Optimizing the Pore Structure of Lotus-Type Porous Copper Fabricated by Continuous Casting
Abstract
1. Introduction
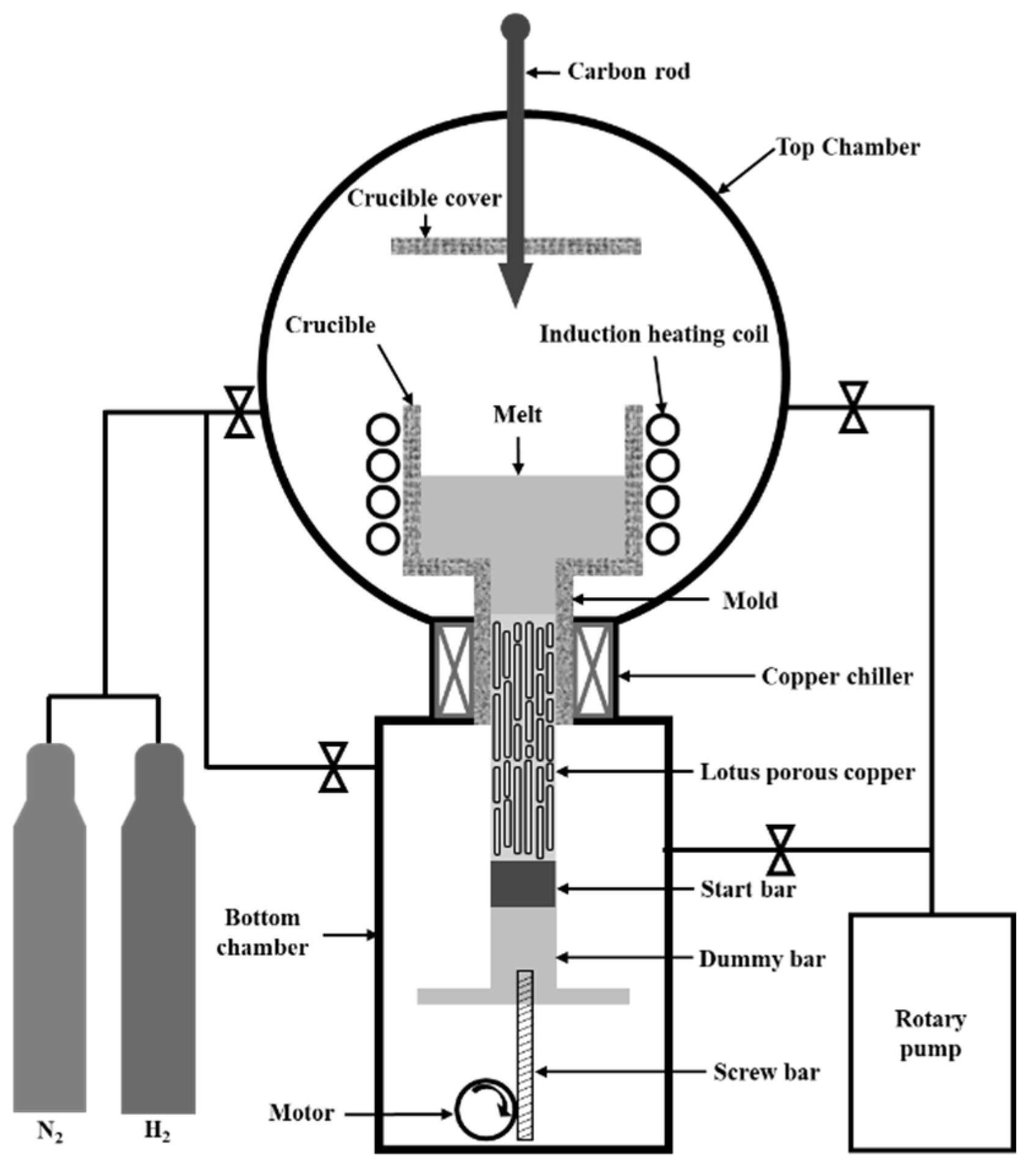
2. Materials and Methods
3. Results
3.1. Single Process Parameter
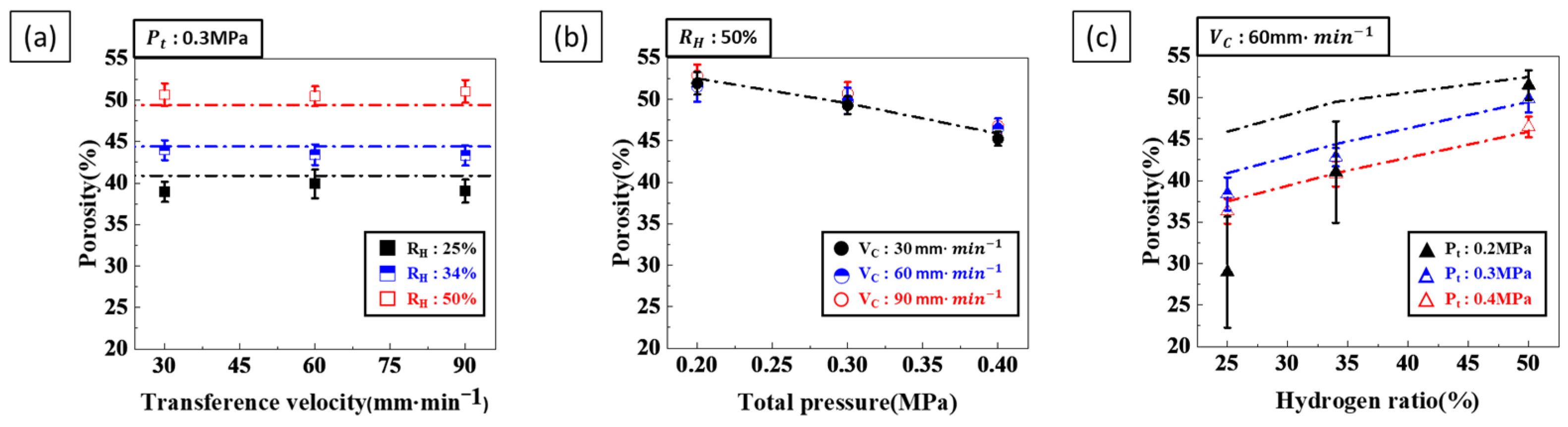
3.1.1. Effect of Single Process Parameter on Porosity
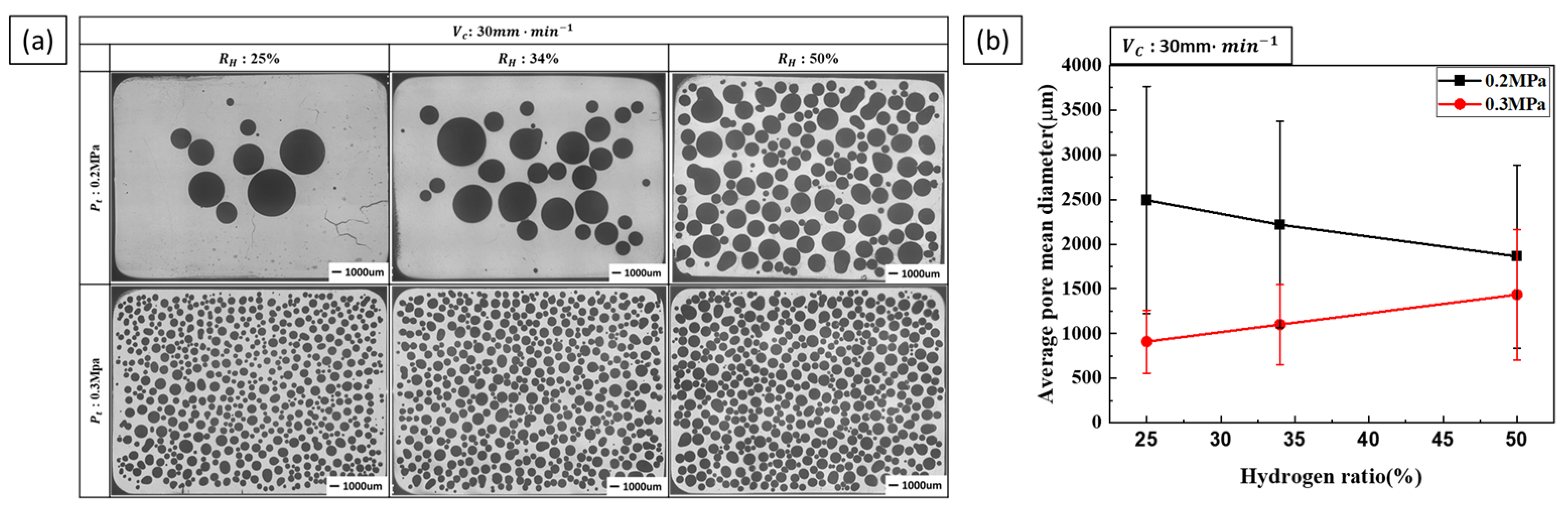
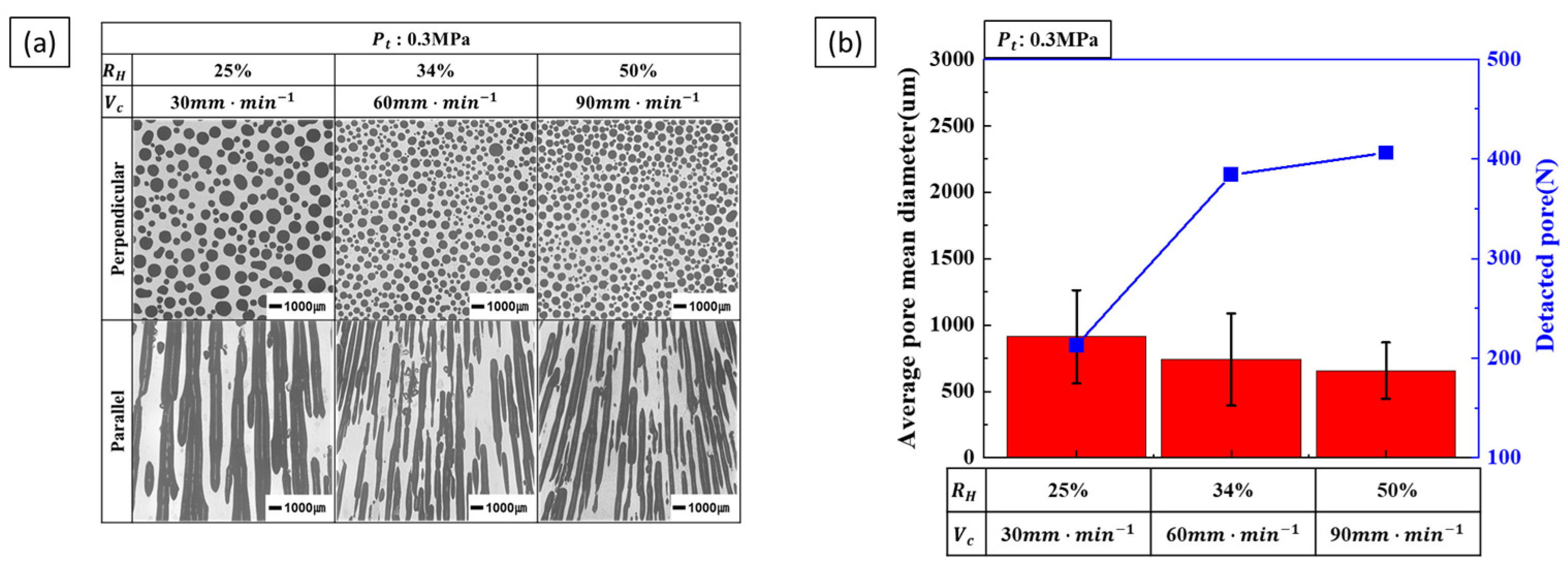
3.1.2. Effect of Single Process Parameter on Pore Diameter
3.2. Composite Process Parameter
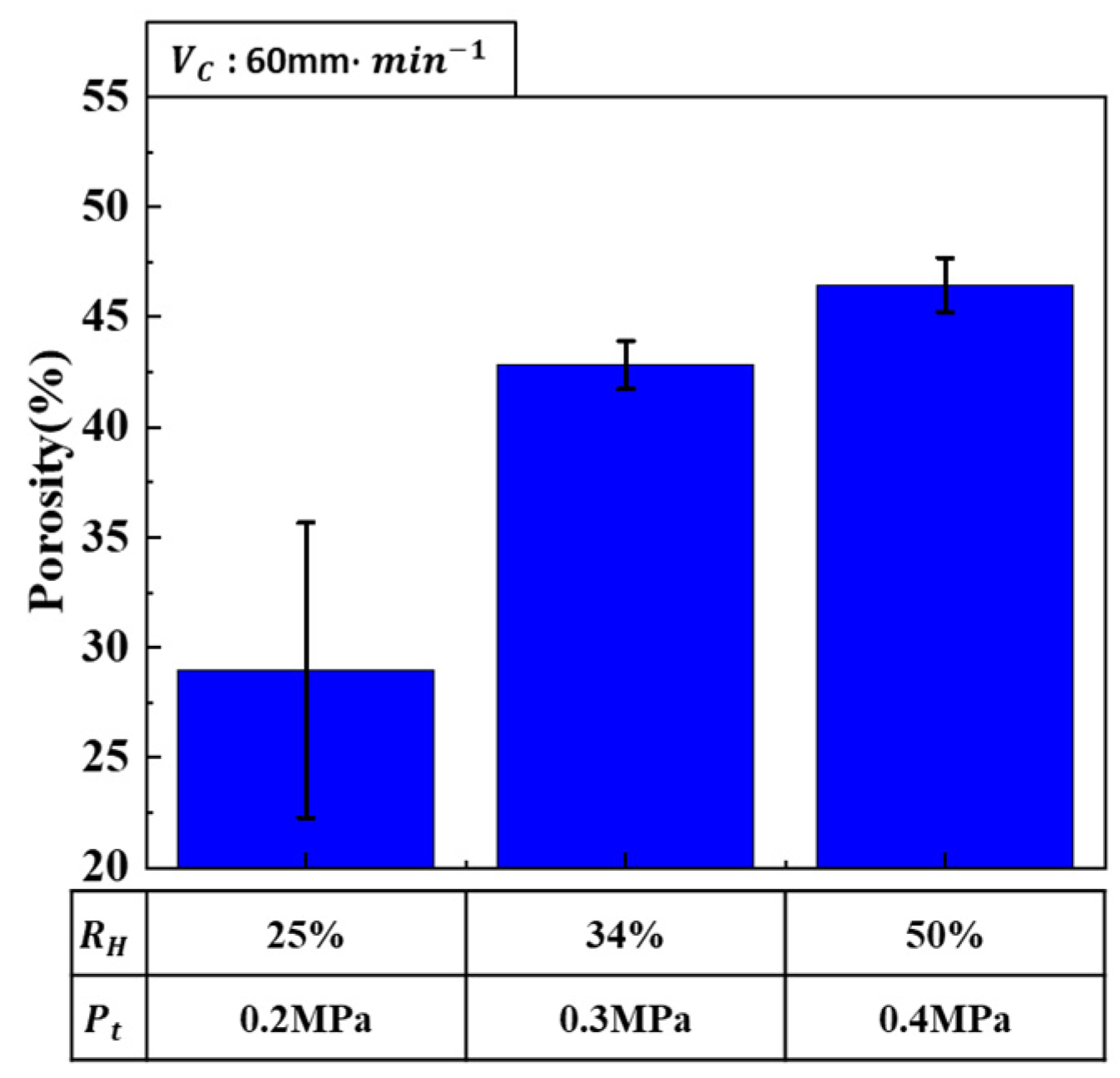
3.2.1. Effect of Composite Process Parameter on Porosity
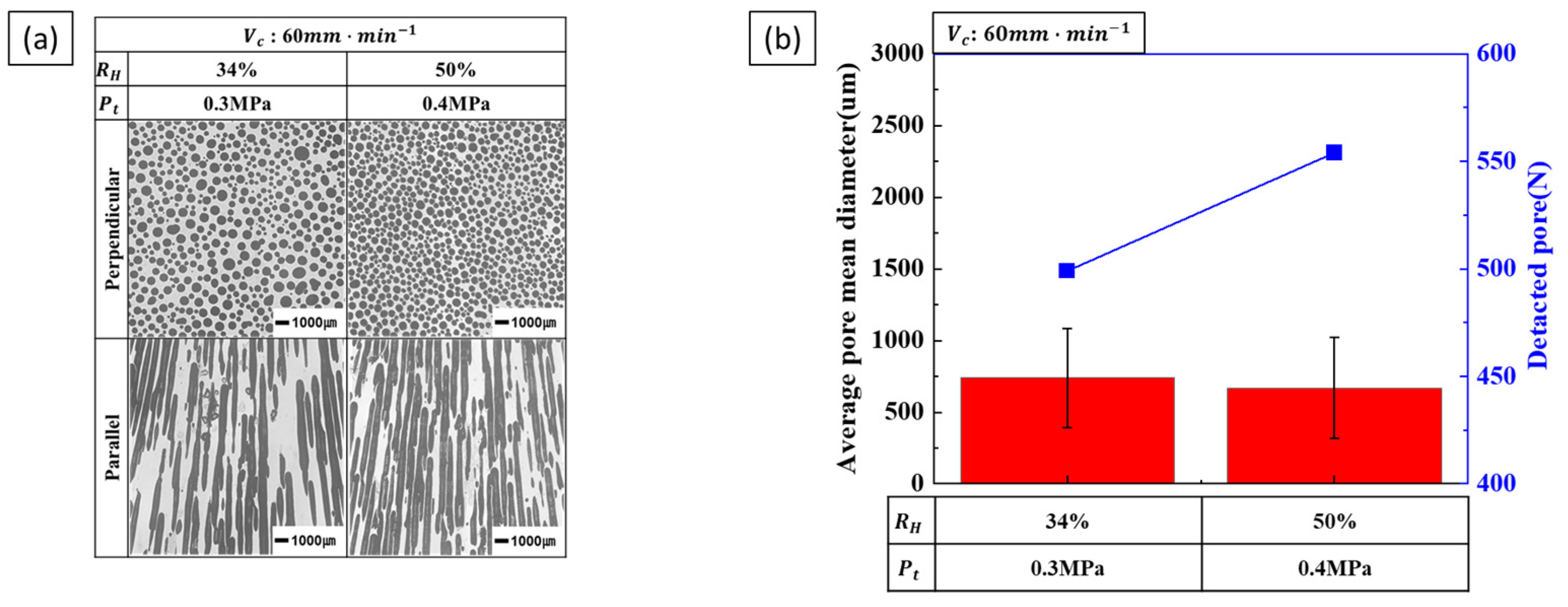
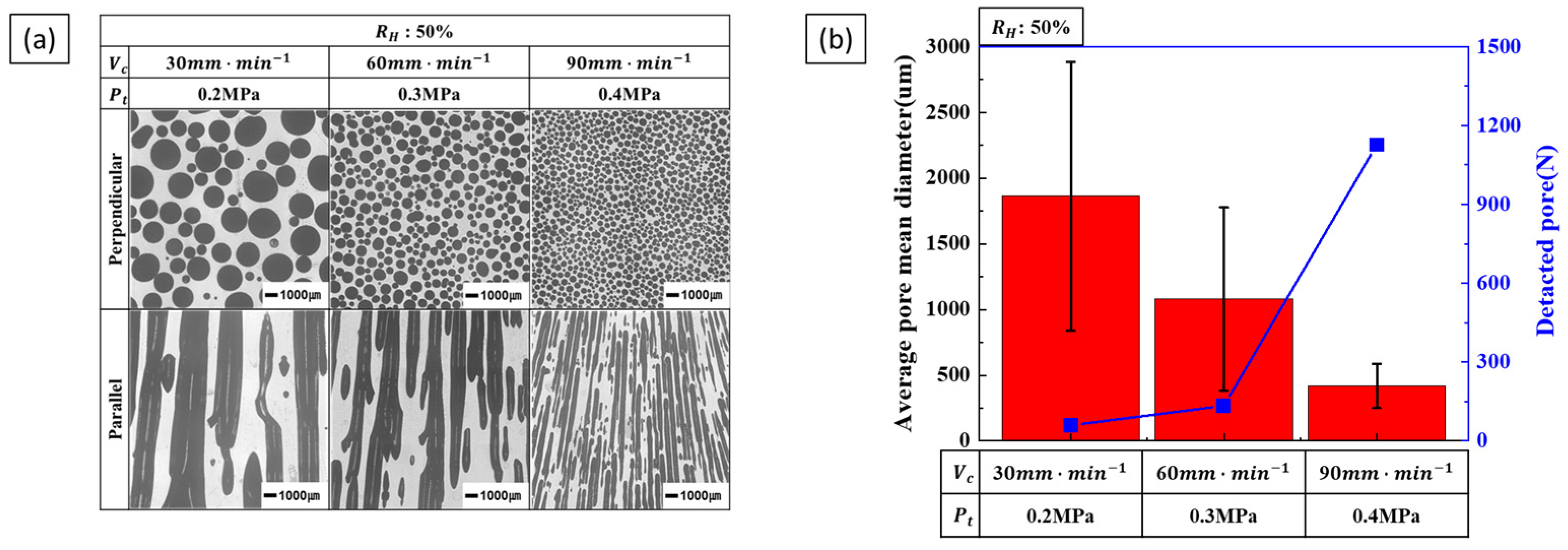
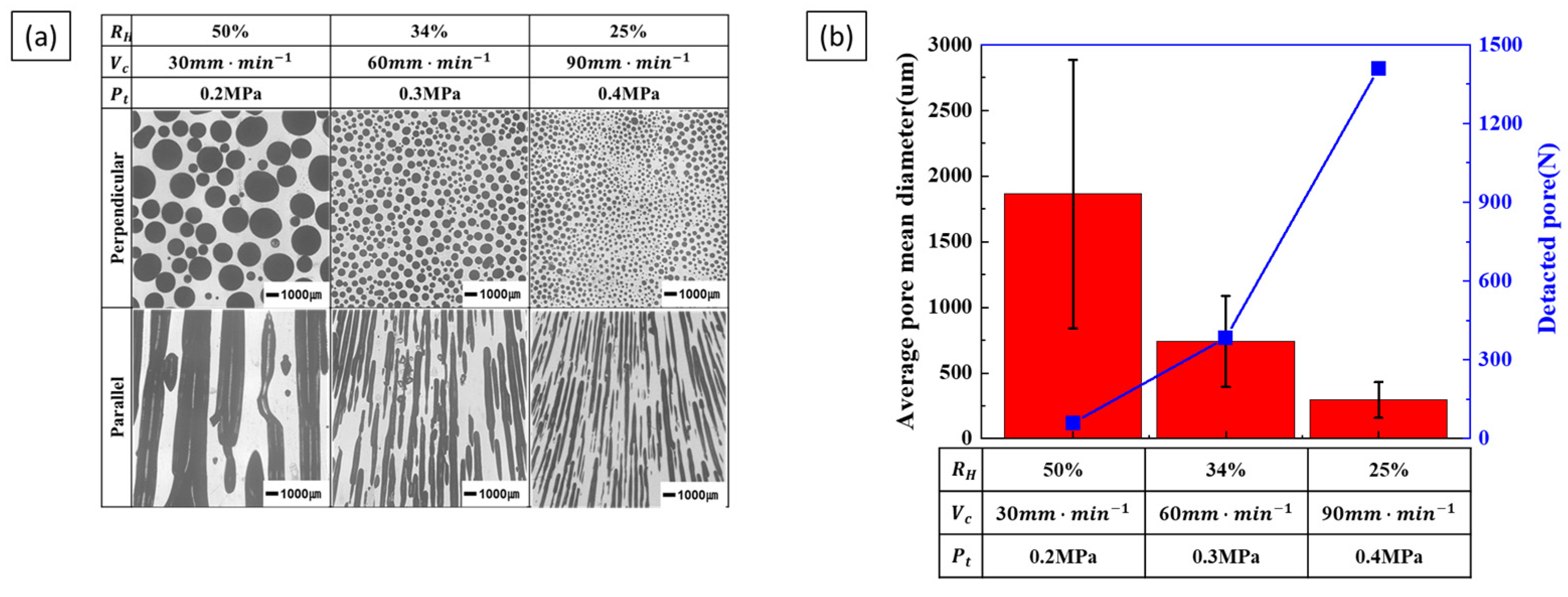
3.2.2. Effect of Composite Process Parameter on Pore Diameter
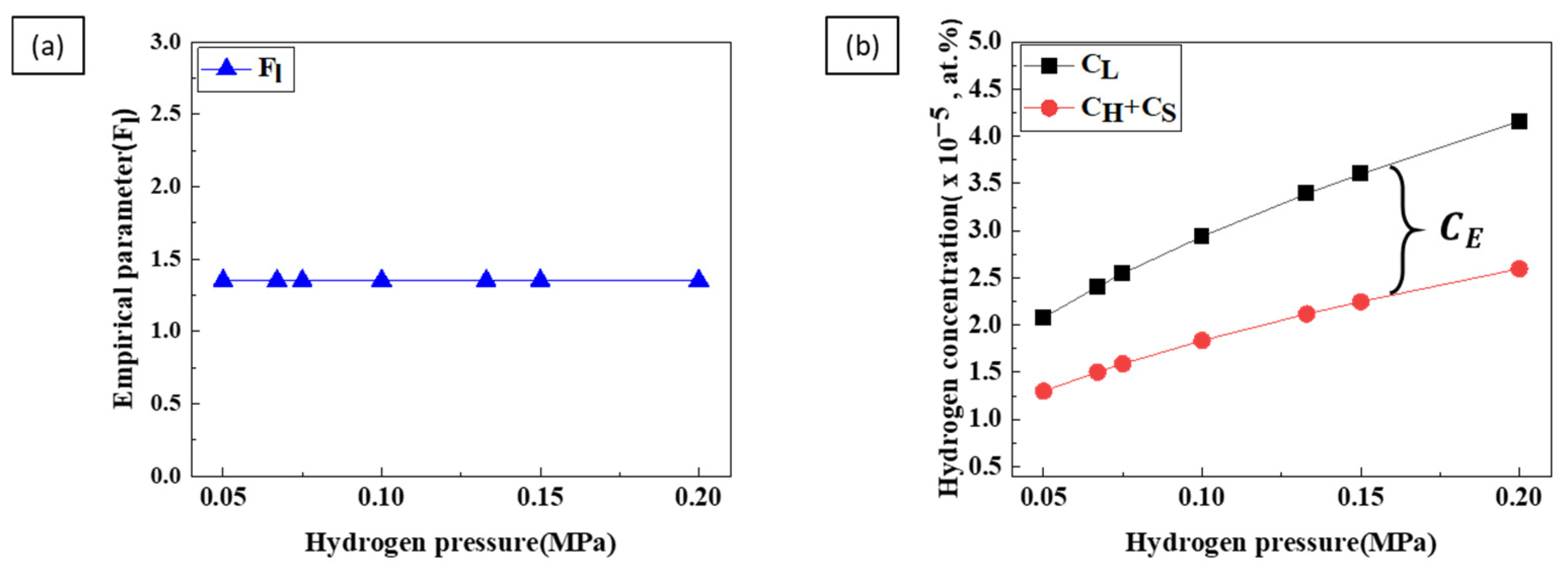
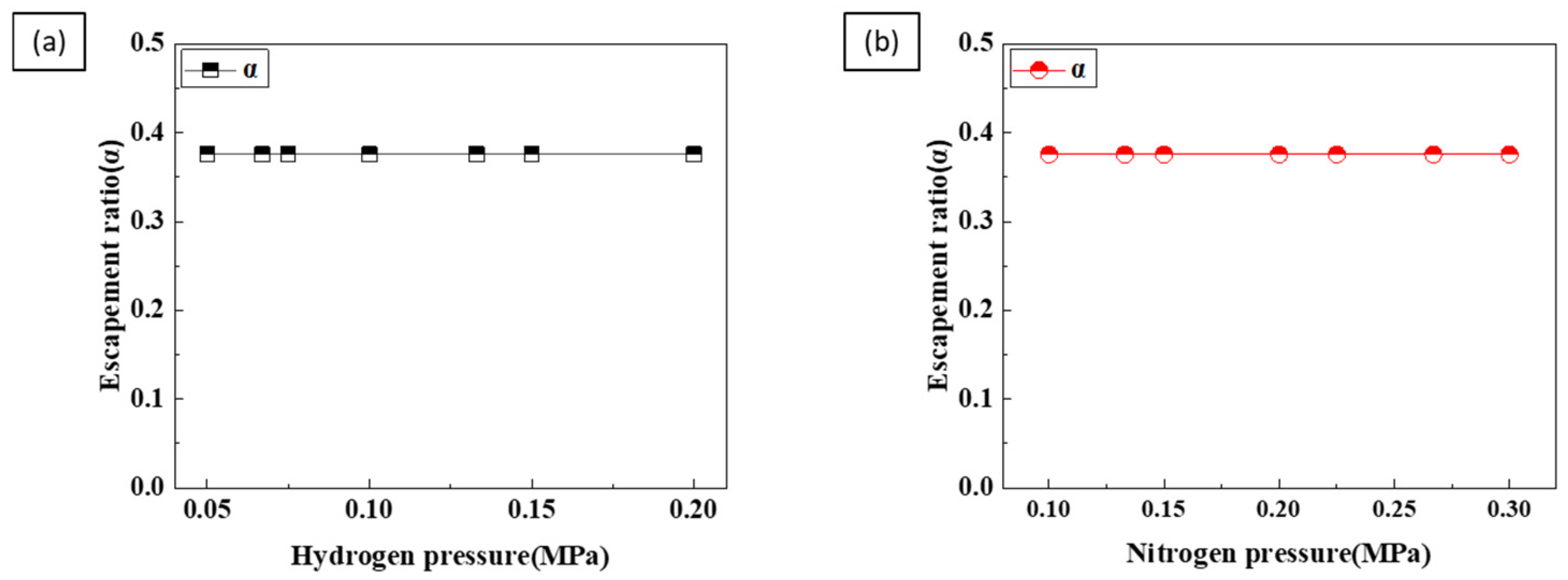
4. Discussion
4.1. Porosity
4.2. Pore Diameter
5. Conclusions
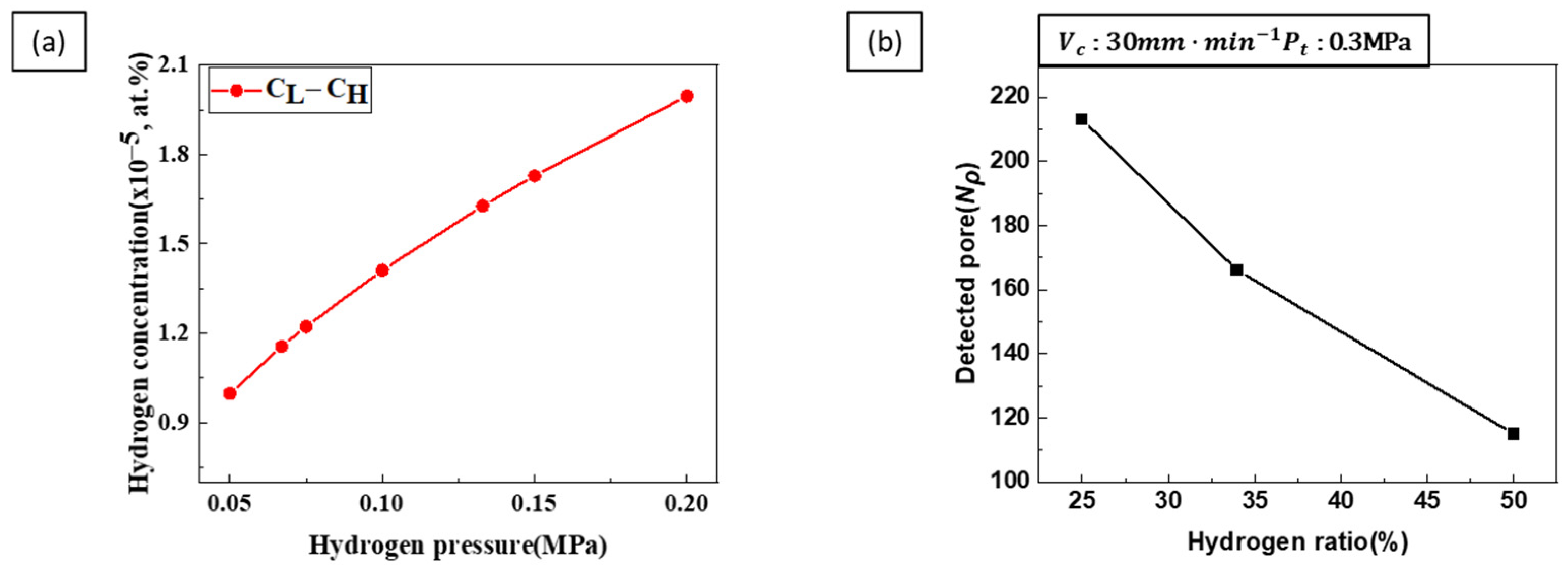
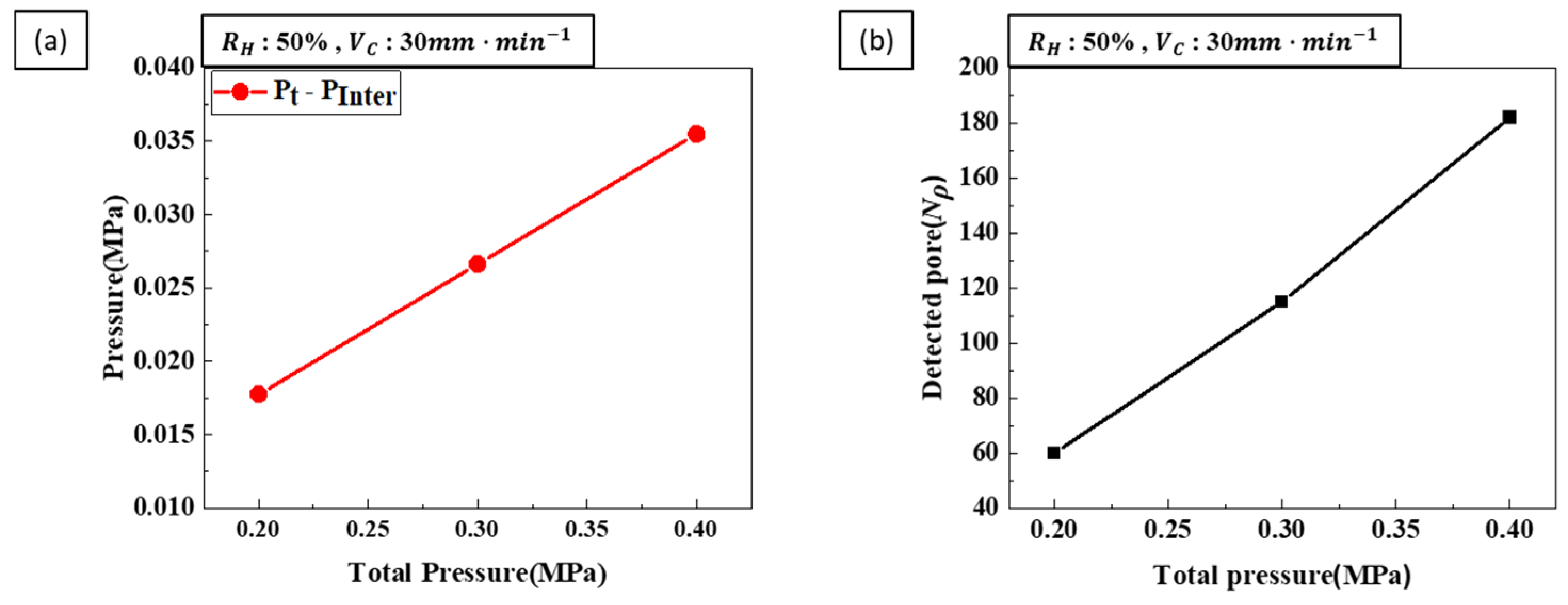
- Porosity control: Hydrogen ratio was identified as a critical factor influencing porosity. Higher hydrogen ratios resulted in increased porosity as more hydrogen gas contributed to pore formation. Conversely, increasing the total pressure decreased porosity by inhibiting pore expansion. When both hydrogen ratio and total pressure were increased simultaneously, the porosity-increasing effect of the hydrogen ratio was more dominant than the porosity-decreasing effect of the total pressure. Therefore, achieving the desired porosity requires a careful balance between these two parameters.
- Pore diameter and number: Pore diameter increased at higher hydrogen ratios due to increased pore growth. However, increasing the transference velocity and total pressure caused the pore diameter to decrease while the number of detected pores increased. This indicates that higher transference velocities and total pressures promote pore nucleation over pore growth. The internal pressure of the pores increases with hydrogen concentration, while the external pressure applied during the process reduces pore expansion, resulting in smaller pores and higher pore density.
- Pore nucleation and growth: Pore nucleation and growth are affected by several factors. Increasing the hydrogen ratio enhances both pore nucleation and growth, but the experimental results suggest that pore growth is more dominant under these conditions. On the other hand, increasing the transference velocity and total pressure favored nucleation over growth, resulting in a greater number of smaller pores. This balance between pore nucleation and growth is critical for controlling pore size and distribution.
- Process parameter optimization: The optimization of process parameters is essential to controlling the pore structure of lotus-type porous copper. To achieve the smallest pore size, a hydrogen ratio of 25%, a feed rate of 90 mm/min, and a total pressure of 0.4 MPa resulted in a porosity of about 36% and a pore size of 300 µm. By systematically adjusting the process parameters within the ranges of a hydrogen ratio of 25–50%, a transference velocity of 30–90 mm/min, and a total pressure of 0.2–0.4 MPa, it is possible to precisely control the porosity (36–55%) and pore size (300–1500 µm) of lotus-type porous copper. This makes it possible to produce materials with properties tailored to specific requirements.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Nakajima, H. Fabrication, properties and application of porous metals with directional pores. Prog. Mater. Sci. 2007, 52, 1091–1173. [Google Scholar] [CrossRef]
- Shapovalov, V. Porous metals. Mrs. Bull. 1994, 19, 24–28. [Google Scholar] [CrossRef]
- Drenchev, L.; Sobczak, J.; Sobczak, N.; Sha, W.; Malinov, S. A comprehensive model of ordered porosity formation. Acta Mater. 2007, 55, 6459–6471. [Google Scholar] [CrossRef]
- Ogushi, T.; Chiba, H.; Nakajima, H. Development of lotus-type porous copper heat sink. Mater. Trans. 2006, 47, 2240–2247. [Google Scholar] [CrossRef]
- Du, H.; Lu, D.; Qi, J.; Shen, Y.; Yin, L.; Wang, Y.; Xiong, T. Heat dissipation performance of porous copper with elongated cylindrical pores. J. Mater. Sci. Technol. 2014, 30, 934–938. [Google Scholar] [CrossRef]
- Liu, X.; Li, Y.; Zhang, H.; Liu, Y.; Chen, X. Effect of pore structure on heat transfer performance of lotus-type porous copper heat sink. Int. J. Heat Mass Transf. 2019, 144, 118641. [Google Scholar] [CrossRef]
- Alvarez, K.; Hyun, S.K.; Nakano, T.; Umakoshi, Y.; Nakajima, H. In vivo osteocompatibility of lotus-type porous nickel-free stainless steel in rats. Mater. Sci. Eng. C 2009, 29, 1182–1190. [Google Scholar] [CrossRef]
- Kim, S.W.; Son, K.T.; Hyun, S.K. Mechanical behaviors of lotus-type porous Cu/Cu joint soldered by Sn-3.0 Ag-0.5 Cu alloy. Mater. Sci. Eng. A 2021, 822, 141655. [Google Scholar] [CrossRef]
- Joison, R.; Babu, V.; Kannan, A.; Muhammed, A.; Harikrishnan, G. Numerical study on thermal performance of lotus type copper porous heat sink. AIP. Conf. Proc. 2021, 2358, 070005. [Google Scholar] [CrossRef]
- Ogushi, T.; Ide, T. Thermal Solution for Cooling of Electronic Equipment using Lotus-type Porous Copper Heat Sink. In Proceedings of the 2021 Third International Symposium on 3D Power Electronics Integration and Manufacturing (3D-PEIM), Osaka, Japan, 21–23 June 2021; pp. 1–6. [Google Scholar] [CrossRef]
- Prasad, V.R. (Ed.) Porous Fluids: Advances in Fluid Flow and Transport Phenomena in Porous Media. 2021, pp. 85–102. BoD–Books on Demand. Available online: https://www.intechopen.com/books/10284 (accessed on 2 September 2024).
- Terada, M.; Yuki, K.; Unno, N.; Kibushi, R.; Ogushi, T.; Murakami, M.; Nomura, H. Heat transfer performance of two-phase immersion cooling by breathing phenomena with different pore structures of lotus copper. In Proceedings of the 2022 International Conference on Electronics Packaging (ICEP), Sapporo, Japan, 11–14 May 2022; pp. 103–104. [Google Scholar] [CrossRef]
- Kibushi, R.; Yuki, K.; Unno, N.; Ogushi, T.; Murakami, M.; Numata, T.; Nomura, H. Enhancement of the critical heat flux of saturated pool boiling by the breathing phenomenon induced by lotus copper in combination with a grooved heat transfer surface. Int. J. Heat Mass Transf. 2021, 179, 121663. [Google Scholar] [CrossRef]
- Hyun, S.K.; Nakajima, H. Fabrication of porous iron by unidirectional solidification in nitrogen atmosphere. Mater. Trans. 2002, 43, 526–531. [Google Scholar] [CrossRef]
- Hyun, S.K.; Nakajima, H. Effect of solidification velocity on pore morphology of lotus-type porous copper fabricated by unidirectional solidification. Mater. Lett. 2003, 57, 3149–3154. [Google Scholar] [CrossRef]
- Kim, S.W.; Lee, Y.S.; Lee, J.W.; Lim, S.S.; Jung, T.K.; Hyun, S.K. Pore characteristics of Lotus-Type Porous Copper Fabricated by Centrifugal Casting. Met. Mater. Int. 2020, 26, 660–667. [Google Scholar] [CrossRef]
- Park, J.S.; Hyun, S.K.; Suzuki, S.; Nakajima, H. Effect of transference velocity and hydrogen pressure on porosity and pore morphology of lotus-type porous copper fabricated by a continuous casting technique. Acta Mater. 2007, 55, 5646–5654. [Google Scholar] [CrossRef]
- Ikeda, T.; Aoki, T.; Nakazima, H. Fabrication of lotus-type porous stainless steel by continuous zone melting technique. Tetsu--Hagané 2004, 90, 9–16. [Google Scholar] [CrossRef][Green Version]
- Wang, L.; Lee, B.Y.; Wei, P.S.; Quan, M. Parametric Control via the Algebraic Expression of Lotus-Type Pore Shapes in Metals. Materials 2024, 17, 3013. [Google Scholar] [CrossRef]
- Shapovalov, V.I. Formation of ordered gas-solid structures via solidification in metal-hydrogen systems. MRS Online Proc. Libr. 1998, 521, 281. [Google Scholar] [CrossRef]
- Yamamura, S.; Shiota, H.; Murakami, K.; Nakajima, H. Evaluation of porosity in porous copper fabricated by unidirectional solidification under pressurized hydrogen. Mater. Sci. Eng. A 2001, 318, 137–143. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, H.W.; Li, Y.X. Effect of melt superheat on structural uniformity of lotus-type porous metals prepared by unidirectional solidification. Trans. Non. Met. Soc. China 2015, 25, 1004–1010. [Google Scholar] [CrossRef]
- Yuan, L.; Yanxiang, L.; Jiang, W.; Huawei, Z. Evaluation of porosity in lotus-type porous magnesium fabricated by metal/gas eutectic unidirectional solidification. Mater. Sci. Eng. A 2005, 402, 47–54. [Google Scholar] [CrossRef]
- Fromm, E.; Gebhardt, E. Gases and Carbon in Metals; Springer: Berlin, Germany, 1976; p. 747. [Google Scholar]
- Drenchev, L.; Sobczak, J.J. Gasars: A Specific Class of Porous Materials. 2009. pp. 24–45. Available online: https://www.researchgate.net/publication/262009039_GASARS_A_SPECIFIC_CLASS_OF_POROUS_MATERIALS (accessed on 2 September 2024).
- Nakahata, T. Nakajima, Fabrication of lotus-type porous silicon by unidirectional solidification in hydrogen. Mater. Sci. Eng. A 2004, 384, 373–376. [Google Scholar] [CrossRef]
- Song, Q.L.; Jin, Q.L.; Li, Z.J.; Zhou, R. Effects of pore nucleation, growth and solidification mode on pore structure and distribution of lotus type porous copper. Trans. Indian Ins. Met. 2017, 70, 1437–1445. [Google Scholar] [CrossRef]
- Wei, P.S.; Luo, C.W.; Hsieh, I.C. Scaling of inter-pore spacing of lotus-type pores. Phys. Scr. 2023, 98, 085943. [Google Scholar] [CrossRef]
- Campbell, J. Castings Practice: The Ten Rules of Castings; Elsevier: Amsterdam, The Netherlands, 2004; pp. 108–113. [Google Scholar]
- Fisher, J.C. The fracture of liquids. J. Appl. Phys. 1948, 19, 1062. [Google Scholar] [CrossRef]
- Flemings, M.C. Solidification processing. Metal. Mater. Trans. B 1974, 5, 2121–2134. [Google Scholar] [CrossRef]
- Varotsos, P.; Alexopoulos, K. Calculation of diffusion coefficients at any temperature and pressure from a single measurement. I. Self diffusion. Phys. Rev. B 1980, 22, 3130. [Google Scholar] [CrossRef]


| No. | Total Pressure (MPa) | Hydrogen Ratio (%) | Transference Velocity (mm·min−1) | No. | Total Pressure (MPa) | Hydrogen Ratio (%) | Transference Velocity (mm·min−1) | No. | Total Pressure (MPa) | Hydrogen Ratio (%) | Transference Velocity (mm·min−1) |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 0.2 | 25 | 30 | 10. | 0.3 | 25 | 30 | 19. | 0.4 | 25 | 30 |
| 2 | 60 | 11. | 60 | 20. | 60 | ||||||
| 3 | 90 | 12. | 90 | 21. | 90 | ||||||
| 4 | 34 | 30 | 13. | 34 | 30 | 22. | 34 | 30 | |||
| 5 | 60 | 14. | 60 | 23. | 60 | ||||||
| 6 | 90 | 15. | 90 | 24. | 90 | ||||||
| 7 | 50 | 30 | 16. | 50 | 30 | 25. | 50 | 30 | |||
| 8 | 60 | 17. | 60 | 26. | 60 | ||||||
| 9 | 90 | 18. | 90 | 27. | 90 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shin, B.-S.; Hyun, S.-K. Optimizing the Pore Structure of Lotus-Type Porous Copper Fabricated by Continuous Casting. Materials 2024, 17, 5015. https://doi.org/10.3390/ma17205015
Shin B-S, Hyun S-K. Optimizing the Pore Structure of Lotus-Type Porous Copper Fabricated by Continuous Casting. Materials. 2024; 17(20):5015. https://doi.org/10.3390/ma17205015
Chicago/Turabian StyleShin, Byung-Sue, and Soong-Keun Hyun. 2024. "Optimizing the Pore Structure of Lotus-Type Porous Copper Fabricated by Continuous Casting" Materials 17, no. 20: 5015. https://doi.org/10.3390/ma17205015
APA StyleShin, B.-S., & Hyun, S.-K. (2024). Optimizing the Pore Structure of Lotus-Type Porous Copper Fabricated by Continuous Casting. Materials, 17(20), 5015. https://doi.org/10.3390/ma17205015






