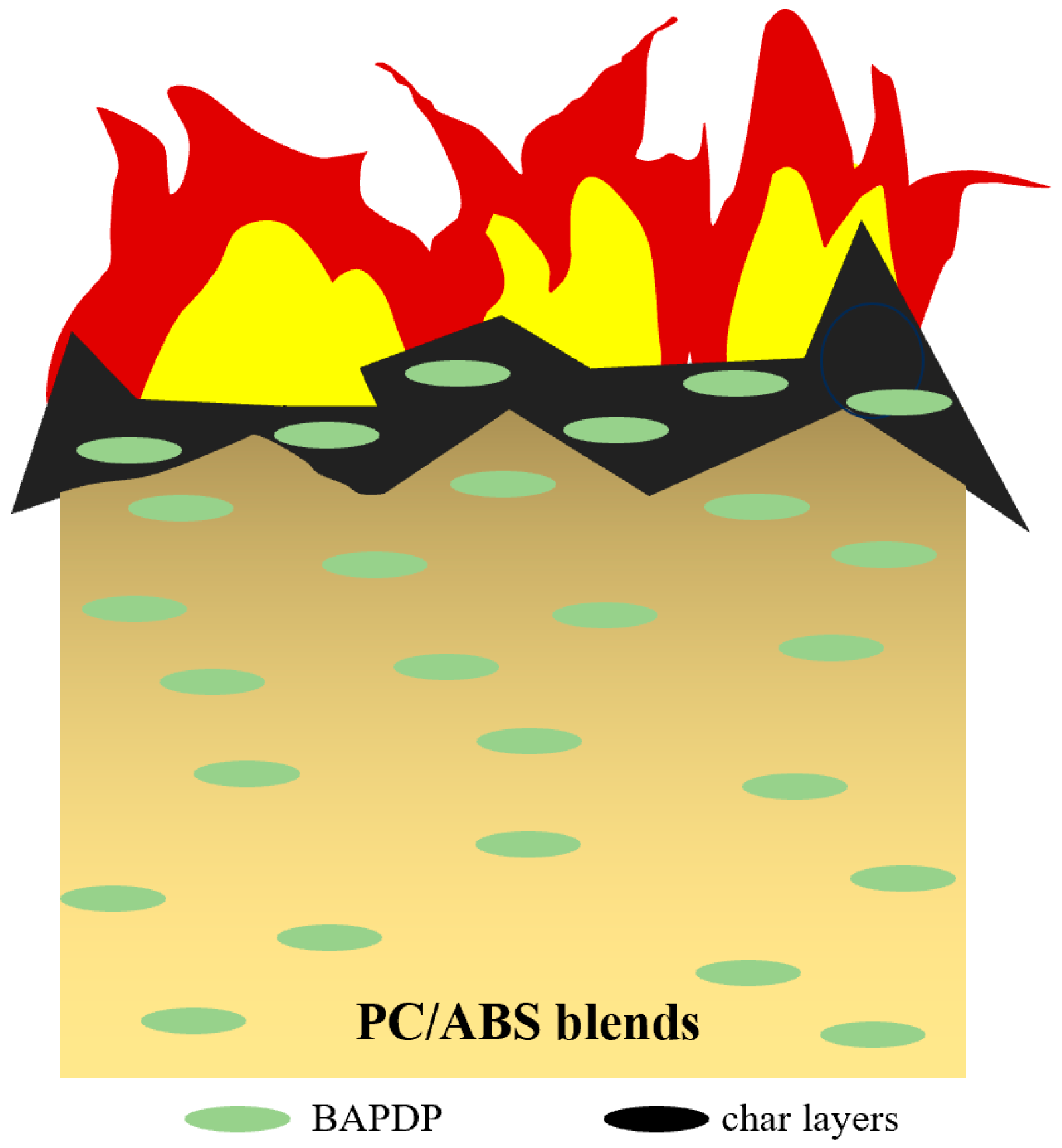
3.5. Flame-Retardant Mode of Action for BAPDP in PC/ABS Blends
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, L.; Huang, H.Z.; Wang, Y.Z.; Jow, J.; Su, K. Transesterification-controlled compatibility and microfibrillation in PC-ABS composites reinforced by phosphorus-containing thermotropic liquid crystalline polyester. Polymer 2009, 50, 3037–3046. [Google Scholar] [CrossRef]
- Bee, S.T.; Lim, K.S.; Sin, L.T.; Ratnam, C.T.; Bee, S.L.; Rahmat, A.R. Interactive effect of ammonium polyphosphate and montmorillonite on enhancing flame retardancy of polycarbonate/acrylonitrile butadiene styrene composites. Iran. Polym. J. 2018, 27, 899–911. [Google Scholar] [CrossRef]
- Wang, J.C.; Wang, H.; Yue, D.B. Optimization of Surface Treatment Using Sodium Hypochlorite Facilitates Coseparation of ABS and PC from WEEE Plastics by Flotation. Environ. Sci. Technol. 2019, 53, 2086–2094. [Google Scholar] [CrossRef] [PubMed]
- Alaee, M.; Arias, P.; Sjödin, A.; Bergman, Å. An overview of commercially used brominated flame retardants, their applications, their use patterns in different countries/regions and possible modes of release. Environ. Int. 2003, 29, 683–689. [Google Scholar] [CrossRef] [PubMed]
- Aquino, F.W.B.; Paranhos, C.M.; Pereira, E.R. Method for the production of acrylonitrile-butadiene-styrene (ABS) and polycarbonate (PC)/ABS standards for direct Sb determination in plastics from e-waste using laser-induced breakdown spectroscopy. J. Anal. At. Spectrom. 2016, 31, 1228–1233. [Google Scholar] [CrossRef]
- Wang, W.H.; Zhao, G.Q.; Wu, X.H.; Li, X.H.; Wang, C.G. Investigation on phosphorus halogen-free flame-retardancy systems in short glass fiber-reinforced PC/ABS composites under rapid thermal cycle molding process condition. Polym. Compos. 2015, 36, 1653–1663. [Google Scholar] [CrossRef]
- Darnerud, P.O. Brominated flame retardants as possible endocrine disrupters. Int. J. Androl. 2008, 31, 152–160. [Google Scholar] [CrossRef]
- Kim, J.; Lee, K.; Lee, K.; Bae, J.; Yang, J.; Hong, S. Studies on the thermal stabilization enhancement of ABS; synergistic effect of triphenyl phosphate nanocomposite, epoxy resin, and silane coupling agent mixtures. Polym. Degrad. Stabil. 2003, 79, 201–207. [Google Scholar] [CrossRef]
- Wu, Q.; Qu, B.J. Synergistic effects of silicotungistic acid on intumescent flame-retardant polypropylene. Polym. Degrad. Stabil. 2001, 74, 255–261. [Google Scholar] [CrossRef]
- Chen, L.; Wang, Y.Z. Aryl Polyphosphonates: Useful Halogen-Free Flame Retardants for Polymers. Materials 2010, 3, 4746–4760. [Google Scholar] [CrossRef]
- Liu, Q.Y.; Wang, D.H.; Li, Z.K.; Li, Z.F.; Peng, X.L.; Liu, C.B.; Zhang, Y.; Zheng, P.L. Recent Developments in the Flame-Retardant System of Epoxy Resin. Materials 2020, 13, 2145. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Li, Y.G.; Ma, H.J.; Song, W.P.; Lin, T.; Lu, C.F.; Nie, J.Q.; Yang, G.C.; Chen, Z.X. Novel phosphorus-nitrogen-silicon copolymers with double-decker silsesquioxane in the main chain and their flame retardancy application in PC/ABS. Fire Mater. 2018, 42, 946–957. [Google Scholar] [CrossRef]
- Rakotomalala, M.; Wagner, S.; Döring, M. Recent Developments in Halogen Free Flame Retardants for Epoxy Resins for Electrical and Electronic Applications. Materials 2010, 3, 4300–4327. [Google Scholar] [CrossRef] [PubMed]
- Seraji, S.M.; Gan, H.L.; Swan, S.R.; Varley, R.J. Phosphazene as an effective flame retardant for rapid curing epoxy resins. React. Funct. Polym. 2021, 164, 11. [Google Scholar] [CrossRef]
- Yan, C.; Yan, P.W.; Xu, H.B.; Liu, D.; Chen, G.; Cai, G.B.; Zhu, Y.D. Preparation of continuous glass fiber/polyamide 6 composites containing hexaphenoxycyclotriphosphazene: Mechanical properties, thermal stability, and flame retardancy. Polym. Compos. 2022, 43, 1022–1037. [Google Scholar] [CrossRef]
- Hoang, D.Q.; Kim, J.W. Synthesis and applications of biscyclic phosphorus flame retardants. Polym. Degrad. Stabil. 2008, 93, 36–42. [Google Scholar] [CrossRef]
- Zong, R.W.; Hu, Y.; Wang, S.F.; Song, L. Thermogravimetric evaluation of PC/ABS/montmorillonite nanocomposite. Polym. Degrad. Stabil. 2004, 83, 423–428. [Google Scholar] [CrossRef]
- Wang, J.B.; Xin, Z. Non-isothermal degradation kinetics for polycarbonate/polymethylphenylsilsesquioxane composites. e-Polymers 2010, 10, 1–10. [Google Scholar] [CrossRef][Green Version]
- Zhang, W.C.; Li, X.M.; Yang, R.J. Flame retardancy mechanisms of phosphorus-containing polyhedral oligomeric silsesquioxane (DOPO-POSS) in polycarbonate/acrylonitrile-butadiene-styrene blends. Polym. Adv. Technol. 2012, 23, 588–595. [Google Scholar] [CrossRef]
- Pawlowski, K.H.; Schartel, B. Flame retardancy mechanisms of aryl phosphates in combination with boehmite in bisphenol A polycarbonate/acrylonitrile-butadiene-styrene blends. Polym. Degrad. Stabil. 2008, 93, 657–667. [Google Scholar] [CrossRef]
- Pour, R.H.; Soheilmoghaddam, M.; Hassan, A.; Bourbigot, S. Flammability and thermal properties of polycarbonate/acrylonitrile-butadiene-styrene nanocomposites reinforced with multilayer graphene. Polym. Degrad. Stabil. 2015, 120, 88–97. [Google Scholar] [CrossRef]
- Ma, H.Y.; Tong, L.F.; Xu, Z.B.; Fang, Z.P. Functionalizing carbon nanotubes by grafting on intumescent flame retardant: Nanocomposite synthesis, morphology, rheology, and flammability. Adv. Funct. Mater. 2008, 18, 414–421. [Google Scholar] [CrossRef]
- Kiliaris, P.; Papaspyrides, C.D. Polymer/layered silicate (clay) nanocomposites: An overview of flame retardancy. Prog. Polym. Sci. 2010, 35, 902–958. [Google Scholar] [CrossRef]
- Huang, G.B.; Liang, H.D.; Wang, Y.; Wang, X.; Gao, J.R.; Fei, Z.D. Combination effect of melamine polyphosphate and graphene on flame retardant properties of poly(vinyl alcohol). Mater. Chem. Phys. 2012, 132, 520–528. [Google Scholar] [CrossRef]
- Despinasse, M.C.; Schartel, B. Aryl phosphate-aryl phosphate synergy in flame-retarded bisphenol A polycarbonate/acrylonitrile-butadiene-styrene. Thermochim. Acta 2013, 563, 51–61. [Google Scholar] [CrossRef]
- Nguyen, C.; Kim, A. Synthesis of a Novel Nitrogen-Phosphorus Flame Retardant Based on Phosphoramidate and Its Application to PC, PBT, EVA, and ABS. Macromol. Res. 2008, 16, 620–625. [Google Scholar] [CrossRef]
- Wendels, S.; Chavez, T.; Bonnet, M.; Salmeia, K.A.; Gaan, S. Recent Developments in Organophosphorus Flame Retardants Containing P-C Bond and Their Applications. Materials 2017, 10, 784. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Hu, Y.; Song, L.; Xing, W.Y.; Lu, H.D.A.; Lv, P.; Jie, G.X. Flame retardancy and thermal degradation mechanism of epoxy resin composites based on a DOPO substituted organophosphorus oligomer. Polymer 2010, 51, 2435–2445. [Google Scholar] [CrossRef]
- Artner, J.; Ciesielski, M.; Walter, O.; Döring, M.; Perez, R.M.; Sandler, J.K.W.; Altstädt, V.; Schartel, B. A novel DOPO-based diamine as hardener and flame retardant for epoxy resin systems. Macromol. Mater. Eng. 2008, 293, 503–514. [Google Scholar] [CrossRef]
- Nguyen, H.K.; Sakai, W.; Nguyen, C. Preparation of a Novel Flame Retardant Formulation for Cotton Fabric. Materials 2020, 13, 54. [Google Scholar] [CrossRef]
- Liu, Y.L. Flame-retardant epoxy resins from novel phosphorus-containing novolac. Polymer 2001, 42, 3445–3454. [Google Scholar] [CrossRef]
- Levchik, S.V.; Bright, D.A.; Alessio, G.R.; Dashevsky, S. New halogen-free fire retardant for engineering plastic applications. J. Vinyl Addit. Technol. 2001, 7, 98–103. [Google Scholar] [CrossRef]
- Levchik, S.V.; Bright, D.A.; Moy, P.; Dashevsky, S. New developments in fire retardant non-halogen aromatic phosphates. J. Vinyl Addit. Technol. 2000, 6, 123–128. [Google Scholar] [CrossRef]
- ASTMD3801-20; Standard Test Method for Mearsuring the Comparative Burning Characteristics of Solid Plastics in a Vertical Position. ASTM International: West Conshohocken, PA, USA, 2020.
- Fang, F.; Ran, S.Y.; Fang, Z.P.; Song, P.A.; Wang, H. Improved flame resistance and thermo-mechanical properties of epoxy resin nanocomposites from functionalized graphene oxide via self-assembly in water. Compos. Pt. B Eng. 2019, 165, 406–416. [Google Scholar] [CrossRef]
- Meinier, R.; Sonnier, R.; Zavaleta, P.; Suard, S.; Ferry, L. Fire behavior of halogen-free flame retardant electrical cables with the cone calorimeter. J. Hazard. Mater. 2018, 342, 306–316. [Google Scholar] [CrossRef]
- Howell, B.A. Thermal Degradation of Organophosphorus Flame Retardants. Polymers 2022, 14, 4929. [Google Scholar] [CrossRef]
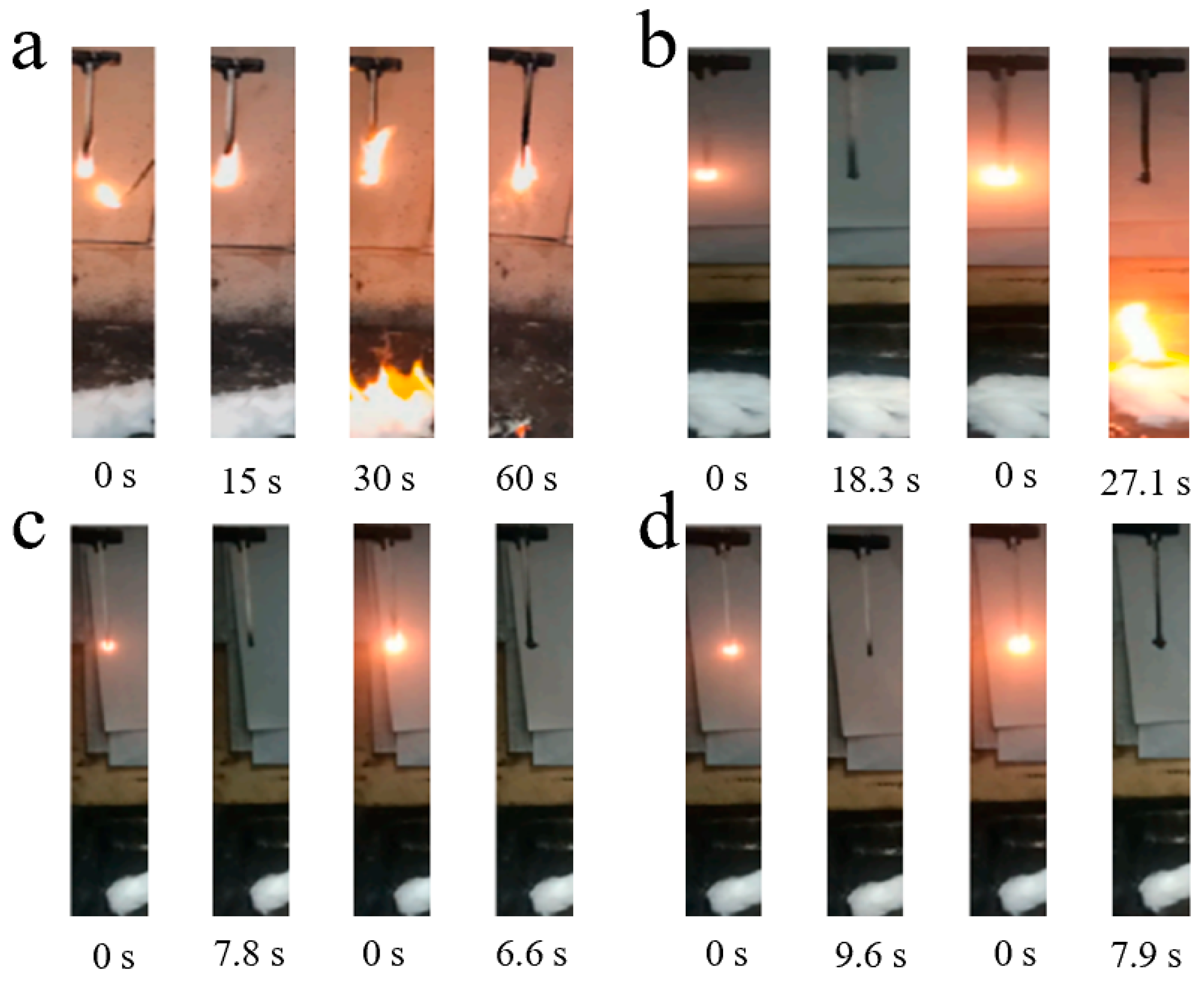
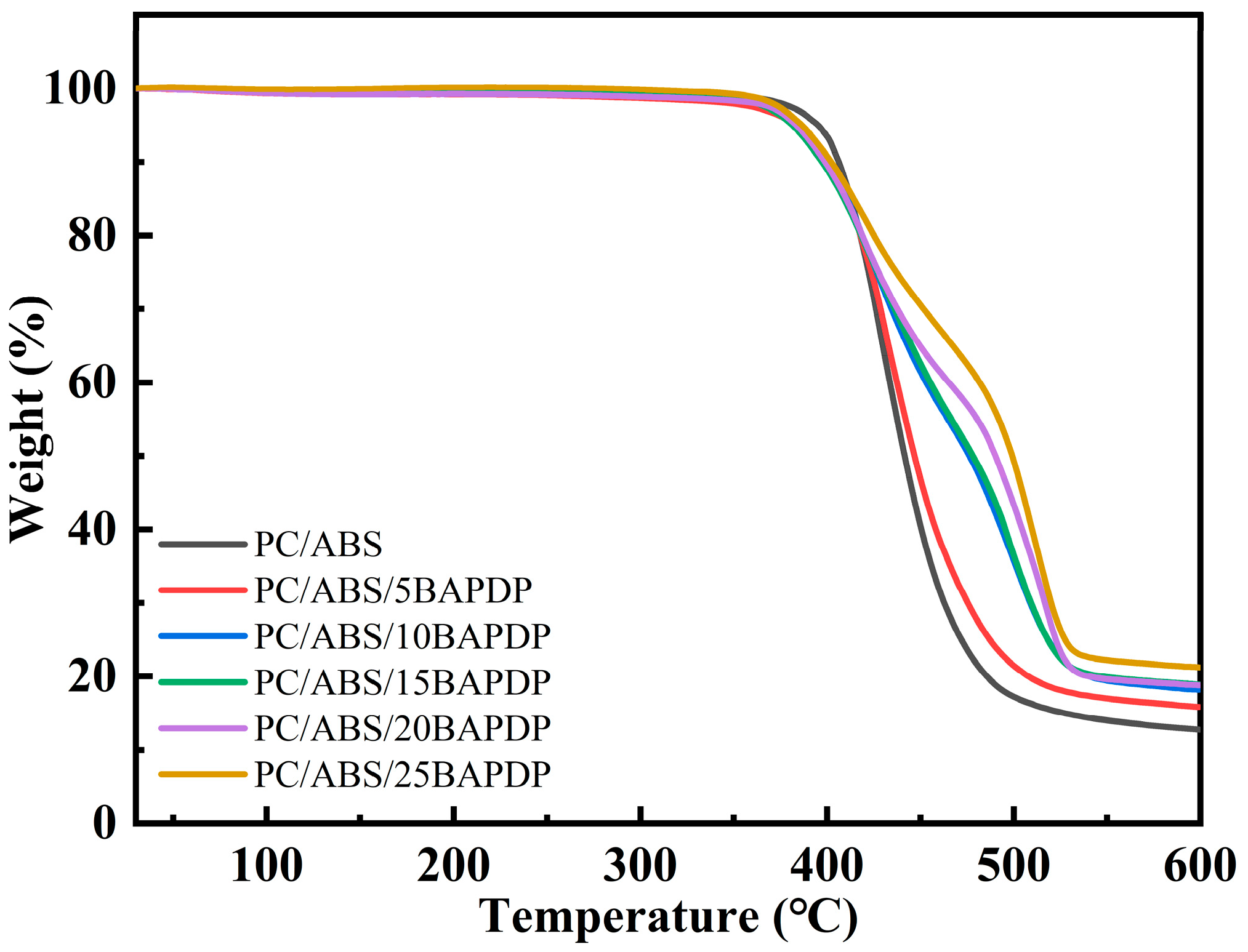
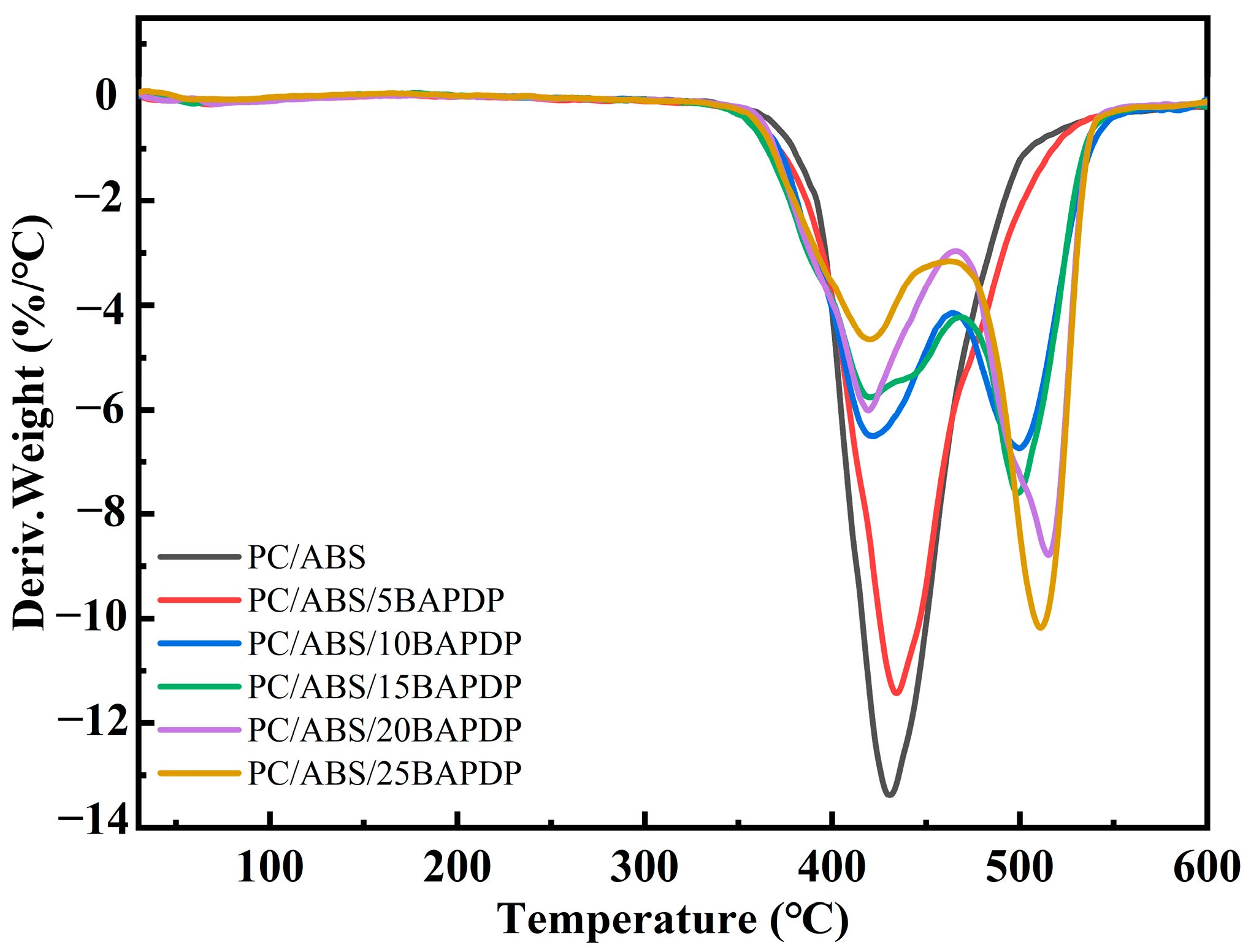
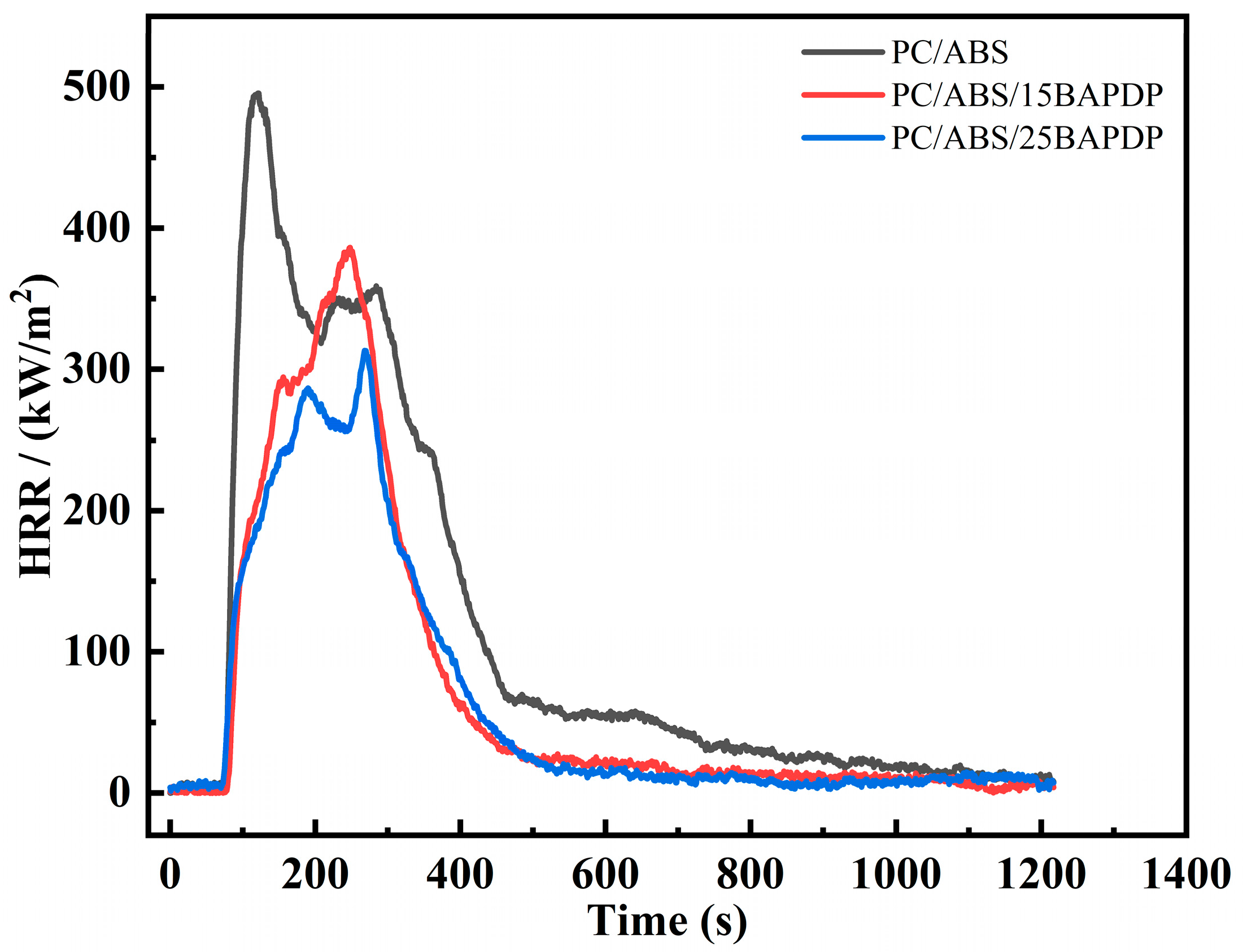

| Sample | PC/ABS (wt%) | BAPDP (wt%) | RDP (wt%) | PTFE (wt%) |
|---|---|---|---|---|
| PC/ABS | 100 | 0 | 0 | 0 |
| PC/ABS/5BAPDP | 95 | 5 | 0 | 0.4 |
| PC/ABS/10BAPDP | 90 | 10 | 0 | 0.4 |
| PC/ABS/15BAPDP | 85 | 15 | 0 | 0.4 |
| PC/ABS/20BAPDP | 80 | 20 | 0 | 0.4 |
| PC/ABS/25BAPDP | 75 | 25 | 0 | 0.4 |
| PC/ABS/15RDP | 85 | 0 | 15 | 0.4 |
| PC/ABS/20RDP | 80 | 0 | 20 | 0.4 |
| PC/ABS/25RDP | 75 | 0 | 25 | 0.4 |
| Element | Experimental Content (%) | Theoretical Content (%) |
|---|---|---|
| C | 68.2 | 70.0 |
| H | 5.0 | 4.8 |
| P | 8.1 | 8.2 |
| Sample | BAPDP (wt%) | RDP (wt%) | LOI (%) | UL-94 | t1/s | t2/s | Dripping |
|---|---|---|---|---|---|---|---|
| PC/ABS | 0 | 0 | 21.1 ± 0.1 | NR | >60 | >60 | Yes |
| PC/ABS/5BAPDP | 5 | 0 | 22.3 ± 0.1 | NR | >60 | >60 | Yes |
| PC/ABS/10BAPDP | 10 | 0 | 23.1 ± 0.2 | V-2 | 18.3 | 27.1 | Yes |
| PC/ABS/15BAPDP | 15 | 0 | 24.7 ± 0.1 | V-1 | 15.6 | 19.1 | No |
| PC/ABS/20BAPDP | 20 | 0 | 25.4 ± 0.1 | V-0 | 9.8 | 8.3 | No |
| PC/ABS/25BAPDP | 25 | 0 | 26.3 ± 0.1 | V-0 | 7.8 | 6.6 | No |
| PC/ABS/15RDP | 0 | 15 | 24.1 ± 0.1 | V-2 | 19.2 | 28.7 | Yes |
| PC/ABS/20RDP | 0 | 20 | 24.8 ± 0.1 | V-1 | 16.3 | 19.8 | No |
| PC/ABS/25RDP | 0 | 25 | 25.7 ± 0.1 | V-0 | 9.6 | 7.9 | No |
| Sample | Td5 (°C) | Tmax (°C) | Char Yield at 600 °C (%) |
|---|---|---|---|
| PC/ABS | 395.2 | 426.8 | 12.8 |
| PC/ABS/5BAPDP | 383.5 | 431.1 | 15.8 |
| PC/ABS/10BAPDP | 385.8 | 499.5 | 18.2 |
| PC/ABS/15BAPDP | 381.4 | 495.3 | 18.9 |
| PC/ABS/20BAPDP | 383.5 | 516.5 | 18.8 |
| PC/ABS/25BAPDP | 386.5 | 508.2 | 21.2 |
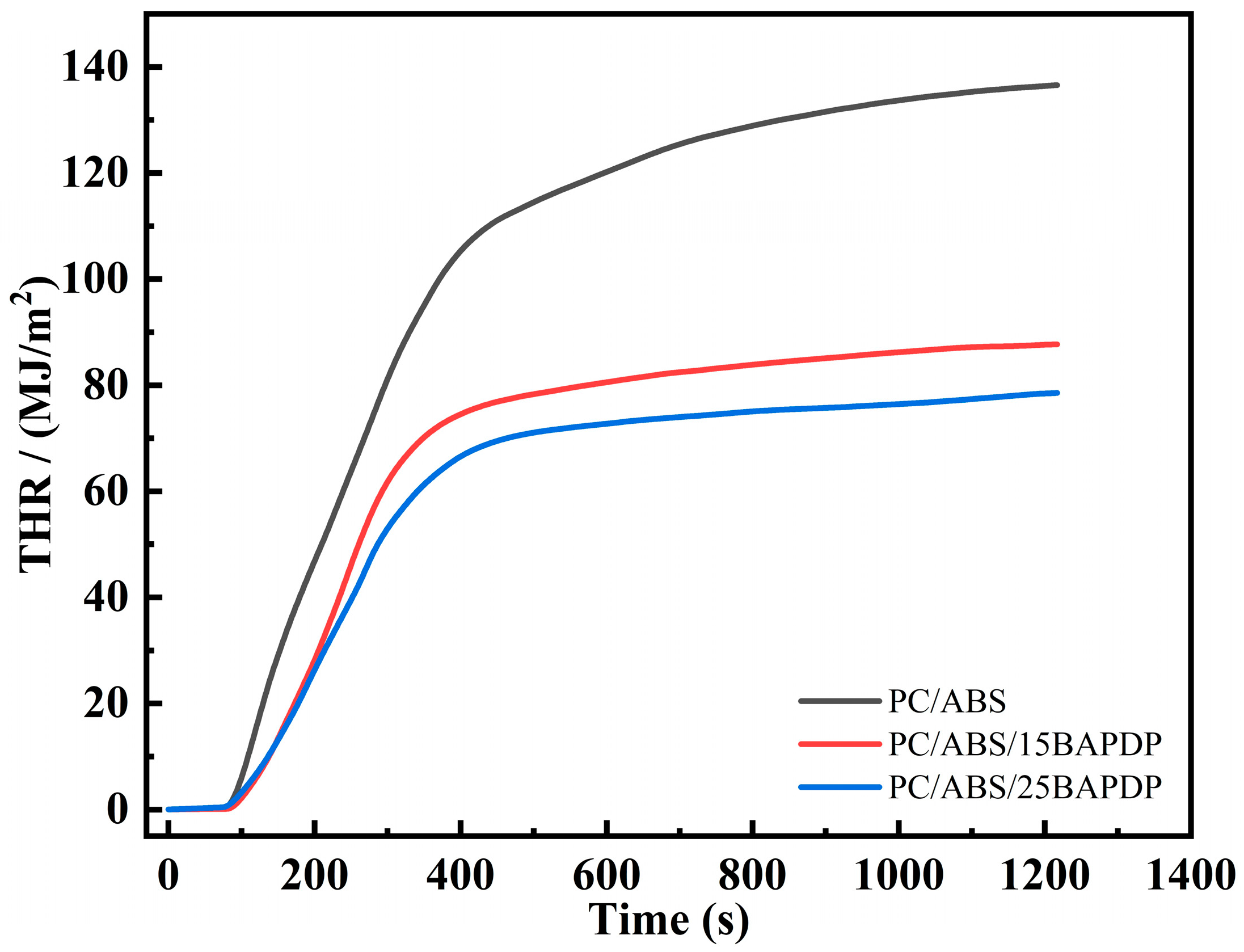
| Specimen | TTI (s) | PHRR (kW/m2) | THR (MJ/m2) | TSP (m2) | TSR (m2/m2) | Mean EHC (MJ/kg) |
|---|---|---|---|---|---|---|
| PC/ABS | 75 | 495.5 | 136.6 | 19.7 | 2209.8 | 32.1 |
| PC/ABS/15BAPDP | 80 | 386.2 | 87.9 | 24.0 | 2691.0 | 24.9 |
| PC/ABS/25BAPDP | 72 | 313.2 | 78.7 | 28.0 | 2824.5 | 21.6 |
| Sample | Tensile Strength, MPa | Flexural Strength, MPa | Izod Impact Strength, kJ/m2 |
|---|---|---|---|
| PC/ABS | 63.9 ± 1.9 | 93.4 ± 1.2 | 25.5 ± 2.8 |
| PC/ABS/5BAPDP | 58.3 ± 1.4 | 86.7 ± 0.9 | 15.2 ± 1.7 |
| PC/ABS/10BAPDP | 57.6 ± 1.1 | 85.2 ± 0.9 | 13.1 ± 1.8 |
| PC/ABS/15BAPDP | 52.6 ± 1.3 | 83.1 ± 0.8 | 10.6 ± 0.8 |
| PC/ABS/20BAPDP | 47.8 ± 1.2 | 78.7 ± 0.7 | 8.5 ± 0.7 |
| PC/ABS/25BAPDP | 40.7 ± 2.2 | 75.9 ± 0.9 | 7.3 ± 0.8 |
| PC/ABS/15RDP | 34.2 ± 0.4 | 80.1 ± 1.1 | 6.4 ± 0.3 |
| PC/ABS/20RDP | 28.2 ± 1.2 | 73.2 ± 0.8 | 4.9 ± 0.4 |
| PC/ABS/25RDP | 22.7 ± 1.3 | 70.9 ± 0.9 | 3.6 ± 0.3 |
| Sample | HDT (°C) |
|---|---|
| PC/ABS | 111.6 ± 2.0 |
| PC/ABS/5BAPDP | 87.0 ± 0.6 |
| PC/ABS/10BAPDP | 83.8 ± 0.3 |
| PC/ABS/15BAPDP | 81.3 ± 0.7 |
| PC/ABS/20BAPDP | 72.6 ± 0.7 |
| PC/ABS/25BAPDP | 69.7 ± 1.3 |
| PC/ABS/15RDP | 69.2 ± 0.6 |
| PC/ABS/20RDP | 60.7 ± 0.6 |
| PC/ABS/25RDP | 57.2 ± 0.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, Y.; Wang, C.; Guan, Y.; Wei, D.; Xu, X. Synthesis of Polyphosphate Flame Retardant Bisphenol AP Bis(Diphenyl Phosphate) and Its Application in Polycarbonate/Acrylonitrile-Butadiene-Styrene. Materials 2024, 17, 5682. https://doi.org/10.3390/ma17235682
Yang Y, Wang C, Guan Y, Wei D, Xu X. Synthesis of Polyphosphate Flame Retardant Bisphenol AP Bis(Diphenyl Phosphate) and Its Application in Polycarbonate/Acrylonitrile-Butadiene-Styrene. Materials. 2024; 17(23):5682. https://doi.org/10.3390/ma17235682
Chicago/Turabian StyleYang, Yang, Chunzhi Wang, Yong Guan, Dafu Wei, and Xiang Xu. 2024. "Synthesis of Polyphosphate Flame Retardant Bisphenol AP Bis(Diphenyl Phosphate) and Its Application in Polycarbonate/Acrylonitrile-Butadiene-Styrene" Materials 17, no. 23: 5682. https://doi.org/10.3390/ma17235682
APA StyleYang, Y., Wang, C., Guan, Y., Wei, D., & Xu, X. (2024). Synthesis of Polyphosphate Flame Retardant Bisphenol AP Bis(Diphenyl Phosphate) and Its Application in Polycarbonate/Acrylonitrile-Butadiene-Styrene. Materials, 17(23), 5682. https://doi.org/10.3390/ma17235682






