The Effect of Calcium Sulfate on the Hydration and Properties of Red Mud-Based Calcium Ferroaluminate Cement Clinker
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Sample Preparation
2.3. Methodology
2.3.1. Mechanical Properties
2.3.2. Isothermal Calorimetry
2.3.3. X-ray Powder Diffraction
2.3.4. Differential Scanning Calorimetry–Thermogravimetric Analysis
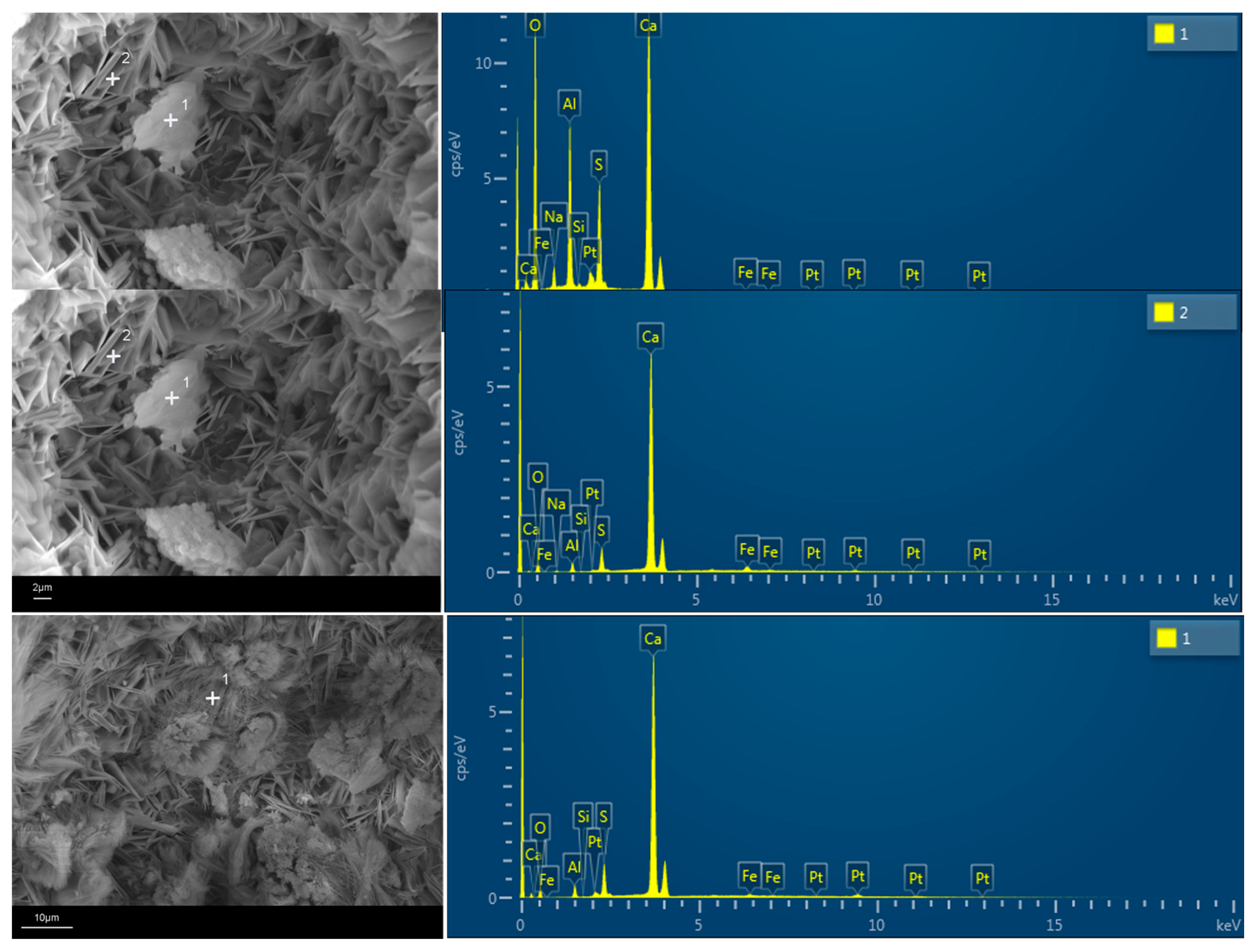
2.3.5. Morphology Analysis
3. Results and Discussion
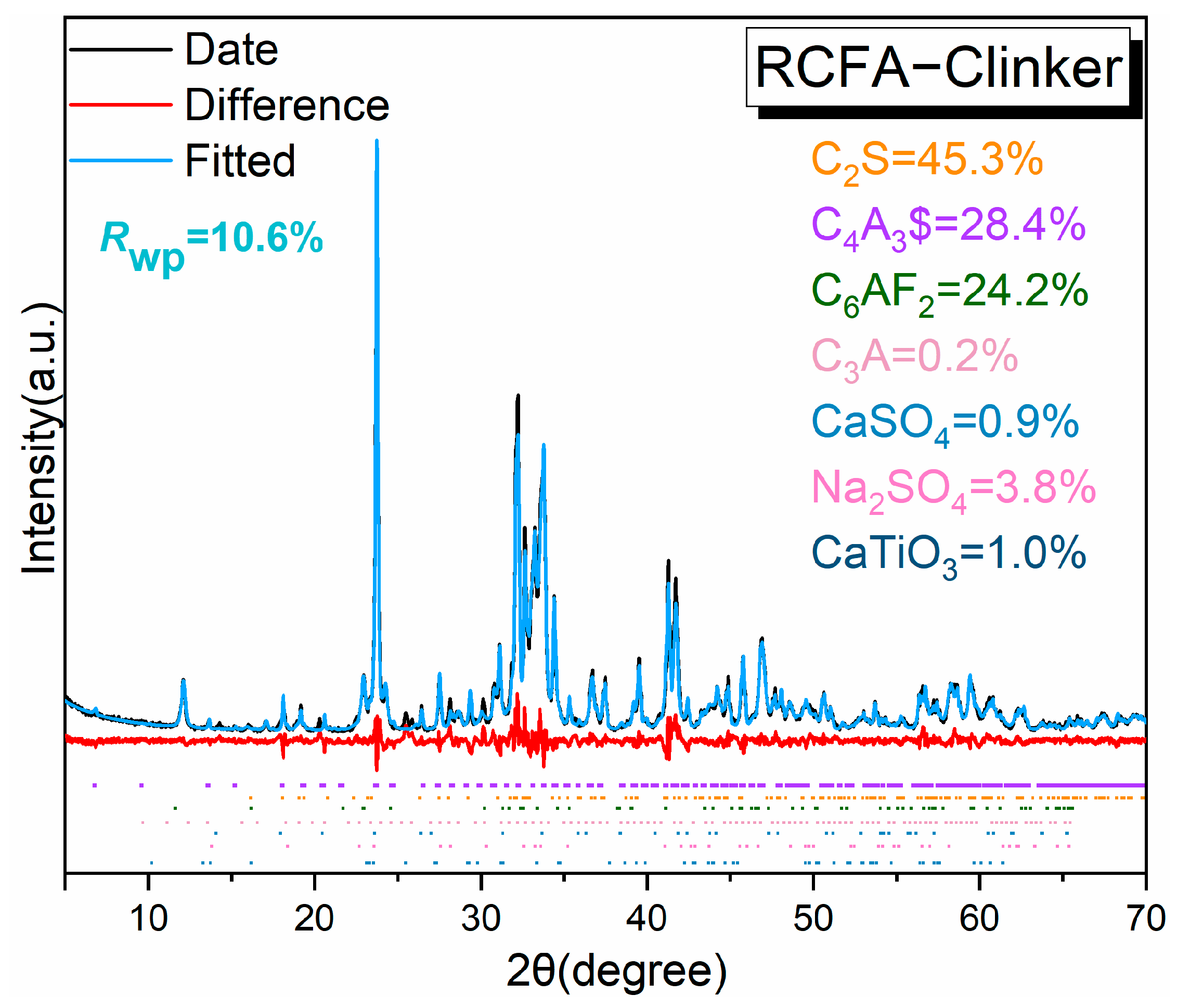
3.1. The Phase Analysis of RCFA Clinker
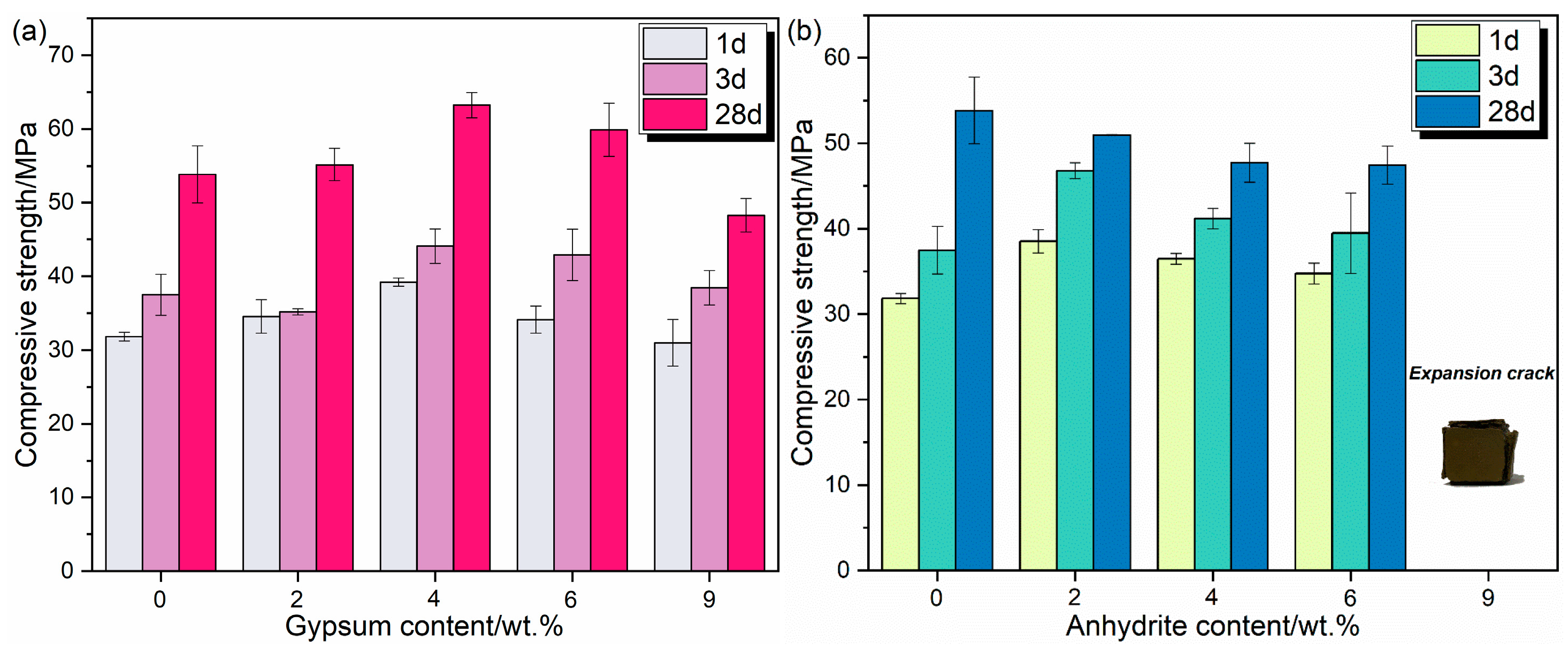
3.2. Compressive Strength
3.3. Hydration Heat
3.4. Analysis of Hydration Products
3.4.1. XRD Analysis
+ (2 + 2x) FH3 + (2 − 2x) AH3
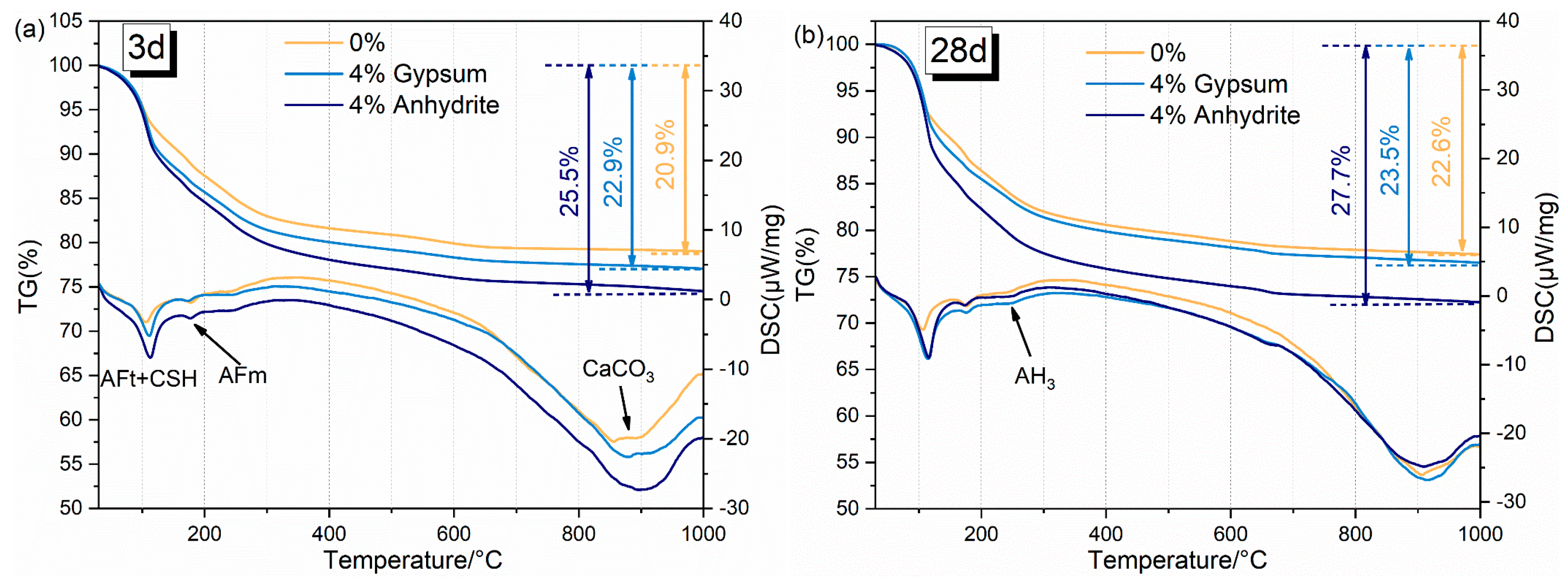
3.4.2. DSC-TG Analysis
3.4.3. SEM Analysis
4. Conclusions
- (1)
- XRD analysis reveals the presence of the main minerals like C2S, C4A3$, and ferrite phase (C6AF2) and trace minerals like sulfate in the RCFA clinker. The cement paste is 61.6% stronger when 4% gypsum is added, with a 3 d compressive strength of 39.1 MPa and a 28 d compressive strength of 63.2 MPa. Excessive anhydrite content can precipitate deleterious effects on the properties of cement paste.
- (2)
- RCFA clinker is heated by both gypsum and anhydrite during the initial phase of hydration, but anhydrite contributes more to the initial stage. As the gypsum content increases, the longer it takes to reach equilibrium in the hydration heat release curve.
- (3)
- The RCFA main hydration products are AFt and AFm, along with non-hydrated C2S and CaTiO3. A sufficient sulfur source can promote the hydration of C4A3$, thereby increasing the AFt in the hydration product.
- (4)
- The mass loss of hydration products from RCFA is mainly due to the dehydration of AFt, AFm, and AH3. The calcium sulfate group lost more mass than the blank group. AFm and non-hydrated cement particles contained most of the Na in the hardened cement paste.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Nomenclature
| C4A3$ | 3CaO·Al2O3·CaSO4 |
| C2S | 2CaO·SiO2 |
| AFt | 3CaO·Al2O3·3CaSO4·32H2O |
| AFm | 3CaO·3Al2O3·CaSO4·12H2O |
| AH3 | Al2O3·3H2O(gel) |
| FH3 | Fe2O3·3H2O(gel) |
| CH | Ca(OH)2 |
References
- Negrão, L.B.A.; da Costa, M.L.; Pöllmann, H. Waste clay from bauxite beneficiation to produce calcium sulphoaluminate eco-cements. Constr. Build. Mater. 2022, 340, 127703. [Google Scholar] [CrossRef]
- Hertel, T.; Van den Bulck, A.; Onisei, S.; Sivakumar, P.P.; Pontikes, Y. Boosting the use of bauxite residue (red mud) in cement-Production of an Fe-rich calciumsulfoaluminate-ferrite clinker and characterisation of the hydration. Cem. Concr. Res. 2021, 145, 106463. [Google Scholar] [CrossRef]
- Shah, S.S.; Palmieri, M.C.; Sponchiado, S.R.P.; Bevilaqua, D. Environmentally sustainable and cost-effective bioleaching of aluminum from low-grade bauxite ore using marine-derived Aspergillus niger. Hydrometallurgy 2020, 195, 105368. [Google Scholar] [CrossRef]
- Ram Kumar, B.; Ramakrishna, G. Performance evaluation of red mud as a construction material—A review. Mater. Today Proc. 2023. [Google Scholar] [CrossRef]
- Khairu, L.M.; Zanganeh, J.; Moghtaderi, B. The composition, recycling and utilisation of Bayer red mud. Resour. Conserv. Recycl. 2019, 141, 483–498. [Google Scholar] [CrossRef]
- Zhang, X.; Lu, X.L.; Feng, J.R.; Zheng, G.F.; Yang, Q.C.; Du, P.; Zhu, J.; Cheng, X. Calcium ferroaluminate cements made with red mud: Synthetic issues and mechanical properties. Ceramics-Silikáty 2024, 68, 195–204. [Google Scholar] [CrossRef]
- Hanic, F.; Kaprálik, I.; Gabrisová, A. Mechanism of hydration reactions in the system C4A3S-CS-CaO-H2O referred to hydration of sulphoaluminate cements. Cem. Concr. Res. 1989, 19, 671–682. [Google Scholar] [CrossRef]
- Sahu, S.; Havlica, J.; Tomková, V.; Majling, J. Hydration behaviour of sulphoaluminate belite cement in the presence op various calcium sulphates. Thermochim. Acta 1991, 175, 45–52. [Google Scholar] [CrossRef]
- Péra, J.; Ambroise, J.; Holard, E.; Beauvent, G. Influence of the type of calcium sulfate on the properties of calcium sulfoaluminate cement. In Proceedings of the Eleventh International Congress on the Chemistry of Cements, Durban, South Africa, 11–16 May 2003; Volume 3, pp. 1129–1135. [Google Scholar]
- Glasser, F.P.; Zhang, L. High-performance cement matrices based on calcium sulfoaluminate–belite compositions. Cem. Concr. Res. 2001, 31, 1881–1886. [Google Scholar] [CrossRef]
- Zhang, L.; Glasser, F.P. Hydration of calcium sulfoaluminate cement at less than 24 h. Adv. Cem. Res. 2002, 14, 141–155. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Calorimetric and thermogravimetric study on the influence of calcium sulfate on the hydration of ye′elimite. J. Therm. Anal. Calorim. 2010, 101, 949–957. [Google Scholar] [CrossRef]
- Álvarez-Pinazo, G.; Santacruz, I.; Aranda, M.A.G.; De la Torre, A.G. Hydration of belite–ye′elimite–ferrite cements with different calcium sulfate sources. Adv. Cem. Res. 2016, 28, 529–543. [Google Scholar] [CrossRef]
- Gallardo-Heredia, M.; Almanza-Robles, J.M.; Magallanes-Rivera, R.X.; Cortes-Hernández, D.A.; Escobedo-Bocardo, J.C.; Avila-López, U. Calcium sulfoaluminate cement pastes from industrial wastes: Effect of hemihydrate content. Mater. Struct. 2017, 50, 93. [Google Scholar] [CrossRef]
- Winnefeld, F.; Lothenbach, B. Phase equilibria in the system Ca4Al6O12SO4–Ca2SiO4–CaSO4–H2O referring to the hydration of calcium sulfoaluminate cements. RILEM Tech. Lett. 2016, 1, 10–16. [Google Scholar] [CrossRef]
- Jansen, D.; Spies, A.; Neubauer, J.; Ectors, D.; Goetz-Neunhoeffer, F. Studies on the early hydration of two modifications of ye’elimite with gypsum. Cem. Concr. Res. 2017, 91, 106–116. [Google Scholar] [CrossRef]
- Zhang, W.; Cao, L.; Ren, X.; Ye, J.; Liu, L.; Wu, Y. Influence of free calcium sulfate levels on the early hydration of sulfate-rich belite sulfoaluminate clinkers: A comparative study with anhydrite and gypsum. Cem. Concr. Res. 2024, 181, 107522. [Google Scholar] [CrossRef]
- Szabados, M.; Pásztor, K.; Csendes, Z.; Muráth, S.; Kónya, Z.; Kukovecz, Á.; Carlson, S.; Sipos, P.; Pálinkó, I. Synthesis of high-quality, well-characterized CaAlFe-layered triple hydroxide with the combination of dry-milling and ultrasonic irradiation in aqueous solution at elevated temperature. Ultrason. Sonochem. 2016, 32, 173–180. [Google Scholar] [CrossRef]
- Mogashane, T.M.; Maree, J.P.; Mujuru, M.; Mphahlele-Makgwane, M.M.; Modibane, K.D. Ferric hydroxide recovery from iron-rich acid mine water with calcium carbonate and a gypsum scale inhibitor. Minerals 2023, 13, 167. [Google Scholar] [CrossRef]
- Yang, L.; Chen, M.; Lu, Z.; Huang, Y.; Wang, J.; Lu, L.; Cheng, X. Synthesis of CaFeAl layered double hydroxides 2D nanosheets and the adsorption behaviour of chloride in simulated marine concrete. Cem. Concr. Comp. 2020, 114, 103817. [Google Scholar] [CrossRef]
- Yao, B.W.; Mei, S.G.; Song, S.M. Effect of Gypsum on Hydration of High Belite Sulphoaluminate Cement. J. Wuhan Univ. Technol. 2009, 31, 1–4. [Google Scholar]
- Jia, H.X.; Lv, S.Z.; Hu, J.L.; Gao, Y.; Duan, X.Y. Influence of Gypsum on Performance of High Belite-sulphoaluminate Cement. J. Wuhan Univ. Technol. 2014, 36, 24–28. [Google Scholar]
- Winnefeld, F.; Martin, L.H.J.; Müller, C.J.; Lothenbach, B. Using gypsum to control hydration kinetics of CSA cements. Constr. Build. Mater. 2017, 155, 154–163. [Google Scholar] [CrossRef]
- Majling, J.; Znášik, P.; Gabrišová, A.; Svetík, Š. The influence of anhydrite activity upon the hydration of calcium sulphoaluminate cement clinker. Thermochim. Acta 1985, 92, 349–352. [Google Scholar] [CrossRef]
- Winnefeld, F.; Barlag, S. Influence of calcium sulfate and calcium hydroxide on the hydration of calcium sulfoaluminate clinker. Zkg Int. 2009, 62, 42–53. [Google Scholar]
- Winnefeld, F.; Lothenbach, B. Hydration of calcium sulfoaluminate cements-Experimental findings and thermodynamic modelling. Cem. Concr. Res. 2010, 40, 1239–1247. [Google Scholar] [CrossRef]
- García-Maté, M.; Angeles, G.D.L.T.; León-Reina, L.; Losilla, E.R.; Aranda, M.A.; Santacruz, I. Effect of calcium sulfate source on the hydration of calcium sulfoaluminate eco-cement. Cem. Concr. Comp. 2015, 55, 53–61. [Google Scholar] [CrossRef]
- Allevi, S.; Marchi, M.; Scotti, F.; Bertini, S.; Cosentino, C. Hydration of calcium sulphoaluminate clinker with additions of different calcium sulphate sources. Mater. Struct. 2016, 49, 453–466. [Google Scholar] [CrossRef]
- Han, S.W.; Zhong, J.; Ding, W.J.; Ou, J.P. Strength, hydration, and microstructure of seawater sea-sand concrete using high-ferrite Portland cement. Constr. Build. Mater. 2021, 295, 123703. [Google Scholar] [CrossRef]
- Jansen, E.; Schafer, W.; Will, G. R values in analysis of powder diffraction data using Rietveld refinement. J. Appl. Crystallogr. 1994, 27, 492–496. [Google Scholar] [CrossRef]
- Von Dreele, R. Quantitative texture analysis by Rietveld refinement. J. Appl. Crystallogr. 1997, 30, 517–525. [Google Scholar] [CrossRef]
- Morsli, K.; de la Torre, Á.G.; Zahir, M.; Aranda, M.A. Mineralogical phase analysis of alkali and sulfate bearing belite rich laboratory clinkers. Cem. Concr. Res. 2007, 37, 639–646. [Google Scholar] [CrossRef]
- Xu, L.L.; Zhou, X.Z.; Li, N.; Wang, P.M. Impact of Calcium Sulfate on Hydration Features of Calcium Sulfoaluminate. Cem. J. Tongji Univ. (Nat. Sci.) 2017, 45, 885–890. [Google Scholar]




| Chemical Composition | CaO | SiO2 | Al2O3 | Fe2O3 | Na2O | TiO2 | K2O | Others |
|---|---|---|---|---|---|---|---|---|
| Red mud | 3.56 | 15.33 | 22.50 | 33.56 | 8.68 | 6.45 | 0.06 | 9.86 |
| Sample | Mineral Composition | Chemical Composition | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| RCFA | C4A3$ | C2F | C2S | CaSO4 | CaO | SiO2 | Al2O3 | Fe2O3 | SO3 | Na2O |
| 35 | 20 | 35 | 10 | 48.0 | 12.2 | 17.6 | 11.8 | 10.5 | 2.8 | |
| Type | Content | |||
|---|---|---|---|---|
| Gypsum | 2 | 4 | 6 | 9 |
| Anhydrite | 2 | 4 | 6 | 9 |
| Phase | Space Group | ICSD Codes |
|---|---|---|
| c-C4A3$ | I-43 m | 9560 |
| o-C4A3$ | Pcc2 | 80361 |
| β-C2S | P21/n | 79553 |
| C6AF2 | Ibm2 | 1000040 |
| C3A | Pa-3 | 1841 |
| Na2SO4 | Fddd-70 | 1903926 |
| CaSO4 | Amma | 40043 |
| CaTiO3 | Pbnm | 163528 |
| AFt | P31c | 155395 |
| AFm | R-3 | 24461 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, N.; Ma, Y.; Zhang, X.; Li, J.; Lu, X.; Zhang, L.; Cheng, X. The Effect of Calcium Sulfate on the Hydration and Properties of Red Mud-Based Calcium Ferroaluminate Cement Clinker. Materials 2024, 17, 5064. https://doi.org/10.3390/ma17205064
Shi N, Ma Y, Zhang X, Li J, Lu X, Zhang L, Cheng X. The Effect of Calcium Sulfate on the Hydration and Properties of Red Mud-Based Calcium Ferroaluminate Cement Clinker. Materials. 2024; 17(20):5064. https://doi.org/10.3390/ma17205064
Chicago/Turabian StyleShi, Nan, Ya Ma, Xiang Zhang, Jun Li, Xiaolei Lu, Lina Zhang, and Xin Cheng. 2024. "The Effect of Calcium Sulfate on the Hydration and Properties of Red Mud-Based Calcium Ferroaluminate Cement Clinker" Materials 17, no. 20: 5064. https://doi.org/10.3390/ma17205064
APA StyleShi, N., Ma, Y., Zhang, X., Li, J., Lu, X., Zhang, L., & Cheng, X. (2024). The Effect of Calcium Sulfate on the Hydration and Properties of Red Mud-Based Calcium Ferroaluminate Cement Clinker. Materials, 17(20), 5064. https://doi.org/10.3390/ma17205064





