Difficulties in Determining the Pozzolanic Activity of Thermally Activated Lower-Grade Clays
Abstract
1. Introduction
2. Experimental Methods
2.1. Raw Material Characterisation
2.2. Direct Methods for Pozzolanic Activity Assessment
2.2.1. Frattini Test
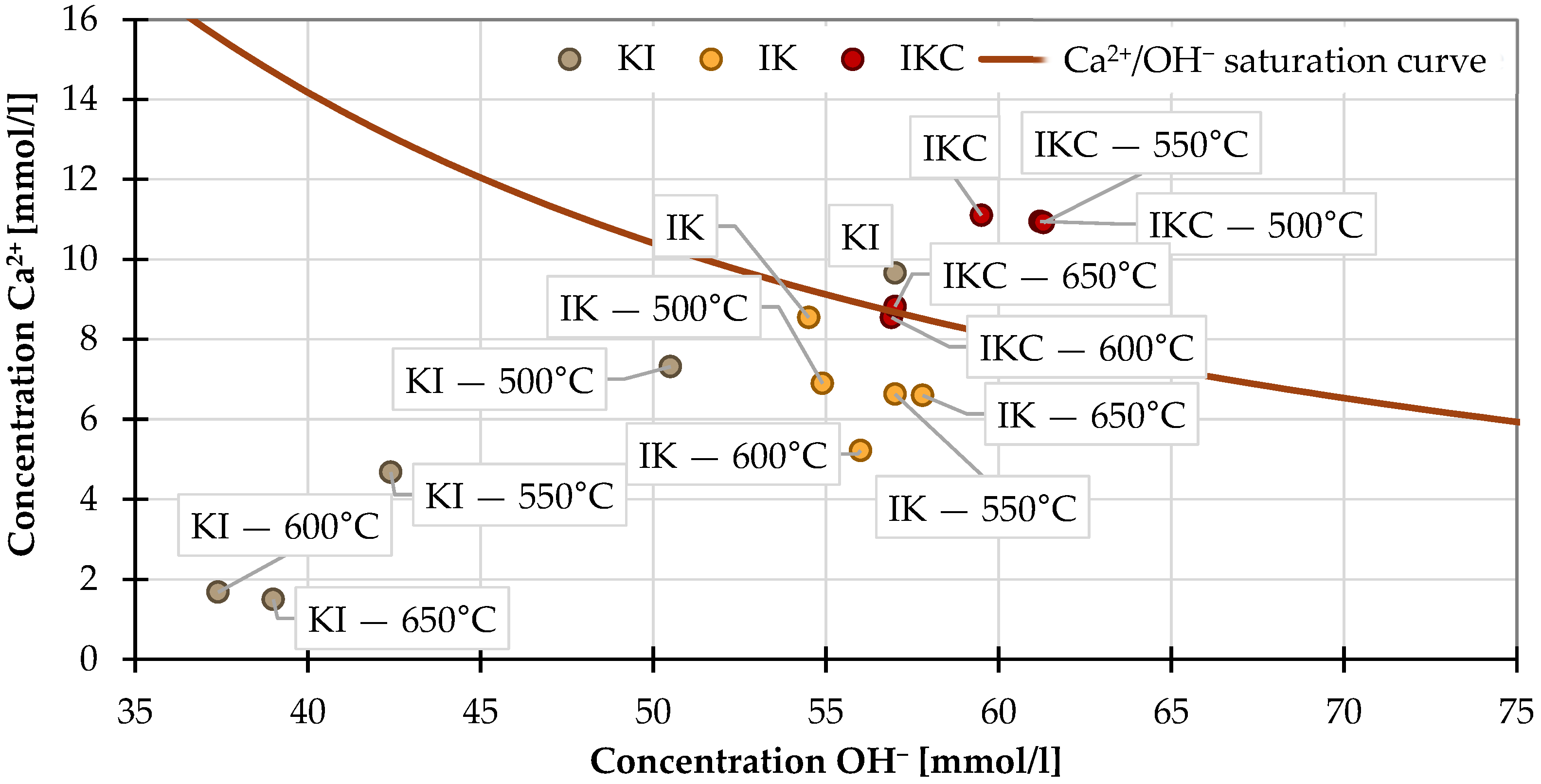
2.2.2. Lime Saturation Test
2.2.3. Isothermal Calorimetry
2.2.4. XRD Method
2.3. Indirect Methods for Pozzolanic Activity Assessment
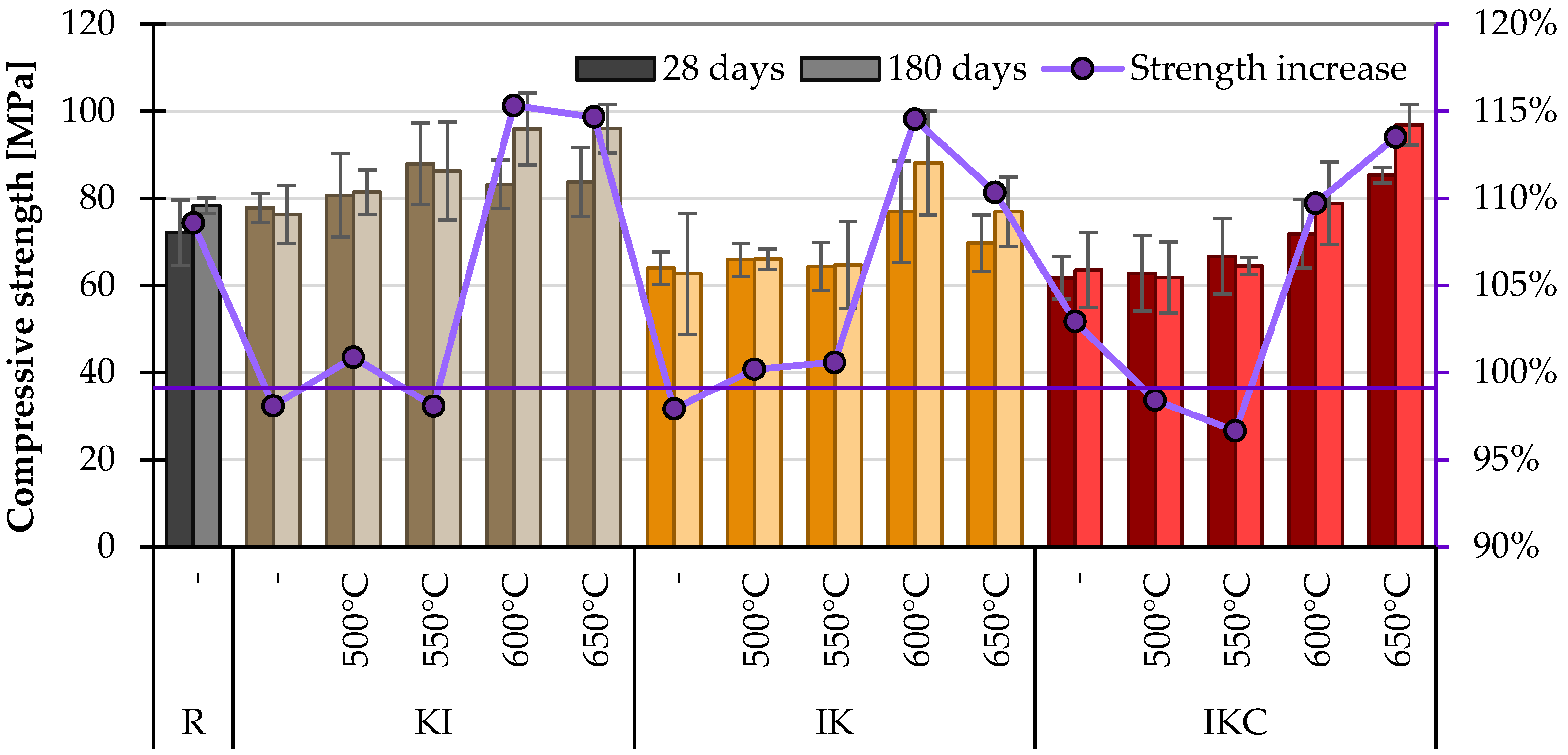
2.3.1. Mechanical Parameters
2.3.2. pH Value
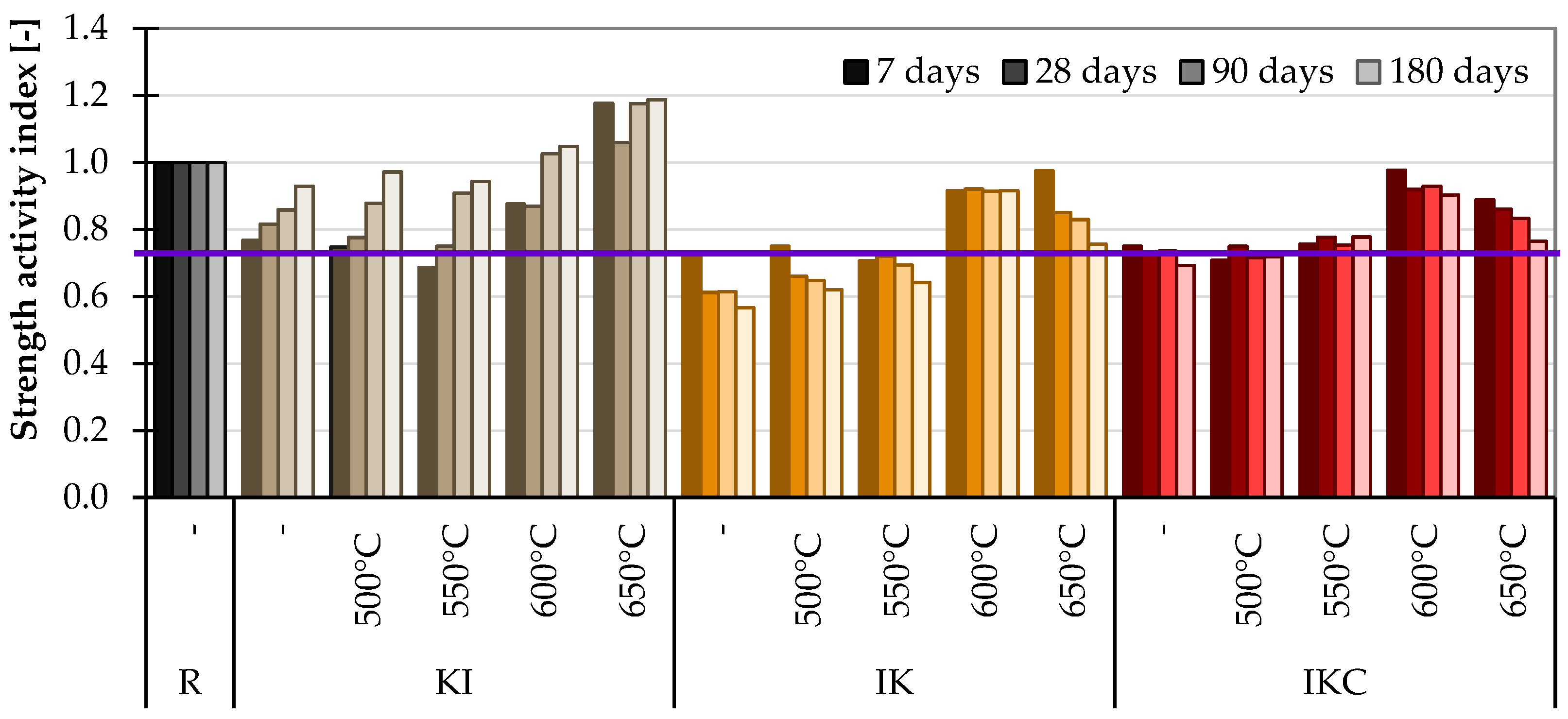
2.3.3. Strength Activity Index (SAI)
2.3.4. Specific Surface Area
3. Studied Materials
3.1. Raw Materials and Their Characterisation
3.2. Thermal Activation
4. Results and Discussion
4.1. Direct Methods
4.1.1. Frattini Test
4.1.2. Lime Saturation Test
4.1.3. Isothermal Calorimetry
4.1.4. XRD Method
4.2. Indirect Methods
4.2.1. Mechanical Properties of Cement Pastes
4.2.2. pH Value of Cement Pastes
4.2.3. Strength Activity Index
4.2.4. Specific Surface Area
4.3. Summary of Reached Results
5. Conclusions
- When measuring the pozzolanic activity of thermally activated clay materials, it is advisable to include the raw material in the measurement. The result is then more comprehensive, and the development of the onset of pozzolanic activity can be deduced. Examining the raw material also checks the method’s suitability, as the raw material should show no or minimal pozzolanic activity.
- Especially in the case of the Frattini experiment, it is of great importance to also study raw clay. On the first view, this test proposed applicable results; however, due to the ability of raw clay to fix Ca2+, the results are distorted. Thus, more reasonable is to assess the shift of the TAC compared to a raw state than just consider if the sample is above/below the limit given by the saturation curve.
- The lime saturated test is unsuitable for investigating TAC reactivity, as the raw clays seemed to be reactive due to ionic exchange, and almost all clays reached the same Ca(OH)2 bound values after 168 h of testing. The most reactive clays, in this case, were completely different from all other tests.
- Isothermal calorimetry applied to samples containing lime instead of cement ranked among the most suitable types of tests used to determine pozzolanic activity. The raw clays were found to be non-reactive, and the amount of hydration heat released allowed the material’s reactivity to be reliably determined.
- The XRD method can serve as an excellent complementary direct method in this type of measurement, showing the reactivity of clays based on the amount of amorphous phase and the consumption of the clay minerals contained. However, it should be noted that phase quantification is somehow difficult in the case of clay minerals.
- Measurements of the mechanical properties of cement pastes containing TACs at one age are unreliable. In this case, more credible results proposed searching the strength development, which in the case of pozzolana should be over 10% per 180 days.
- SAI should be carried out on specimens aged for at least 28 days but more likely for longer. In addition, the limit of 0.75 for pozzolanic activity proof of TACs is insufficient because the raw clays can easily exceed this value. On that account, it should be higher, at least 0.85.
- Monitoring the specific surface area is somewhat inaccurate because it is affected by clay sintering. Still, as an additional measurement, it can be helpful for a more detailed understanding of the effect of grinding fineness on the reactivity of the clay under test.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hewlett, P. Lea’s Chemistry of Cement and Concrete, 4th ed.; Elsevier Science & Technology: Amsterdam, The Netherlands, 2003; ISBN 978-0-7506-6256-7. [Google Scholar]
- Aydin, A.C.; Öz, A.; Polat, R.; Mindivan, H. Effects of the Different Atmospheric Steam Curing Processes on the Properties of Self-Compacting-Concrete Containing Microsilica. Sadhana 2015, 40, 1361–1371. [Google Scholar] [CrossRef]
- Rais, M.S.; Khan, R.A. Strength and Durability Characteristics of Binary Blended Recycled Coarse Aggregate Concrete Containing Microsilica and Metakaolin. Innov. Infrastruct. Solut. 2020, 5, 114. [Google Scholar] [CrossRef]
- Narattha, C.; Thongsanitgarn, P.; Chaipanich, A. Thermogravimetry Analysis, Compressive Strength and Thermal Conductivity Tests of Non-Autoclaved Aerated Portland Cement–Fly Ash–Silica Fume Concrete. J. Therm. Anal. Calorim. 2015, 122, 11–20. [Google Scholar] [CrossRef]
- Bai, W.; Song, Z.; Yuan, C.; Guan, J.; Xie, C.; Huang, H.; Ma, Y. Study on Mechanical Properties and Damage Mechanism of Recycled Concrete Containing Silica Fume in Freeze–Thaw Environment. Constr. Build. Mater. 2023, 375, 130872. [Google Scholar] [CrossRef]
- Özbay, E.; Erdemir, M.; Durmuş, H.İ. Utilization and Efficiency of Ground Granulated Blast Furnace Slag on Concrete Properties—A Review. Constr. Build. Mater. 2016, 105, 423–434. [Google Scholar] [CrossRef]
- Zhu, X.; Tang, D.; Yang, K.; Zhang, Z.; Li, Q.; Pan, Q.; Yang, C. Effect of Ca(OH)2 on Shrinkage Characteristics and Microstructures of Alkali-Activated Slag Concrete. Constr. Build. Mater. 2018, 175, 467–482. [Google Scholar] [CrossRef]
- Rivera, F.; Martínez, P.; Castro, J.; López, M. Massive Volume Fly-Ash Concrete: A More Sustainable Material with Fly Ash Replacing Cement and Aggregates. Cem. Concr. Compos. 2015, 63, 104–112. [Google Scholar] [CrossRef]
- Kayali, O.; Sharfuddin Ahmed, M. Assessment of High Volume Replacement Fly Ash Concrete—Concept of Performance Index. Constr. Build. Mater. 2013, 39, 71–76. [Google Scholar] [CrossRef]
- Melichar, J.; Žižková, N.; Brožovský, J.; Mészárosová, L.; Hermann, R. Study of the Interaction of Cement-Based Materials for 3D Printing with Fly Ash and Superabsorbent Polymers. Buildings 2022, 12, 2008. [Google Scholar] [CrossRef]
- Sabir, B.B.; Wild, S.; Bai, J. Metakaolin and Calcined Clays as Pozzolans for Concrete: A Review. Cem. Concr. Compos. 2001, 23, 441–454. [Google Scholar] [CrossRef]
- Engbert, A.; Gruber, S.; Plank, J. The Effect of Alginates on the Hydration of Calcium Aluminate Cement. Carbohydr. Polym. 2020, 236, 116038. [Google Scholar] [CrossRef] [PubMed]
- Koňáková, D.; Pommer, V.; Jerman, M.; Keppert, M.; Černý, R.; Vejmelková, E. Utilization of Ceramic Powder, Calcined Shale and Sintered Mullite as Partial Replacements of Calcium Aluminate Cement. Constr. Build. Mater. 2022, 326, 126824. [Google Scholar] [CrossRef]
- Donatello, S.; Tyrer, M.; Cheeseman, C.R. Comparison of Test Methods to Assess Pozzolanic Activity. Cem. Concr. Compos. 2010, 32, 121–127. [Google Scholar] [CrossRef]
- Tseng, Y.-S.; Huang, C.-L.; Hsu, K.-C. The Pozzolanic Activity of a Calcined Waste FCC Catalyst and Its Effect on the Compressive Strength of Cementitious Materials. Cem. Concr. Res. 2005, 35, 782–787. [Google Scholar] [CrossRef]
- Shi, C.; Grattan-Bellew, P.E.; Stegemann, J.A. Conversion of a Waste Mud into a Pozzolanic Material. Constr. Build. Mater. 1999, 13, 279–284. [Google Scholar] [CrossRef]
- Cao, Y.; Wang, Y.; Zhang, Z.; Ma, Y.; Wang, H. Turning Sandstone Clay into Supplementary Cementitious Material: Activation and Pozzolanic Reactivity Evaluation. Compos. Part B Eng. 2021, 223, 109137. [Google Scholar] [CrossRef]
- Rahhal, V.; Talero, R. Calorimetry of Portland Cement with Silica Fume, Diatomite and Quartz Additions. Constr. Build. Mater. 2009, 23, 3367–3374. [Google Scholar] [CrossRef]
- Trusilewicz, L.; Fernández-Martínez, F.; Rahhal, V.; Talero, R. TEM and SAED Characterization of Metakaolin. Pozzolanic Activity. J. Am. Ceram. Soc. 2012, 95, 2989–2996. [Google Scholar] [CrossRef]
- Talero, R.; Rahhal, V. Calorimetric Comparison of Portland Cements Containing Silica Fume and Metakaolin. J. Therm. Anal. Calorim. 2009, 96, 383–393. [Google Scholar] [CrossRef]
- Argın, G.; Uzal, B. Enhancement of Pozzolanic Activity of Calcined Clays by Limestone Powder Addition. Constr. Build. Mater. 2021, 284, 122789. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Pontikes, Y.; Elsen, J.; Cizer, Ö. Comparing the Reactivity of Different Natural Clays under Thermal and Alkali Activation. RILEM Tech. Lett. 2019, 4, 74–80. [Google Scholar] [CrossRef]
- Padilla-Ortega, E.; Medellín-Castillo, N.; Robledo-Cabrera, A. Comparative Study of the Effect of Structural Arrangement of Clays in the Thermal Activation: Evaluation of Their Adsorption Capacity to Remove Cd(II). J. Environ. Chem. Eng. 2020, 8, 103850. [Google Scholar] [CrossRef]
- Avet, F.; Snellings, R.; Alujas Diaz, A.; Ben Haha, M.; Scrivener, K. Development of a New Rapid, Relevant and Reliable (R3) Test Method to Evaluate the Pozzolanic Reactivity of Calcined Kaolinitic Clays. Cem. Concr. Res. 2016, 85, 1–11. [Google Scholar] [CrossRef]
- Tironi, A.; Trezza, M.A.; Scian, A.N.; Irassar, E.F. Assessment of Pozzolanic Activity of Different Calcined Clays. Cem. Concr. Compos. 2013, 37, 319–327. [Google Scholar] [CrossRef]
- Kaminskas, R.; Kubiliute, R.; Prialgauskaite, B. Smectite Clay Waste as an Additive for Portland Cement. Cem. Concr. Compos. 2020, 113, 103710. [Google Scholar] [CrossRef]
- Musie, W.; Gonfa, G. Thermal Activation, Characterization and Performance Evaluation of Ethiopian Bentonite for Sodium Removal. Water Sci. Technol. 2023, 87, 998–1008. [Google Scholar] [CrossRef]
- Balczár, I.; Korim, T.; Kovács, A.; Makó, É. Mechanochemical and Thermal Activation of Kaolin for Manufacturing Geopolymer Mortars—Comparative Study. Ceram. Int. 2016, 42, 15367–15375. [Google Scholar] [CrossRef]
- Dang, J.; Zhao, J.; Du, H.; Pang, S.D. Calcined Marine Clay as Cement Replacement to Make Low-Carbon Mortar. ACI Mater. J. 2022, 119, 189–198. [Google Scholar] [CrossRef]
- Yan, R.; Peng, J.; Shen, Y.; Zhang, Y.; Gu, L.; Wang, S. Evaluation on Activation Energy of Deposited Clay Based on Thermo-Gravimetric Analysis (TGA) and Four Kinetics Models. Bull. Eng. Geol. Envrion. 2020, 79, 371–382. [Google Scholar] [CrossRef]
- Payá, J.; Borrachero, M.V.; Monzó, J.; Peris-Mora, E.; Amahjour, F. Enhanced Conductivity Measurement Techniques for Evaluation of Fly Ash Pozzolanic Activity. Cem. Concr. Res. 2001, 31, 41–49. [Google Scholar] [CrossRef]
- Luxán, M.P.; Madruga, F.; Saavedra, J. Rapid Evaluation of Pozzolanic Activity of Natural Products by Conductivity Measurement. Cem. Concr. Res. 1989, 19, 63–68. [Google Scholar] [CrossRef]
- Yu, Q.; Sawayama, K.; Sugita, S.; Shoya, M.; Isojima, Y. The Reaction between Rice Husk Ash and Ca(OH)2 Solution and the Nature of Its Product. Cem. Concr. Res. 1999, 29, 37–43. [Google Scholar] [CrossRef]
- Sinthaworn, S.; Nimityongskul, P. Quick Monitoring of Pozzolanic Reactivity of Waste Ashes. Waste Manag. 2009, 29, 1526–1531. [Google Scholar] [CrossRef] [PubMed]
- Damineli, B.L.; Kemeid, F.M.; Aguiar, P.S.; John, V.M. Measuring the Eco-Efficiency of Cement Use. Cem. Concr. Compos. 2010, 32, 555–562. [Google Scholar] [CrossRef]
- Bazhenova, O.; Bazhenova, S.; Nemirova, V.; Bazhenov, D. The Clays after Heat Treatment as the Concrete Active Mineral Additive. MATEC Web Conf. 2018, 170, 03021. [Google Scholar] [CrossRef]
- Torgal, F.P.; Jalali, S. Eco-Efficient Construction and Building Materials; Springer London, Limited: London, UK, 2011; ISBN 978-1-4471-6132-5. [Google Scholar]
- Souri, A.; Kazemi-Kamyab, H.; Snellings, R.; Naghizadeh, R.; Golestani-Fard, F.; Scrivener, K. Pozzolanic Activity of Mechanochemically and Thermally Activated Kaolins in Cement. Cem. Concr. Res. 2015, 77, 47–59. [Google Scholar] [CrossRef]
- Mohammed, S.; Elhem, G.; Mekki, B. Valorization of Pozzolanicity of Algerian Clay: Optimization of the Heat Treatment and Mechanical Characteristics of the Involved Cement Mortars. Appl. Clay Sci. 2016, 132–133, 711–721. [Google Scholar] [CrossRef]
- Ilic, B.; Mitrovic, A.; Milicic, L. Thermal Treatment of Kaolin Clay to Obtain Metakaolin. Hem. Ind. 2010, 64, 351–356. [Google Scholar] [CrossRef]
- Chakchouk, A.; Trifi, L.; Samet, B.; Bouaziz, S. Formulation of Blended Cement: Effect of Process Variables on Clay Pozzolanic Activity. Constr. Build. Mater. 2009, 23, 1365–1373. [Google Scholar] [CrossRef]
- Samet, B.; Mnif, T.; Chaabouni, M. Use of a Kaolinitic Clay as a Pozzolanic Material for Cements: Formulation of Blended Cement. Cem. Concr. Compos. 2007, 29, 741–749. [Google Scholar] [CrossRef]
- Fernández, R.; Nebreda, B.; De La Villa, R.V.; García, R.; Frías, M. Mineralogical and Chemical Evolution of Hydrated Phases in the Pozzolanic Reaction of Calcined Paper Sludge. Cem. Concr. Compos. 2010, 32, 775–782. [Google Scholar] [CrossRef]
- Murat, M. Hydration Reaction and Hardening of Calcined Clays and Related Minerals. I. Preliminary Investigation on Metakaolinite. Cem. Concr. Res. 1983, 13, 259–266. [Google Scholar] [CrossRef]
- Shvarzman, A.; Kovler, K.; Grader, G.S.; Shter, G.E. The Effect of Dehydroxylation/Amorphization Degree on Pozzolanic Activity of Kaolinite. Cem. Concr. Res. 2003, 33, 405–416. [Google Scholar] [CrossRef]
- Kakali, G.; Perraki, T.; Tsivilis, S.; Badogiannis, E. Thermal Treatment of Kaolin: The Effect of Mineralogy on the Pozzolanic Activity. Appl. Clay Sci. 2001, 20, 73–80. [Google Scholar] [CrossRef]
- Arikan, M.; Sobolev, K.; Ertün, T.; Yeğinobali, A.; Turker, P. Properties of Blended Cements with Thermally Activated Kaolin. Constr. Build. Mater. 2009, 23, 62–70. [Google Scholar] [CrossRef]
- BS 3892-1:1997; Pulverized-Fuel Ash. Specification for Pulverized-Fuel Ash for Use with Portland Cement. British Standards Institution: London, UK, 1997.
- ČSN EN 196-5; Standard Methods for Testing Cement. Part 5: Pozzolanicity Test for Pozzolanic Cements. Czech Office for Standards, Metrology and Testing: Prague, Czech Republic, 2011.
- ASTM C311-11; Standard Test Methods for Sampling and Testing Fly Ash or Natural Pozzolans for Use in Portland-Cement Concrete. ASTM International: West Conshohocken, PA, USA, 2011.
- Kaljuvee, T.; Štubňa, I.; Húlan, T.; Kuusik, R. Heating Rate Effect on the Thermal Behavior of Some Clays and Their Blends with Oil Shale Ash Additives. J. Therm. Anal. Calorim. 2017, 127, 33–45. [Google Scholar] [CrossRef]
- Irassar, E.F.; Tironi, A.; Bonavetti, V.L.; Trezza, M.A.; Castellano, C.C.; Rahhal, V.F.; Donza, H.A.; Scian, A.N. Thermal Treatment and Pozzolanic Activity of Calcined Clay and Shale. ACI Mater. J. 2019, 116, 133–143. [Google Scholar] [CrossRef]
- Šídlová, M.; Šulc, R.; Škvára, F.; Pulcová, K.; Formáček, P.; Snop, R. Pozzolanic Activity of Stockpile Ash: Comparison of Test Methods. Case Stud. Constr. Mater. 2023, 19, e02396. [Google Scholar] [CrossRef]
- ČSN EN 196-11; Methods of Testing Cement—Part 11: Heat of Hydration—Isothermal Conduction Calorimetry Method. Czech Office for Standards, Metrology and Testing: Prague, Czech Republic, 2018.
- ASTM C1897-20; Standard Test Methods for Measuring the Reactivity of Supplementary Cementitious Materials by Isothermal Calorimetry and Bound Water Measurements. ASTM International: West Conshohocken, PA, USA, 2020.
- Döbelin, N.; Kleeberg, R. Profex: A Graphical User Interface for the Rietveld Refinement Program BGMN. J. Appl. Crystallogr. 2015, 48 Pt 5, 1573–1580. [Google Scholar] [CrossRef]
- Tak, S.; Gupta, P.; Kumar, A.; Sofi, A.; Mei Yun, C. Effect of Using Silica Fume as a Partial Replacement of Cement in Concrete. Mater. Today Proc. 2023, S2214785323021016. [Google Scholar] [CrossRef]
- Khankhaje, E.; Kim, T.; Jang, H.; Kim, C.-S.; Kim, J.; Rafieizonooz, M. A Review of Utilization of Industrial Waste Materials as Cement Replacement in Pervious Concrete: An Alternative Approach to Sustainable Pervious Concrete Production. Heliyon 2024, 10, e26188. [Google Scholar] [CrossRef] [PubMed]
- Sathiparan, N.; Dassanayake, D.H.H.P.; Subramaniam, D.N. Utilization of Supplementary Cementitious Materials in Pervious Concrete: A Review. Int. J. Environ. Sci. Technol. 2024, 21, 5883–5918. [Google Scholar] [CrossRef]
- Kina, C. Sustainable Binary/Ternary Blended Mortars with Recycled Water Treatment Sludge Using Fly Ash or Blast Slag: Characterization and Environmental-Economical Impacts. Envrion. Sci. Pollut. Res. 2024, 31, 15126–15152. [Google Scholar] [CrossRef] [PubMed]
- Moukannaa, S.; Alzeer, M.; Ramteke, D.D.; Ohenoja, K.; Roppo, J.; Kinnunen, P.; Illikainen, M. Investigation of Different Paper Mill Ashes as Potential Supplementary Cementitious Materials. J. Clean. Prod. 2022, 363, 132583. [Google Scholar] [CrossRef]
- Kameche, Z.e.-A.; Djelil, M.; Dahmani, B. Effects of Incorporating Silica Glass Powder as a Supplementary Cementitious Material on Selected Properties of Portland Cement Mortar. J. Build. Eng. 2023, 78, 107550. [Google Scholar] [CrossRef]
- ČSN EN 1015-11 (722400); Methods of Test for Mortar for Masonry—Part 11: Determination of Flexural and Compressive Strength of Hardened Mortar. Czech Office for Standards, Metrology and Testing: Prague, Czech Republic, 2020.
- BS 7755-3.2; Soil Quality. Chemical Methods Determination of pH. British Standards Institution: London, UK, 2005. [CrossRef]
- ČSN EN 12390-3; Testing of Hardened Concrete—Part 3: Compressive Strength of Test Specimens. Czech Office for Standards, Metrology and Testing: Prague, Czech Republic, 2020.
- EN-450-1; Fly Ash for Concrete. Definition, Specifications and Conformity Criteria. European Committee for Standardization: Brussels, Belgium, 2005.
- ASTM C618; Standard Specification for Fly Ash and Raw or Calcined Natural Pozzolan for Use as a Mineral Admixture in Portland Cement Concrete. ASTM International: West Conshohocken, PA, USA, 2003.
- EN 196-6; Methods of Testing Cement—Part 6: Determination of Fineness. European Committee for Standardization: Brussels, Belgium, 2019.
- Földvári, M. Handbook of Thermogravimetric System of Minerals and Its Use in Geological Practice; Occasional papers of the Geological Institute of Hungary; Geological Inst. of Hungary: Budapest, Hungary, 2011; ISBN 978-963-671-288-4. [Google Scholar]
- Keppert, M.; Pommer, V.; Šádková, K.; Krejsová, J.; Vejmelková, E.; Černý, R.; Koňáková, D. Thermal Activation of Illitic-Kaolinitic Mixed Clays. J. Therm. Anal. Calorim. 2024. [Google Scholar] [CrossRef]
- Vejmelková, E.; Pommer, V.; Šádková, K.; Koňáková, D. The Nature of Kaolinitic Clays and Their Impact on the Performance of SCM. J. Phys. Conf. Ser. 2023, 2628, 012032. [Google Scholar] [CrossRef]
- Avet, F.; Boehm-Courjault, E.; Scrivener, K. Investigation of C-A-S-H Composition, Morphology and Density in Limestone Calcined Clay Cement (LC3). Cem. Concr. Res. 2019, 115, 70–79. [Google Scholar] [CrossRef]
- Barbhuiya, S.; Nepal, J.; Das, B.B. Properties, Compatibility, Environmental Benefits and Future Directions of Limestone Calcined Clay Cement (LC3) Concrete: A Review. J. Build. Eng. 2023, 79, 107794. [Google Scholar] [CrossRef]
- Mousavi, S.S.; Bhojaraju, C.; Ouellet-Plamondon, C. Clay as a Sustainable Binder for Concrete—A Review. Constr. Mater. 2021, 1, 134–168. [Google Scholar] [CrossRef]
- Norvell, J.K.; Stewart, J.G.; Juenger, M.C.; Fowler, D.W. Influence of Clays and Clay-Sized Particles on Concrete Performance. J. Mater. Civ. Eng. 2007, 19, 1053–1059. [Google Scholar] [CrossRef]







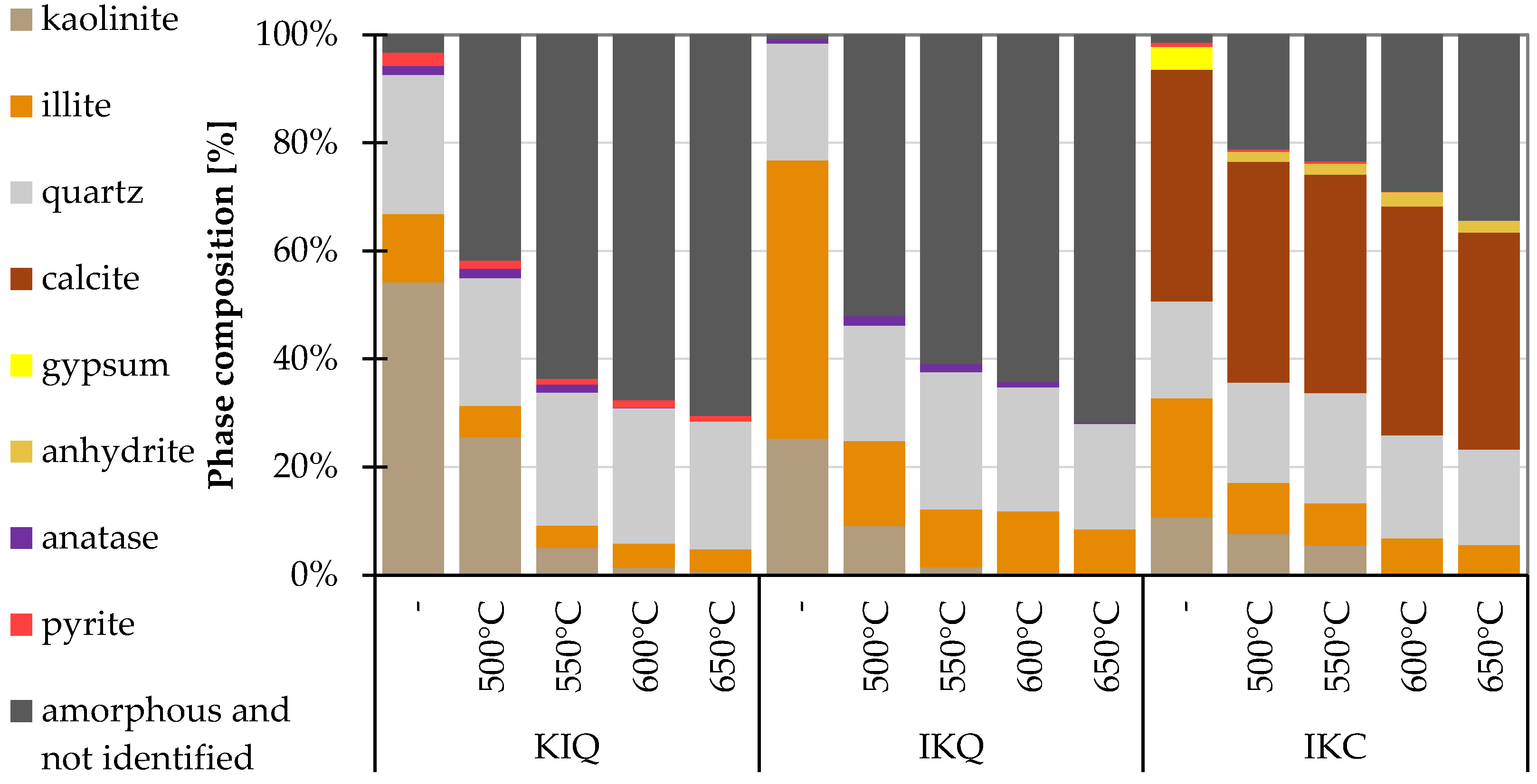
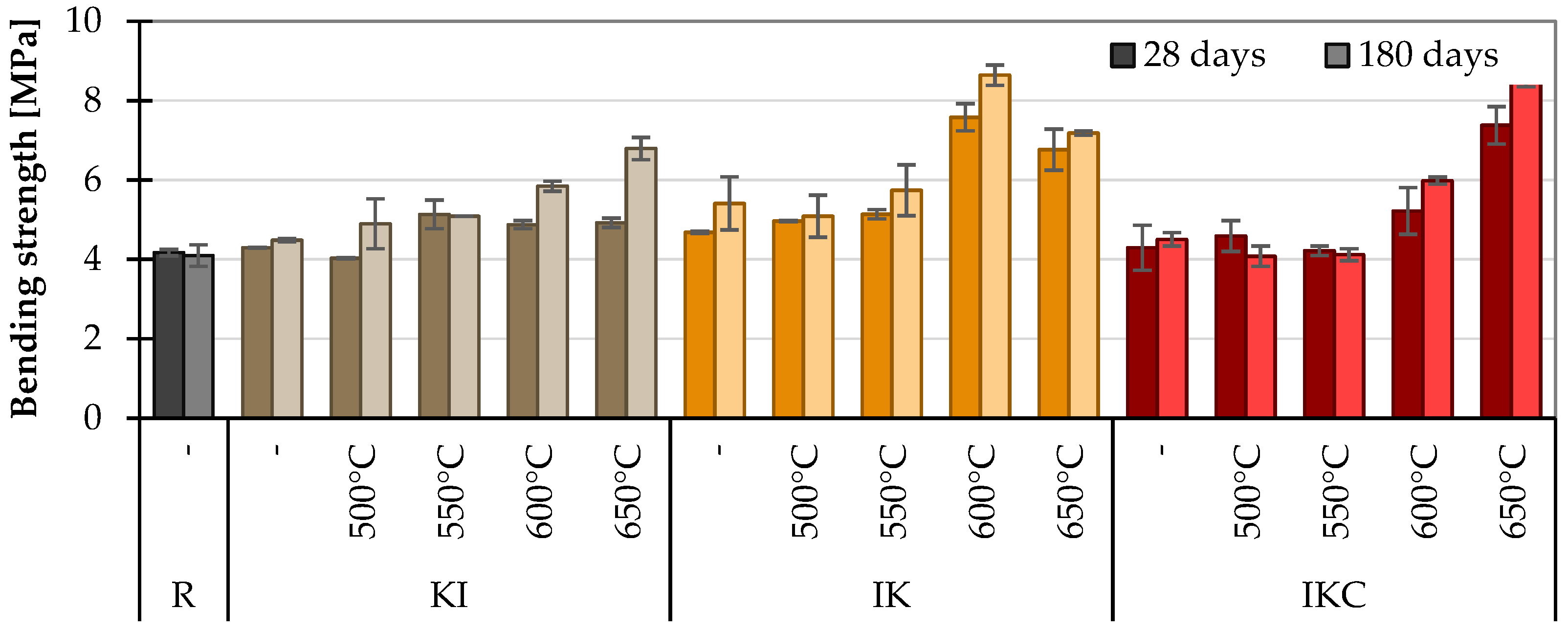

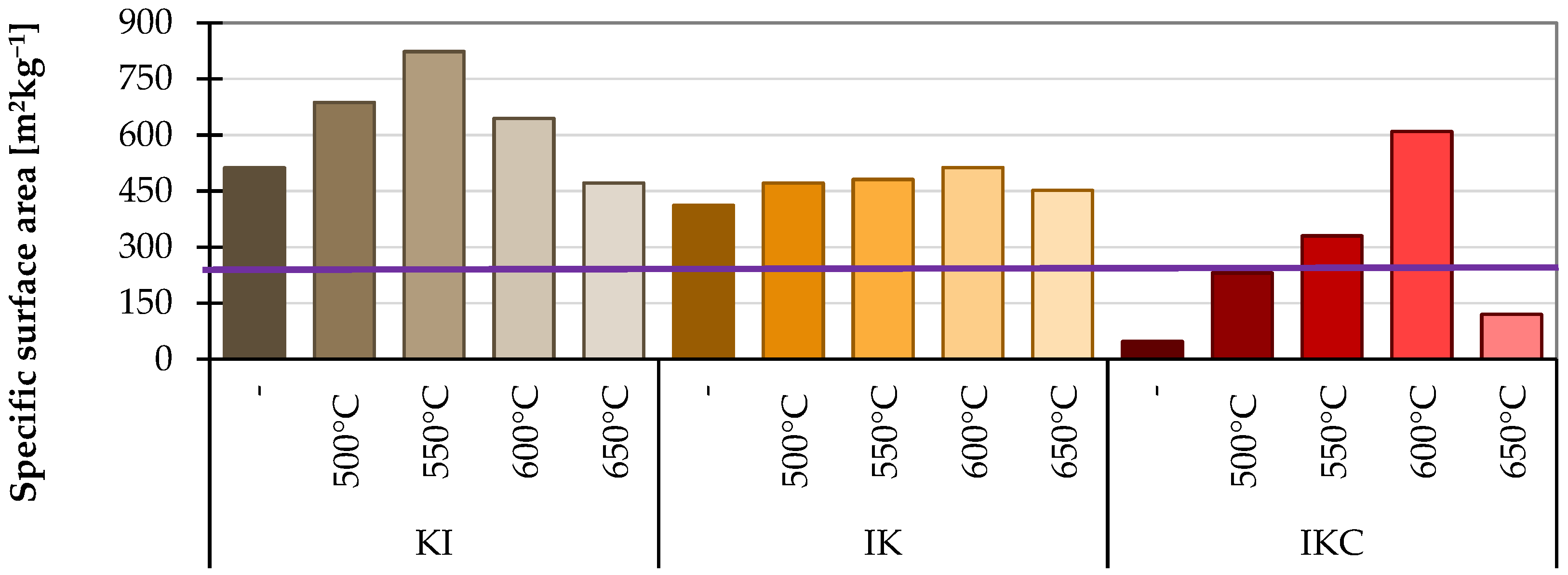
| Label | Locality | Density [kg m−3] | Specific Surface Area [cm2 g−1] | D10 [µm] | D50 [µm] | D90 [µm] |
|---|---|---|---|---|---|---|
| KI | CZ—Brník | 2520 | 5123 | 1.33 | 6.65 | 29.03 |
| IK | CZ—Horní Bříza | 2397 | 1038 | 1.99 | 6.45 | 21.08 |
| IKC | CZ—Libochovice | 2598 | 477 | 1.97 | 13.5 | 47.17 |
| LOI [wt.%] | Chemical Composition [wt.%] | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| SiO2 | Al2O3 | Fe2O3 | CaO | K2O | MgO | TiO2 | SO3 | Na2O | ||
| KI | 23.8 | 52.2 | 38.8 | 2.3 | 0.0 | 1.3 | 0.8 | 1.7 | 2.6 | 0.0 |
| IK | 13.7 | 52.8 | 33.7 | 4.8 | 0.1 | 5.2 | 1.4 | 1.2 | 0.0 | 0.4 |
| IKC | 26.9 | 36.8 | 14.3 | 4.4 | 36.6 | 3.1 | 2.4 | 0.8 | 1.3 | 0.0 |
| Test | Age [Days] | Pozzolanic Activity | ||||||
|---|---|---|---|---|---|---|---|---|
| Best Results | Worst Results | |||||||
| Direct methods | FT | 8 | KI 650 | KI 600 | KI 550 | IKC 500 | IKC 550 | IKC |
| LST | 3 | KI 500 | IK 600 | IKC 550 | IKC | IK 500 | IK | |
| 7 | IK 550 | IK 500 | IK 650 | IKC 500 | KI 650 | IK | ||
| IC—lime | 5 | KI 650 | KI 600 | KI 550 | IKC 500 | IK | IKC | |
| XRD | 0 | IK 650 | KI 650 | KI 600 | KI | IKC | IK | |
| Indirect methods | cement paste | 28 | KI 550 | IKC 650 | KI 650 | IK | IKC 500 | IKC |
| 180 | IKC 650 | KI 650 | KI 600 | IKC | IK | IKC 500 | ||
| CSD | 180 | KI 600 | KI 650 | IK 600 | KI 550 | IK | IKC 550 | |
| pH | 180 | KI 600 | IK 600 | KI 650 | IK 550 | IKC 500 | IK 500 | |
| SAI | 28 | KI 650 | IK 600 | IKC 600 | IK 550 | IK 500 | IK | |
| 180 | KI 650 | KI 600 | KI 500 | IK 550 | IK 500 | IK | ||
| SSA | 0 | KI 550 | KI 500 | KI 600 | IKC 500 | IKC 650 | IKC | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Šádková, K.; Pommer, V.; Keppert, M.; Vejmelková, E.; Koňáková, D. Difficulties in Determining the Pozzolanic Activity of Thermally Activated Lower-Grade Clays. Materials 2024, 17, 5093. https://doi.org/10.3390/ma17205093
Šádková K, Pommer V, Keppert M, Vejmelková E, Koňáková D. Difficulties in Determining the Pozzolanic Activity of Thermally Activated Lower-Grade Clays. Materials. 2024; 17(20):5093. https://doi.org/10.3390/ma17205093
Chicago/Turabian StyleŠádková, Kateřina, Vojtěch Pommer, Martin Keppert, Eva Vejmelková, and Dana Koňáková. 2024. "Difficulties in Determining the Pozzolanic Activity of Thermally Activated Lower-Grade Clays" Materials 17, no. 20: 5093. https://doi.org/10.3390/ma17205093
APA StyleŠádková, K., Pommer, V., Keppert, M., Vejmelková, E., & Koňáková, D. (2024). Difficulties in Determining the Pozzolanic Activity of Thermally Activated Lower-Grade Clays. Materials, 17(20), 5093. https://doi.org/10.3390/ma17205093








