Synthesis and Thermal Properties of Bio-Based Janus Ring Siloxanes Incorporating Terpenes and Terpenoids
Abstract
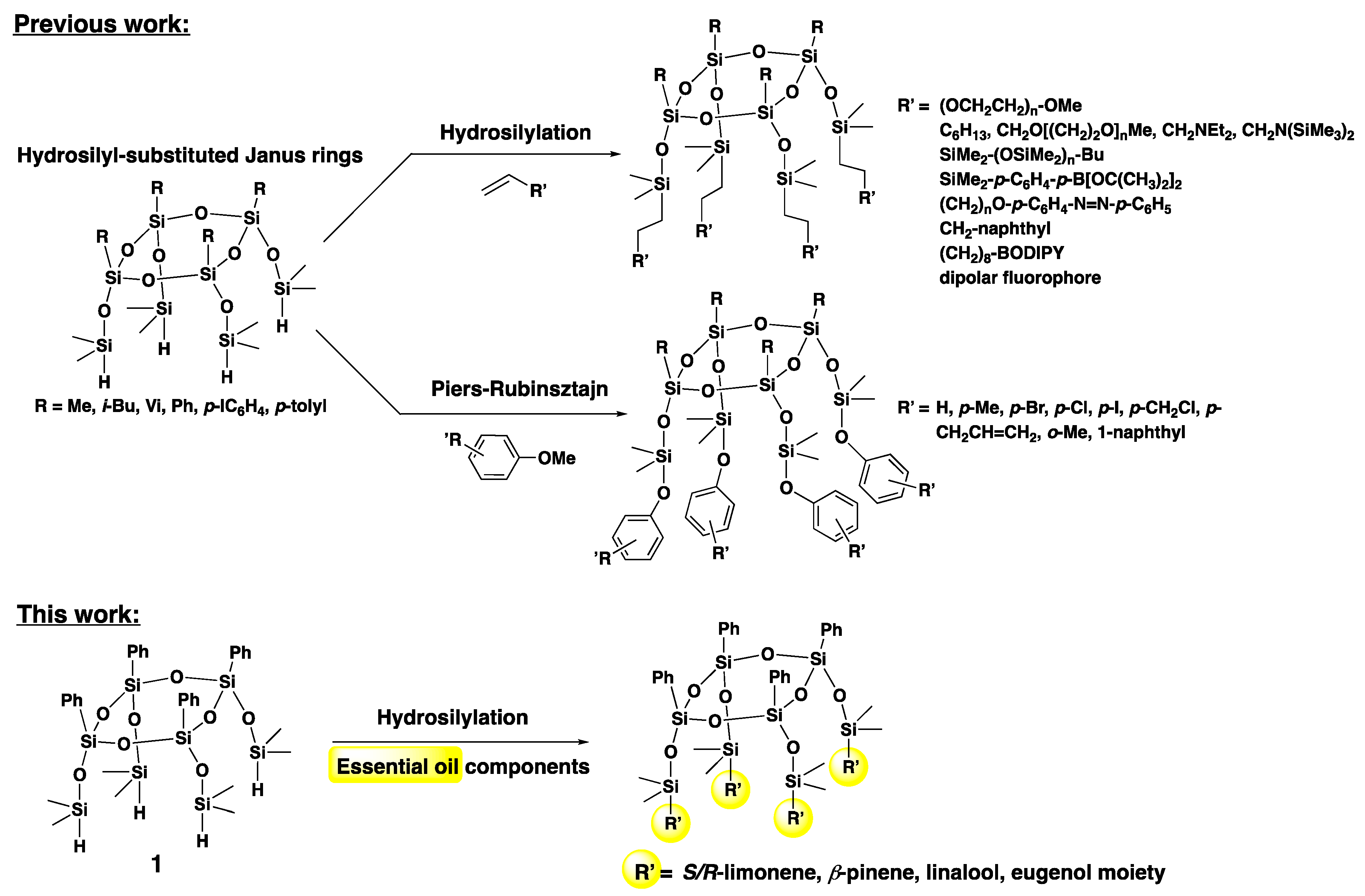
:1. Introduction
2. Materials and Methods
2.1. General Consideration
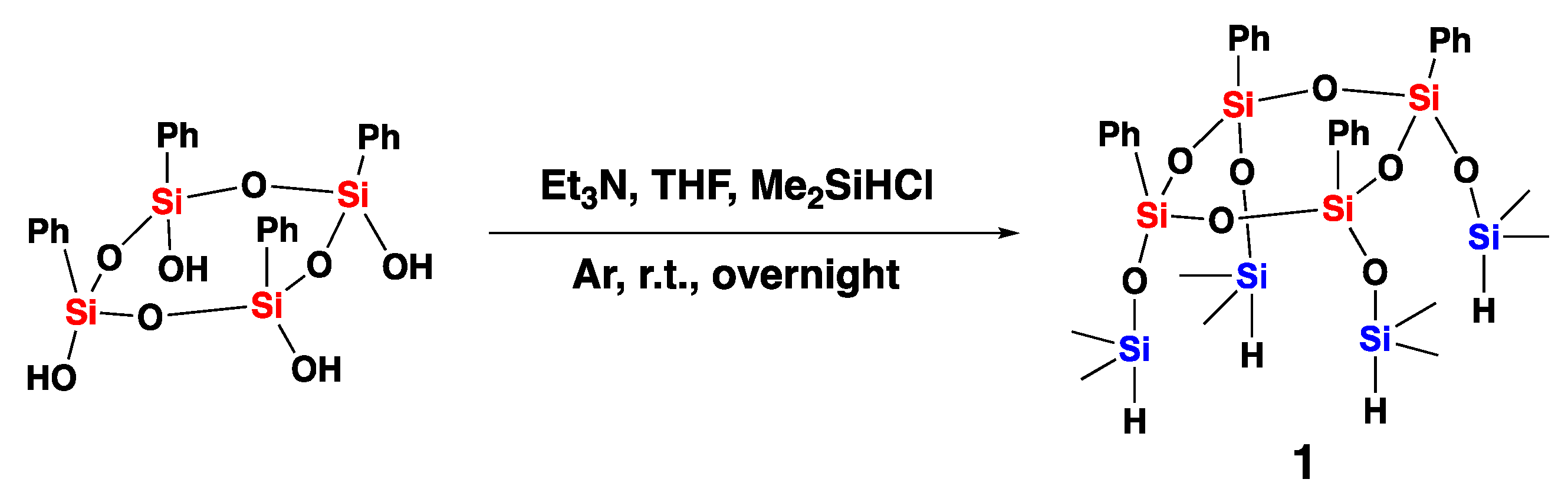
2.2. Synthesis of Compound 1 Starting from All-Cis Tetraphenylcyclotetrasiloxanetetraol
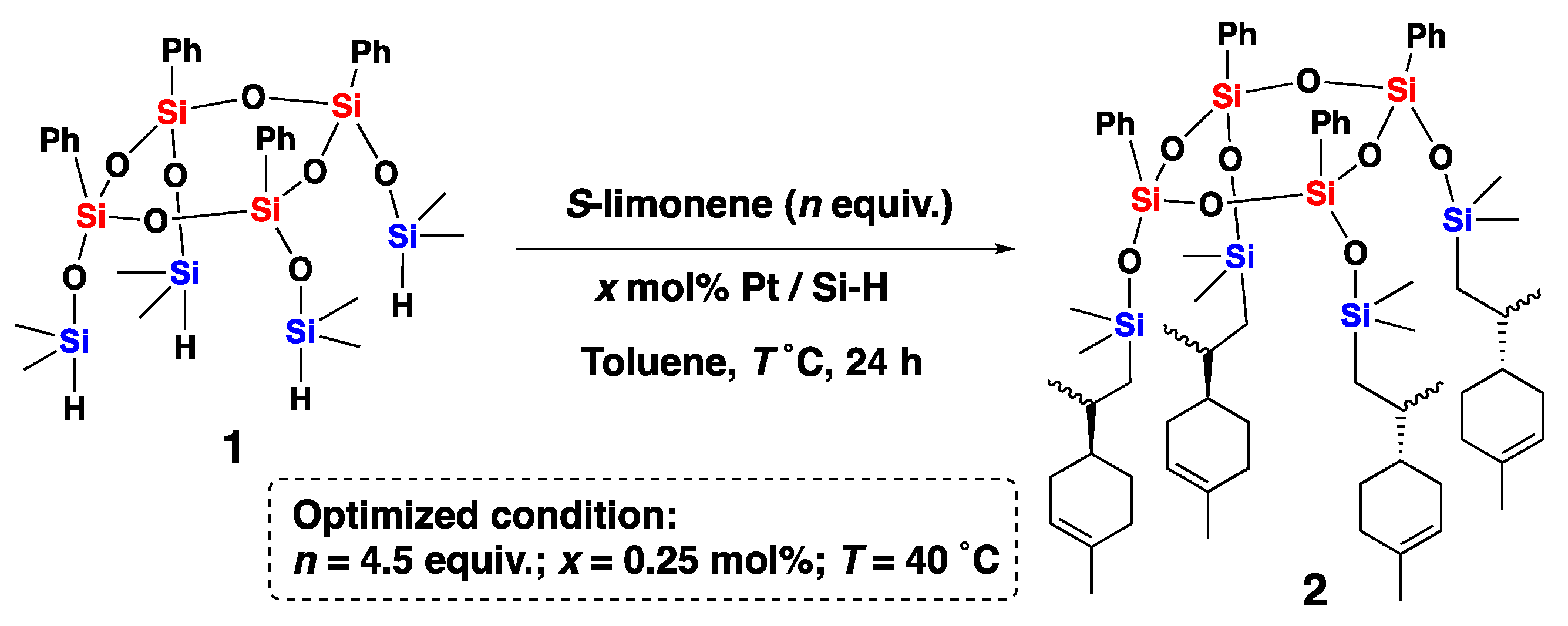
2.3. Synthesis of Compounds 2–6
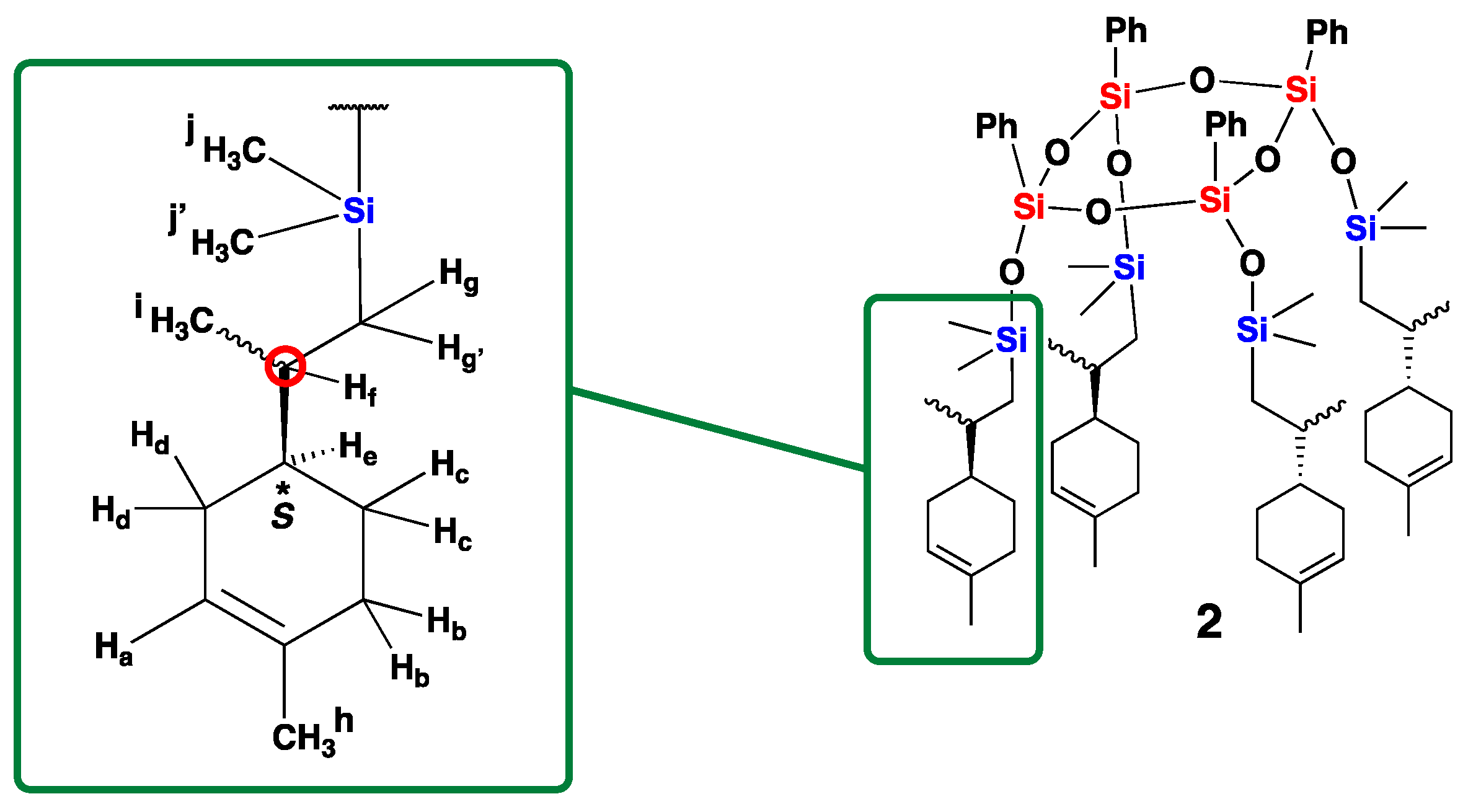
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Baney, R.H.; Itoh, M.; Sakakibara, A.; Suzuki, T. Silsesquioxanes. Chem. Rev. 1995, 95, 1409–1430. [Google Scholar] [CrossRef]
- Cordes, D.B.; Lickiss, P.D.; Rataboul, F. Recent Developments in the Chemistry of Cubic Polyhedral Oligosilsesquioxane. Chem. Rev. 2010, 110, 2081–2173. [Google Scholar] [PubMed]
- Cordes, D.B.; Lickiss, P.D. Preparation and Characterization of Polyhedral Oligosilsesquioxanes. In Applications of Polyhedral Oligomeric Silsesquioxanes; Matisons, J., Hartmann-Thompson, C., Eds.; Advances in Silicon Science Series; Springer: Dordrecht, The Netherlands, 2011; Volume 3, pp. 47–133. [Google Scholar]
- Laine, R.M.; Roll, M.F. Polyhedral phenylsilsesquioxanes. Macromolecules 2011, 44, 1073–1109. [Google Scholar] [CrossRef]
- Zhou, H.; Ye, Q.; Xu, J. Polyhedral oligomeric silsesquioxane-based hybrid materials and their applications. Mater. Chem. Front. 2017, 1, 212–230. [Google Scholar] [CrossRef]
- Chen, F.; Lin, F.; Zhang, Q.; Cai, R.; Wu, Y.; Ma, X. Polyhedral Oligomeric Silsesquioxane Hybrid Polymers: Well-Defined Architectural Design and Potential Functional Applications. Macromol. Rapid Commun. 2019, 40, 1900101. [Google Scholar] [CrossRef]
- Du, Y.; Liu, H. Cage-like Silsesquioxanes-based hybrid materials. Dalton Trans. 2020, 49, 5396–5405. [Google Scholar] [CrossRef]
- Liu, Y.; Chaiprasert, T.; Ouali, A.; Unno, M. Well-defined cyclic silanol derivatives. Dalton Trans. 2022, 51, 4227–4245. [Google Scholar] [CrossRef]
- Panisch, R.; Bassindale, A.R.; Korlyukov, A.A.; Pitak, M.B.; Coles, S.J.; Taylor, P.G. Selective Derivatization and Characterization of Bifunctional “Janus-type” Cyclotetrasiloxanes. Organometallics 2013, 32, 1732–1742. [Google Scholar] [CrossRef]
- Unno, M.; Endo, H.; Takeda, N. Synthesis and Structures of Extended Cyclic Siloxanes. Heteroatom. Chem. 2014, 25, 525–532. [Google Scholar] [CrossRef]
- Anisimov, A.A.; Kononevich, Y.N.; Buzin, M.I.; Peregudov, A.S.; Shchegolikhina, O.I.; Muzafarov, A.M. Convenient Synthesis of New Si-H and Si-Vinyl Functionalized Stereospecific 8-, 12- and 24-Membered Cyclosiloxanes. Macroheterocycles 2016, 9, 442–452. [Google Scholar] [CrossRef]
- Vysochinskaya, Y.S.; Anisimov, A.A.; Milenin, S.A.; Korlyukov, A.A.; Dolgushin, F.M.; Kononova, E.G.; Peregudov, A.S.; Buzin, M.I.; Shchegolikhina, O.I.; Muzafarov, A.M. New all-cis-tetra(p-tolyl)cyclotetrasiloxanetetraol and its functionalization. Mendeleev Commun. 2018, 28, 418–420. [Google Scholar] [CrossRef]
- Chaiprasert, T.; Liu, Y.; Intaraprecha, P.; Kunthom, R.; Takeda, N.; Unno, M. Synthesis of Tricyclic Laddersiloxane with Various Ring Sizes (Bat Siloxane). Macromol. Rapid Commun. 2021, 42, 2000608. [Google Scholar] [CrossRef] [PubMed]
- Zhemchugov, P.V.; Peregudov, A.S.; Malakhova, Y.N.; Buzin, A.I.; Buzin, M.I.; Shchegolikhina, O.I.; Muzafarov, A.M. Synthesis of siloxane analogs of calixarenes. Russ. Chem. Bull. Int. Ed. 2015, 64, 1394–1399. [Google Scholar] [CrossRef]
- Kholodkov, D.N.; Eremchuk, K.I.; Soldatkin, Y.V.; Volodin, A.D.; Korlyukov, A.A.; Anisimov, A.A.; Novikov, R.A.; Arzumanyan, A.V. Stereoregular cyclic p-tolyl-siloxanes with alkyl, O- and N-containing groups as promising reagents for the synthesis of functionalized organosiloxanes. New J. Chem. 2021, 45, 9805–9810. [Google Scholar] [CrossRef]
- Vysochinskaya, Y.S.; Gorodov, V.V.; Anisimov, A.A.; Boldyrev, K.L.; Buzin, M.I.; Naumkin, A.V.; Maslakov, K.I.; Peregudov, A.S.; Shchegolikhina, O.I.; Muzafarov, A.M. New star-like polydimethylsiloxanes: Synthesis, properties, and application. Russ. Chem. Bull. Int. Ed. 2017, 66, 1094–1098. [Google Scholar] [CrossRef]
- Vysochinskaya, Y.S.; Anisimov, A.A.; Peregudov, A.S.; Dubovik, A.S.; Orlov, V.N.; Malakhova, Y.N.; Stupnikov, A.A.; Buzin, M.I.; Nikiforova, G.G.; Vasil’ev, V.G.; et al. Star-Shaped Siloxane Polymers with Various Cyclic Cores: Synthesis and Properties. J. Polym. Sci. Part A Polym. Chem. 2019, 57, 1233–1246. [Google Scholar] [CrossRef]
- Dyuzhikova, Y.S.; Anisimov, A.A.; Peregudov, A.S.; Buzin, M.I.; Nikiforova, G.G.; Vasil’ev, V.G.; Kostrov, S.A.; Buzin, A.I.; Stupnikov, A.A.; Malakhova, Y.N.; et al. Star-Shaped Polydimethylsiloxanes with Organocyclotetrasilsesquioxane Branching-Out Centers: Synthesis and Properties. Polymers 2022, 14, 285. [Google Scholar] [CrossRef]
- Dyuzhikova, Y.S.; Anisimov, A.A.; Gorodov, V.V.; Olenich, E.A.; Buzin, M.I.; Nikiforova, G.G.; Kostrov, S.A.; Shchegolikhina, O.I.; Muzafarov, A.M. The effect of the polydimethylsiloxane chain length on the properties of four-arm siloxane stars. J. Organomet. Chem. 2023, 989, 122650. [Google Scholar] [CrossRef]
- Anisimov, A.A.; Drozdov, F.V.; Vysochinskaya, Y.S.; Minyaylo, E.O.; Peregudov, A.S.; Dolgushin, F.M.; Shchegolikhina, O.I.; Muzafarov, A.M. Organoboron Derivatives of Stereoregular Phenylcyclosilsesquioxanes. Chem. Eur. J. 2020, 26, 11404–11407. [Google Scholar] [CrossRef]
- Migulin, D.; Vysochinskaya, Y.; Buzin, M.; Bakirov, A.; Cherkaev, G.; Shchegolikhina, O.I. Stereoregular hybrid azobenzene-cyclosiloxanes with photoinduced reversible solid to liquid transition properties. J. Photochem. Photobio. A Chem. 2021, 407, 113033. [Google Scholar] [CrossRef]
- Belova, A.S.; Kononevich, Y.N.; Sazhnikov, V.A.; Safonov, A.A.; Ionov, D.S.; Anisimov, A.A.; Shchegolikhina, O.I.; Alfimov, M.V.; Muzafarov, A.M. Solvent-controlled intramolecular excimer emission from organosilicon derivatives of naphthalene. Tetrahedron 2021, 93, 132287. [Google Scholar] [CrossRef]
- Pakhomov, A.A.; Kononevich, Y.N.; Stukalova, M.V.; Svidchenko, E.A.; Surin, N.M.; Cherkaev, G.V.; Shchegolikhina, O.I.; Martynov, V.I.; Muzafarov, A.M. Synthesis and photophysical properties of a new BODIPY-based siloxane dye. Tetrahedron Lett. 2016, 57, 979–982. [Google Scholar] [CrossRef]
- Pakhomov, A.A.; Kim, E.E.; Kononevich, Y.N.; Ionov, D.S.; Maksimova, M.A.; Khalchenia, V.B.; Maksimov, E.G.; Anisimov, A.A.; Shchegolikhina, O.I.; Martynov, V.I.; et al. Modulation of the photophysical properties of multi-BODIPY-siloxane conjugates by varying the number of fluorophores. Dyes Pigment. 2022, 203, 110371. [Google Scholar] [CrossRef]
- Belova, A.S.; Kononevich, Y.N.; Ionov, D.S.; Sazhnikov, V.A.; Anisimov, A.A.; Safonov, A.A.; Surin, N.M.; Svidchenko, E.A.; Fedorov, Y.V.; Shchegolikhina, O.I.; et al. Tetrachromophoric Derivatives of Dibenzoylmethanatoboron Difluoride Based on Stereoregular Cyclotetrasiloxanes: Synthesis and Properties. J. Phys. Chem. B 2023, 127, 5881–5898. [Google Scholar] [CrossRef] [PubMed]
- Chaiprasert, T.; Liu, Y.; Takeda, N.; Unno, M. Janus ring siloxane: Versatile precursor of extended Janus ring and tricyclic laddersiloxanes. Dalton Trans. 2020, 49, 13533–13537. [Google Scholar] [CrossRef]
- Chaiprasert, T.; Liu, Y.; Takeda, N.; Unno, M. Vinyl-Functionalized Janus Ring Siloxane: Potential Precursors to Hybrid Functional Materials. Materials 2021, 14, 2014. [Google Scholar] [CrossRef]
- Chaiprasert, T.; Chanmungkalakul, S.; Liu, Y.; Bureerug, T.; Silpcharu, K.; Unno, M.; Liu, X.; Ervithayasuporn, V.; Chang, Y.; Rashatasakhon, P. Fluorescent Janus ring siloxanes for detection of Au(III) and L-cysteine. Dyes Pigment. 2022, 208, 110793. [Google Scholar] [CrossRef]
- Baptiste Hzounda Fokou, J.; Michel Jazet Dongmo, P.; Fekam Boyom, F. Essential Oil’s Chemical Composition and Pharmacological Properties. In Essential Oils—Oils of Nature; EI-Shemy, H.A., Ed.; IntechOpen: London, UK, 2020; Available online: https://www.intechopen.com/chapters/68008 (accessed on 8 January 2020).
- Bakkali, F.; Averbeck, S.; Averbeck, D.; Idaomar, M. Biological effects of essential oils—A review. Food Chem. Toxicol. 2008, 46, 446–475. [Google Scholar] [CrossRef]
- Adorjan, B.; Buchbauer, G. Biological properties of essential oils: An updated review. Flavour. Fragr. J. 2010, 25, 407–426. [Google Scholar] [CrossRef]
- Dhifi, W.; Bellili, S.; Jazi, S.; Bahloul, N.; Mnif, W. Essential Oil’s Chemical Characterization and Investigation of Some Biological Activities: A Critical Review. Medicines 2016, 3, 25. [Google Scholar] [CrossRef]
- de Sousa, D.P.; Damasceno, R.O.S.; Amorati, R.; Elshabrawy, H.A.; de Castro, R.D.; Bezerra, D.P.; Nunes, V.R.V.; Gomes, R.C.; Lima, T.C. Essential Oils: Chemistry and Pharmacological Activities. Biomolecules 2023, 13, 1144. [Google Scholar] [CrossRef]
- Masyita, A.; Sari, R.M.; Astuti, A.D.; Yasir, B.; Rumata, N.R.; Emran, T.B.; Mainu, F.; Simal-Gandara, J. Terpenes and Terpenoids as main bioactive compounds of essential oils, their roles in human health and potential application as natural food preservatives. Food Chem. 2022, 13, 100217. [Google Scholar] [CrossRef] [PubMed]
- Mosquera, M.E.G.; Jiménez, G.; Tabernero, V.; Vinueza-Vaca, J.; García-Estrada, C.; Kosalková, K.; Sola-Landa, A.; Monje, B.; Acosta, C.; Alonso, R.; et al. Terpenes and Terpenoids: Building Blocks to Produce Biopolymers. Sustain. Chem. 2021, 2, 467–492. [Google Scholar] [CrossRef]
- Chen, G.; Feng, J.; Qiu, W.; Zhao, Y. Eugenol-modified polysiloxanes as effective anticorrosion additives for epoxy resin coatings. RSC Adv. 2017, 7, 55967–55976. [Google Scholar] [CrossRef]
- Walczak, M.; Januszewski, R.; Franczyk, A.; Marciniec, B. Synthesis of monofunctionalized POSS through hydrosilylation. J. Organomet. Chem. 2018, 872, 73–78. [Google Scholar] [CrossRef]
- Dai, J.; Yang, S.; Teng, N.; Liu, Y.; Liu, X.; Zhu, J.; Zhao, J. Synthesis of Eugenol-Based Silicon-Containing Benzoxazines and Their Applications as Bio-Based Organic Coatings. Coatings 2018, 8, 88. [Google Scholar] [CrossRef]
- Walczak, M.; Franczyk, A.; Dutkiewicz, M.; Marciniec, B. Synthesis of Bifunctional Silsesquioxanes (RSiMe2O)~4(R’SiMe2O)~4Si8O12 via Hydrosilylation of Alkenes. Organometallics 2019, 38, 3018–3024. [Google Scholar] [CrossRef]
- Ji, J.; Ge, X.; Liang, W.; Liang, R.; Pang, X.; Liu, R.; Wei, S.; Sun, J.; Chen, X.; Ge, J. A Simple Preparation Route for Bio-Phenol MQ Silicone Resin via the Hydrosilylation Method and its Autonomic Antibacterial Property. Polymers 2019, 11, 1389. [Google Scholar] [CrossRef]
- Brzakalski, D.; Sztorch, B.; Frydrych, M.; Pakula, D.; Dydek, K.; Kozera, R.; Boczkowska, A.; Marciniec, B.; Przekop, B.E. Limonene Derivative of Spherosiliate as a Polylactide Modifier for Applications in 3D Printing Technology. Molecules 2020, 25, 5882. [Google Scholar] [CrossRef]
- Yi, M.; Chen, X.; Shuttleworth, P.S.; Tan, L.; Ruan, Y.; Xu, Y.; Zheng, J.; Wu, S.; Hu, S.; Xie, S.; et al. Facile fabrication of eugenol-containing polysiloxane films with good optical properties and excellent thermal stability via Si-H chemistry. J. Mater. Chem. C 2021, 9, 8020. [Google Scholar] [CrossRef]
- Zhang, X.; Cai, Y.; Zhang, X.; Aziz, T.; Fan, H.; Bittencourt, C. Synthesis and characterization of eugenol-based silicone modified waterborne polyurethane with excellent properties. J. Appl. Polym. Sci. 2021, 138, e50515. [Google Scholar] [CrossRef]
- Sokolnicki, T.; Franczyk, A.; Janowski, B.; Walkowiak, J. Synthesis of Bio-Based Silane Coupling Agents by the Modification of Eugenol. Adv. Synth. Catal. 2021, 363, 5493–5500. [Google Scholar] [CrossRef]
- Ryzhkov, A.I.; Drozdov, F.V.; Cherkaev, G.V.; Muzafarov, A.M. Synthesis of Carbosilane and Carbosilane-Siloxane Dendrons Based on Limonene. Polymers 2022, 14, 3279. [Google Scholar] [CrossRef] [PubMed]
- Nowacka, M.; Kowalewska, A.; Rygala, A.; Kregiel, D.; Kaczorowski, W. Bio-Based Silicone Coatings with Anti-adhesive Properties. Materials 2023, 16, 1381. [Google Scholar] [CrossRef] [PubMed]
- Nakajima, Y.; Shimada, S. Hydrosilylation reaction of olefins: Recent advances and perspectives. RSC Adv. 2015, 5, 20603–20616. [Google Scholar] [CrossRef]
- Erasto, P.; Viljoen, A.M. Limonene—A Review: Biosynthetic, Ecological and Pharmacological Relevance. Nat. Prod. Commun. 2008, 3, 1193–1202. [Google Scholar] [CrossRef]
- Frydrych, M.; Sztorch, B.; Brzakalski, D.; Kozera, R.; Konieczna, R.; Osiecki, T.; Przekop, R.E. Silsesquioxane-Doped Electrospun Nanofibrillar Membranes for Separation Systems. Polymers 2022, 14, 3569. [Google Scholar] [CrossRef]


| Compound | Isolated Yield (%) | 29Si NMR, ppm | |
|---|---|---|---|
| M-Unit | T-Unit | ||
| 1 [26] | 80 | −3.16 | −78.08 |
| 2 | 75 | 11.06, 11.12 | −79.87 |
| 3 | 77 | 11.04, 11.10 | −79.87 |
| 4 | 65 | 10.66 | −79.89 |
| 5 | 70 | 11.72 | −79.36 |
| 6 | 53 | 10.79 | −79.54 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yagafarov, N.; Kuang, J.; Takeda, N.; Liu, Y.; Ouali, A.; Unno, M. Synthesis and Thermal Properties of Bio-Based Janus Ring Siloxanes Incorporating Terpenes and Terpenoids. Materials 2024, 17, 5348. https://doi.org/10.3390/ma17215348
Yagafarov N, Kuang J, Takeda N, Liu Y, Ouali A, Unno M. Synthesis and Thermal Properties of Bio-Based Janus Ring Siloxanes Incorporating Terpenes and Terpenoids. Materials. 2024; 17(21):5348. https://doi.org/10.3390/ma17215348
Chicago/Turabian StyleYagafarov, Niyaz, Jiaorong Kuang, Nobuhiro Takeda, Yujia Liu, Armelle Ouali, and Masafumi Unno. 2024. "Synthesis and Thermal Properties of Bio-Based Janus Ring Siloxanes Incorporating Terpenes and Terpenoids" Materials 17, no. 21: 5348. https://doi.org/10.3390/ma17215348
APA StyleYagafarov, N., Kuang, J., Takeda, N., Liu, Y., Ouali, A., & Unno, M. (2024). Synthesis and Thermal Properties of Bio-Based Janus Ring Siloxanes Incorporating Terpenes and Terpenoids. Materials, 17(21), 5348. https://doi.org/10.3390/ma17215348








