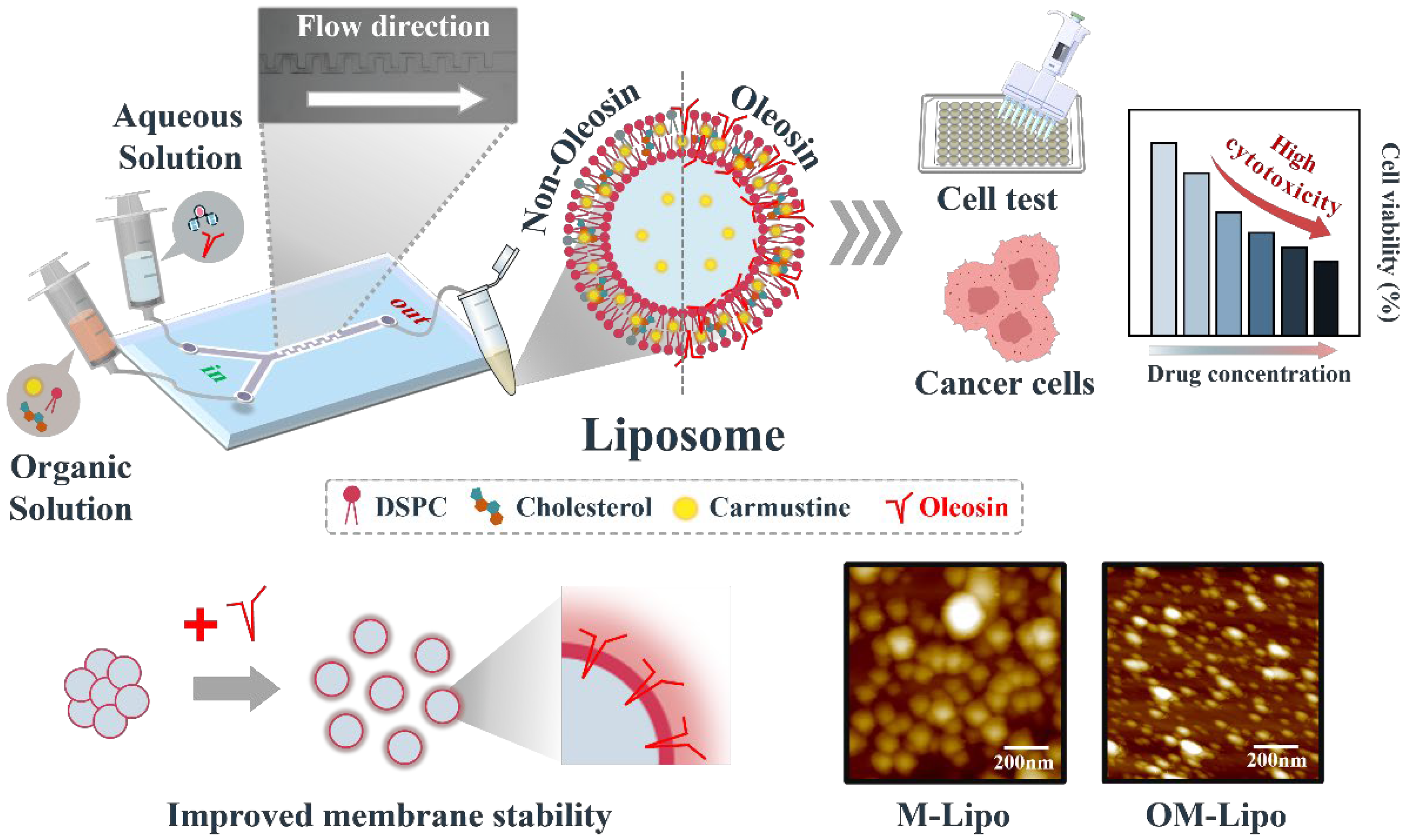
Microfluidic Fabrication of Oleosin-Coated Liposomes as Anticancer Drug Carriers with Enhanced Sustained Drug Release
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Oleosin Extraction
2.3. SDS-PAGE
2.4. Manufacturing of Microfluidic Chip for M-Lipo
2.5. M-Lipo Preparation
2.6. M-Lipo Characterization
2.7. Drug Encapsulation Efficiency (EE) and In Vitro Drug Release Profile
2.8. Cell Line and Cell Culture
2.9. Cytotoxicity Test
2.10. Drug Delivery Efficiency Test
3. Results
3.1. SDS-PAGE Analysis for Extracted Oleosin
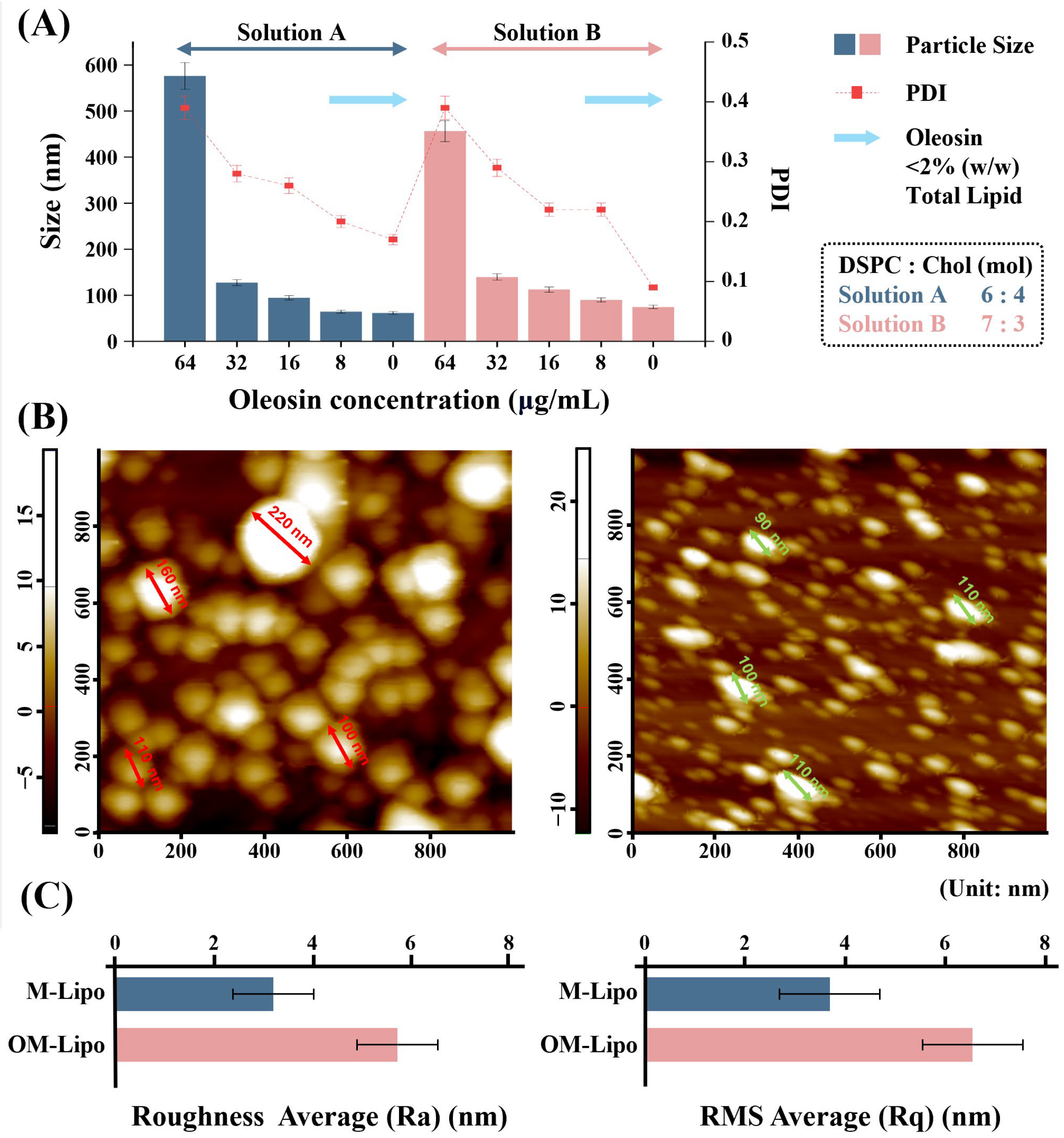
3.2. Optimization of OM-Lipo Synthesis Conditions
3.3. Particle Stability Test
3.4. In Vitro Release Kinetics
3.5. In Vitro Cytotoxicity Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef] [PubMed]
- Baskar, R.; Lee, K.A.; Yeo, R.; Yeoh, K.W. Cancer and radiation therapy: Current advances and future directions. Int. J. Med. Sci. 2012, 9, 193–199. [Google Scholar] [CrossRef] [PubMed]
- Pearce, T.R.; Shroff, K.; Kokkoli, E. Peptide targeted lipid nanoparticles for anticancer drug delivery. Adv. Mater. 2012, 24, 3803–3822. [Google Scholar] [CrossRef] [PubMed]
- Pourjavadi, A.; Amin, S.S.; Hosseini, S.H. Delivery of Hydrophobic Anticancer Drugs by Hydrophobically Modified Alginate Based Magnetic Nanocarrier. Ind. Eng. Chem. Res. 2018, 57, 822–832. [Google Scholar] [CrossRef]
- Glassman, P.M.; Muzykantov, V.R. Pharmacokinetic and pharmacodynamic properties of drug delivery systems. J. Pharmacol. Exp. Ther. 2019, 370, 570–580. [Google Scholar] [CrossRef]
- Dang, Y.; Guan, J. Nanoparticle-based drug delivery systems for cancer therapy. Smart Mater. Med. 2020, 1, 10–19. [Google Scholar] [CrossRef]
- Mujahid, K.; Rana, I.; Suliman, I.H.; Li, Z.; Wu, J.; He, H.; Nam, J. Biomaterial-Based Sustained-Release Drug Formulations for Localized Cancer Immunotherapy. ACS Appl. Bio Mater. 2023, 7, 4944–4961. [Google Scholar] [CrossRef]
- Din, F.U.; Aman, W.; Ullah, I.; Qureshi, O.S.; Mustapha, O.; Shafique, S.; Zeb, A. Effective use of nanocarriers as drug delivery systems for the treatment of selected tumors. Int. J. Nanomed. 2017, 12, 7291–7309. [Google Scholar] [CrossRef]
- Nasirizadeh, S.; Malaekeh-Nikouei, B. Solid lipid nanoparticles and nanostructured lipid carriers in oral cancer drug delivery. J. Drug Deliv. Sci. Technol. 2020, 55, 101458. [Google Scholar] [CrossRef]
- Souto, E.B.; Müller, R.H. Lipid nanoparticles: Effect on bioavailability and pharmacokinetic changes. Handb. Exp. Pharmacol. 2010, 197, 115–141. [Google Scholar] [CrossRef]
- Wong, H.L.; Bendayan, R.; Rauth, A.M.; Li, Y.; Wu, X.Y. Chemotherapy with anticancer drugs encapsulated in solid lipid nanoparticles. Adv. Drug Deliv. Rev. 2007, 59, 491–504. [Google Scholar] [CrossRef] [PubMed]
- Bhagya, N.; Chandrashekar, K.R. Liposome encapsulated anticancer drugs on autophagy in cancer cells—Current and future perspective. Int. J. Pharm. 2023, 642, 123105. [Google Scholar] [CrossRef] [PubMed]
- Mougenot, M.F.; Pereira, V.S.; Costa, A.L.R.; Lancellotti, M.; Porcionatto, M.A.; da Silveira, J.C.; de la Torre, L.G. Biomimetic nanovesicles—Sources, design, production methods, and applications. Pharmaceutics 2022, 14, 2008. [Google Scholar] [CrossRef] [PubMed]
- Baldassari, S.; Balboni, A.; Drava, G.; Donghia, D.; Canepa, P.; Ailuno, G.; Caviglioli, G. Phytochemicals and Cancer Treatment: Cell-Derived and Biomimetic Vesicles as Promising Carriers. Pharmaceutics 2023, 15, 1445. [Google Scholar] [CrossRef] [PubMed]
- Shan, H.; Sun, X.; Liu, X.; Sun, Q.; He, Y.; Chen, Z.; Lin, Q.; Jiang, Z.; Chen, X.; Chen, Z.; et al. One-Step Formation of Targeted Liposomes in a Versatile Microfluidic Mixing Device. Small 2023, 19, 2205498. [Google Scholar] [CrossRef]
- Maeki, M.; Saito, T.; Sato, Y.; Yasui, T.; Kaji, N.; Ishida, A.; Tani, H.; Baba, Y.; Harashima, H.; Tokeshi, M. A strategy for synthesis of lipid nanoparticles using microfluidic devices with a mixer structure. RSC Adv. 2015, 5, 46181–46185. [Google Scholar] [CrossRef]
- Maeki, M.; Fujishima, Y.; Sato, Y.; Yasui, T.; Kaji, N.; Ishida, A.; Tani, H.; Baba, Y.; Harashima, H.; Tokeshi, M. Understanding the formation mechanism of lipid nanoparticles in microfluidic devices with chaotic micromixers. PLoS ONE 2017, 12, e0187962. [Google Scholar] [CrossRef]
- Roces, C.B.; Lou, G.; Jain, N.; Abraham, S.; Thomas, A.; Halbert, G.W.; Perrie, Y. Manufacturing considerations for the development of lipid nanoparticles using microfluidics. Pharmaceutics 2020, 12, 1095. [Google Scholar] [CrossRef]
- Matsuura-Sawada, Y.; Maeki, M.; Nishioka, T.; Niwa, A.; Yamauchi, J.; Mizoguchi, M.; Wada, K.; Tokeshi, M. Microfluidic Device-Enabled Mass Production of Lipid-Based Nanoparticles for Applications in Nanomedicine and Cosmetics. ACS Appl. Nano Mater. 2022, 5, 7867–7876. [Google Scholar] [CrossRef]
- Shepherd, S.J.; Warzecha, C.C.; Yadavali, S.; El-Mayta, R.; Alameh, M.G.; Wang, L.; Weissman, D.; Wilson, J.M.; Issadore, D.; Mitchell, M.J. Scalable mRNA and siRNA Lipid Nanoparticle Production Using a Parallelized Microfluidic Device. Nano Lett. 2021, 21, 5671–5680. [Google Scholar] [CrossRef]
- Lopes, C.; Cristóvão, J.; Silvério, V.; Lino, P.R.; Fonte, P. Microfluidic production of mRNA-loaded lipid nanoparticles for vaccine applications. Expert Opin. Drug Deliv. 2022, 19, 1381–1395. [Google Scholar] [CrossRef] [PubMed]
- Sopyan, I.; Gozali, D. A Review: A Novel of Efforts to Enhance Liposome Stability as Drug Delivery Approach. Syst. Rev. Pharm. 2020, 11, 555. [Google Scholar]
- Leung, A.K.; Hafez, I.M.; Baoukina, S.; Belliveau, N.M.; Zhigaltsev, I.V.; Afshinmanesh, E.; Tieleman, D.P.; Hansen, C.L.; Hope, M.J.; Cullis, P.R. Lipid nanoparticles containing siRNA synthesized by microfluidic mixing exhibit an electron-dense nanostructured core. J. Phys. Chem. C 2012, 116, 18440–18450. [Google Scholar] [CrossRef] [PubMed]
- Okuda, K.; Sato, Y.; Iwakawa, K.; Sasaki, K.; Okabe, N.; Maeki, M.; Tokeshi, M.; Harashima, H. On the size-regulation of RNA-loaded lipid nanoparticles synthesized by microfluidic device. J. Control Release 2022, 348, 648–659. [Google Scholar] [CrossRef]
- Seo, Y.; Lim, H.; Park, H.; Yu, J.; An, J.; Yoo, H.Y.; Lee, T. Recent Progress of Lipid Nanoparticles-Based Lipophilic Drug Delivery: Focus on Surface Modifications. Pharmaceutics 2023, 15, 772. [Google Scholar] [CrossRef]
- Maurer, S.; Waschatko, G.; Schach, D.; Zielbauer, B.I.; Dahl, J.; Weidner, T.; Bonn, M.; Vilgis, T.A. The role of intact oleosin for stabilization and function of oleosomes. J. Phys. Chem. B 2013, 117, 13872–13883. [Google Scholar] [CrossRef]
- Şen, A.; Acevedo-Fani, A.; Dave, A.; Ye, A.; Husny, J.; Singh, H. Plant oil bodies and their membrane components: New natural materials for food applications. Crit. Rev. Food Sci. Nutr. 2024, 64, 256–279. [Google Scholar] [CrossRef]
- Li, R.; Pu, C.; Sun, Y.; Sun, Q.; Tang, W. Interaction between soybean oleosome-associated proteins and phospholipid bilayer and its influence on environmental stability of luteolin-loaded liposomes. Food Hydrocoll. 2022, 130, 107721. [Google Scholar] [CrossRef]
- Cho, H.Y.; Lee, T.; Yoon, J.; Han, Z.; Rabie, H.; Lee, K.B.; Choi, J.W. Magnetic oleosome as a functional lipophilic drug carrier for cancer therapy. ACS Appl. Mater. Interfaces 2018, 10, 9301–9309. [Google Scholar] [CrossRef]
- Plankensteiner, L.; Yang, J.; Bitter, J.H.; Vincken, J.P.; Hennebelle, M.; Nikiforidis, C.V. High yield extraction of oleosins, the proteins that plants developed to stabilize oil droplets. Food Hydrocoll. 2023, 137, 108419. [Google Scholar] [CrossRef]
- Song, F.; Yang, G.; Wang, Y.; Tian, S. Effect of phospholipids on membrane characteristics and storage stability of liposomes. Innov. Food Sci. Emerg. Technol. 2022, 81, 103155. [Google Scholar] [CrossRef]
- Dejeu, I.L.; Vicaș, L.G.; Jurca, T.; Teușdea, A.C.; Mureșan, M.E.; Fritea, L.; Svera, P.; Gabor, G.A.; Dejeu, G.E.; Maghiar, O.A.; et al. Liposomes with caffeic acid: Morphological and structural characterisation, their properties and stability in time. Processes 2021, 9, 912. [Google Scholar] [CrossRef]
- Bi, Y.; Liu, L.; Lu, Y.; Sun, T.; Shen, C.; Chen, X.; Chen, Q.; An, S.; He, X.; Ruan, C.; et al. T7 Peptide-Functionalized PEG-PLGA Micelles Loaded with Carmustine for Targeting Therapy of Glioma. ACS Appl. Mater. Interfaces 2016, 8, 27465–27473. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Zhang, S.; Xie, F.; Zhong, M.; Jiang, L.; Qi, B.; Li, Y. Effects of covalent modification with epigallocatechin-3-gallate on oleosin structure and ability to stabilize artificial oil body emulsions. Food Chem. 2021, 341, 128272. [Google Scholar] [CrossRef]
- Wolski, P.; Narkiewicz-Michalek, J.; Panczyk, M.; Pastorin, G.; Panczyk, T. Molecular Dynamics Modeling of the Encapsulation and De-encapsulation of the Carmustine Anticancer Drug in the Inner Volume of a Carbon Nanotube. J. Phys. Chem. C 2017, 121, 18922–18934. [Google Scholar] [CrossRef]
- Briuglia, M.L.; Rotella, C.; McFarlane, A.; Lamprou, D.A. Influence of cholesterol on liposome stability and on in vitro drug release. Drug Deliv. Transl. Res. 2015, 5, 231–242. [Google Scholar] [CrossRef]
- Bae, J.E.; Huh, M.I.; Ryu, B.K.; Do, J.Y.; Jin, S.U.; Moon, M.J.; Jung, J.C.; Chang, Y.; Kim, E.; Chi, S.G.; et al. The effect of static magnetic fields on the aggregation and cytotoxicity of magnetic nanoparticles. Biomaterials 2011, 32, 9401–9414. [Google Scholar] [CrossRef]
- Kaźmierczak, Z.; Szostak-Paluch, K.; Przybyło, M.; Langner, M.; Witkiewicz, W.; Jędruchniewicz, N.; Dąbrowska, K. Endocytosis in cellular uptake of drug delivery vectors: Molecular aspects in drug development. Bioorg. Med. Chem. 2020, 28, 115556. [Google Scholar] [CrossRef]
- Wang, G.; Wang, J.; Wu, W.; Tony To, S.S.; Zhao, H.; Wang, J. Advances in lipid-based drug delivery: Enhancing efficiency for hydrophobic drugs. Expert Opin. Drug Deliv. 2015, 12, 1475–1499. [Google Scholar] [CrossRef]
- Cullis, P.R.; Hope, M.J. Lipid Nanoparticle Systems for Enabling Gene Therapies. Mol. Ther. 2017, 25, 1467–1475. [Google Scholar] [CrossRef]
- Maeki, M.; Okada, Y.; Uno, S.; Niwa, A.; Ishida, A.; Tani, H.; Tokeshi, M. Production of siRNA-loaded lipid nanoparticles using a microfluidic device. J. Vis. Exp. 2022, e62999. [Google Scholar] [CrossRef]
- Al-amin, M.D.; Bellato, F.; Mastrotto, F.; Garofalo, M.; Malfanti, A.; Salmaso, S.; Caliceti, P. Dexamethasone loaded liposomes by thin-film hydration and microfluidic procedures: Formulation challenges. Int. J. Mol. Sci. 2020, 21, 1611. [Google Scholar] [CrossRef] [PubMed]
- Andra, V.V.S.N.L.; Pammi, S.V.N.; Bhatraju, L.V.K.P.; Ruddaraju, L.K. A Comprehensive Review on Novel Liposomal Methodologies, Commercial Formulations, Clinical Trials and Patents. BioNanoScience 2022, 12, 274–291. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Kiselev, M.A. Methods of Liposomes Preparation: Formation and Control Factors of Versatile Nanocarriers for Biomedical and Nanomedicine Application. Pharmaceutics 2022, 14, 543. [Google Scholar] [CrossRef]
- Pattni, B.S.; Chupin, V.V.; Torchilin, V.P. New Developments in Liposomal Drug Delivery. Chem. Rev. 2015, 115, 10938–10966. [Google Scholar] [CrossRef]
- Elsana, H.; Olusanya, T.O.B.; Carr-wilkinson, J.; Darby, S.; Faheem, A.; Elkordy, A.A. Evaluation of novel cationic gene based liposomes with cyclodextrin prepared by thin film hydration and microfluidic systems. Sci. Rep. 2019, 9, 15120. [Google Scholar] [CrossRef]
- Scott, H.L.; Skinkle, A.; Kelley, E.G.; Waxham, M.N.; Levental, I.; Heberle, F.A. On the Mechanism of Bilayer Separation by Extrusion, or Why Your LUVs Are Not Really Unilamellar. Biophys. J. 2019, 117, 1381–1386. [Google Scholar] [CrossRef]
- Shah, S.; Dhawan, V.; Holm, R.; Nagarsenker, M.S.; Perrie, Y. Liposomes: Advancements and innovation in the manufacturing process. Adv. Drug Deliv. Rev. 2020, 154, 102–122. [Google Scholar] [CrossRef]
- Chen, Z.; Han, J.Y.; Shumate, L.; Fedak, R.; DeVoe, D.L. High Throughput Nanoliposome Formation Using 3D Printed Microfluidic Flow Focusing Chips. Adv. Mater. Technol. 2019, 4, 1800511. [Google Scholar] [CrossRef]
- Hood, R.R.; Devoe, D.L. High-Throughput Continuous Flow Production of Nanoscale Liposomes by Microfluidic Vertical Flow Focusing. Small 2015, 11, 5790–5799. [Google Scholar] [CrossRef]
- Patil, Y.P.; Jadhav, S. Novel methods for liposome preparation. Chem. Phys. Lipids 2014, 177, 8–18. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, K.; Yamashita, K.; Itoh, Y.; Yoshino, K.; Nozawa, S.; Kasukawa, H. Comparative studies of polyethylene glycol-modified liposomes prepared using different PEG-modification methods. Biochim. Biophys. Acta Biomembr. 2012, 1818, 2801–2807. [Google Scholar] [CrossRef] [PubMed]
- Ding, J.; Dong, Y.; Huang, G.; Zhang, Y.; Jiang, L.; Sui, X. Fabrication and characterization of β-carotene emulsions stabilized by soy oleosin and lecithin mixtures with a composition mimicking natural soy oleosomes. Food Funct. 2021, 12, 10875–10886. [Google Scholar] [CrossRef]
- Guzha, A.; Whitehead, P.; Ischebeck, T.; Chapman, K.D. Lipid droplets: Packing hydrophobic molecules within the aqueous cytoplasm. Annu. Rev. Plant Biol. 2023, 74, 195–223. [Google Scholar] [CrossRef]
- Kapchie, V.N.; Yao, L.; Hauck, C.C.; Wang, T.; Murphy, P.A. Oxidative stability of soybean oil in oleosomes as affected by pH and iron. Food Chem. 2013, 141, 2286–2293. [Google Scholar] [CrossRef]
- Qin, C.; Han, M.; Fu, R.; Mei, Y.; Wen, X.; Ni, Y.; Boom, R.M.; Nikiforidis, C.V. Influence of extraction pH and homogenization on soybean oleosome emulsion stability. LWT 2024, 203, 116404. [Google Scholar] [CrossRef]
- Chavanpatil, M.; Jain, P.; Chaudhari, S.; Shear, R.; Vavia, P. Development of sustained release gastroretentive drug delivery system for ofloxacin: In vitro and in vivo evaluation. Int. J. Pharm. 2005, 304, 178–184. [Google Scholar] [CrossRef]
- Desfrançois, C.; Auzély, R.; Texier, I. Lipid nanoparticles and their hydrogel composites for drug delivery: A review. Pharmaceuticals 2018, 11, 118. [Google Scholar] [CrossRef]
- Chen, S.Q.; Song, Y.Q.; Wang, C.; Tao, S.; Yu, F.Y.; Lou, H.Y.; Hu, F.Q.; Yuan, H. Chitosan-modified lipid nanodrug delivery system for the targeted and responsive treatment of ulcerative colitis. Carbohydr. Polym. 2020, 230, 115613. [Google Scholar] [CrossRef]
- Abdullah; Weiss, J.; Zhang, H. Recent advances in the composition, extraction and food applications of plant-derived oleosomes. Trends Food Sci. Technol. 2020, 106, 322–332. [Google Scholar] [CrossRef]
- Keseru, G.M.; Makara, G.M. Hit discovery and hit-to-lead approaches. Drug Discov. Today 2006, 11, 741–748. [Google Scholar] [CrossRef] [PubMed]
- Majumder, R.; Karmakar, S.; Mishra, S.; Mallick, A.B.; Das Mukhopadhyay, C. Functionalized Carbon Nano-Onions as a Smart Drug Delivery System for the Poorly Soluble Drug Carmustine for the Management of Glioblastoma. ACS Appl. Bio Mater. 2023, 7, 154–167. [Google Scholar] [CrossRef] [PubMed]
- Abasian, P.; Shakibi, S.; Maniati, M.S.; Nouri Khorasani, S.; Khalili, S. Targeted delivery, drug release strategies, and toxicity study of polymeric drug nanocarriers. Polym. Adv. Technol. 2021, 32, 931–944. [Google Scholar] [CrossRef]
- Vatansever, O.; Bahadori, F.; Bulut, S.; Eroglu, M.S. Coating with cationic inulin enhances the drug release profile and in vitro anticancer activity of lecithin-based nano drug delivery systems. Int. J. Biol. Macromol. 2023, 237, 123955. [Google Scholar] [CrossRef]
- Verhoeckx, K.; Broekman, H.; Knulst, A.; Houben, G. Allergenicity assessment strategy for novel food proteins and protein sources. Regul. Toxicol. Pharm. 2016, 79, 118–124. [Google Scholar] [CrossRef]
- Schwager, C.; Kull, S.; Krause, S.; Schocker, F.; Petersen, A.; Becker, W.M.; Jappe, U. Development of a novel strategy to isolate lipophilic allergens (oleosins) from peanuts. PLoS ONE 2015, 10, e0123419. [Google Scholar] [CrossRef]
- Vojdani, A. The evolution of food immune reactivity testing: Why immunoglobulin g or immunoglobulin a antibody for food may not be reproducible from one lab to another. Altern. Ther. Health Med. 2015, 21, 8–22. [Google Scholar]



| Conventional Production Method | Microfluidic System | |||
|---|---|---|---|---|
| Thin-Film Hydration | Ethanol/Ether Injection | M-Lipo | OM-Lipo | |
| Particle Size | >1000 nm | >200 nm | 5~200 nm | >100 nm |
| For Nanoscale Synthesis | Sonication and Extrusion | Self-assembly | Self-assembly | |
| Synthesis Speed | Slow | Normal | Fast | Fast |
| Encapsulation Efficiency | Low | Normal | High | High |
| Particle Size Distribution | Low Consistent | Low Consistent | Consistent | High Consistent |
| Particle Stability | Low | Low | Low | High |
| Quantity Production | Relatively Low | Normal | High | High |
| Organic Solvent Removal Method | Dry | Dialysis | Dialysis | Dialysis |
| Reference | [43,44,45] | [46,47,48] | [49,50,51] | This study |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Seo, Y.; Woo, Y.; Oh, B.; Yoo, D.; Kwon, H.K.; Park, C.; Cho, H.-Y.; Kim, H.S.; Lee, T. Microfluidic Fabrication of Oleosin-Coated Liposomes as Anticancer Drug Carriers with Enhanced Sustained Drug Release. Materials 2024, 17, 5550. https://doi.org/10.3390/ma17225550
Seo Y, Woo Y, Oh B, Yoo D, Kwon HK, Park C, Cho H-Y, Kim HS, Lee T. Microfluidic Fabrication of Oleosin-Coated Liposomes as Anticancer Drug Carriers with Enhanced Sustained Drug Release. Materials. 2024; 17(22):5550. https://doi.org/10.3390/ma17225550
Chicago/Turabian StyleSeo, Yoseph, Yeeun Woo, Byeolnim Oh, Daehyeon Yoo, Hyeok Ki Kwon, Chulhwan Park, Hyeon-Yeol Cho, Hyun Soo Kim, and Taek Lee. 2024. "Microfluidic Fabrication of Oleosin-Coated Liposomes as Anticancer Drug Carriers with Enhanced Sustained Drug Release" Materials 17, no. 22: 5550. https://doi.org/10.3390/ma17225550
APA StyleSeo, Y., Woo, Y., Oh, B., Yoo, D., Kwon, H. K., Park, C., Cho, H.-Y., Kim, H. S., & Lee, T. (2024). Microfluidic Fabrication of Oleosin-Coated Liposomes as Anticancer Drug Carriers with Enhanced Sustained Drug Release. Materials, 17(22), 5550. https://doi.org/10.3390/ma17225550










