In Vivo Study of Organ and Tissue Stability According to the Types of Bioresorbable Bone Screws
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials Preparation
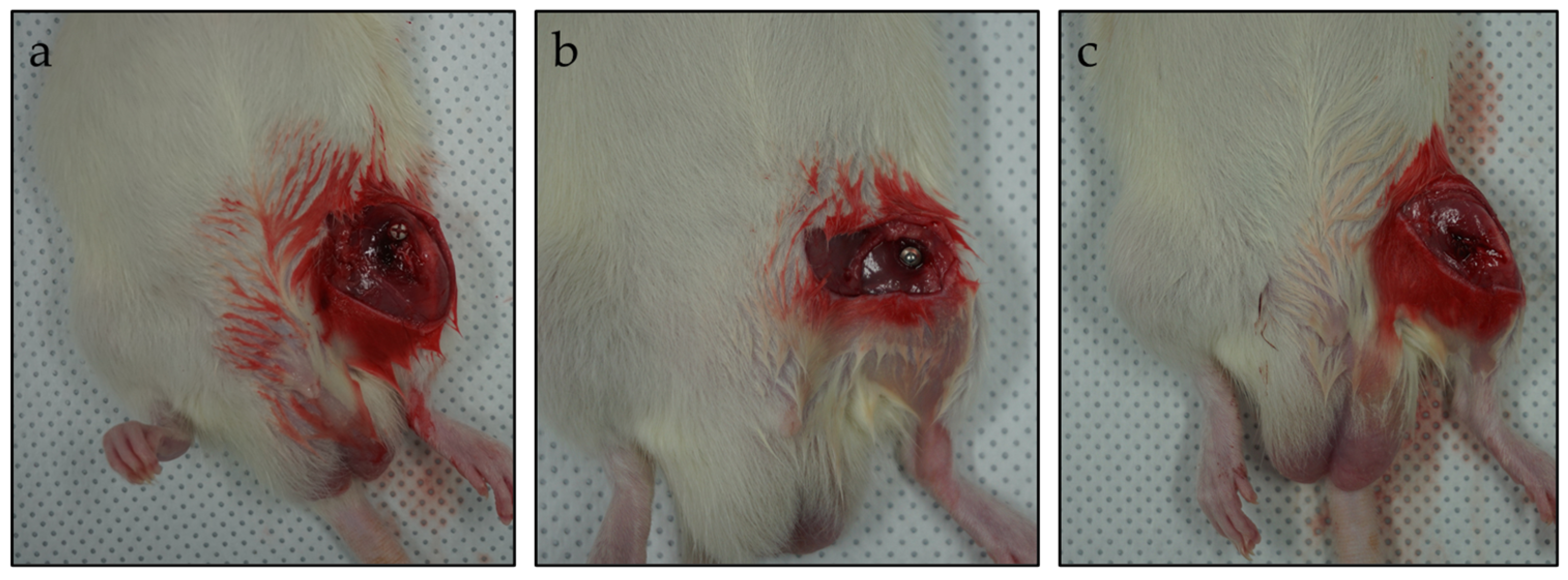
2.2. Animals and Surgery
2.3. Serum Assay of Biochemical Parameter
2.4. Assessment of Bone Microstructure Using Micro-Computed Tomography
2.5. Immunoblotting and Real-Time PCR Analysis
2.6. Hematoxylin and Eosin Staining
2.7. Statistical Analysis
3. Results
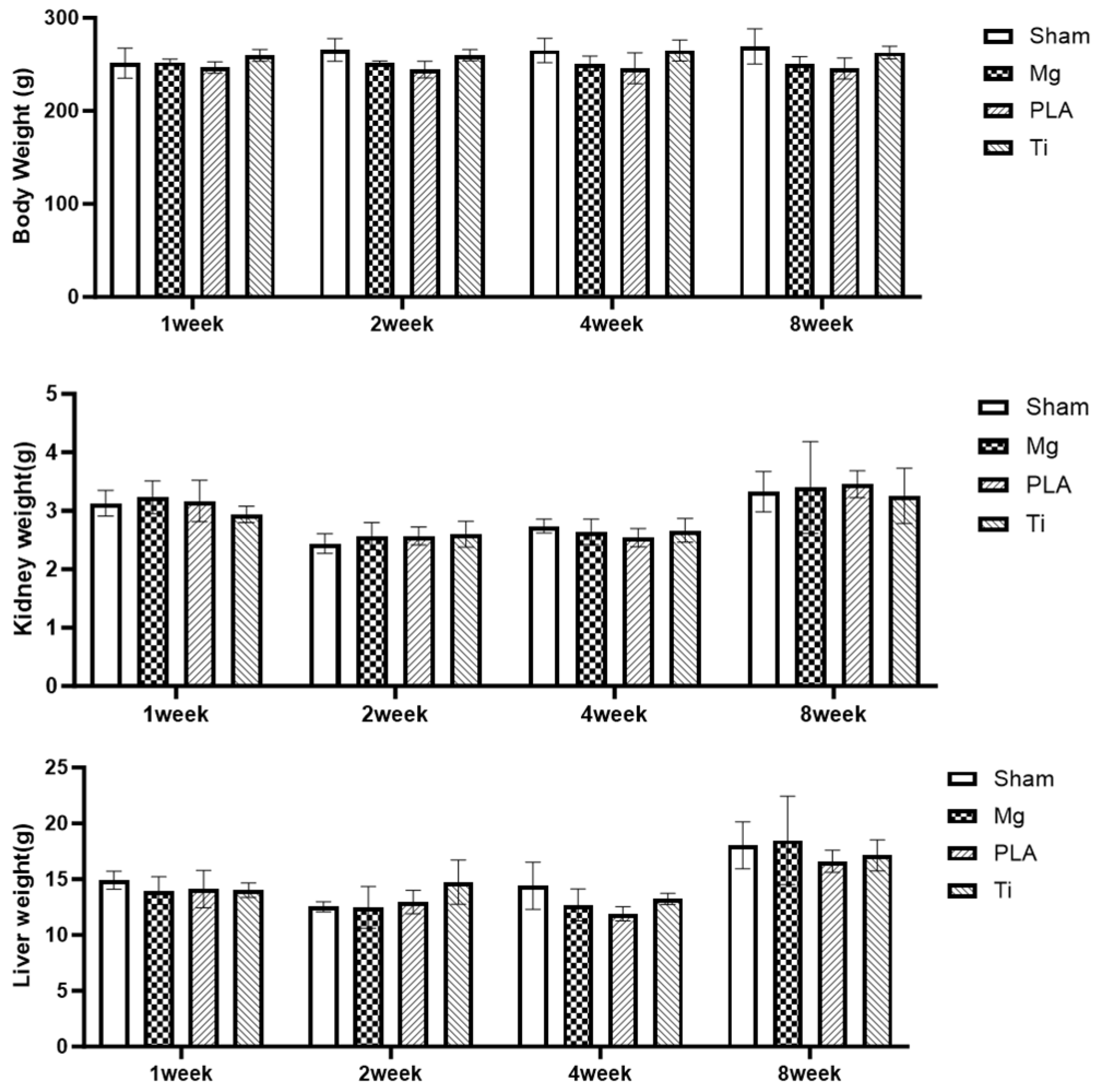
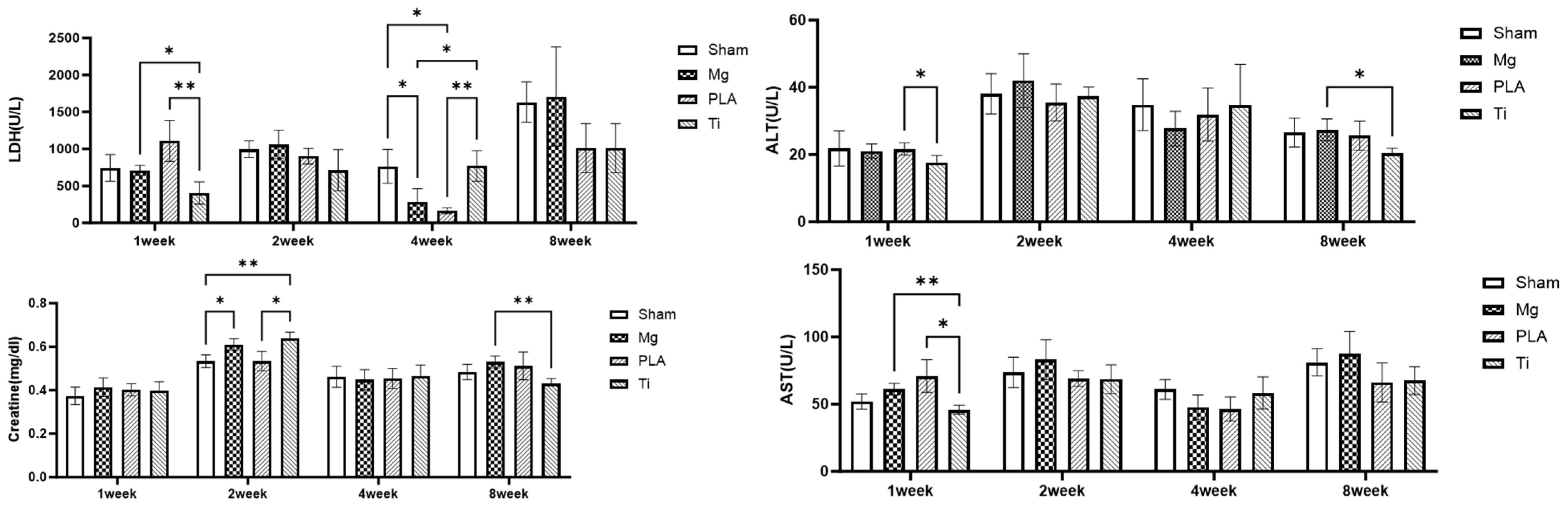
3.1. Systemic Toxicity Evaluation
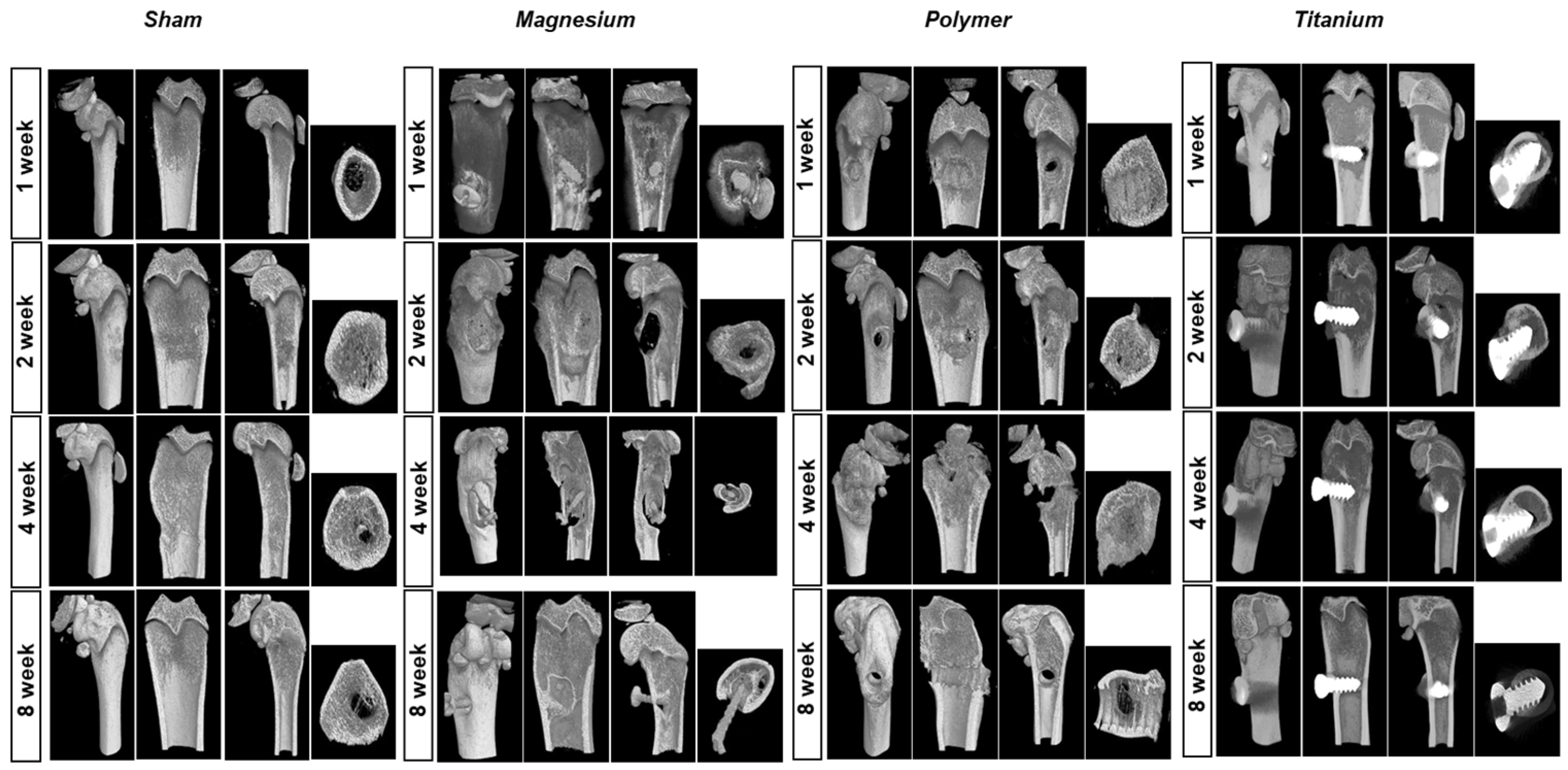
3.2. Micro-CT Evaluation
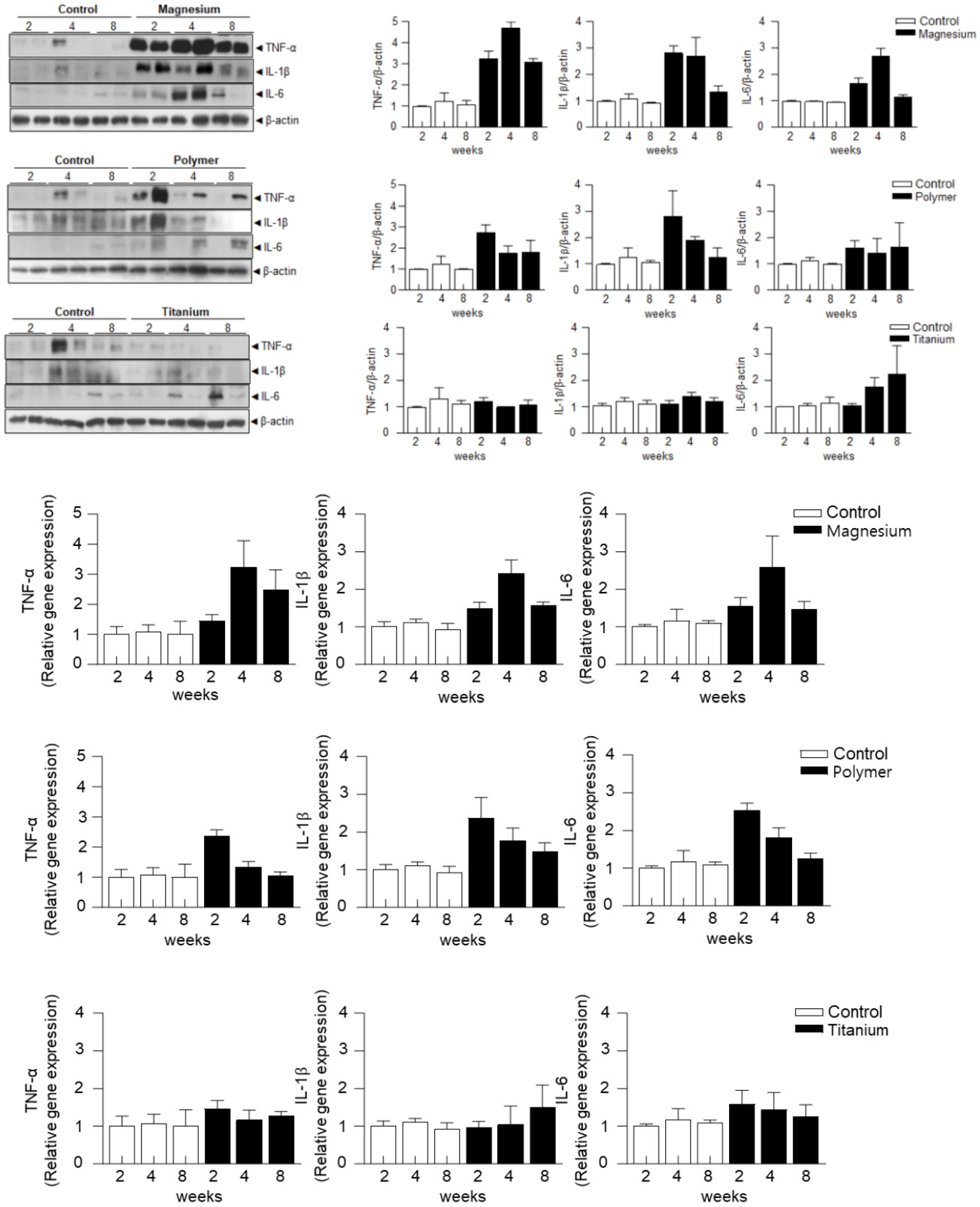
3.3. Analysis of Protein and Gene Expression
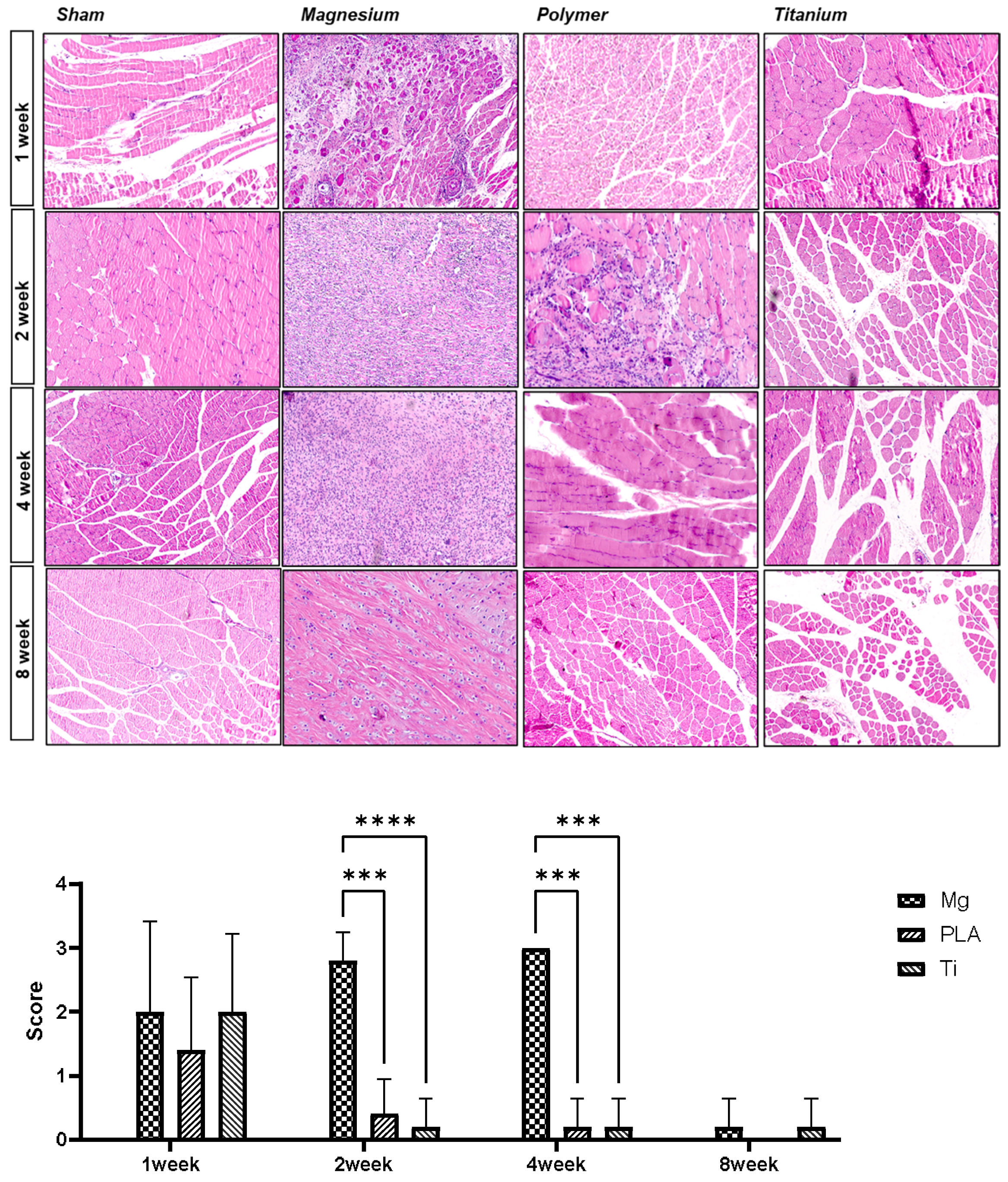
3.4. Histologic Analysis for the Surrounding Bone Tissue by H and E Staining
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Navarro, M.; Michiardi, A.; Castano, O.; Planell, J. Biomaterials in orthopaedics. J. R. Soc. Interface 2008, 5, 1137–1158. [Google Scholar] [CrossRef] [PubMed]
- Uhthoff, H.K.; Poitras, P.; Backman, D.S. Internal plate fixation of fractures: Short history and recent developments. J. Orthop. Sci. 2006, 11, 118–126. [Google Scholar] [CrossRef]
- Nair, L.S.; Laurencin, C.T. Biodegradable polymers as biomaterials. Prog. Polym. Sci. 2007, 32, 762–798. [Google Scholar] [CrossRef]
- Wang, N.; Ma, Y.; Shi, H.; Song, Y.; Guo, S.; Yang, S. Mg-, Zn-, and Fe-Based Alloys with Antibacterial Properties as Orthopedic Implant Materials. Front. Bioeng. Biotechnol. 2022, 10, 888084. [Google Scholar] [CrossRef]
- Li, G.; Zhao, M.; Xu, F.; Yang, B.; Li, X.; Meng, X.; Teng, L.; Sun, F.; Li, Y. Synthesis and Biological Application of Polylactic Acid. Molecules 2020, 25, 5023. [Google Scholar] [CrossRef]
- Kawamura, N.; Nakao, Y.; Ishikawa, R.; Tsuchida, D.; Iijima, M. Degradation and Biocompatibility of AZ31 Magnesium Alloy Implants In Vitro and In Vivo: A Micro-Computed Tomography Study in Rats. Materials 2020, 13, 473. [Google Scholar] [CrossRef]
- Ballerini, G.; Bardi, U.; Bignucolo, R.; Ceraolo, G. About some corrosion mechanisms of AZ91D magnesium alloy. Corros. Sci. 2005, 47, 2173–2184. [Google Scholar] [CrossRef]
- Kim, Y.K.; Park, I.S.; Lee, S.J.; Lee, M.H. Biodegradation and cytotoxic properties of pulse anodized Mg alloys. Met. Mater. Int. 2013, 19, 353–360. [Google Scholar] [CrossRef]
- Shaw, B.A. Corrosion resistance of magnesium alloys. In ASM Handbook; Pennsylvania State University: University Park, PA, USA, 2003; pp. 692–696. [Google Scholar]
- Adetunla, A.; Fide-Akwuobi, A.; Benjamin, H.; Adeyinka, A.; Kolawole, A. A study of degradable orthopedic implant: An insight in magnesium metal matrix composites. Heliyon 2022, 8, e10503. [Google Scholar] [CrossRef] [PubMed]
- Fayzullin, A.; Churbanov, S.; Ignatieva, N.; Zakharkina, O.; Tokarev, M.; Mudryak, D.; Khristidis, Y.; Balyasin, M.; Kurkov, A.; Golubeva, E.N.; et al. Local Delivery of Pirfenidone by PLA Implants Modifies Foreign Body Reaction and Prevents Fibrosis. Biomedicines 2021, 9, 853. [Google Scholar] [CrossRef]
- Pérez Davila, S.; González Rodríguez, L.; Chiussi, S.; Serra, J.; González, P. How to Sterilize Polylactic Acid Based Medical Devices? Polymers 2021, 13, 2115. [Google Scholar] [CrossRef] [PubMed]
- Rutala, W.A.; Weber, D.J. Infection control: The role of disinfection and sterilization. J. Hosp. Infect. 1999, 43, S43–S55. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.-K.; Lee, G.-H.; Bhattarai, K.R.; Junjappa, R.P.; Lee, H.-Y.; Handigund, M.; Marahatta, A.; Bhandary, B.; Baek, I.-H.; Pyo, J.S. PI3Kδ contributes to ER stress-associated asthma through ER-redox disturbances: The involvement of the RIDD–RIG-I–NF-κB axis. Exp. Mol. Med. 2018, 50, e444. [Google Scholar] [CrossRef]
- Liu, Z.; Yao, X.; Jiang, W.; Zhou, Z.; Yang, M. Sodium butyrate enhances titanium nail osseointegration in ovariectomized rats by inhibiting the PKCα/NOX4/ROS/NF-κB pathways. J. Orthop. Surg. Res. 2023, 18, 556. [Google Scholar] [CrossRef]
- Chagnon, M.; Guy, L.-G.; Jackson, N. Evaluation of magnesium-based medical devices in preclinical studies: Challenges and points to consider. Toxicol. Pathol. 2019, 47, 390–400. [Google Scholar] [CrossRef]
- Kamata, M.; Sakamoto, Y.; Kishi, K. Foreign-body reaction to bioabsorbable plate and screw in craniofacial surgery. J. Craniofacial Surg. 2019, 30, e34–e36. [Google Scholar] [CrossRef]
- Gerlach, K.; Eitenmuller, J. Biomaterials and Clinical Applications; Elsevier: Amsterdam, The Netherlands, 1987. [Google Scholar]
- Lee, B.Y.; Park, J.Y.; Kim, Y.C. Effect of polycarbonate structure and reduction time on graphene oxide dispersion. Polym. Adv. Technol. 2015, 26, 1241–1246. [Google Scholar] [CrossRef]
- Chen, J.; Du, Y.; Que, W.; Xing, Y.; Chen, X.; Lei, B. Crack-free polydimethylsiloxane–bioactive glass–poly (ethylene glycol) hybrid monoliths with controlled biomineralization activity and mechanical property for bone tissue regeneration. Colloids Surf. B Biointerfaces 2015, 136, 126–133. [Google Scholar] [CrossRef]
- Laughlin, R.M.; Block, M.S.; Wilk, R.; Malloy, R.B.; Kent, J.N. Resorbable plates for the fixation of mandibular fractures: A prospective study. J. Oral Maxillofac. Surg. 2007, 65, 89–96. [Google Scholar] [CrossRef]
- Shikinami, Y.; Matsusue, Y.; Nakamura, T. The complete process of bioresorption and bone replacement using devices made of forged composites of raw hydroxyapatite particles/poly l-lactide (Fu-HA/PLLA). Biomaterials 2005, 26, 5542–5551. [Google Scholar] [CrossRef]
- Feng, P.; Jia, J.; Liu, M.; Peng, S.; Zhao, Z.; Shuai, C. Degradation mechanisms and acceleration strategies of poly (lactic acid) scaffold for bone regeneration. Mater. Des. 2021, 210, 110066. [Google Scholar] [CrossRef]
- Godard, H.P. The Corrosion of Light Metals; John Wiley & Sons: Hoboken, NJ, USA, 1967. [Google Scholar]
- Song, G.; Atrens, A.; Stjohn, D.; Nairn, J.; Li, Y. The electrochemical corrosion of pure magnesium in 1 N NaCl. Corros. Sci. 1997, 39, 855–875. [Google Scholar] [CrossRef]
- Okutan, B.; Schwarze, U.Y.; Berger, L.; Martinez, D.C.; Herber, V.; Suljevic, O.; Plocinski, T.; Swieszkowski, W.; Santos, S.G.; Schindl, R. The combined effect of zinc and calcium on the biodegradation of ultrahigh-purity magnesium implants. Biomater. Adv. 2023, 146, 213287. [Google Scholar] [CrossRef] [PubMed]
- Chou, D.T.; Hong, D.; Oksuz, S.; Schweizer, R.; Roy, A.; Lee, B.; Shridhar, P.; Gorantla, V.; Kumta, P.N. Corrosion and bone healing of Mg-Y-Zn-Zr-Ca alloy implants: Comparative in vivo study in a non-immobilized rat femoral fracture model. J. Biomater. Appl. 2019, 33, 1178–1194. [Google Scholar] [CrossRef] [PubMed]
- Shunmugasamy, V.C.; Abdel Gawad, M.; Sohail, M.U.; Ibrahim, T.; Khan, T.; Seers, T.D.; Mansoor, B. In vitro and in vivo study on fine-grained Mg-Zn-RE-Zr alloy as a biodegradeable orthopedic implant produced by friction stir processing. Bioact. Mater. 2023, 28, 448–466. [Google Scholar] [CrossRef]
- Kim, Y.K.; Kim, S.Y.; Lee, S.H.; Lee, M.H.; Lee, K.B. Stabilized Loading of Hyaluronic Acid-Containing Hydrogels into Magnesium-Based Cannulated Screws. ACS Biomater. Sci. Eng. 2020, 6, 715–726. [Google Scholar] [CrossRef]
- Hohlinger, M.; Christa, D.; Zimmermann, V.; Heise, S.; Boccaccini, A.R.; Virtanen, S. Influence of proteins on the corrosion behavior of a chitosan-bioactive glass coated magnesium alloy. Mater. Sci. Eng. C Mater. Biol. Appl. 2019, 100, 706–714. [Google Scholar] [CrossRef]
- Tran, N.T.; Kim, Y.K.; Kim, S.Y.; Lee, M.H.; Lee, K.B. Comparative Osteogenesis and Degradation Behavior of Magnesium Implant in Epiphysis and Diaphysis of the Long Bone in the Rat Model. Materials 2022, 15, 5630. [Google Scholar] [CrossRef]
- Wang, Y.; Liang, W.; Liu, X.; Li, Q.; Xie, Y.; Jiang, Y. Osteogenesis and degradation behavior of magnesium alloy plate in vivo. Eur. J. Inflamm. 2021, 19, 20587392211034078. [Google Scholar] [CrossRef]
- Kim, Y.K.; Lee, K.B.; Kim, S.Y.; Bode, K.; Jang, Y.S.; Kwon, T.Y.; Jeon, M.H.; Lee, M.H. Gas formation and biological effects of biodegradable magnesium in a preclinical and clinical observation. Sci. Technol. Adv. Mater. 2018, 19, 324–335. [Google Scholar] [CrossRef]
- Noviana, D.; Paramitha, D.; Ulum, M.F.; Hermawan, H. The effect of hydrogen gas evolution of magnesium implant on the postimplantation mortality of rats. J. Orthop. Transl. 2016, 5, 9–15. [Google Scholar] [CrossRef] [PubMed]
- Schaller, B.; Burkhard, J.P.M.; Chagnon, M.; Beck, S.; Imwinkelried, T.; Assad, M. Fracture healing and bone remodeling with human standard-sized magnesium versus polylactide–Co-glycolide plate and screw systems using a mini-swine craniomaxillofacial osteotomy fixation model. J. Oral Maxillofac. Surg. 2018, 76, 2138–2150. [Google Scholar] [CrossRef]
- Eppley, B.; Prevel, C.; Sadove, A.; Sarver, D. Resorbable bone fixation: Its potential role in cranio-maxillofacial trauma. J. Cranio-Maxillofac. Trauma 1996, 2, 56–60. [Google Scholar]
- Suuronen, R.; Lindqvist, C. Bioresorbable materials for bone fixation: Review of biological concepts and mechanical aspects. In Craniomaxillofacial Reconstructive and Corrective Bone Surgery: Principles of Internal Fixation Using the AO/ASIF Technique; Springer: New York, NY, USA, 2002; pp. 113–123. [Google Scholar]
- Chen, Y.; Xu, Z.; Smith, C.; Sankar, J. Recent advances on the development of magnesium alloys for biodegradable implants. Acta Biomater. 2014, 10, 4561–4573. [Google Scholar] [CrossRef] [PubMed]
- Zwawi, M. Recent advances in bio-medical implants; mechanical properties, surface modifications and applications. Eng. Res. Express 2022, 4, 032003. [Google Scholar] [CrossRef]
- Teo, A.J.T.; Mishra, A.; Park, I.; Kim, Y.J.; Park, W.T.; Yoon, Y.J. Polymeric Biomaterials for Medical Implants and Devices. ACS Biomater. Sci. Eng. 2016, 2, 454–472. [Google Scholar] [CrossRef]
- Nieminen, T.; Rantala, I.; Hiidenheimo, I.; Keränen, J.; Kainulainen, H.; Wuolijoki, E.; Kallela, I. Degradative and mechanical properties of a novel resorbable plating system during a 3-year follow-up in vivo and in vitro. J. Mater. Sci. Mater. Med. 2008, 19, 1155–1163. [Google Scholar] [CrossRef]
| Gene | Forward Primer (5′–3′) | Reverse Primer (5′–3′) |
|---|---|---|
| TNF-α | CAGGGGCCACCACGCTCTTC | CTTGGGGCAGGGGCTCTTGA |
| IL-6 | AGGGCATGTTAAGGAGC | CATCAGAGGCAAGGAGGA |
| IL-1β | GCAACTGTTCCTGAACTCAACT | ATCTTTTGGGGT CCGTCAACT |
| β-actin | TTCAACACCCCAGCCATGT | CAGTGGTACGACCAGAGGCATA |
| Group | Implantation Time | Body Weight (g) | Kidney Weight (g) | Liver Weight (g) | LDH (U/L) | Creatine (mg/dl) | ALT (U/L) | AST (U/L) |
|---|---|---|---|---|---|---|---|---|
| Sham | 1 week | 251.3 ± 16.1 | 3.13 ± 0.22 | 14.92 ± 0.79 | 742.2 ± 180.58 | 0.37 ± 0.04 | 21.8 ± 5.23 | 51.9 ± 5.71 |
| 2 weeks | 265.5 ± 12.2 | 2.43 ± 0.17 | 12.52 ± 0.45 | 996.00 ± 112.59 | 0.53 ± 0.03 | 38.08 ± 5.98 | 73.64 ± 11.34 | |
| 4 weeks | 265.1 ± 13.1 | 2.74 ± 0.12 | 14.42 ± 2.12 | 764.40 ± 229.34 | 0.46 ± 0.05 | 34.86 ± 7.71 | 60.92 ± 7.37 | |
| 8 weeks | 269.4 ± 18.8 | 3.32 ± 0.35 | 18.03 ± 2.11 | 1632.40 ± 274.03 | 0.48 ± 0.03 | 26.54 ± 5.27 | 81.10 ± 10.17 | |
| Magnesium Alloy | 1 week | 252.2 ± 2.5 | 3.23 ± 0.28 | 13.97 ± 1.23 | 709.80 ± 69.35 | 0.41 ± 0.04 | 21.00 ± 2.18 | 61.04 ± 4.41 |
| 2 weeks | 252.1 ± 1.4 | 2.56 ± 0.23 | 12.48 ± 1.87 | 1061.40 ± 194.65 | 0.61 ± 0.03 | 42.00 ± 8.00 | 83.08 ± 14.79 | |
| 4 weeks | 251.1 ± 8.2 | 2.64 ± 0.22 | 12.69 ± 1.43 | 286.20 ± 175.76 | 0.44 ± 0.05 | 27.70 ± 5.15 | 47.68 ± 9.19 | |
| 8 weeks | 250.1 ± 8.4 | 3.39 ± 0.79 | 18.48 ± 3.98 | 1707.80 ± 672.28 | 0.53 ± 0.03 | 27.38 ± 3.24 | 87.44 ± 16.51 | |
| Polylactide | 1 week | 246.7 ± 6.1 | 3.17 ± 0.35 | 14.11 ± 1.68 | 1107.80 ± 177.48 | 0.40 ± 0.03 | 21.70 ± 1.83 | 70.94 ± 12.11 |
| 2 weeks | 244.5 ± 8.9 | 2.56 ± 0.15 | 12.95 ± 1.05 | 902.80 ± 104.98 | 0.53 ± 0.05 | 35.50 ± 5.48 | 69.04 ± 5.87 | |
| 4 weeks | 245.9 ± 16.2 | 2.54 ± 0.18 | 11.80 ± 0.69 | 165.75 ± 46.76 | 0.45 ± 0.05 | 31.10 ± 8.87 | 44.20 ± 8.63 | |
| 8 weeks | 244.5 ± 11.3 | 3.45 ± 0.23 | 16.60 ± 0.99 | 1010.60 ± 333.49 | 0.51 ± 0.06 | 25.62 ± 4.36 | 66.14 ± 14.52 | |
| Ti | 1 week | 259.7 ± 6.3 | 2.93 ± 0.14 | 14.00 ± 0.65 | 403.40 ± 150.95 | 0.39 ± 0.04 | 17.68 ± 2.07 | 45.82 ± 6.40 |
| 2 weeks | 259.9 ± 6.1 | 2.60 ± 0.22 | 14.73 ± 1.97 | 713.20 ± 278.45 | 0.64 ± 0.03 | 37.38 ± 2.69 | 68.58 ± 10.63 | |
| 4 weeks | 265.1 ± 11.3 | 2.66 ± 0.47 | 13.23 ± 0.51 | 771.20 ± 207.11 | 0.46 ± 0.05 | 34.70 ± 12.18 | 58.32 ± 11.99 | |
| 8 weeks | 262.9 ± 6.7 | 3.25 ± 0.45 | 17.13 ± 1.39 | 1010.60 ± 333.49 | 0.43 ± 0.02 | 20.42 ± 1.47 | 67.54 ± 10.45 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kwon, T.-Y.; Lee, G.-H.; Lee, H.; Lee, K.-B. In Vivo Study of Organ and Tissue Stability According to the Types of Bioresorbable Bone Screws. Materials 2024, 17, 5632. https://doi.org/10.3390/ma17225632
Kwon T-Y, Lee G-H, Lee H, Lee K-B. In Vivo Study of Organ and Tissue Stability According to the Types of Bioresorbable Bone Screws. Materials. 2024; 17(22):5632. https://doi.org/10.3390/ma17225632
Chicago/Turabian StyleKwon, Tae-Young, Geum-Hwa Lee, Hyuk Lee, and Kwang-Bok Lee. 2024. "In Vivo Study of Organ and Tissue Stability According to the Types of Bioresorbable Bone Screws" Materials 17, no. 22: 5632. https://doi.org/10.3390/ma17225632
APA StyleKwon, T.-Y., Lee, G.-H., Lee, H., & Lee, K.-B. (2024). In Vivo Study of Organ and Tissue Stability According to the Types of Bioresorbable Bone Screws. Materials, 17(22), 5632. https://doi.org/10.3390/ma17225632







