Study on the Oxidation Behavior of TiB2-CeO2-Modified (Nb,Mo,Cr,W)Si2 Coating on the Surface of Niobium Alloy
Abstract
1. Introduction
2. Experimental Procedure
2.1. Nb Alloy Substrate Preparation
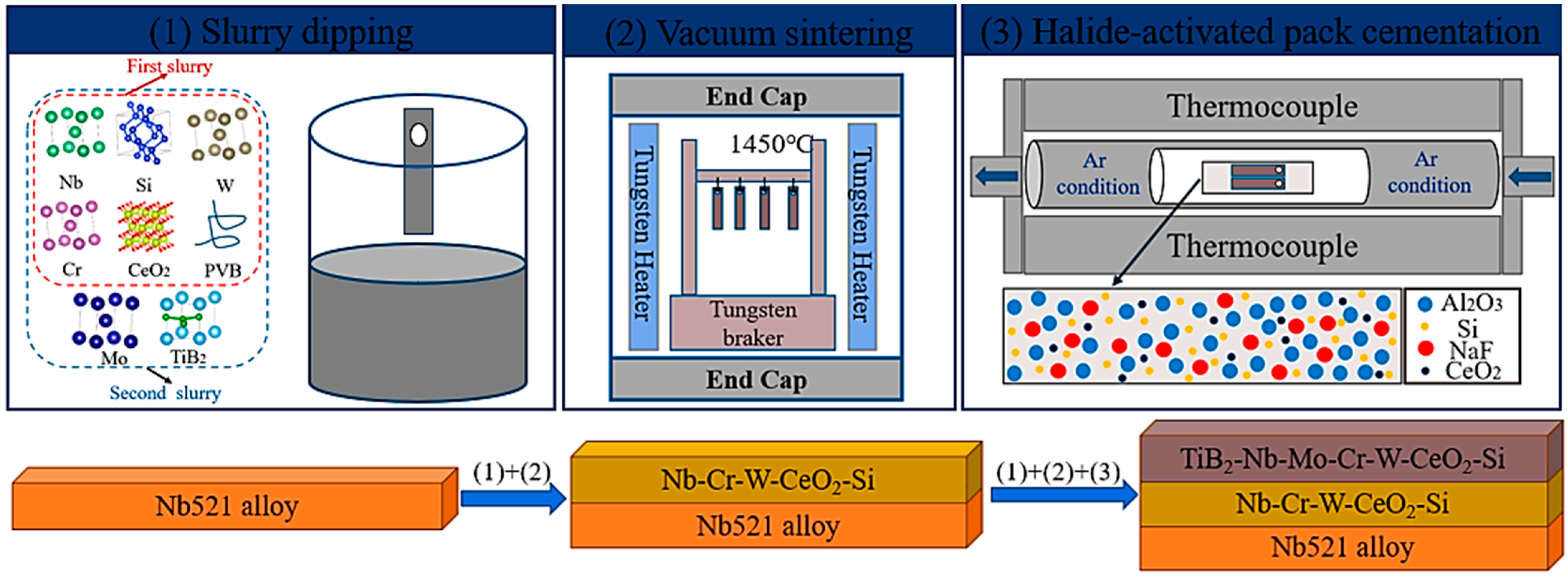
2.2. Preparation of the Coating
2.3. Characterization of Microstructure
2.4. Oxidation Resistance and Thermal Shock Resistance Tests of the Coating
3. Result and Discussion
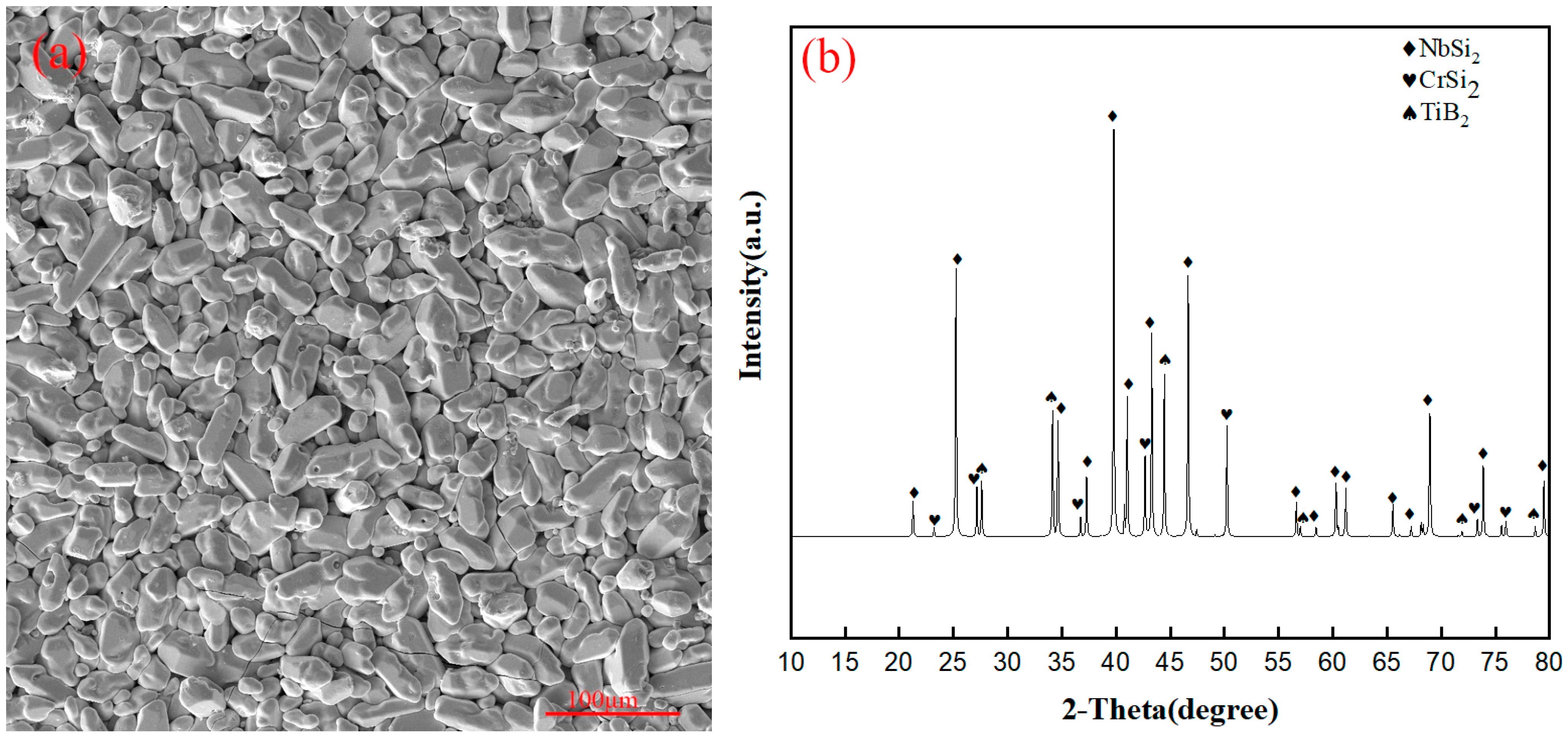
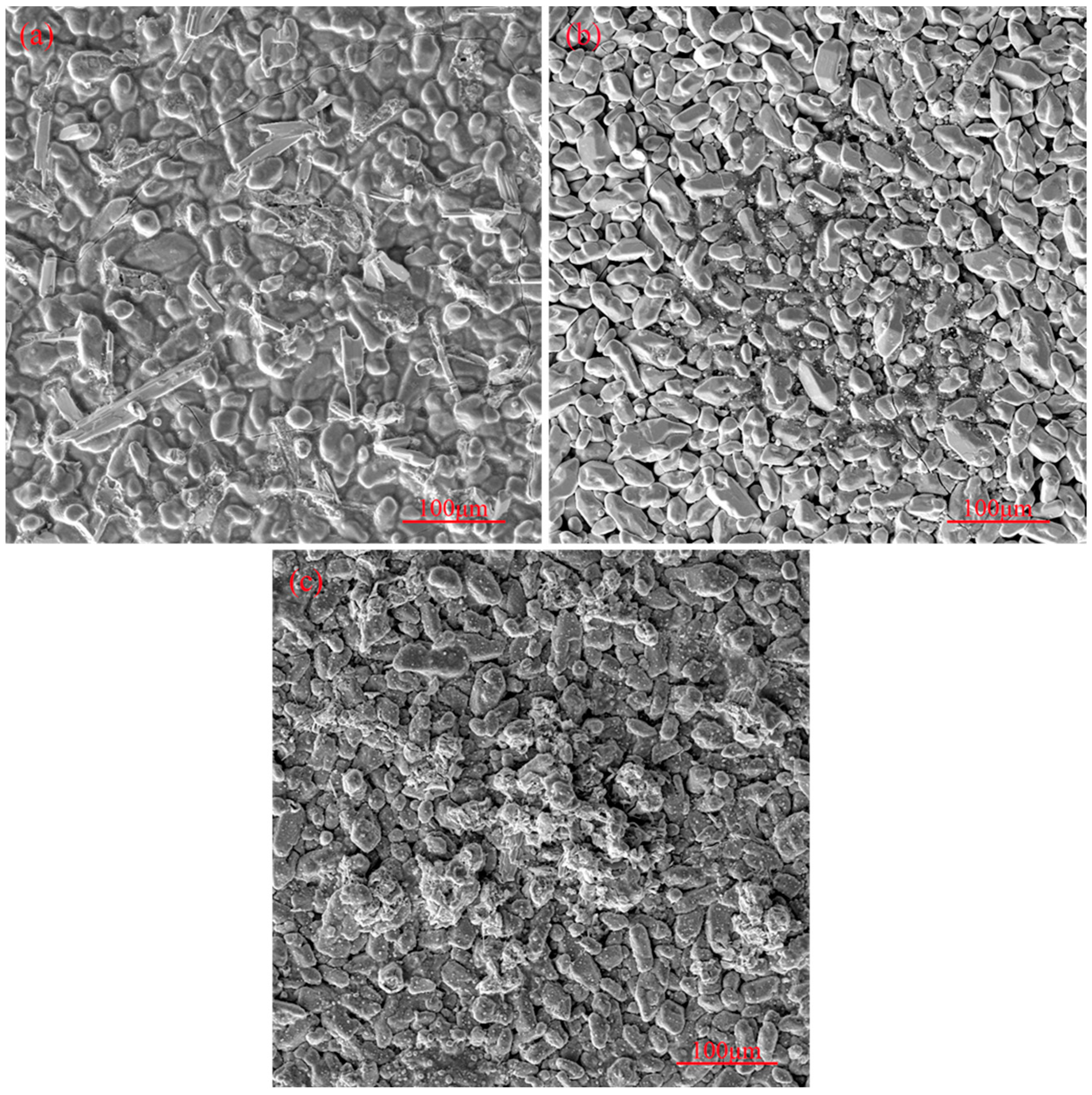
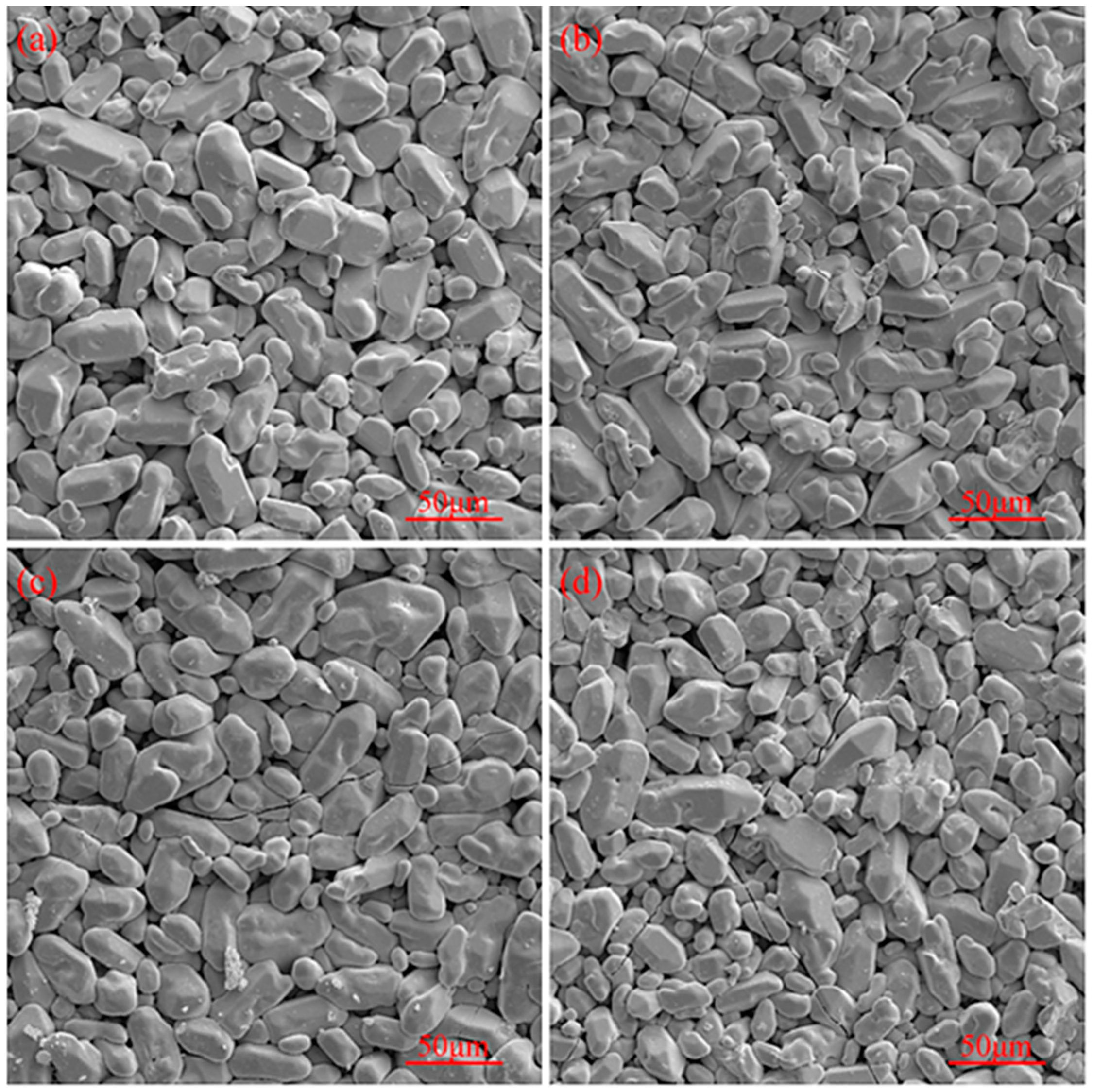
3.1. Surface Morphology of the Original Coating
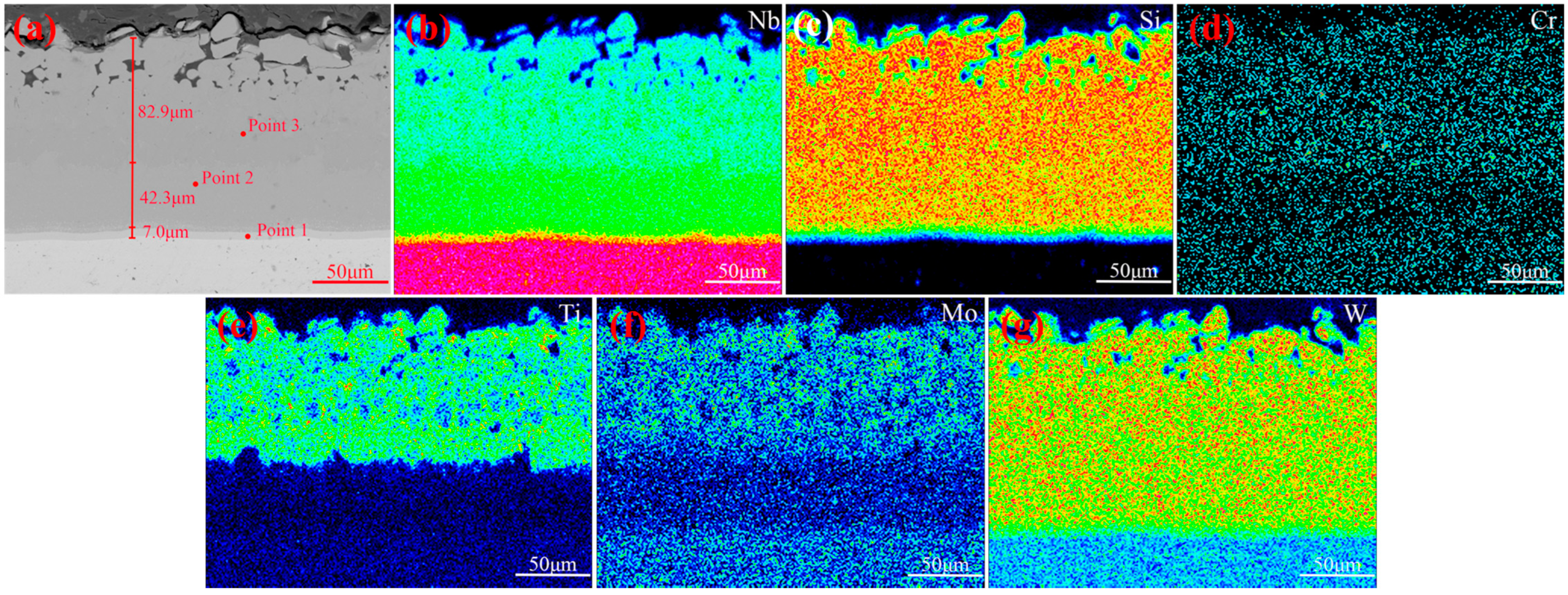
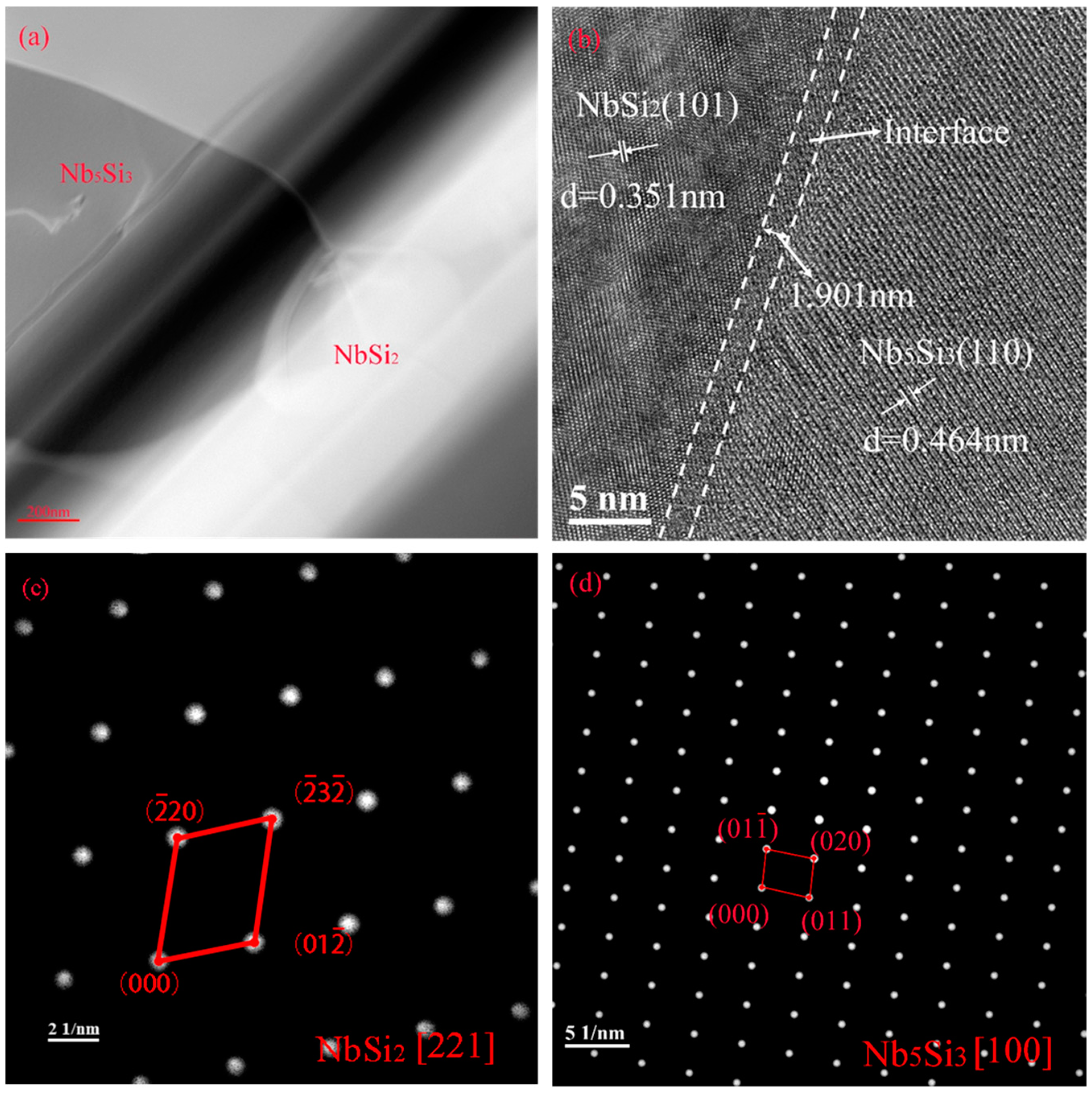
3.2. The Cross-Sectional Morphology and Mechanism of Formation of the Original Coating
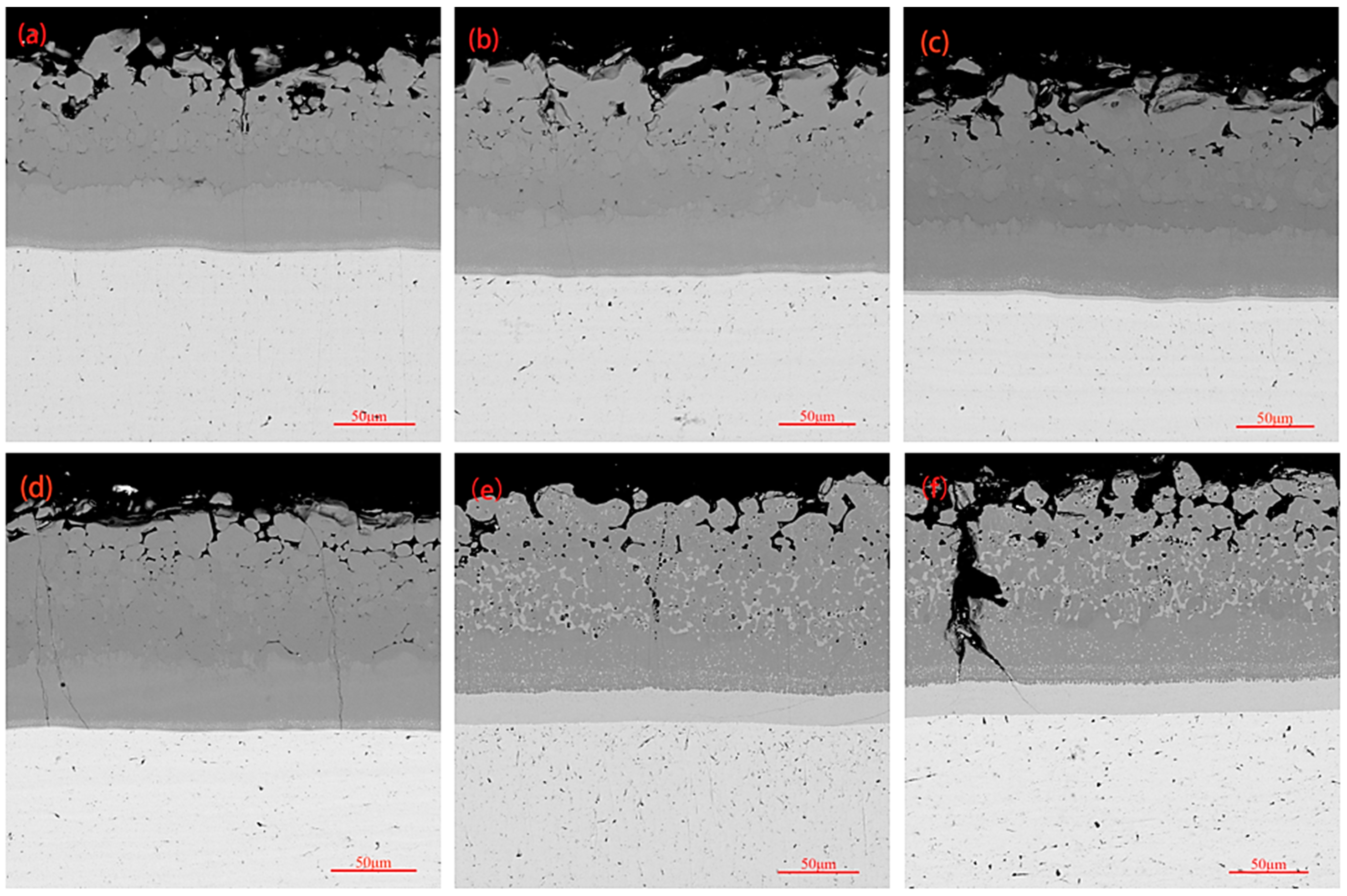
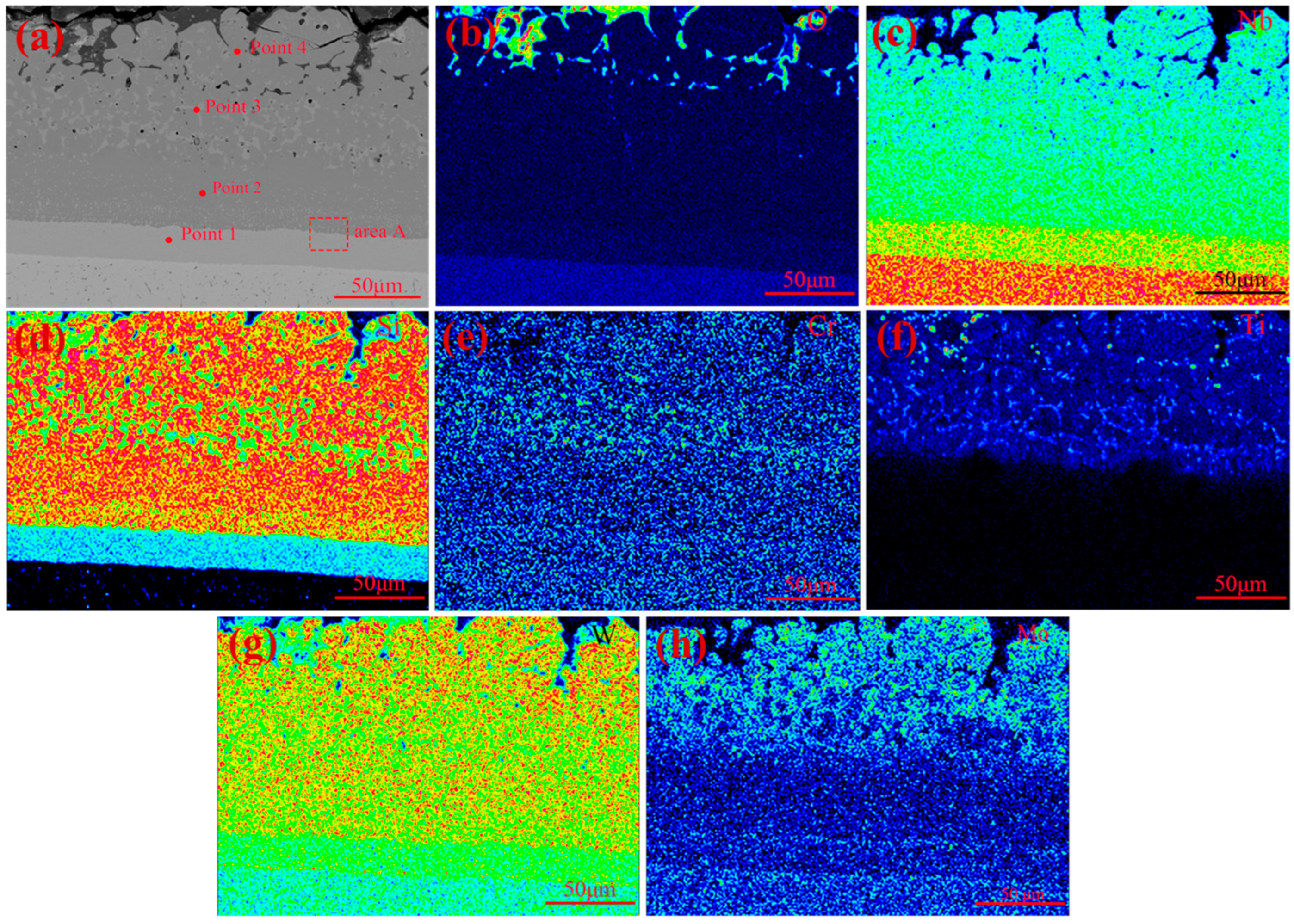
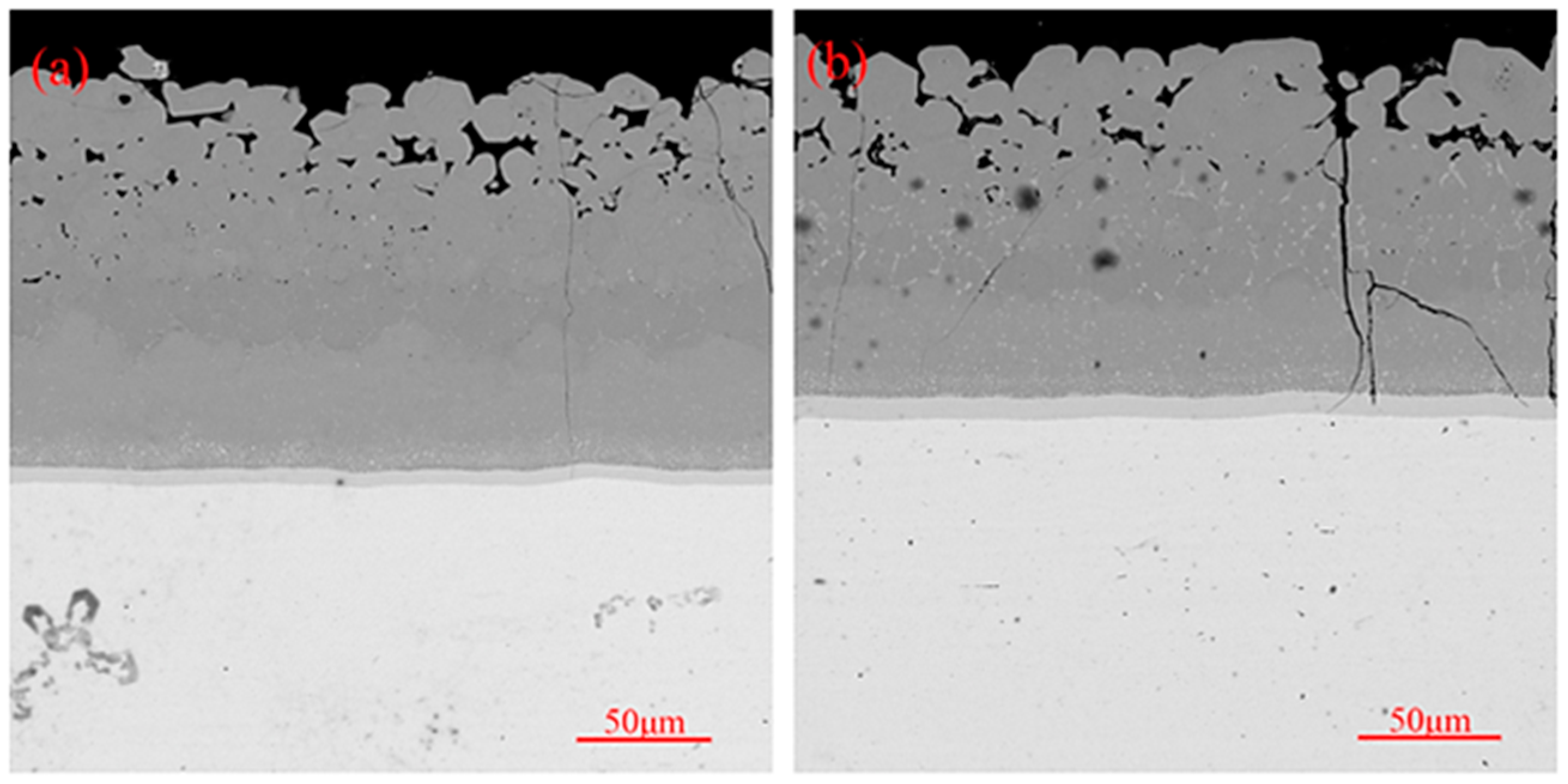
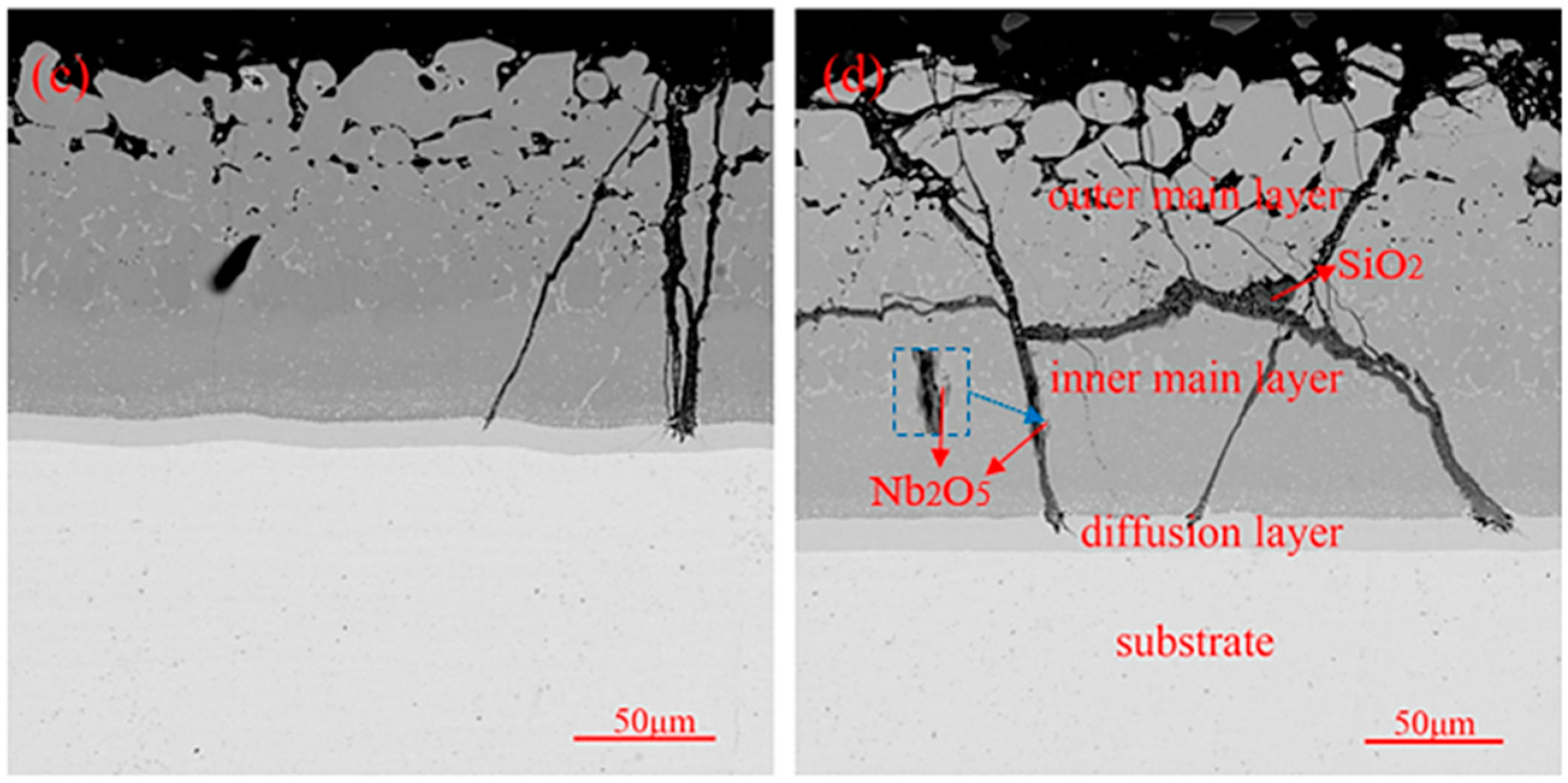
3.3. High-Temperature Oxidation Behavior of Coatings
3.4. Thermal Shock Resistance of Coating
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Knittel, S.; Mathieu, S.; Vilasi, M. Nb4Fe4Si7 coatings to protect niobium and niobium suicide composites against high temperature oxidation. Surf. Coat. Technol. 2013, 235, 144–154. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Y.; Pu, R.; Zhao, G.; Shen, Z.; Huang, Y.; Liu, S.; Cai, Z.; Zhao, X. Formation and oxidation behavior of Ce-modified MoSi2-NbSi2 coating on niobium alloy. Corros. Sci. 2020, 173, 108751. [Google Scholar] [CrossRef]
- Tsakiropoulos, P. On the macrosegregation of silicon in niobium silicide based alloys. Intermetallics 2014, 55, 95–101. [Google Scholar] [CrossRef]
- Li, X.; Guo, X.; Qiao, Y. Structure and Oxidation Behavior of Zr-Y Modified Silicide Coatings Prepared on an Nb-Ti-Si-Cr Based Ultrahigh Temperature Alloy. Oxid. Met. 2015, 83, 253–271. [Google Scholar] [CrossRef]
- Sun, J.; Li, T.; Zhang, G.-P.; Fu, Q.-G. Different oxidation protection mechanisms of HAPC silicide coating on niobium alloy over a large temperature range. J. Alloys Compd. 2019, 790, 1014–1022. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, T.; Yu, L.; Cui, K.; Wang, J.; Shen, F.; Zhang, X.; Zhou, K. Anti-Corrosion Coatings for Protecting Nb-Based Alloys Exposed to Oxidation Environments: A Review. Met. Mater. Int. 2023, 29, 1–17. [Google Scholar] [CrossRef]
- Zhang, X.; Fu, T.; Cui, K.; Zhang, Y.; Shen, F.; Wang, J.; Yu, L.; Mao, H. The Protection, Challenge, and Prospect of Anti-Oxidation Coating on the Surface of Niobium Alloy. Coatings 2021, 11, 742. [Google Scholar] [CrossRef]
- Chen, R.; Chen, D.; Wang, Q.; Wang, S.; Zhou, Z.; Ding, H.; Fu, H. Research Progress on Nb-Si Base Ultrahigh Temperature Alloys and Directional Solidification Technology. Acta Metall. Sin. 2021, 57, 1141–1154. [Google Scholar] [CrossRef]
- Knittel, S.; Mathieu, S.; Portebois, L.; Drawin, S.; Vilasi, M. Development of suicide coatings to ensure the protection of Nb and suicide composites against high temperature oxidation. Surf. Coat. Technol. 2013, 235, 401–406. [Google Scholar] [CrossRef]
- Lokeshkumar, E.; Manojkumar, P.; Saikiran, A.; Premchand, C.; Hariprasad, S.; Rameshbabu, N. Fabrication of Ca and P containing niobium oxide ceramic coatings on niobium by PEO coupled EPD process. Surf. Coat. Technol. 2021, 416, 127161. [Google Scholar] [CrossRef]
- Voyevodin, V.M.; Zmii, V.I.; Rudenkyi, S.G. High-Temperature Heat-Resistant Coatings for Protection of Refractory Metals and Their Alloys (Overview). Powder Metall. Met. Ceram. 2017, 56, 198–209. [Google Scholar] [CrossRef]
- Hou, H.; Ning, X.; Wang, Q.; Liu, Y.; Liu, Y. Anti-ablation behavior of air plasma-sprayed Mo(Si, Al)2 coating. Surf. Coat. Technol. 2015, 274, 60–67. [Google Scholar] [CrossRef]
- Ulrich, A.S.; Galetz, M.C. Protective Aluminide Coatings for Refractory Metals. Oxid. Met. 2016, 86, 511–535. [Google Scholar] [CrossRef]
- Sun, J.; Fu, Q.-G.; Guo, L.-P.; Wang, L. Silicide Coating Fabricated by HAPC/SAPS Combination to Protect Niobium Alloy from Oxidation. ACS Appl. Mater. Interfaces 2016, 8, 15838–15847. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, Y.; Wang, S.; Zou, Y.; Chen, G.; Wen, L.; Zhang, G.; Zhao, L.; Ouyang, J.; Jia, D.; et al. Enhanced high-temperature oxidation resistance of HfSi2-modified silicon based multilayer ceramic coating on Nb alloy prepared by a novel strategy. J. Eur. Ceram. Soc. 2023, 43, 4717–4730. [Google Scholar] [CrossRef]
- Wang, L.; Chen, Z.; Zhang, Y.; Wu, W. Ir coating prepared on Nb substrate by double glow plasma. Int. J. Refract. Met. Hard Mater. 2009, 27, 590–594. [Google Scholar] [CrossRef]
- Fu, T.; Chen, L.; Zhang, Y.; Shen, F.; Zhu, J. Microstructure and oxidation resistant of Si-NbSi2 coating on Nb substrate at 800 °C and 1000 °C. Ceram. Int. 2023, 49, 21222–21233. [Google Scholar] [CrossRef]
- Zhang, G.P.; Sun, J.; Fu, Q.G. Microstructure and oxidation behavior of plasma sprayed WSi2-mullite-MoSi2 coating on niobium alloy at 1500 °C. Surf. Coat. Technol. 2020, 400, 126210. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, C.; Chen, Z.; Shen, C.; Feng, P. Vacuum rapid fabrication of (Nb,Ti,Cr)Si2 coatings with Mo, W addition: Microstructure and oxidation resistance at 1450 °C. Vacuum 2019, 164, 286–292. [Google Scholar] [CrossRef]
- Vishwanadh, B.; Majumdar, S.; Orsborn, J.; Banerjee, R.; Tewari, R.; Fraser, H.; Dey, G. Characterization of suicide phases formed during pack siliconizing coating on the Nb-1Zr-0.1C alloy. Intermetallics 2015, 63, 59–66. [Google Scholar] [CrossRef]
- Zhang, K.; Lei, S.; Yang, R.; Zhang, Y.; Chen, S.; Zhang, X.; Li, W. Formation and oxidation behavior of SiO2/NbSi2 multilayer coating fabricated by one-step method. Surf. Coat. Technol. 2023, 452, 129117. [Google Scholar] [CrossRef]
- Sun, L.; Fu, Q.G.; Sun, J. Effect of SiO2 barrier scale prepared by pre-oxidation on hot corrosion behavior of MoSi2-based coating on Nb alloy. Corros. Sci. 2020, 176, 109051. [Google Scholar] [CrossRef]
- Jiang, H.; Qiao, Y.; Zhang, W.; Guo, X. Characterization of microstructure and oxidation behavior of Al modified MoSi2 coating on Nb-Si based alloy. Surf. Interfaces 2024, 51, 104739. [Google Scholar] [CrossRef]
- Goberis, S.; Yarushyavichyus, K.; Shpokauskas, A. Microstructure and oxidation-resistance of silicide coatings on C-103 niobium alloys. J. Inorg. Mater. 2000, 15, 143–149. [Google Scholar] [CrossRef]
- Wang, C.; Shao, W.; Wang, W.; Zhou, C. Oxidation behaviour of a Ge-modified silicide coating on an Nb-Si based alloy in the moderate temperature range. Corros. Sci. 2020, 163, 108249. [Google Scholar] [CrossRef]
- Wang, W.; Zhou, C. Characterization of microstructure and oxidation resistance of Y and Ge modified silicide coating on Nb-Si based alloy. Corros. Sci. 2016, 110, 114–122. [Google Scholar] [CrossRef]
- Huang, X.X.; Sun, S.C.; Tu, G.F. Investigation of mechanical properties and oxidation resistance of CVD TiB2 ceramic coating on molybdenum. J. Mater. Res. Technol. 2020, 9, 282–290. [Google Scholar] [CrossRef]
- Yue, G.; Guo, X.; Qiao, Y.; Luo, F. Isothermal oxidation and interdiffusion behavior of MoSi2/WSi2 compound coating on Nb-Ti-Si based alloy. Appl. Surf. Sci. 2020, 504, 144477. [Google Scholar] [CrossRef]
- Zhang, H.A.; Gu, S.Y. Preparation and oxidation behavior of MoSi2-CrSi2-Si3N4 composite coating on Mo substrate. Int. J. Refract. Met. Hard Mater. 2013, 41, 128–132. [Google Scholar] [CrossRef]
- Kuang, J.; Zhang, P.; Wang, Q.; Hu, Z.; Liang, X.; Shen, B. Formation and oxidation behavior of refractory high-entropy silicide (NbMoTaW)Si2 coating. Corros. Sci. 2022, 198, 110134. [Google Scholar] [CrossRef]
- Chu, Y.-H.; Fu, Q.-G.; Cao, C.-W.; Li, H.-J.; Li, K.-Z.; Lei, Q. Microstructure and oxidation resistant property of C/C composites modified by SiC-MoSi2-CrSi2 coating. Surf. Eng. 2011, 27, 355–361. [Google Scholar] [CrossRef]
- Ramasesha, S.K.; Shobu, K. Oxidation of MoSi2 and MoSi2-based materials. Bull. Mater. Sci. 1999, 22, 769–773. [Google Scholar] [CrossRef]
- Murthy, T.; Sonber, J.; Subramanian, C.; Hubli, R.; Suri, A. Densification, characterization and oxidation studies of TiB2-WSi2 composite. Int. J. Refract. Met. Hard Mater. 2012, 33, 10–21. [Google Scholar] [CrossRef]
- Zhang, B.; Yi, M.; Xie, A.; Zhou, Y.; Ning, Y.; Feng, Z. A novel gradient CrSi2-ZrSi2-SiC-Si coating for long-term oxidation protection of C/C composites at 1773 K. J. Eur. Ceram. Soc. 2024, 44, 1534–1542. [Google Scholar] [CrossRef]
- Sharif, A.A. High-temperature oxidation of MoSi2. J. Mater. Sci. 2010, 45, 865–870. [Google Scholar] [CrossRef]
- Murthy, T.; Balasubramaniam, R.; Basu, B.; Suri, A.; Mungole, M. Oxidation of monolithic TiB2 and TiB2-20 wt% MoSi2 composite at 850 °C. J. Eur. Ceram. Soc. 2006, 26, 187–192. [Google Scholar] [CrossRef]
- An, D.; Liu, C.; Zhao, S.; Zhang, H.; Tang, Z.; Xiao, P.; Dai, J.; Wang, Y. High-Temperature Thermal Shock Performance of Si-Ti-Cr Silicide Coating on Nb-Hf Alloy Surface in Atmosphere and Vacuum. Rare Met. Mater. Eng. 2022, 51, 3974–3980. [Google Scholar] [CrossRef]
- Sun, L.; Fu, Q.-G.; Fang, X.-Q.; Sun, J. AMoSi2-based composite coating by supersonic atmospheric plasma spraying to protect Nb alloy against oxidation at 1500 °C. Surf. Coat. Technol. 2018, 352, 182–190. [Google Scholar] [CrossRef]
- Dasgupta, T.; Etourneau, J.; Chevalier, B.; Matar, S.F.; Umarji, A.M. Structural, thermal, and electrical properties of CrSi2. J. Appl. Phys. 2008, 103, 113516. [Google Scholar] [CrossRef]
- Xiao, L.; Xiao, Y.; Zhou, X.; Zhao, G.; Zhong, Q.; Yu, H.; Wang, S.; Peng, Z.; Cai, Z. The high temperature oxidation and thermal shock behavior of a dense WSi2-TaSi2 coating on Ta substrate prepared by a novel two-step process. Ceram. Int. 2023, 49, 26767–26777. [Google Scholar] [CrossRef]
- Liu, S.; Shen, H.; Xu, J.; Liu, J.; Cai, Z.; Zhao, X.; Xiao, L. Preparation of a tantalum-based MoSi2-Mo coating resistant to ultra-high-temperature thermal shock by a new two-step process. J. Mater. Sci. Technol. 2021, 81, 117–122. [Google Scholar] [CrossRef]
- Paul, T.R.; Mondal, M.K.; Mallik, M. Thermal shock behavior of ZrB2-MoSi2-SiCw composites. J. Alloys Compd. 2022, 924, 166443. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Sinha, R.; Mitra, R.; Ray, K. Effect of Mo and Si on morphology and volume fraction of eutectic in Nb-Si-Mo alloys. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2007, 456, 358–363. [Google Scholar] [CrossRef]
- Kang, H.-S.; Shon, I.-J. Simultaneous Synthesis and Consolidation of Nanostructured MoSi2-NbSi2 Composite by High-Frequency Induction Heated Sintering and Its Mechanical Properties. Korean J. Mater. Res. 2014, 24, 180–185. [Google Scholar] [CrossRef]
- Hagihara, K.; Nakano, T. Fracture behavior and toughness of NbSi2-based single crystals and MoSi2(C11b)/NbSi2(C40) duplex crystals with a single set of lamellae. Acta Mater. 2011, 59, 4168–4176. [Google Scholar] [CrossRef]
- Jo, H.-G.; Shon, I.-J. Pulsed Current Activated Synthesis and Consolidation of Nanostructured MoSi2-NbSi2 Composite and Its Mechanical Properties. Mater. Trans. 2014, 55, 391–394. [Google Scholar] [CrossRef]
- Kang, H.-S.; Shon, I.-J. Enhanced mechanical properties of nanostructured WSi2-NbSi2 composite synthesized and sintered by high-frequency induction heating. Mater. Sci. Eng. A-Struct. Mater. Prop. Microstruct. Process. 2014, 606, 228–232. [Google Scholar] [CrossRef]
- Yu, C.; Zhao, X.; Xiao, L.; Cai, Z.; Zhang, B.; Guo, L. Microstructure and mechanical properties of in-situ laminated Nb/Nb5Si3 composites. Mater. Lett. 2017, 209, 606–608. [Google Scholar] [CrossRef]
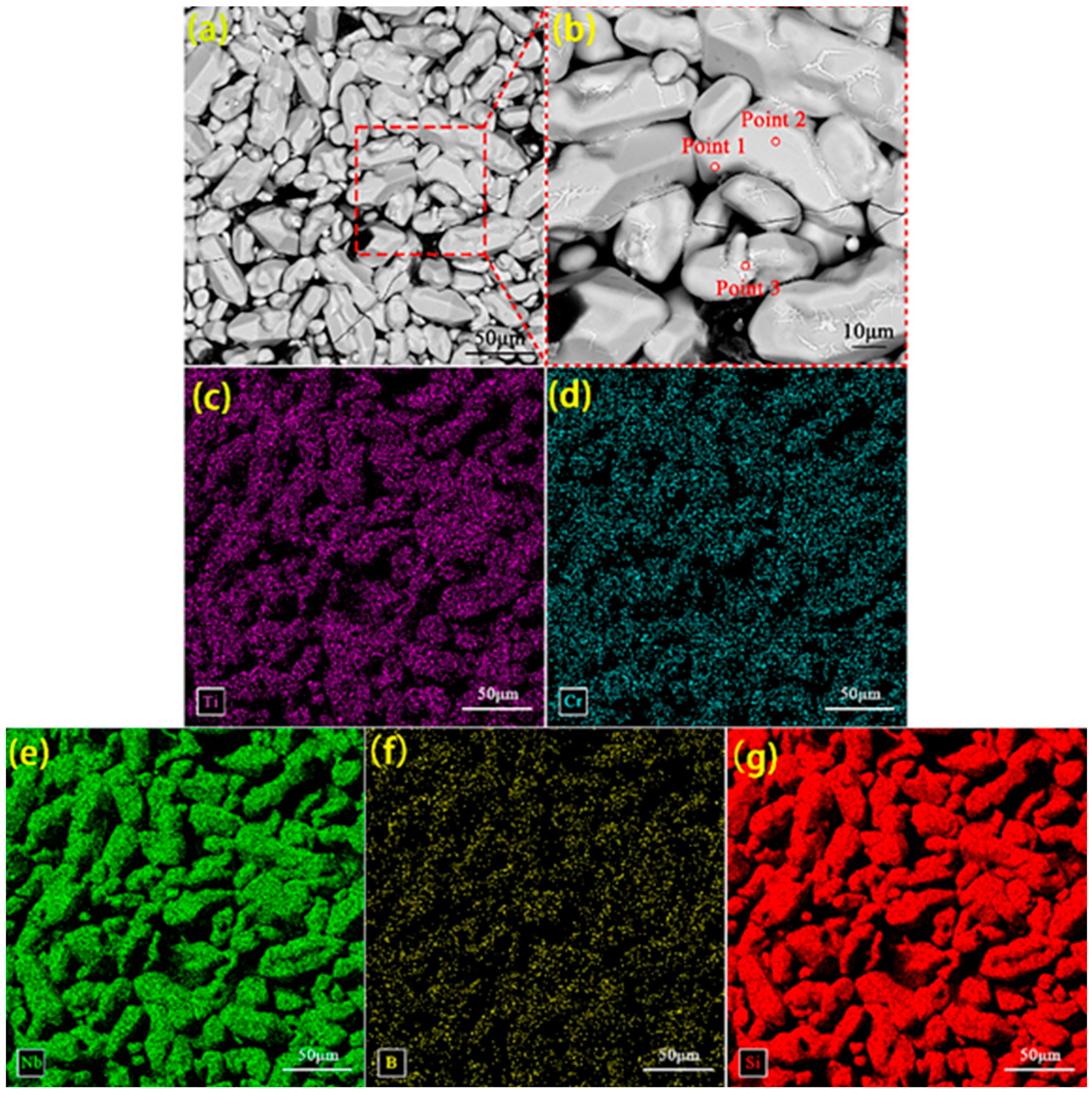
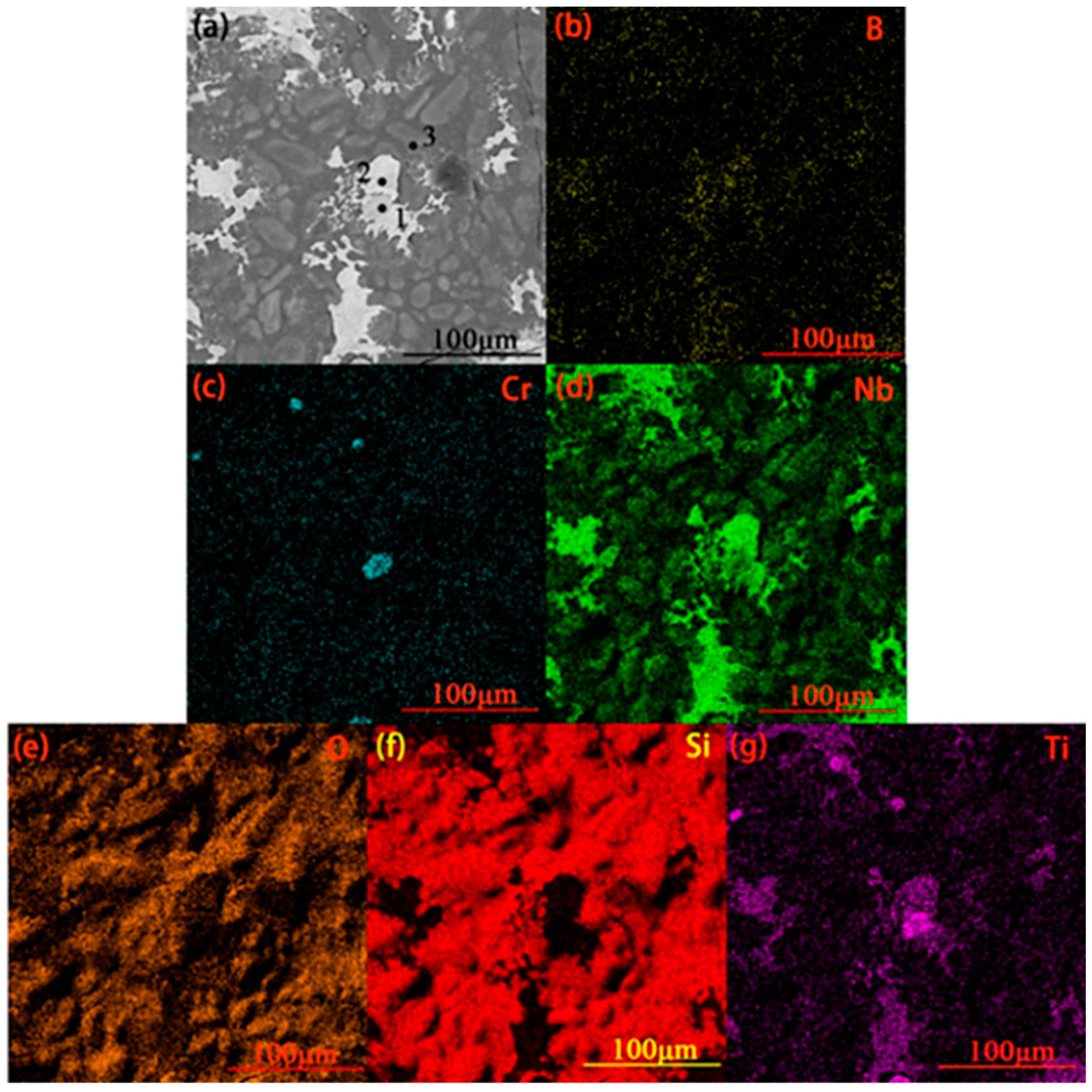
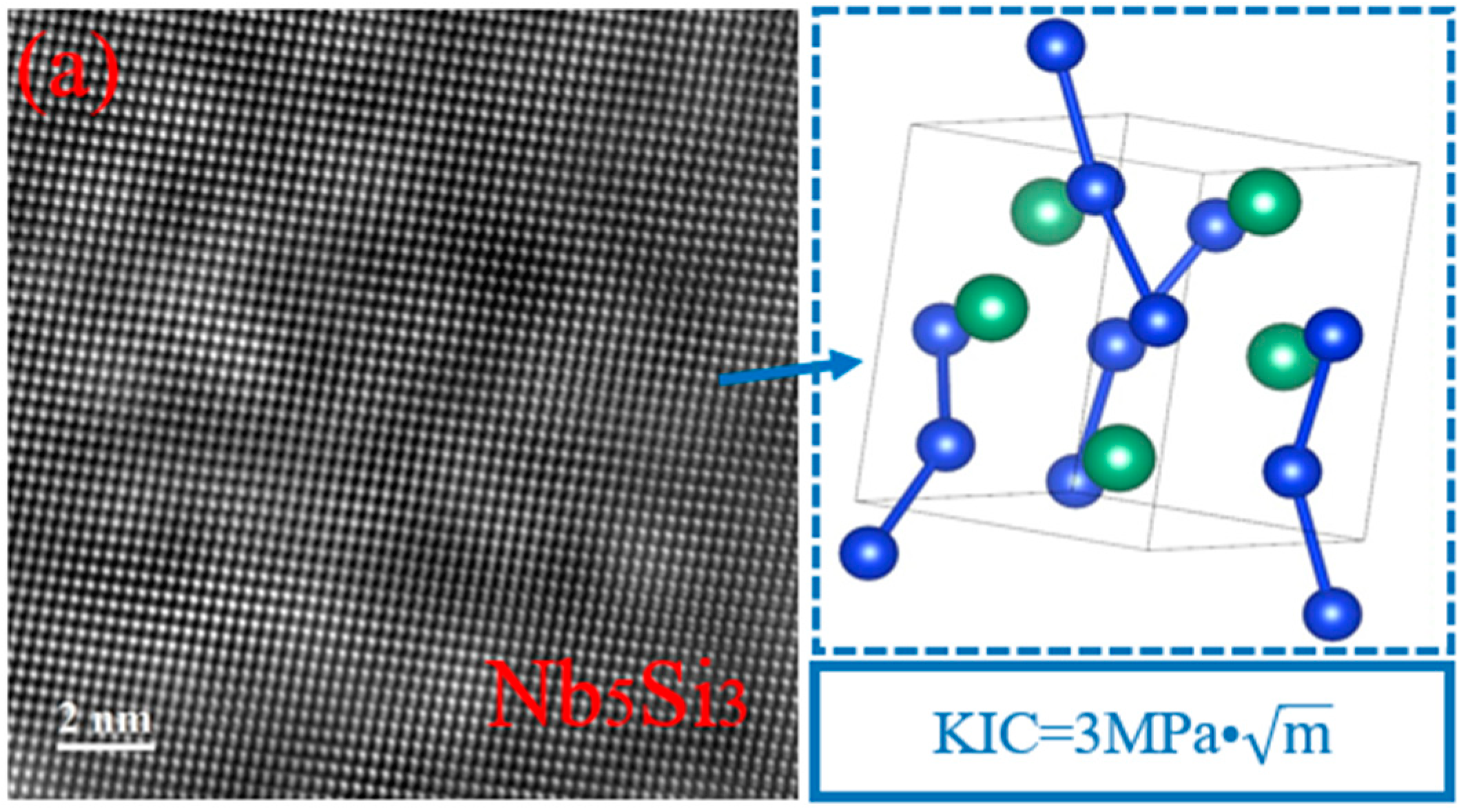
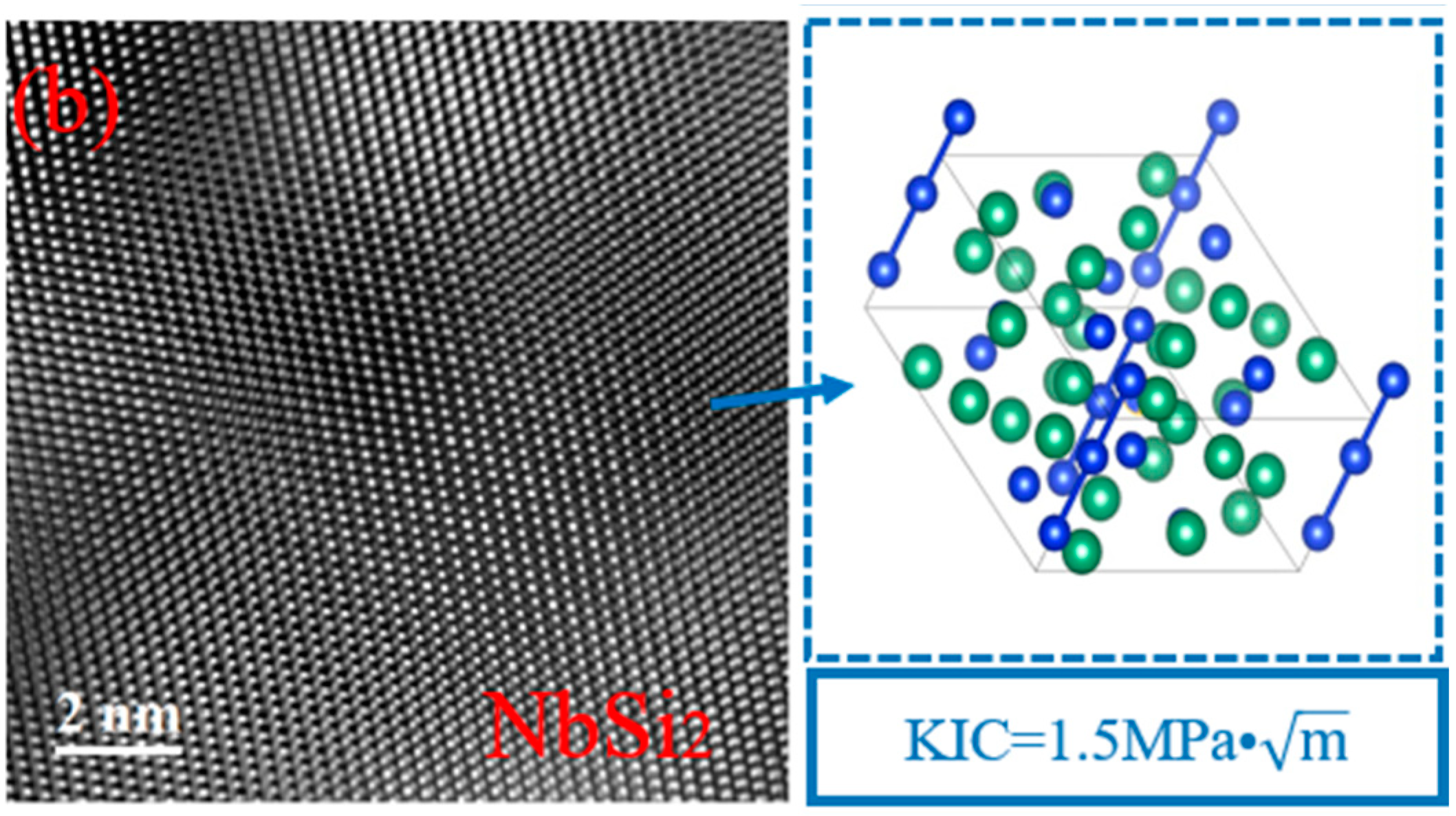
| Position | Composition (at%) | Main Phase | |||
|---|---|---|---|---|---|
| Si | Ti | Cr | Nb | ||
| 1 | 63.54 | 11.26 | 0.52 | 24.68 | (Nb,Cr)Si2, TiB2 |
| 2 | 68.89 | 5.22 | 0.76 | 25.13 | (Nb,Cr)Si2, TiB2 |
| 3 | 62.57 | 6.82 | 1.18 | 29.43 | (Nb,Cr)Si2, TiB2 |
| Position | Composition (at%) | Main Phase | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | W | Cr | Si | Ti | Mo | Nb | Zr | Ce | ||
| 1 | 0.0000 | 2.9718 | 0.0000 | 36.6623 | 0.0000 | 1.1640 | 59.1116 | 0.0903 | 0.0000 | Nb5Si3 |
| 2 | 0.0507 | 3.3061 | 0.1444 | 64.7589 | 0.1950 | 0.6390 | 30.8387 | 0.0360 | 0.0312 | NbSi2 |
| 3 | 0.0885 | 3.8694 | 0.5652 | 64.2653 | 2.6175 | 1.4162 | 27.1306 | 0.0000 | 0.0473 | NbSi2, TiB2 |
| Position | Composition (at%) | Main Phase | ||||
|---|---|---|---|---|---|---|
| O | Si | Ti | Cr | Nb | ||
| 1 | 62.41 | 4.19 | 9.62 | 5.23 | 18.55 | Nb2O5, Cr2O3, SiO2, TiO2 |
| 2 | 60.14 | 4.15 | 6.80 | 0.06 | 28.85 | Nb2O5, TiO2, SiO2 |
| 3 | 67.21 | 28.28 | 0.35 | 0.29 | 3.86 | SiO2, Nb2O5 |
| Position | Composition (at%) | Main Phase | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| O | W | Nb | Si | Ti | Ce | Cr | Mo | Zr | ||
| 1 | 0.0000 | 2.9893 | 60.9344 | 35.9445 | 0.0000 | 0.0000 | 0.1137 | 0.0181 | 0.0000 | Nb5Si3 |
| 2 | 0.0121 | 3.9550 | 31.7541 | 63.7609 | 0.4726 | 0.0065 | 0.0311 | 0.0000 | 0.0077 | NbSi2 |
| 3 | 0.0538 | 3.7226 | 36.5971 | 44.9300 | 10.1253 | 0.0298 | 4.5414 | 0.0000 | 0.0000 | Nb5Si3, TiB2 |
| 4 | 0.0822 | 3.8263 | 29.6296 | 63.4388 | 2.7873 | 0.0412 | 0.1874 | 0.0072 | 0.0000 | NbSi2 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, X.; Xiao, L.; Zha, Y.; Xu, J.; Fang, J.; Deng, G.; Xu, S.; Liu, S.; Zhao, X.; Cai, Z. Study on the Oxidation Behavior of TiB2-CeO2-Modified (Nb,Mo,Cr,W)Si2 Coating on the Surface of Niobium Alloy. Materials 2024, 17, 5244. https://doi.org/10.3390/ma17215244
Zhou X, Xiao L, Zha Y, Xu J, Fang J, Deng G, Xu S, Liu S, Zhao X, Cai Z. Study on the Oxidation Behavior of TiB2-CeO2-Modified (Nb,Mo,Cr,W)Si2 Coating on the Surface of Niobium Alloy. Materials. 2024; 17(21):5244. https://doi.org/10.3390/ma17215244
Chicago/Turabian StyleZhou, Xiaojun, Lairong Xiao, Yitao Zha, Jiawei Xu, Jiashu Fang, Guanzhi Deng, Shaofu Xu, Sainan Liu, Xiaojun Zhao, and Zhenyang Cai. 2024. "Study on the Oxidation Behavior of TiB2-CeO2-Modified (Nb,Mo,Cr,W)Si2 Coating on the Surface of Niobium Alloy" Materials 17, no. 21: 5244. https://doi.org/10.3390/ma17215244
APA StyleZhou, X., Xiao, L., Zha, Y., Xu, J., Fang, J., Deng, G., Xu, S., Liu, S., Zhao, X., & Cai, Z. (2024). Study on the Oxidation Behavior of TiB2-CeO2-Modified (Nb,Mo,Cr,W)Si2 Coating on the Surface of Niobium Alloy. Materials, 17(21), 5244. https://doi.org/10.3390/ma17215244










