Effects of Fly Ash Particle Size and Chemical Activators on the Hydration of High-Volume Fly Ash Mortars
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Mixing Proportions and Testing Method
3. Results and Discussion
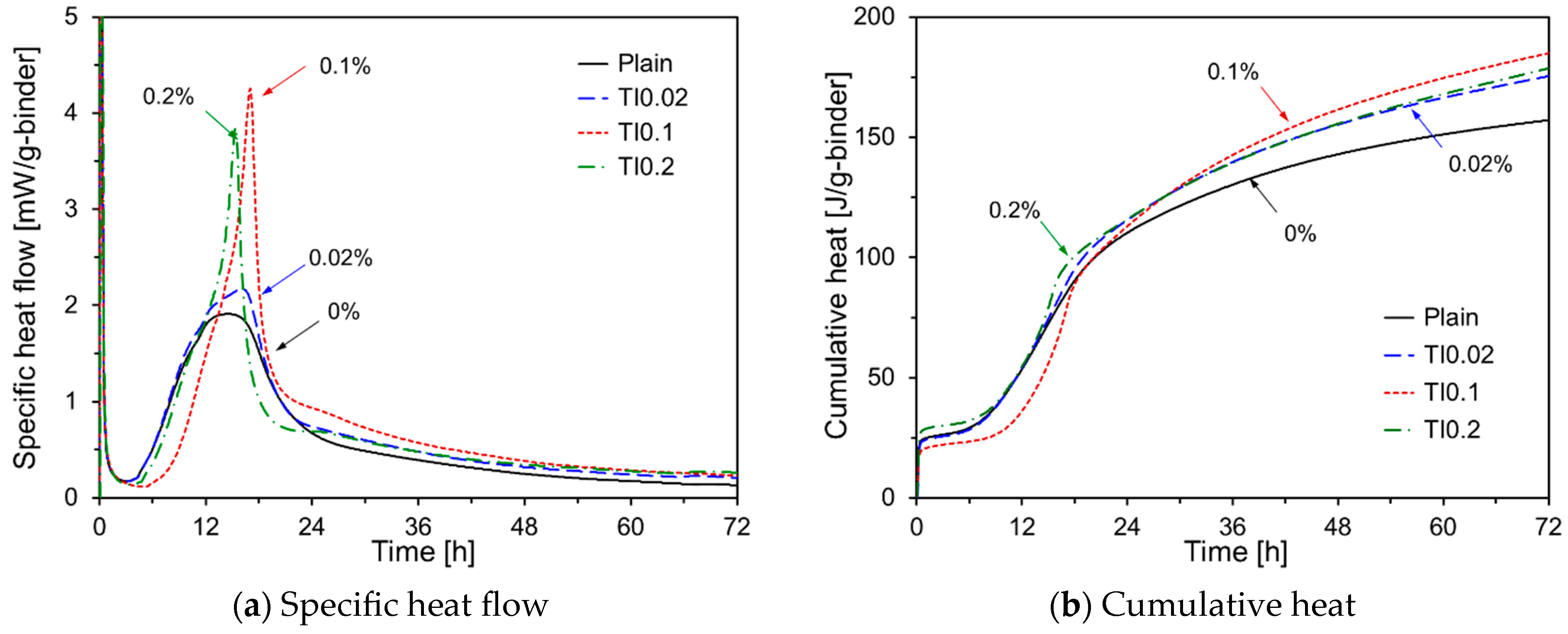
3.1. Hydration Kinetics Characteristics
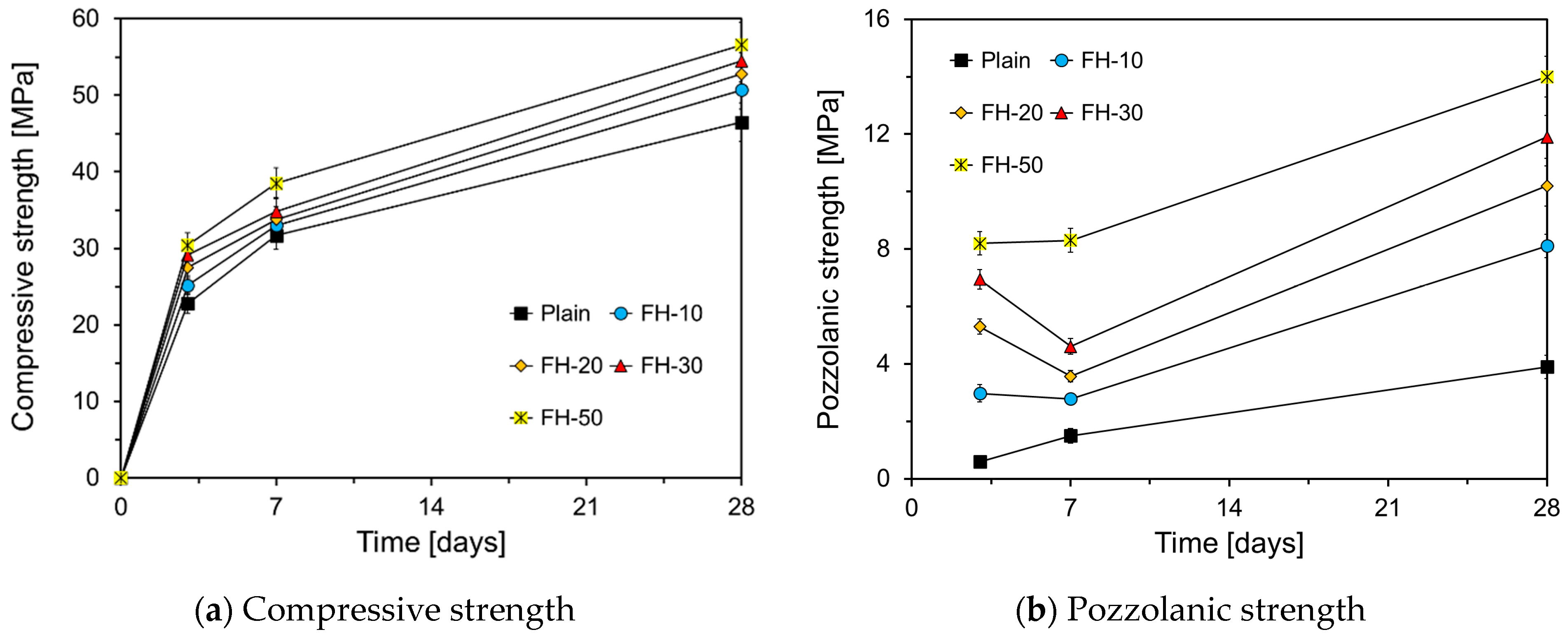
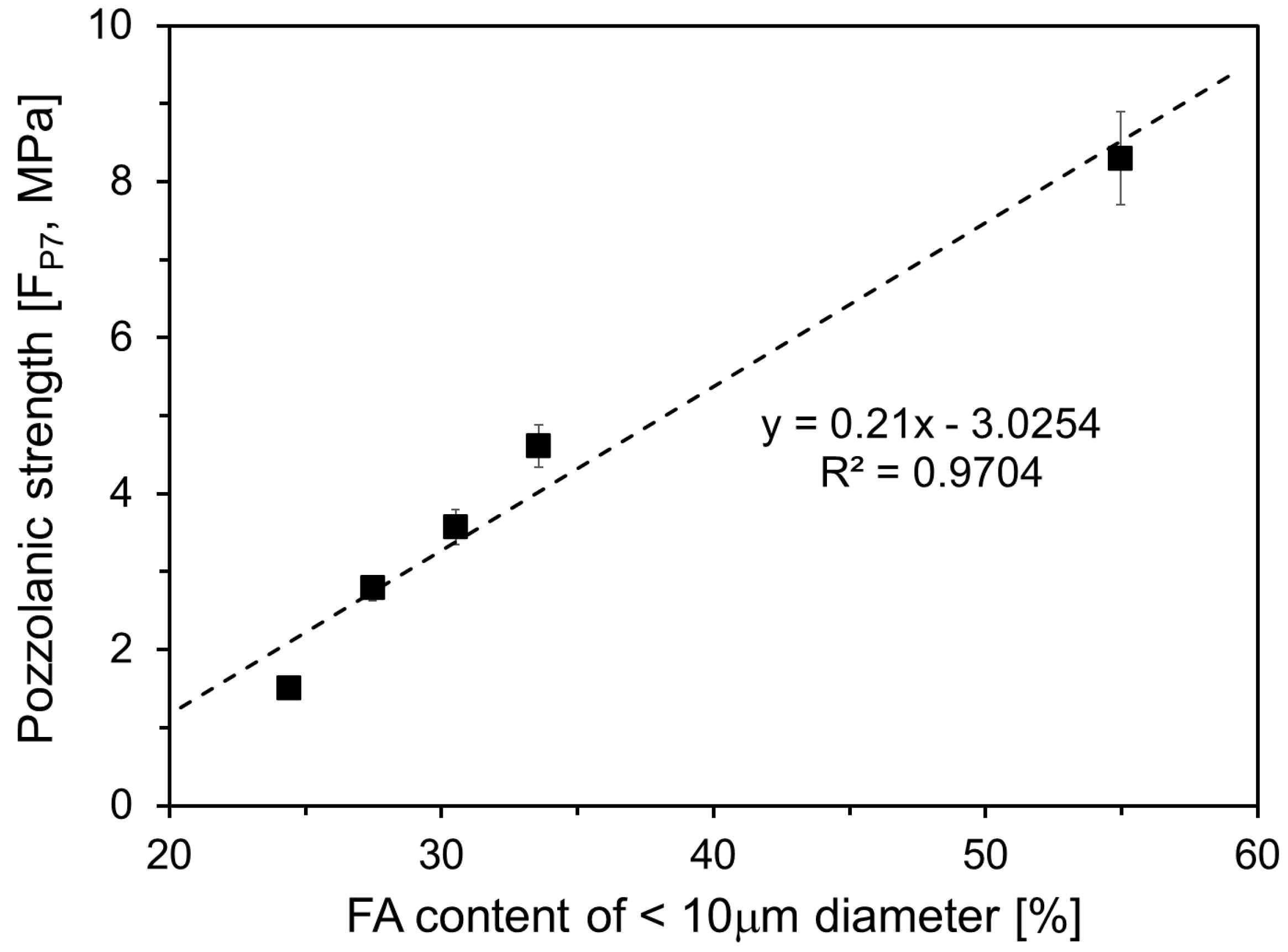
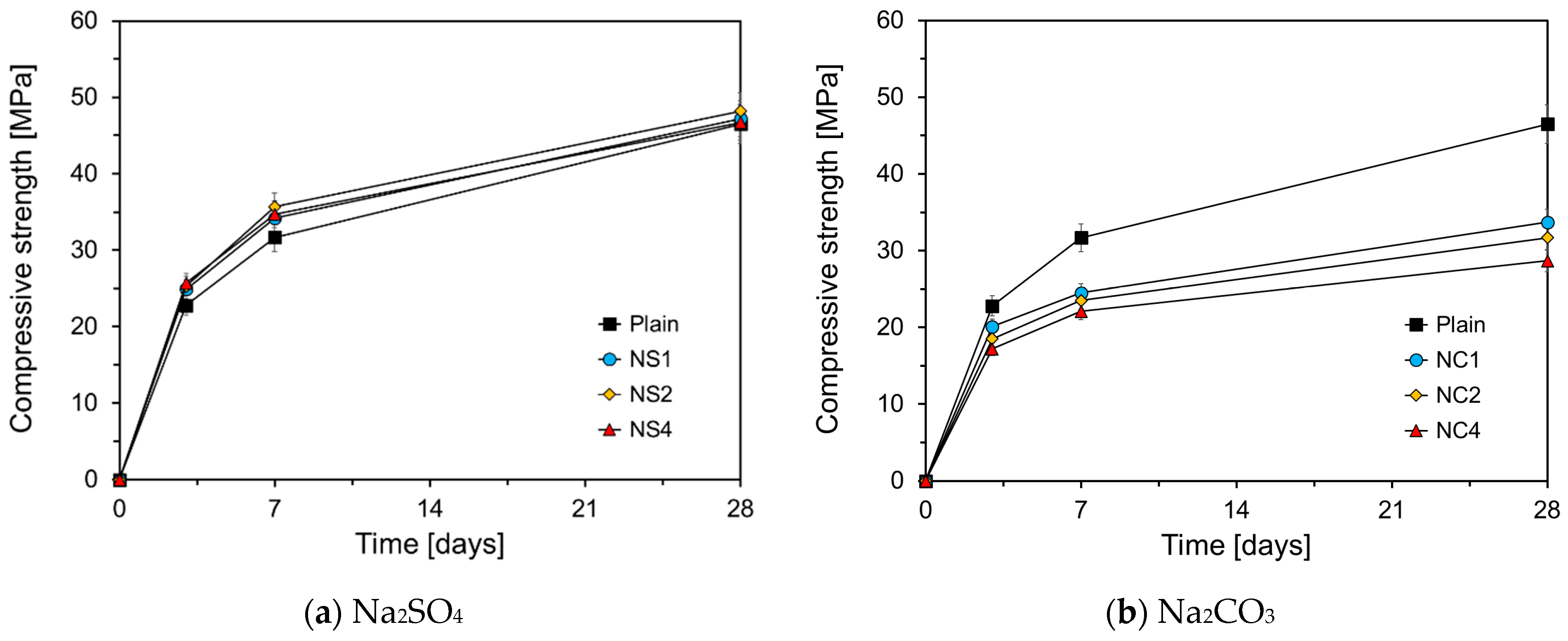
3.2. Compressive Strength
4. Conclusions
- The FA particle size significantly affects the early-age hydration of HVFA cement pastes. An increase in the contents of FA particles with a diameter lower than 10 μm led to higher specific heat flow, indicating that more OPC undergoes hydration due to the filling effect of FA particles.
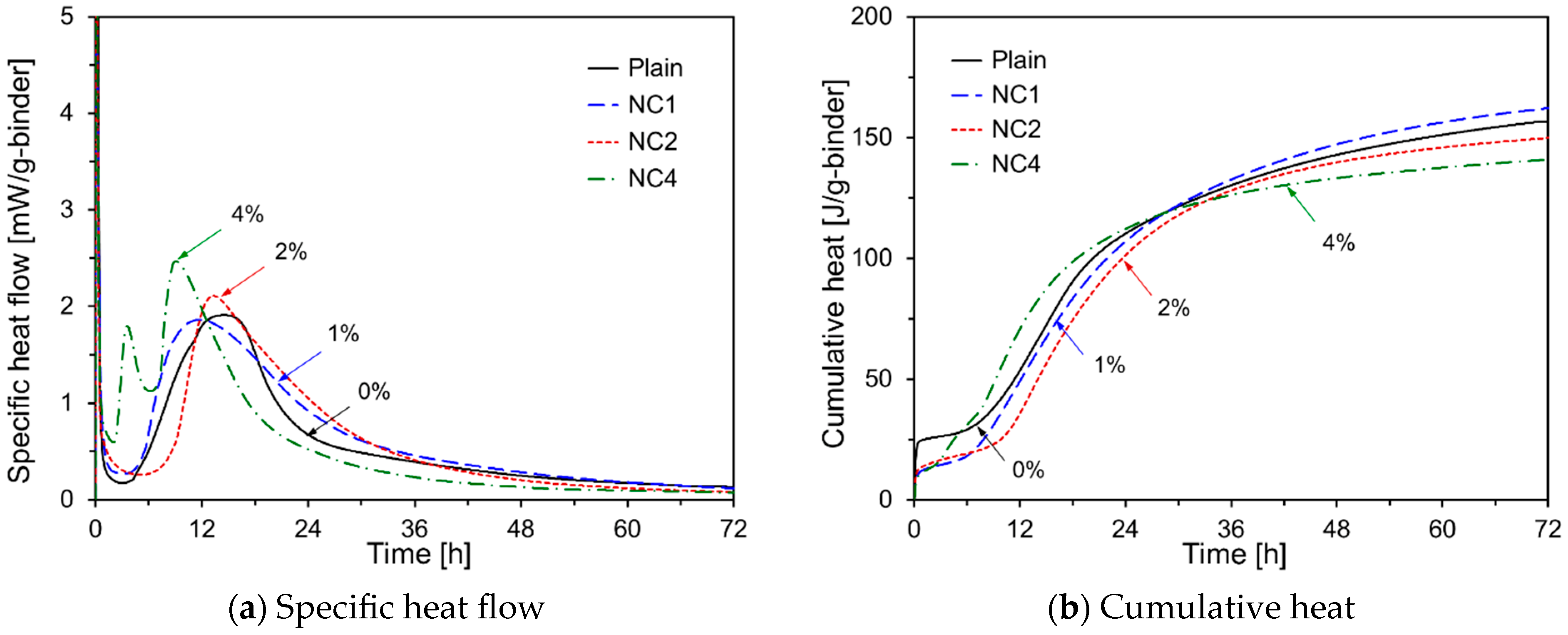
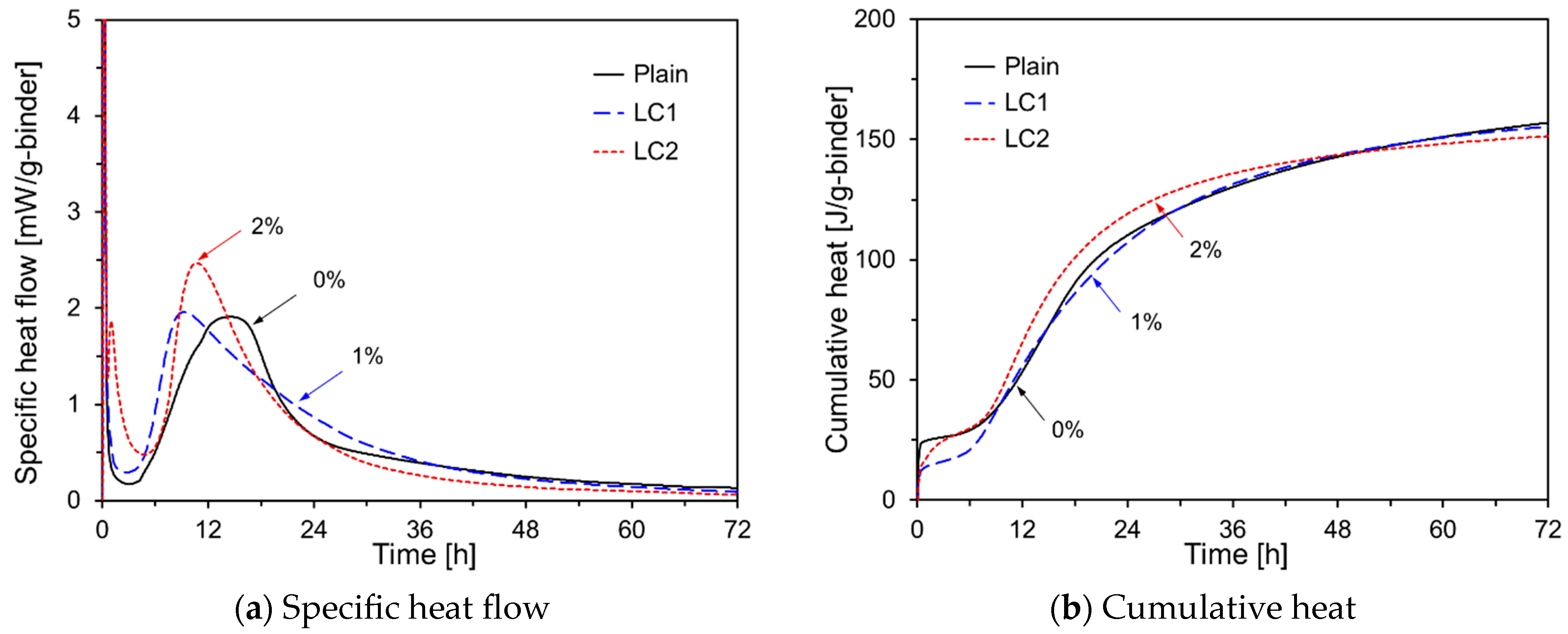
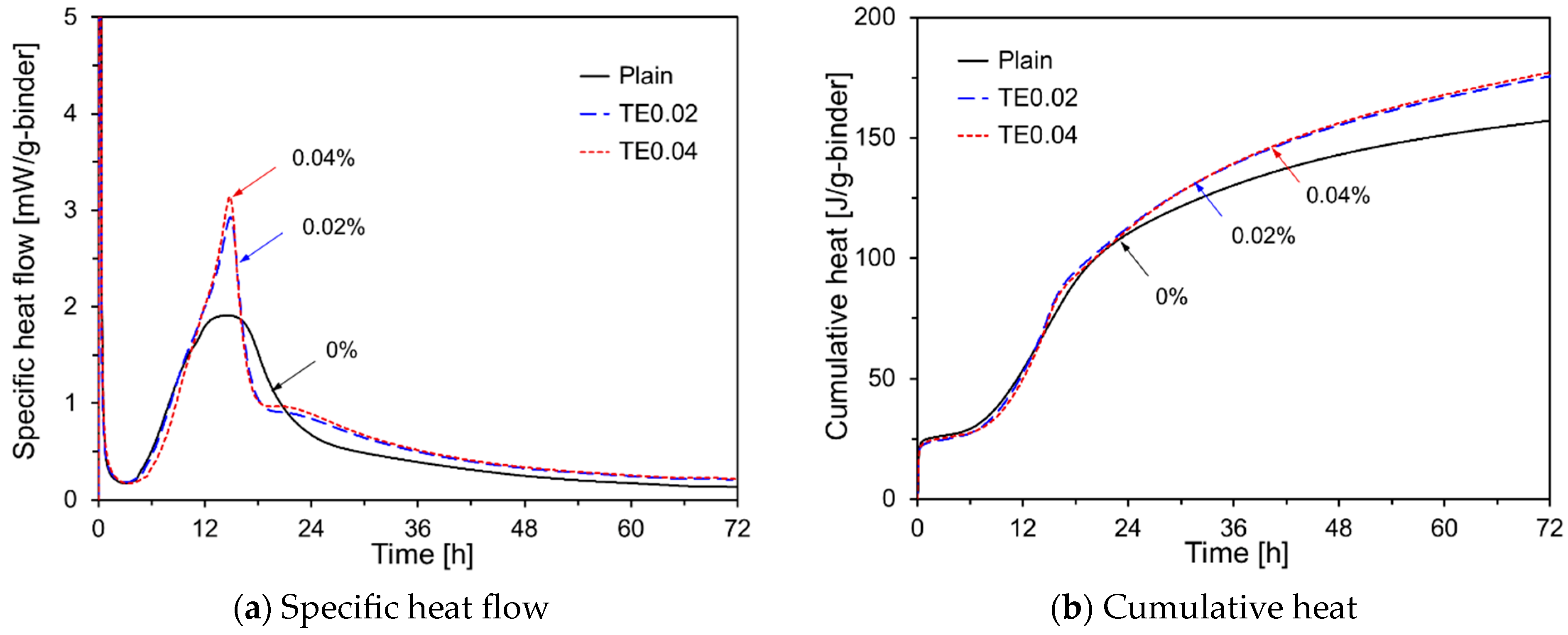
- The early-age hydration of HVFA cement pastes varied depending on the type of chemical activator used. Na2SO4, TEA, and TIPA consistently promoted early hydration, regardless of their concentration. In contrast, Na2CO3 promoted or delayed hydration depending on its concentration. Li2CO3 enhanced the hydration of HVFA cement pastes for up to 48 h but delayed it thereafter. The early-age hydration of HVFA cement pastes was significantly promoted when Na2SO4 was combined with TEA or TIPA.
- The compressive strength of HVFA mortar increased with a higher proportion of FA particles smaller than 10 μm. Specifically, the compressive strength of FH-50 was approximately 30%, 20%, and 20% greater than that of Plain at 3 d, 7 d, and 28 d, respectively. The pozzolanic strength increased linearly until 7 d, correlating with the content of FA particles smaller than 10 μm. Between 7 d and 28 d, the pozzolanic strength increased continuously as the fine particle content increased to 33.6%. However, the pozzolanic strength decreased when the filler content reached 55.0%.
- The compressive strength of the HVFA mortars differed depending on the type of chemical activator used. Na2SO4, TEA, and TIPA enhanced the compressive strength of the HVFA mortars regardless of their concentrations, whereas Na2CO3 and Li2CO3 reduced the compressive strength of the HVFA mortar. The highest compressive strength was achieved in the HVFA mortar using Na2SO4 combined with amine-based activators such as TEA and TIPA.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- ASTM C595-08a; Standard Specification for Blended Hydraulic Cements. ASTM: West Conshohocken, PA, USA, 2010. [CrossRef]
- Kanchanason, V.; Plank, J. Effectiveness of a calcium silicate hydrate—Polycarboxylate ether (C-S-H–PCE) nanocomposite on early strength development of fly ash cement. Constr. Build. Mater. 2018, 169, 20–27. [Google Scholar] [CrossRef]
- Zunino, F.; Bentz, D.P.; Castro, J. Reducing setting time of blended cement paste containing high-SO3 fly ash (HSFA) using chemical/physical accelerators and by fly ash pre-washing. Cem. Concr. Res. 2018, 90, 14–26. [Google Scholar] [CrossRef] [PubMed]
- Da Silva, S.R.; de Oliveira Andrade, J.J. Investigation of mechanical properties and carbonation of concretes with construction and demolition waste and fly ash. Constr. Build. Mater. 2017, 153, 704–715. [Google Scholar] [CrossRef]
- Shen, D.; Shi, X.; Zhu, S.; Duan, X.; Zhang, J. Relationship between tensile Young’s modulus and strength of fly ash high strength concrete at early age. Constr. Build. Mater. 2016, 123, 317–326. [Google Scholar] [CrossRef]
- Yu, J.; Lu, C.; Leung, C.K.Y.; Li, G. Mechanical properties of green structural concrete with ultrahigh-volume fly ash. Constr. Build. Mater. 2017, 147, 510–518. [Google Scholar] [CrossRef]
- De la Varga, I.; Spragg, R.P.; Di Bella, C.; Castro, J.; Bentz, D.P.; Weiss, J. Fluid transport in high volume fly ash mixtures with and without internal curing. Cem. Concr. Compos. 2014, 45, 102–110. [Google Scholar] [CrossRef]
- Siddique, R. Performance characteristics of high-volume Class F fly ash concrete. Cem. Concr. Res. 2004, 34, 487–493. [Google Scholar] [CrossRef]
- De Weerdt, K.; Haha, M.B.; Le Saout, G.; Kjellsen, K.O.; Justnes, H.; Lothenbach, B. Hydration mechanisms of ternary Portland cements containing limestone powder and fly ash. Cem. Concr. Res. 2011, 41, 279–291. [Google Scholar] [CrossRef]
- Rahhal, V.; Talero, R. Influence of two different fly ashes on the hydration of portland cements. J. Therm. Anal. Calorim. 2004, 78, 191–205. [Google Scholar] [CrossRef]
- Rahhal, V.; Talero, R. Early hydration of portland cement with crystalline mineral additions. Cem. Concr. Res. 2005, 35, 1285–1291. [Google Scholar] [CrossRef]
- Baert, G.; Hoste, S.; De Schutter, G.; De Belie, N. Reactivity of fly ash in cement paste studied by means of thermogravimetry and isothermal calorimetry. J. Therm. Anal. Calorim. 2008, 94, 485–492. [Google Scholar] [CrossRef]
- Bentz, D.P. Influence of water-to-cement ratio on hydration kinetics: Simple models based on spatial considerations. Cem. Concr. Res. 2006, 36, 238–244. [Google Scholar] [CrossRef]
- Berry, E.E.; Hemmings, R.T.; Cornelius, B.J. Mechanisms of hydration reactions in high volume fly ash pastes and mortars. Cem. Concr. Compos. 1990, 12, 253–261. [Google Scholar] [CrossRef]
- Lam, L.; Wong, Y.L.; Poon, C.S. Degree of hydration and gel/space ratio of high-volume fly ash/cement systems. Cem. Concr. Res. 2000, 30, 747–756. [Google Scholar] [CrossRef]
- Cui, Y.; Wang, L.; Liu, J.; Liu, R.; Pang, B. Impact of particle size of fly ash on the early compressive strength of concrete: Experimental investigation and modelling. Constr. Build. Mater. 2022, 323, 126444. [Google Scholar] [CrossRef]
- Małolepszy, J.; Tkaczewska, E. Effect of fly ash fineness on the fly ash cement hydration and properties. Cem.-Wapno-Beton Cem. Lime Concr. 2007, 12, 296–302. [Google Scholar]
- Erdogdu, K.; Turker, P. Effects of fly ash particle size on strength of Portland cement fly ash mortars. Cem. Concr. Res. 1998, 28, 1217–1222. [Google Scholar] [CrossRef]
- Kirchberger, I.; Goetz-Neunhoeffer, F.; Neubauer, J. Enhancing the aluminate reaction during OPC hydration by combining increased sulfate content, triethanolamine and tartaric acid. Cem. Concr. Res. 2023, 170, 107188. [Google Scholar] [CrossRef]
- Jung, S.; Kang, H.; Kang, S.-H.; Moon, J. Alkanolamine-based chemically enhanced hydration reaction of ordinary Portland cement. Constr. Build. Mater. 2023, 409, 134045. [Google Scholar] [CrossRef]
- Yaphary, Y.L.; Yu, Z.; Lam, R.H.W.; Lau, D. Effect of triethanolamine on cement hydration toward initial setting time. Constr. Build. Mater. 2017, 141, 94–103. [Google Scholar] [CrossRef]
- Heinz, D.; Gobel, M.; Hilbig, H.; Urbonas, L.; Bujauskaite, G. Effect of TEA on fly ash solubility and early age strength of mortar. Cem. Concr. Res. 2010, 40, 392–397. [Google Scholar] [CrossRef]
- Sandberg, P.J.; Doncaster, F. On the mechanism of strength enhancement of cement paste and mortar with triisopropanolamine. Cem. Concr. Res. 2004, 34, 973–976. [Google Scholar] [CrossRef]
- Lu, Z.; Kong, X.; Jansen, D.; Zhang, C. A comparative study of the effects of two alkanolamines on cement hydration. Adv. Cem. Res. 2022, 34, 47–56. [Google Scholar] [CrossRef]
- Tangpagasit, J.; Cheerarot, R.; Jaturapitakkul, C.; Kiattikomol, K. Packing effect and pozzolanic reaction of fly ash in mortar. Cem. Concr. Res. 2005, 35, 1145–1151. [Google Scholar] [CrossRef]
- Moon, G.D.; Oh, S.; Choi, Y.C. Effects of the physicochemical properties of fly ash on the compressive strength of high-volume fly ash mortar. Constr. Build. Mater. 2016, 124, 1072–1080. [Google Scholar] [CrossRef]
- Park, B.; Choi, Y.C. Hydration and pore-structure characteristics of high-volume fly ash cement pastes. Constr. Build. Mater. 2021, 278, 122390. [Google Scholar] [CrossRef]
- Hefni, Y.; El Zaher, Y.A.; Wahab, M.A. Influence of activation of fly ash on the mechanical properties of concrete. Constr. Build. Mater. 2018, 172, 728–734. [Google Scholar] [CrossRef]
- Krishnan, S.; Singh, A.; Bishnoi, S. Impact of alkali salts on the hydration of ordinary Portland cement and limestone–calcined clay cement. J. Mater. Civ. Eng. 2021, 33, 04021223. [Google Scholar] [CrossRef]
- Wang, Y.-S.; Tae, S.-H.; Lin, R.-S.; Wang, X.-Y. Effects of Na2CO3 on engineering properties of cement–limestone powder–slag ternary blends. J. Build. Eng. 2022, 57, 104937. [Google Scholar] [CrossRef]
- He, H.; Wang, Y.; He, F.; Luo, S.; Liu, S. Effect of Li2CO3 on the properties of Portland cement paste. Mater. Struct. 2021, 54, 33. [Google Scholar] [CrossRef]
- Xia, Y.; Zhao, Y.; Liu, M.; Guo, J.; Du, J.; Du, D. Hydration mechanism and phase assemblage of ternary blended cements based on sewage sludge ash and limestone: Modified by Na2SO4. Constr. Build. Mater. 2023, 364, 129982. [Google Scholar] [CrossRef]
- Lee, S.H.; Sakai, E.; Diamond, M.; Bang, W.K. Characterization of fly ash directly from electrostatic precipitator. Cem. Concr. Res. 1999, 29, 1791–1797. [Google Scholar] [CrossRef]
- Chindaprasirt, P.; Ruangsiriyakul, S.; Cao, H.T.; Bucea, L. Influence of Mae Moh fly ash fineness on characteristics, strength and drying shrinkage development of blended cement mortars. In Proceedings of the The Eighth East Asia-Pacific Conference on Structural Engineering and Construction, Singapore, 5–7 December 2001; p. 6, Paper No. 1191. [Google Scholar]
- Niu, M.; Li, G.; Zhang, J.; Cao, L. Preparation of alkali-free liquid accelerator based on aluminum sulfate and its accelerating mechanism on the hydration of cement pastes. Constr. Build. Mater. 2020, 253, 119246. [Google Scholar] [CrossRef]
- Janotka, I. Hydration of the cement paste with Na2CO3 addition. Ceram.-Silik. 2001, 45, 16–23. [Google Scholar]
- Ramachandran, V.S. Hydration of cement—Role of triethanolamine. Cem. Concr. Res. 1976, 6, 623–631. [Google Scholar] [CrossRef]
- Ramachandran, V.S. Action of triethanolamine on the hydration of tricalcium aluminate. Cem. Concr. Res. 1973, 3, 41–54. [Google Scholar] [CrossRef]
- Ramachandran, V.S. Influence of triethanolamine on the hydration characteristics of tricalcium silicate. J. Appl. Chem. Biotechnol. 1972, 22, 1125–1138. [Google Scholar] [CrossRef]
- Gartner, E.; Myers, D. Influence of tertiary alkanolamines on Portland-cement hydration. J. Am. Ceram. Soc. 1993, 76, 1521–1530. [Google Scholar] [CrossRef]
- Perez, J.-P.; Nonat, A.; Pourchet, S.; Garrault, S.; Mosquet, M.; Canevet, C. Why TIPA Leads to an Increase in the Mechanical Properties of Mortars whereas TEA Does Not. ACI Symp. Publ. 2003, 217, 583–594. [Google Scholar]
- Huang, H.; Li, X.-R.; Shen, X.-D. Hydration of ternary cement in the presence of triisopropanolamine. Constr. Build. Mater. 2016, 111, 513–521. [Google Scholar] [CrossRef]
- Donatello, S.; Fernandez-Jimenez, A.; Palomo, A.; Jantzen, C. Very High Volume Fly Ash Cements. Early Age Hydration Study Using Na2SO4 as an Activator. J. Am. Ceram. Soc. 2013, 96, 900–906. [Google Scholar] [CrossRef]
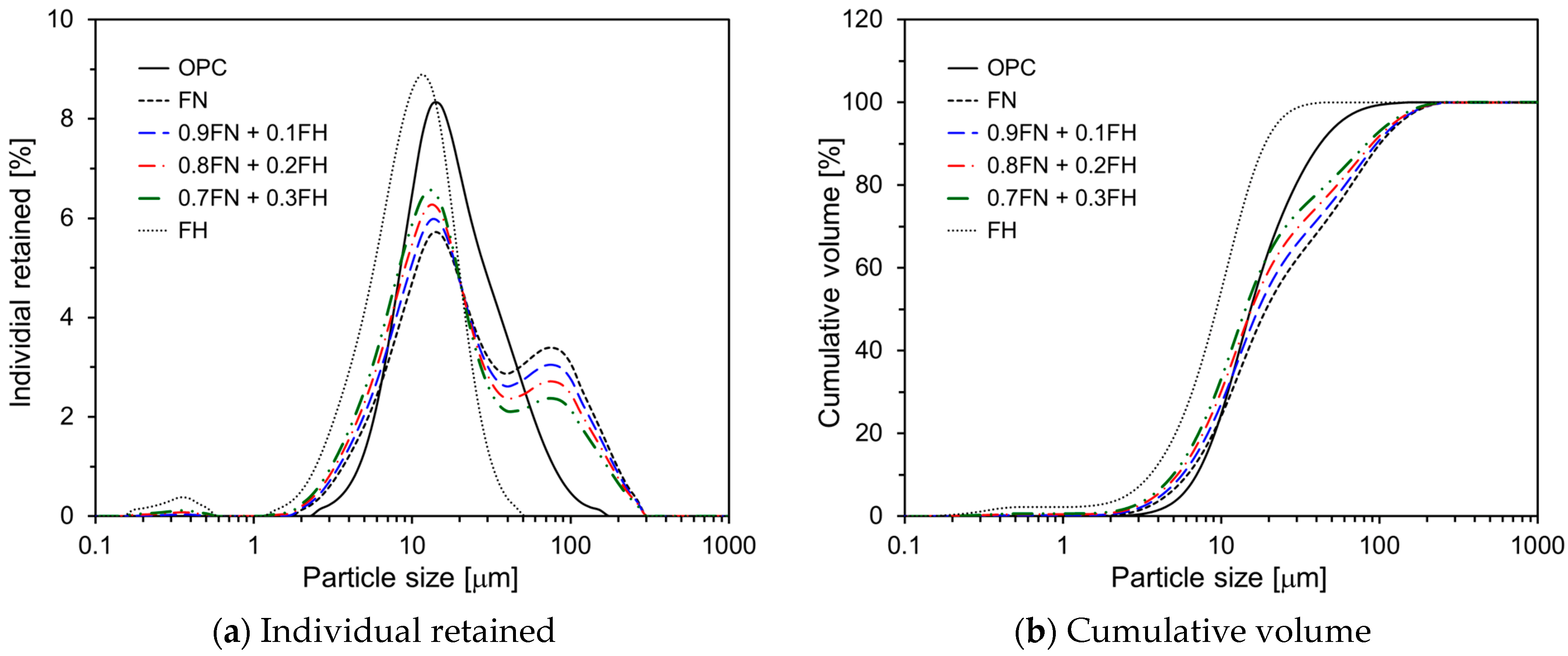







| Chemical Compositions (wt. %) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | Fe2O3 | MgO | K2O | Na2O | SO3 | LOI | |
| OPC | 62.3 | 20.8 | 4.3 | 3.1 | 4.4 | 0.6 | 0.3 | 1.5 | 1.3 |
| FA | 3.9 | 58.7 | 21.8 | 6.7 | 1.8 | 1.2 | 1.1 | 0.4 | 2.2 |
| Content (wt.%) | |||||
|---|---|---|---|---|---|
| FN | 0.9FN + 0.1FH | 0.8FN + 0.2FH | 0.7FN + 0.3FH | FH | |
| <10 μm | 24.4 | 27.5 | 30.5 | 33.6 | 55.0 |
| Binder (wt. %) | Activator (wt.% by Binder) | |||||||
|---|---|---|---|---|---|---|---|---|
| OPC | FN | FH | TEA | TIPA | Na2SO4 | Na2CO3 | LiCO3 | |
| Plain | 50 | 50 | - | - | - | - | - | - |
| Plain-Q * | 50 | 50 * | - | - | - | - | - | - |
| FH-10 | 50 | 45 | 5 | - | - | - | - | - |
| FH-20 | 50 | 40 | 10 | - | - | - | - | - |
| FH-30 | 50 | 35 | 15 | - | - | - | - | - |
| FH-50 | 50 | - | 50 | - | - | - | - | - |
| TE0.02 | 50 | 50 | - | 0.02 | - | - | - | - |
| TE0.04 | 50 | 50 | - | 0.04 | - | - | - | - |
| TI0.02 | 50 | 50 | - | - | 0.02 | - | - | - |
| TI0.1 | 50 | 50 | - | 0.1 | - | - | - | |
| TI0.2 | 50 | 50 | - | - | 0.2 | - | - | - |
| NS1 | 50 | 50 | - | - | - | 1 | - | - |
| NS2 | 50 | 50 | - | - | - | 2 | - | - |
| NS4 | 50 | 50 | - | - | - | 4 | - | - |
| NC1 | 50 | 50 | - | - | - | - | 1 | - |
| NC2 | 50 | 50 | - | - | - | - | 2 | - |
| NC4 | 50 | 50 | - | - | - | - | 4 | - |
| LC1 | 50 | 50 | - | - | - | - | - | 1 |
| LC2 | 50 | 50 | - | - | - | - | - | 2 |
| TE0.02NS2 | 50 | 50 | - | 0.02 | - | 2 | - | - |
| TI0.1NS2 | 50 | 50 | - | - | 0.1 | 2 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Choi, Y.-C.; Park, B. Effects of Fly Ash Particle Size and Chemical Activators on the Hydration of High-Volume Fly Ash Mortars. Materials 2024, 17, 5485. https://doi.org/10.3390/ma17225485
Choi Y-C, Park B. Effects of Fly Ash Particle Size and Chemical Activators on the Hydration of High-Volume Fly Ash Mortars. Materials. 2024; 17(22):5485. https://doi.org/10.3390/ma17225485
Chicago/Turabian StyleChoi, Young-Cheol, and Byoungsun Park. 2024. "Effects of Fly Ash Particle Size and Chemical Activators on the Hydration of High-Volume Fly Ash Mortars" Materials 17, no. 22: 5485. https://doi.org/10.3390/ma17225485
APA StyleChoi, Y.-C., & Park, B. (2024). Effects of Fly Ash Particle Size and Chemical Activators on the Hydration of High-Volume Fly Ash Mortars. Materials, 17(22), 5485. https://doi.org/10.3390/ma17225485






