Effects of Direct and Pulse Plating on the Co-Deposition of Sn–Ni/TiO2 Composite Coatings
Abstract
1. Introduction
2. Materials and Methods
2.1. Electrodeposition Experiments
2.2. Structural and Morphological Analysis
2.3. Photocatalytic Measurements
2.4. Mechanical and Wear Resistance Properties
2.5. Corrosion Tests
- Before each measurement, the sample was immersed in the 3.5 wt.% NaCl solution for ≈30 min to stabilize the open circuit potential.
- LPR (linear polarization resistance) measurement: The polarization resistance (Rp) was calculated from traces of the polarization curve at ±20 mV from the OCP (scan rate of 0.1660 mV/s), from which the Rp slope was estimated after linear fitting. The Rp represents the degree of the passivation layer’s protection of the alloy surface.
- Tafel polarization curves were recorded at least 200 mV below the OCP to 1.2 V vs. Ag/AgCl, with a potential scan rate of 1 mV/s.
3. Results and Discussion
3.1. TiO2 Nanoparticles’ Incorporation into Sn–Ni Matrix
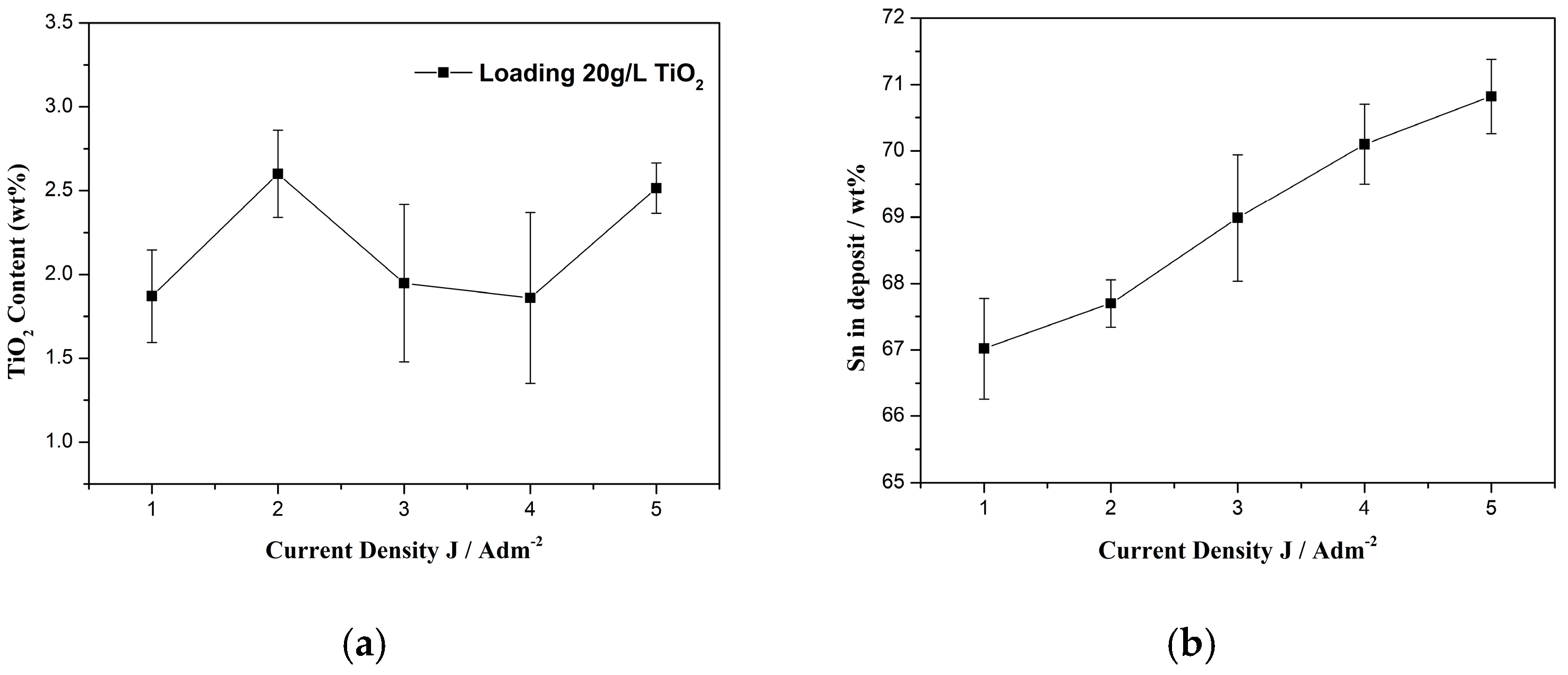
3.1.1. Direct Current Conditions
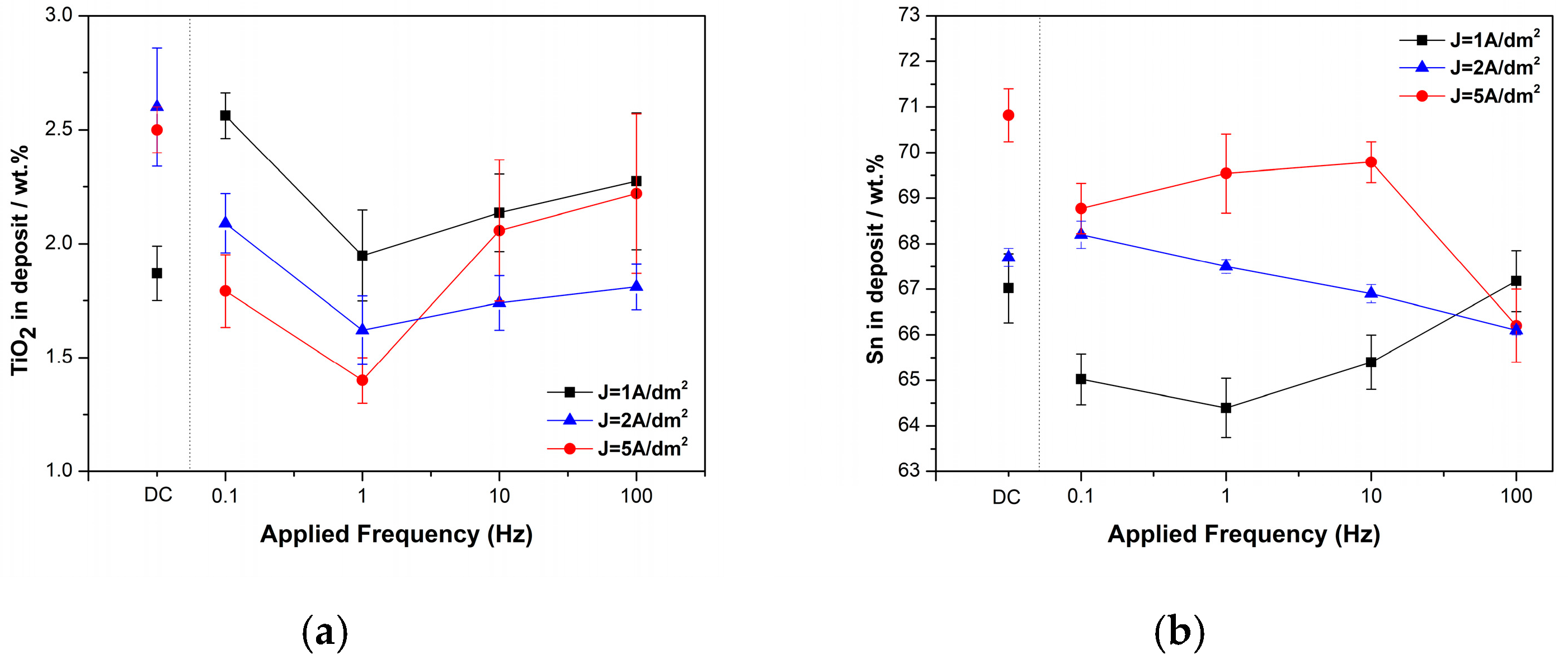
3.1.2. Pulse Current Conditions
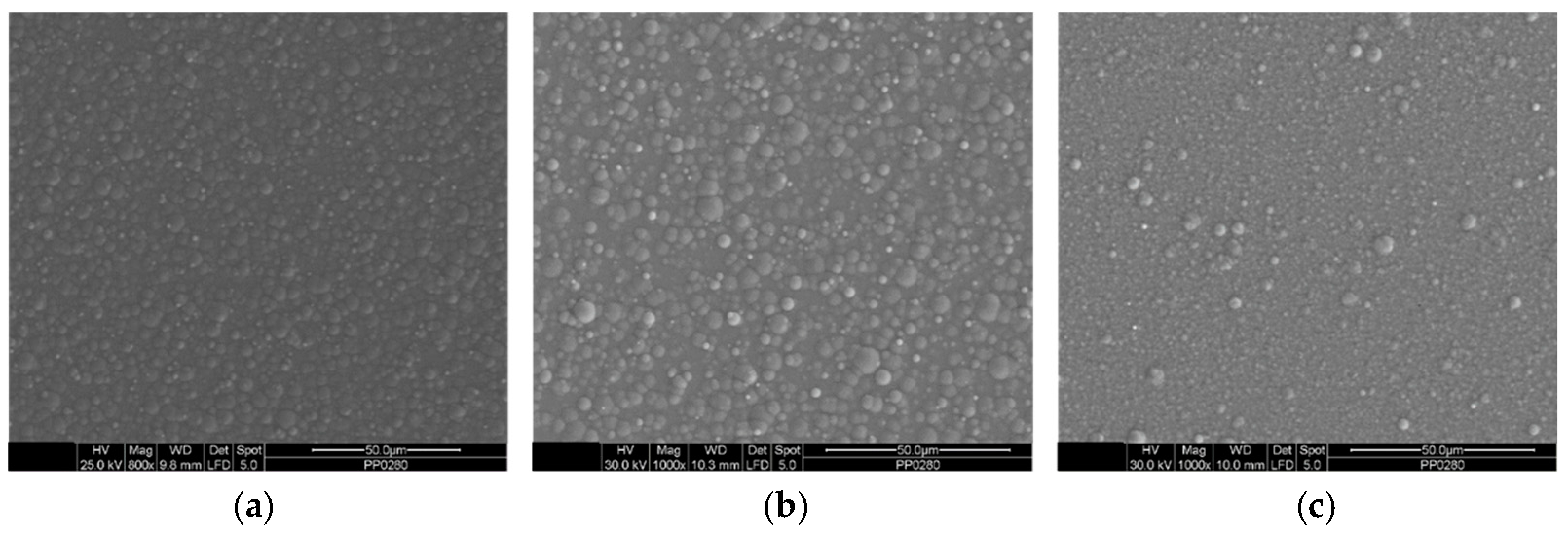
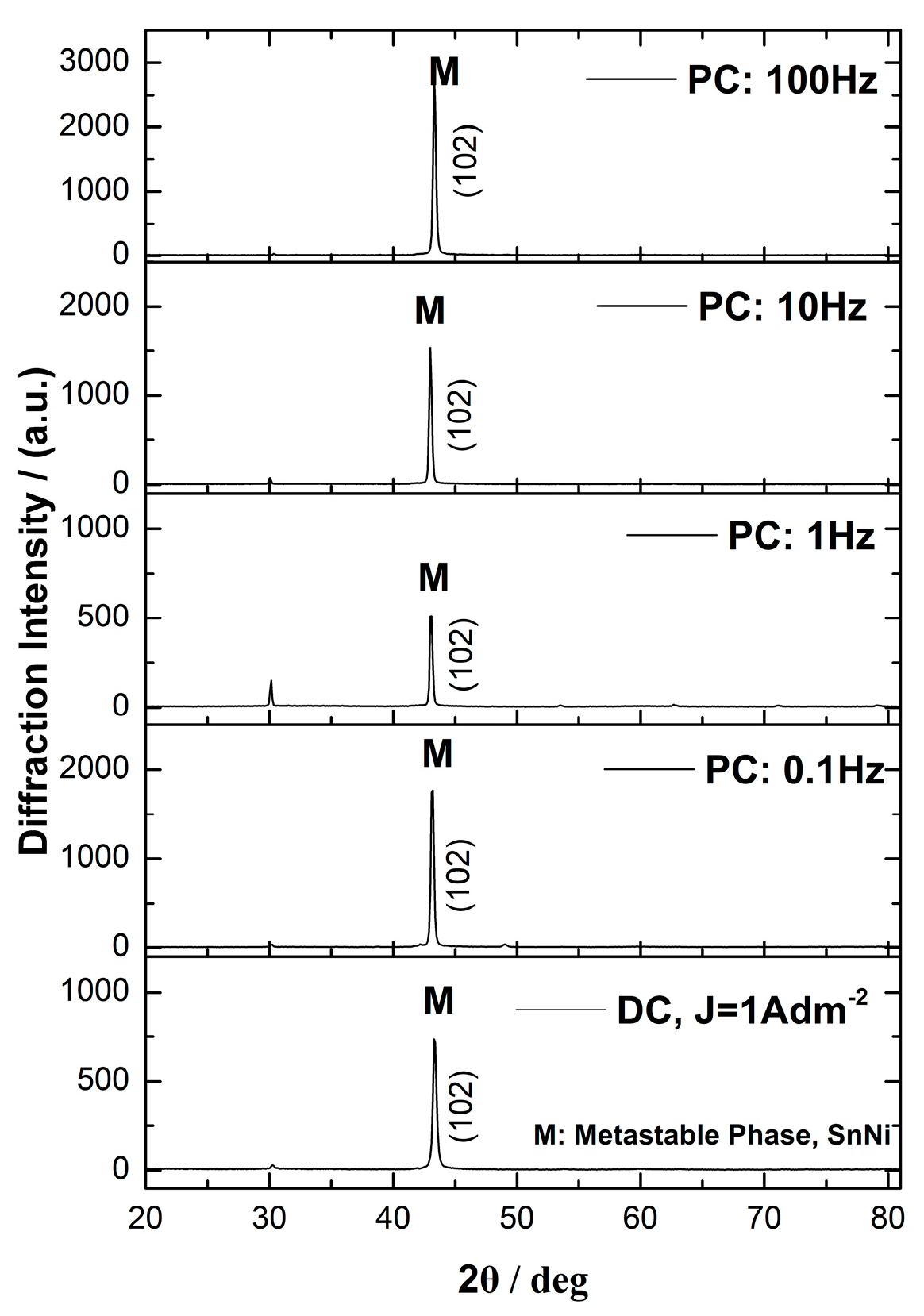
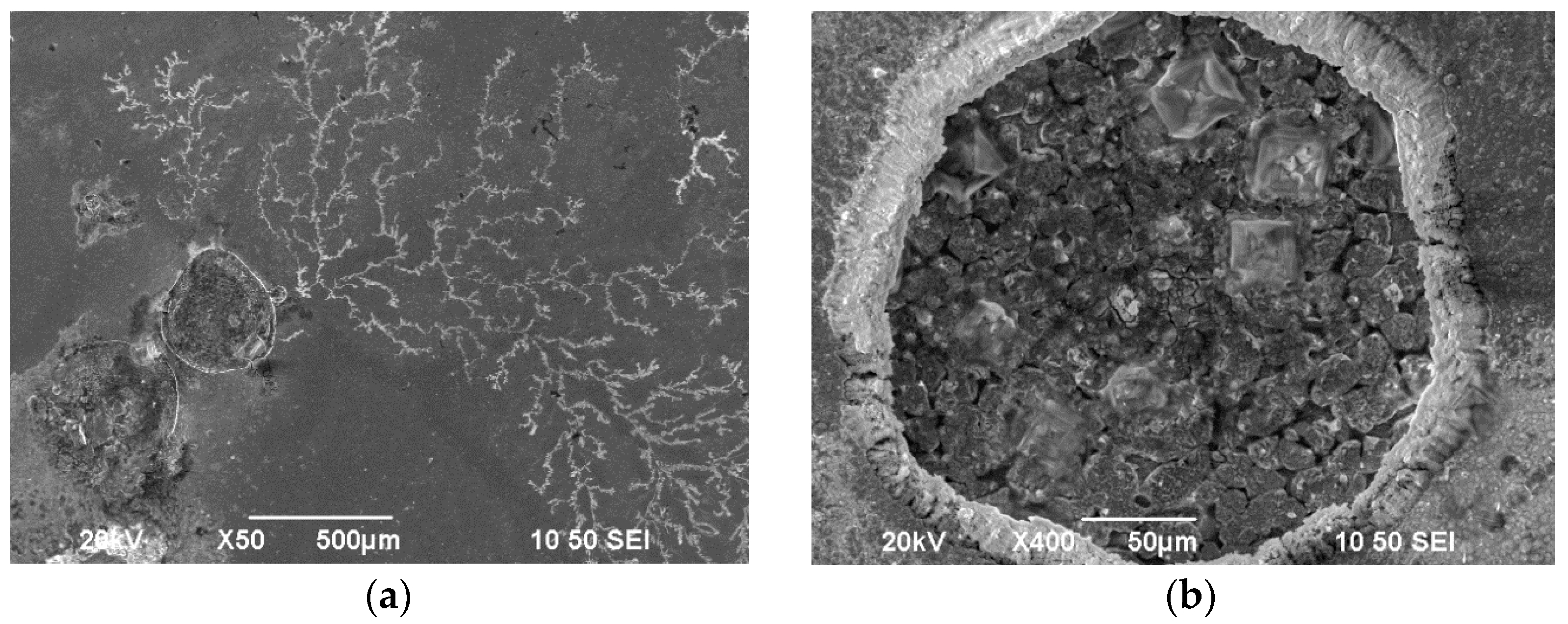
3.2. Surface Morphology and Structural Analysis of Sn–Ni/TiO2 Composite Coatings
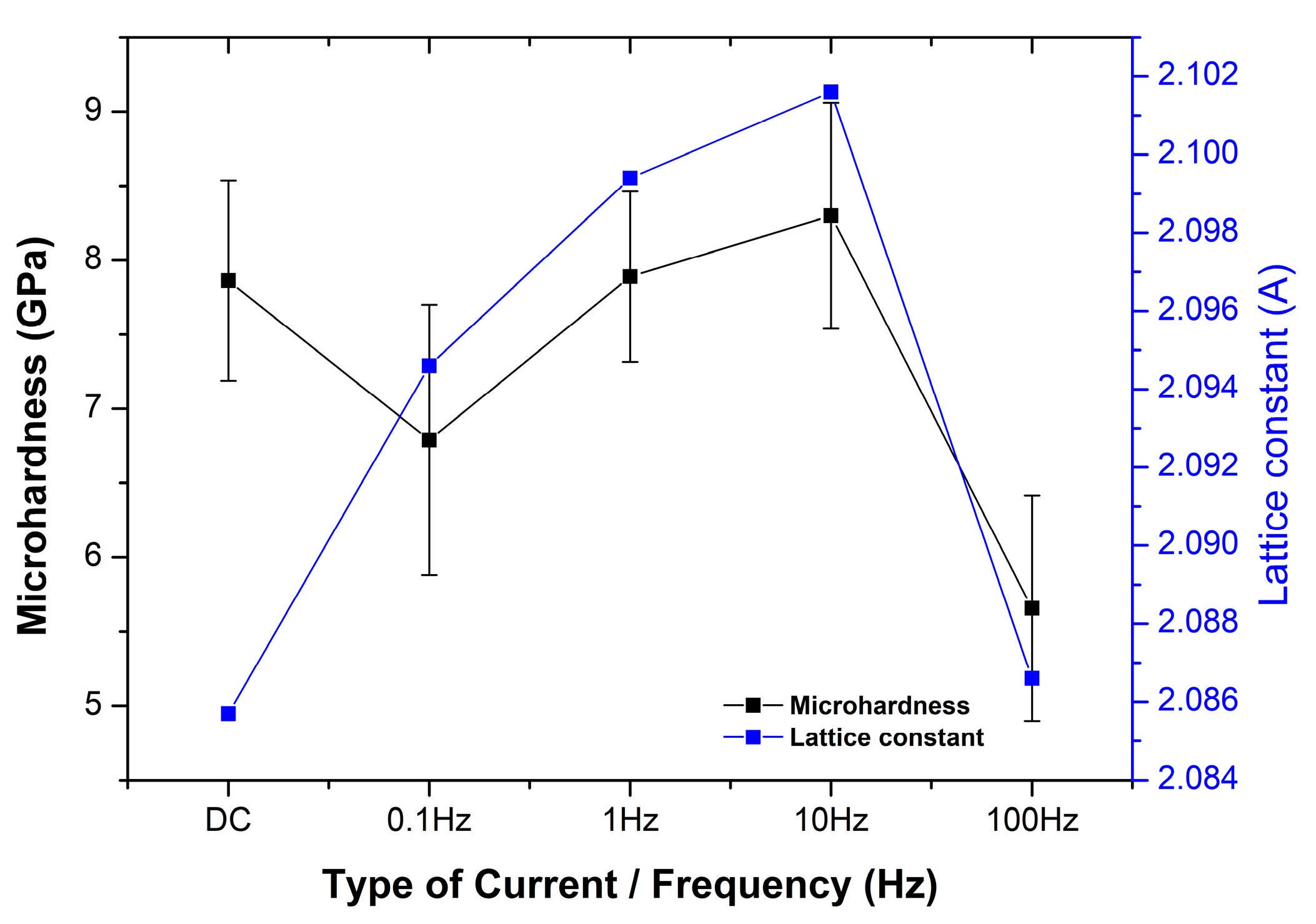
3.3. Microhardness
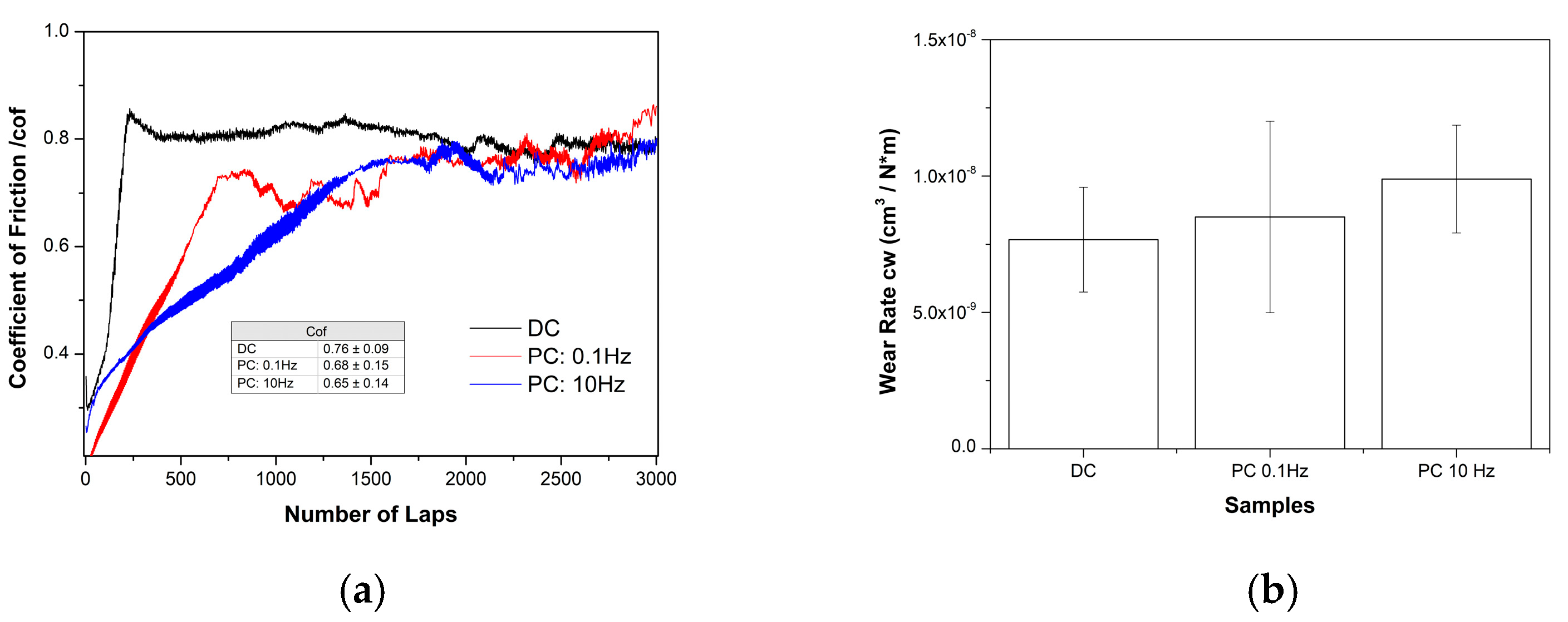
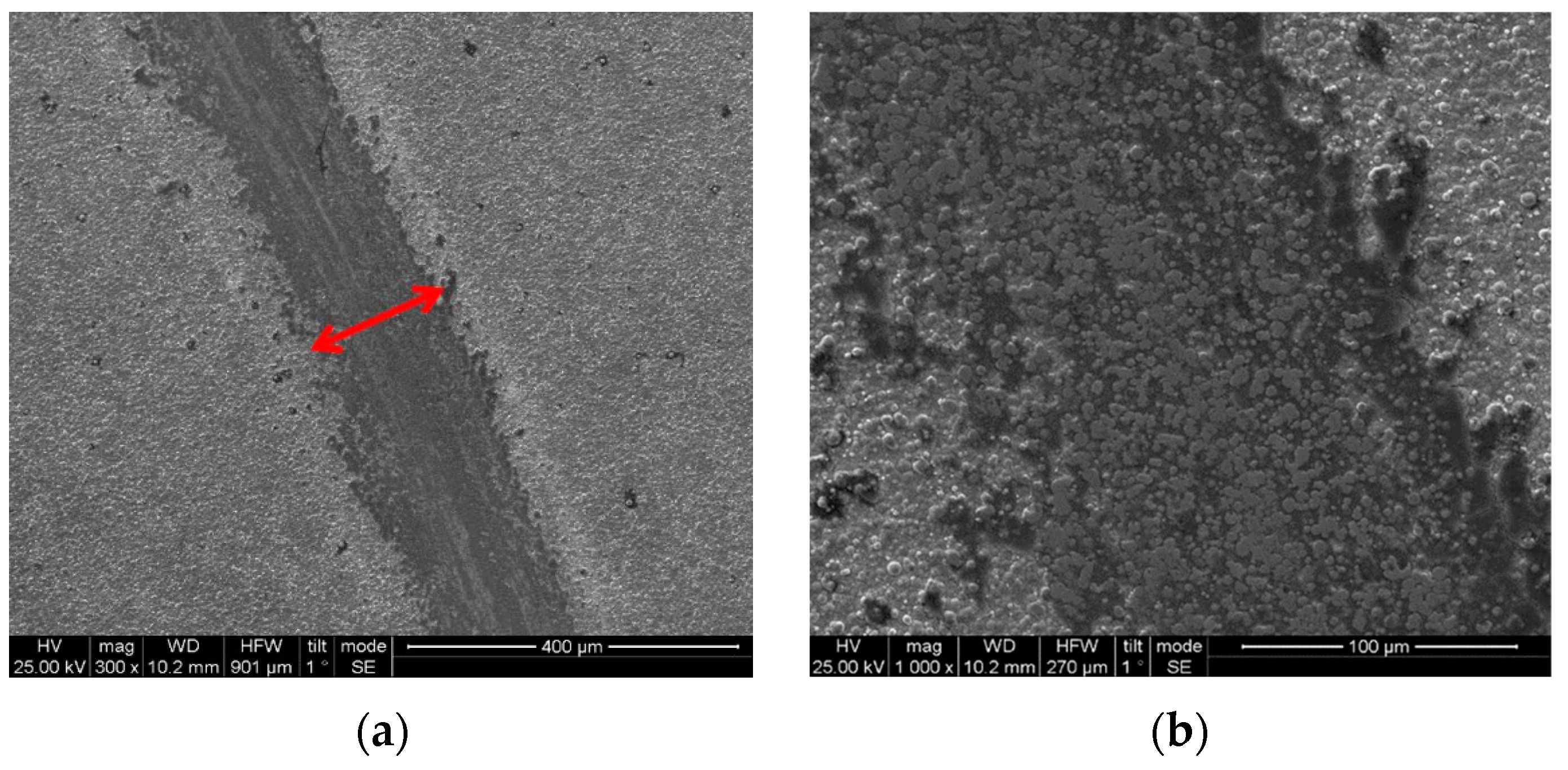
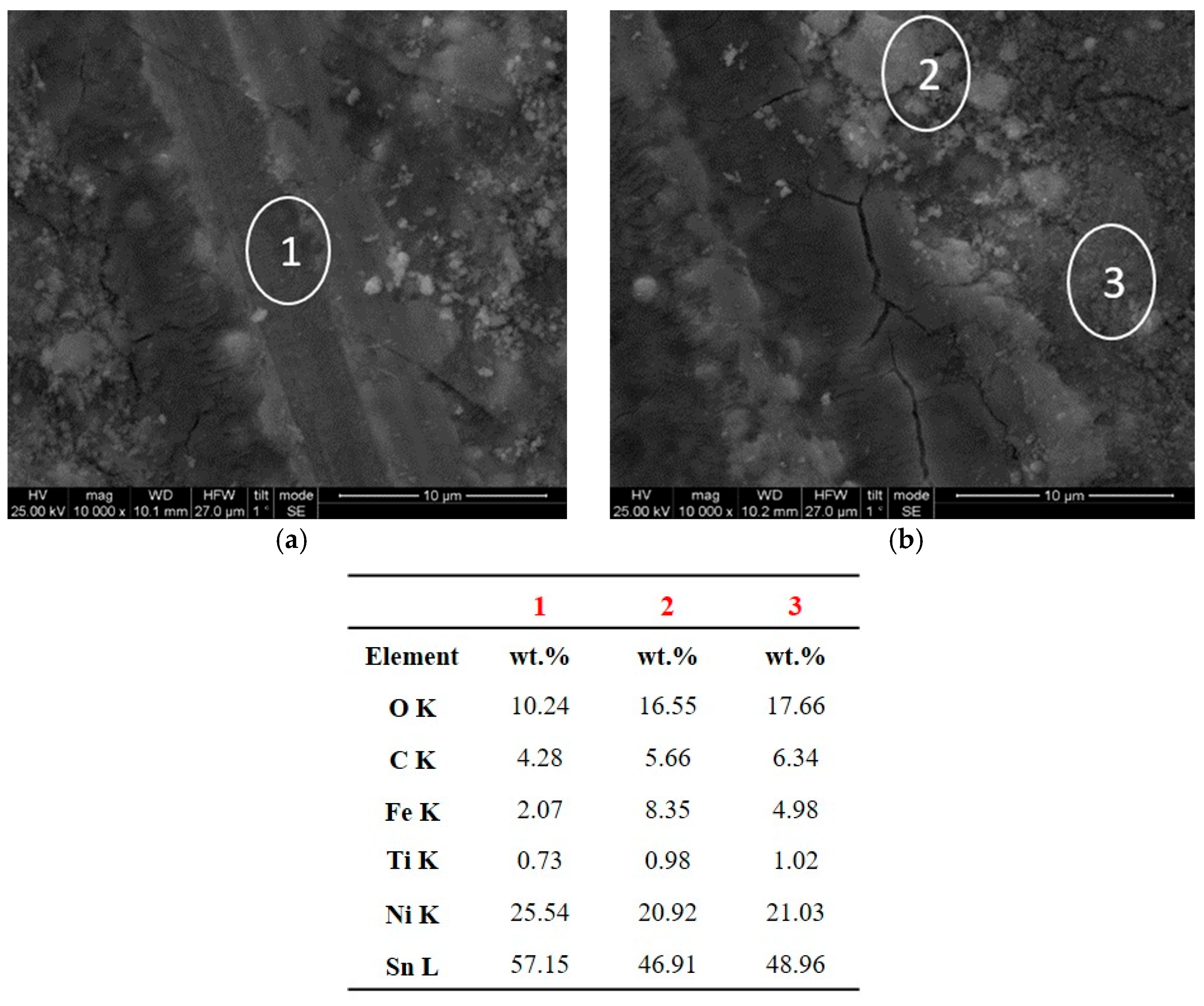
3.4. Wear Behavior of Sn–Ni/TiO2 Composite Coatings
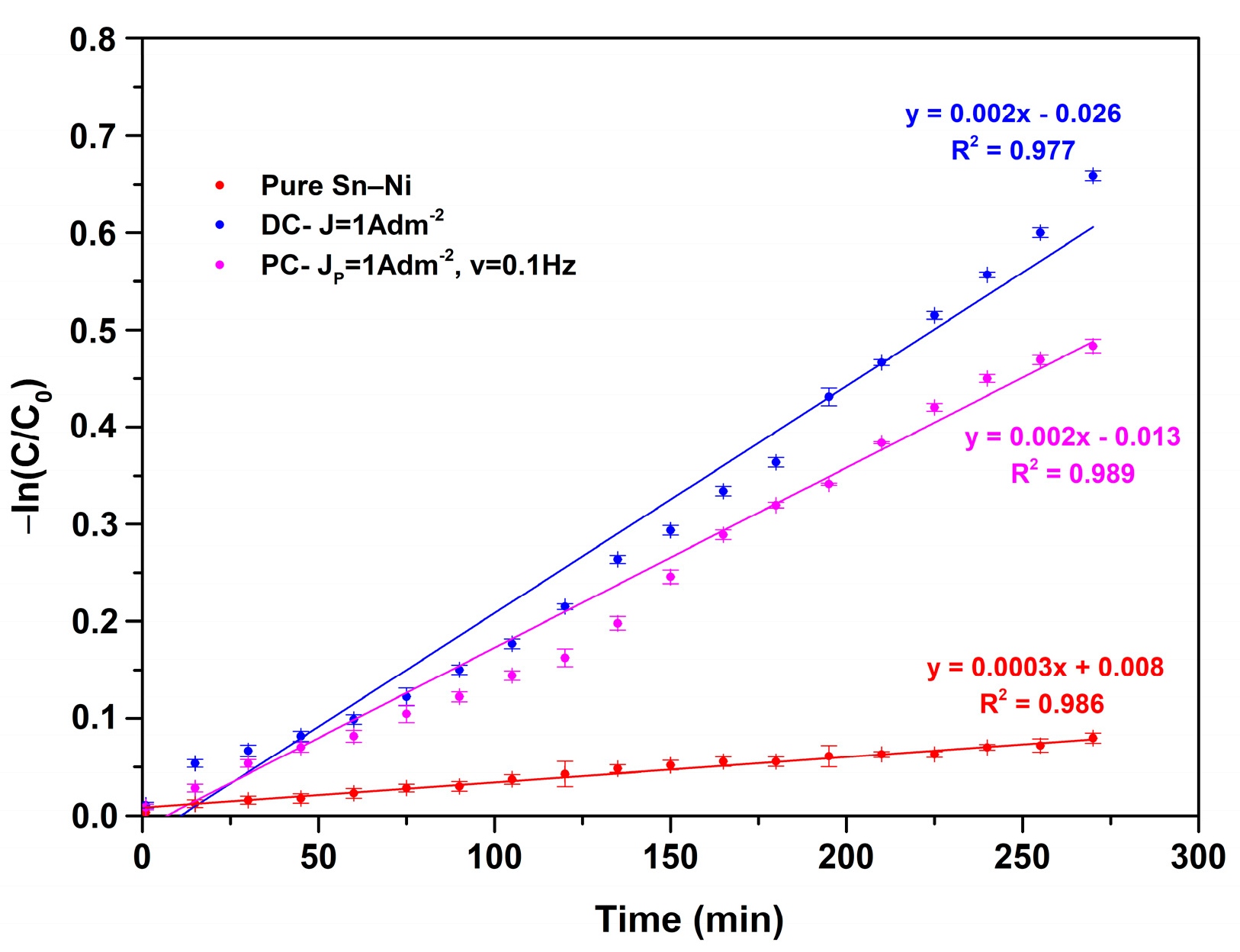
3.5. Photocatalytic Performance of Composite Coatings
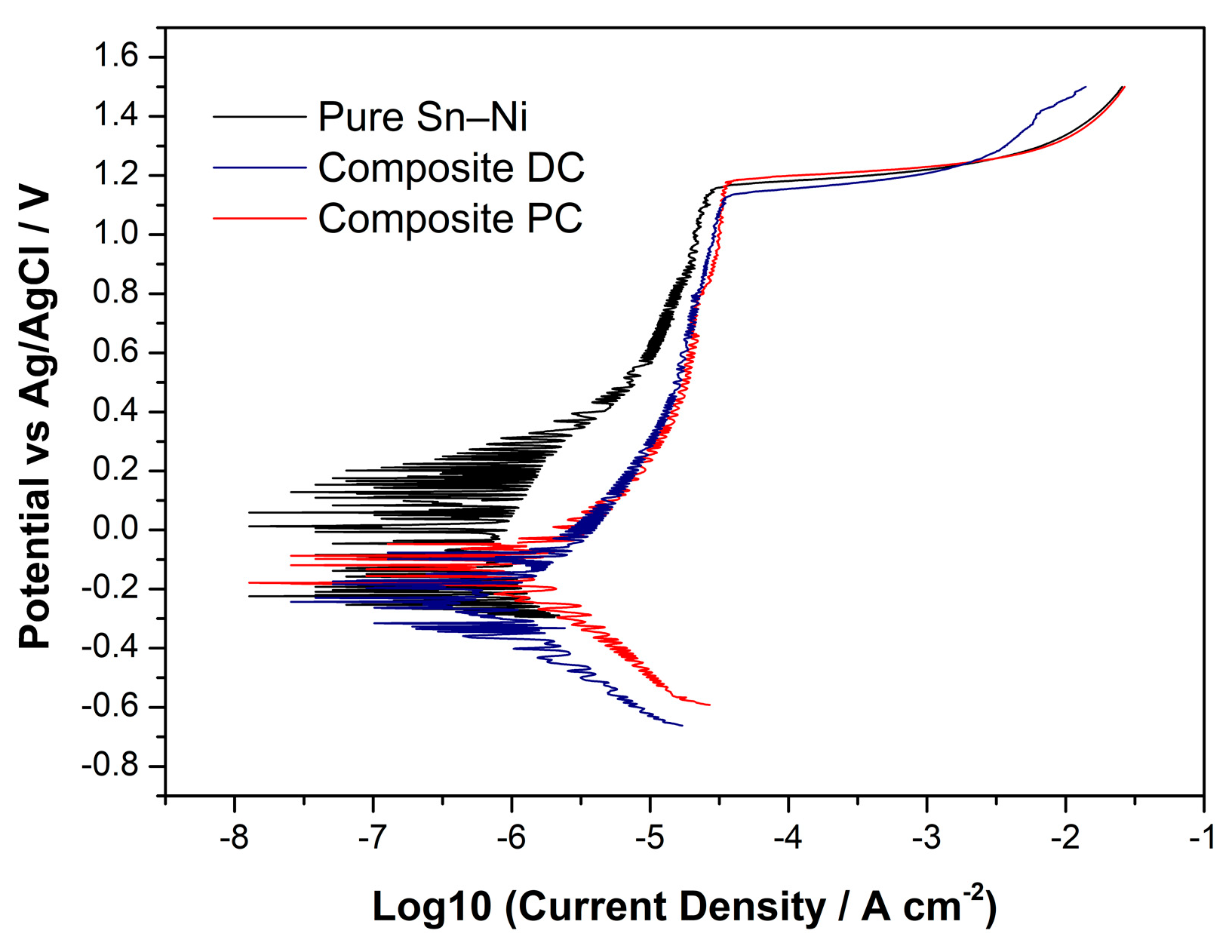
3.6. Corrosion Resistance
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Georgiou, E.P.; Van der Donck, T.; Celis, J.-P. Electrodeposition and structural characteristics of intermetallic nickel-tin based coatings. Trans. IMF 2017, 95, 301–307. [Google Scholar] [CrossRef]
- Jalota, S.K. Tin–nickel alloy plating. Met. Finish. 1999, 97, 319–322. [Google Scholar] [CrossRef]
- Jiang, D.; Ma, X.; Fu, Y. High-performance Sn–Ni alloy nanorod electrodes prepared by electrodeposition for lithium ion rechargeable batteries. J. Appl. Electrochem. 2012, 42, 555–559. [Google Scholar] [CrossRef]
- Rosoiu, S.P.; Pantazi, A.G.; Petica, A.; Cojocaru, A.; Costovici, S.; Zanella, C.; Visan, T.; Anicai, L.; Enachescu, M. Comparative Study of Ni-Sn Alloys Electrodeposited from Choline Chloride-Based Ionic Liquids in Direct and Pulsed Current. Coatings 2019, 9, 801. [Google Scholar] [CrossRef]
- Clarke, M.; Dutta, P.K. The heat of formation of the single-phase NiSn electrodeposit. J. Phys. D Appl. Phys. 1971, 4, 1652–1656. [Google Scholar] [CrossRef]
- Sajjadnejad, M.; Haghshenas, S.M.S.; Badr, P.; Setoudeh, N.; Hosseinpour, S. Wear and tribological characterization of nickel matrix electrodeposited composites: A review. Wear 2021, 486–487, 204098. [Google Scholar] [CrossRef]
- Renganathan, P.; Marimuthu, S.; Kanniah, R.; Karthikeyan, V.; Sundaram, G.A. Synthesis and characterizations of Ni-SnO2-TiO2 nanocomposite for photocatalytic application. Results Chem. 2022, 4, 100557. [Google Scholar] [CrossRef]
- Rosolymou, E.; Spanou, S.; Zanella, C.; Tsoukleris, D.S.; Köhler, S.; Leisner, P.; Pavlatou, E.A. Electrodeposition of photocatalytic Sn–Ni matrix composite coatings embedded with doped TiO2 particles. Coatings 2020, 10, 775. [Google Scholar] [CrossRef]
- Spanou, S.; Siokou, A.; Kontos, A.; Vaenas, N.; Falaras, P.; Pavlatou, E. Self cleaning behaviour of Ni/nano-TiO2 metal matrix composites. Electrochim. Acta 2013, 105, 324–332. [Google Scholar] [CrossRef]
- Benea, L.; Danaila, E.; Celis, J.-P. Influence of electro-co-deposition parameters on nano-TiO2 inclusion into nickel matrix and properties characterization of nanocomposite coatings obtained. Mater. Sci. Eng. A 2014, 610, 106–115. [Google Scholar] [CrossRef]
- Thiemig, D.; Bund, A. Characterization of electrodeposited Ni–TiO2 nanocomposite coatings. Surf. Coat. Technol. 2008, 202, 2976–2984. [Google Scholar] [CrossRef]
- Wang, X.; Wei, S.; Wang, X.; Chang, K.; Yang, H.; Wang, Y. Synergetic Improvements in Surface Hydrophobicity and Wear Resistance of TC4 Materials via the Combination of Rapid Heating and EtOH Quenching Methods. J. Alloys Compd. 2024, 970, 172582. [Google Scholar] [CrossRef]
- Kassalia, M.-E.; Chorianopoulos, N.; Nychas, G.-J.; Pavlatou, E.A. Investigation of the Photoinduced Antimicrobial Properties of N-Doped TiO2 Nanoparticles under Visible-Light Irradiation on Salmonella Typhimurium Biofilm. Appl. Sci. 2023, 13, 4498. [Google Scholar] [CrossRef]
- Pyanko, A.V.; Makarova, I.V.; Kharitonov, D.S.; Makeeva, I.S.; Sergievich, D.S.; Chernik, A.A. Physicochemical and Biocidal Properties of Nickel–Tin and Nickel–Tin—Titania Coatings. Prot. Met. Phys. Chem. Surf. 2021, 57, 88–95. [Google Scholar] [CrossRef]
- Pyanko, A.V.; Makarova, I.V.; Kharitonov, D.S.; Makeeva, I.S.; Alisienok, O.A.; Chernik, A.A. Tin–Nickel–Titania Composite Coatings. Inorg. Mater. 2019, 55, 568–575. [Google Scholar] [CrossRef]
- Møller, P.; Rasmussen, J.; Köhler, S.; Nielsen, D.L. Eltroplated Tin-Nickel Coatings as a Replacement for Nickel to Eliminate Nickel Dermatitis. Nasf Surf. Technol. White Pap. 2013, 78, 15–24. [Google Scholar]
- Jiménez, H.; Gil, L.; Staia, M.H.; Puchi-Cabrera, E.S. Effect of deposition parameters on adhesion, hardness and wear resistance of Sn–Ni electrolytic coatings. Surf. Coat. Technol. 2008, 202, 2072–2079. [Google Scholar] [CrossRef]
- Wan, C.; Liu, X.; Ye, J. Tailorable deposition of Sn Ni alloy from a pyrophosphate bath with an adjustable Sn:Ni molar ratio. Surf. Coat. Technol. 2019, 369, 244–251. [Google Scholar] [CrossRef]
- Stichleutner, S.; Lak, G.B.; Kuzmann, E.; Chisholm, C.U.; El-Sharif, M.; Homonnay, Z.; Sziráki, L. Mössbauer and XRD study of pulse plated Sn-Fe, Sn-Ni and Sn-Ni-Fe electrodeposited alloys. Hyperfine Interact. 2014, 226, 15–25. [Google Scholar] [CrossRef]
- Uysal, M.; Cetinkaya, T.; Alp, A.; Akbulut, H. Active and inactive buffering effect on the electrochemical behavior of Sn–Ni/MWCNT composite anodes prepared by pulse electrodeposition for lithium-ion batteries. J. Alloys Compd. 2015, 645, 235–242. [Google Scholar] [CrossRef]
- Lajevardi, S.A.; Shahrabi, T. Effects of pulse electrodeposition parameters on the properties of Ni–TiO2 nanocomposite coatings. Appl. Surf. Sci. 2010, 256, 6775–6781. [Google Scholar] [CrossRef]
- Gyftou, P.; Pavlatou, E.; Spyrellis, N. Effect of pulse electrodeposition parameters on the properties of Ni/nano-SiC composites. Appl. Surf. Sci. 2008, 254, 5910–5916. [Google Scholar] [CrossRef]
- Stojak, J.L.; Talbot, J.B. Investigation of Electrocodeposition Using a Rotating Cylinder Electrode. J. Electrochem. Soc. 1999, 146, 4504–4513. [Google Scholar] [CrossRef]
- Cuthbertson, J.W.; Parkinson, N.; Rooksby, H.P. Electrodeposition of Tin-Nickel Alloy Plate from Chloride-Fluoride Electrolytes. J. Electrochem. Soc. 1953, 100, 107. [Google Scholar] [CrossRef]
- Beltowska-Lehman, E.; Subiah, J. Kinetics of Electrodeposition of Ni-Sn Alloy Deposits from an Acid Chloride Bath. Surf. Technol. 1982, 15, 191–198. [Google Scholar] [CrossRef]
- Yousefi, E.; Sharafi, S.; Irannejad, A. Microstructure, tribological behavior and magnetic properties of Fe−Ni−TiO2 composite coatings synthesized via pulse frequency variation. Trans. Nonferrous Met. Soc. China 2021, 31, 3800–3813. [Google Scholar] [CrossRef]
- Ibl, N. Some theoretical aspects of pulse electrolysis. Surf. Technol. 1980, 10, 81–104. [Google Scholar] [CrossRef]
- Yousefi, E.; Sharafi, S.; Irannejad, A. The structural, magnetic, and tribological properties of nanocrystalline Fe-Ni permalloy and Fe-Ni-TiO2 composite coatings produced by pulse electro co-deposition. J. Alloys Compd. 2018, 753, 308–319. [Google Scholar] [CrossRef]
- Goldasteh, H.; Rastegari, S. The influence of pulse plating parameters on structure and properties of Ni–W–TiO2 nanocomposite coatings. Surf. Coat. Technol. 2014, 259, 393–400. [Google Scholar] [CrossRef]
- Spanou, S.; Pavlatou, E.A. Pulse electrodeposition of Ni/nano-TiO2 composites: Effect of pulse frequency on deposits properties. J. Appl. Electrochem. 2010, 40, 1325–1336. [Google Scholar] [CrossRef]
- Landolt, D.; Marlot, A. Microstructure and composition of pulse-plated metals and alloys. Surf. Coat. Technol. 2003, 169–170, 8–13. [Google Scholar] [CrossRef]
- Chen, C.J.; Wan, C.C. A Study of the Current Efficiency Decrease Accompanying Short Pulse Time for Pulse Plating. J. Electrochem. Soc. 1989, 136, 2850–2855. [Google Scholar] [CrossRef]
- Gómez, M.J.; Llorente, V.B.; Lacconi, G.I.; Franceschini, E.A. Facile electrodeposition of NiCo-TiO2 composite coatings for enhanced hydrogen evolution reaction. J. Electroanal. Chem. 2021, 895, 115453. [Google Scholar] [CrossRef]
- Allahyarzadeh, M.; Ashrafi, A.; Golgoon, A.; Roozbehani, B. Effect of pulse plating parameters on the structure and properties of electrodeposited Ni Mo films. Mater. Chem. Phys. 2016, 175, 215–222. [Google Scholar] [CrossRef]
- Watanabe, T. Nano-Plating, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004; pp. 630–640. [Google Scholar]
- Gamburg, Y.D.; Zangari, G. Influence of Diffusion on the Rate of Electrochemical Process. In Theory and Practice of Metal Electrodeposition; Springer: New York, NY, USA, 2011; pp. 77–96. [Google Scholar] [CrossRef]
- Rooksby, H.P. An X-ray Study of Tin-Nickel Electrodeposits. Trans. IMF 1950, 27, 153–169. [Google Scholar] [CrossRef]
- He, Z.; Zhou, Y.; Wang, Y.; Guo, P.; Jiang, W.; Yao, C.; Shu, X. Preparation and properties of Ni-W-P-TiO2 nanocomposite coatings developed by a sol-enhanced electroplating method. Chin. J. Chem. Eng. 2022, 44, 369–376. [Google Scholar] [CrossRef]
- Antler, M.; Drozdowicz, M.H.; Hornig, C.F. ChemInform Abstract: The corrosion resistance of worn tin-nickel and gold-coated tin-nickel alloy electrodeposits. J. Electrochem. Soc. 1977, 124, 1069–1075. [Google Scholar] [CrossRef]
- Georgiou, E.; Van der Donck, T.; Peeters, M.; Drees, D.; Celis, J.-P. Proposed method to examine the effect of cycling temperatures on friction illustrated with intermetallic Ni-Sn coatings sliding against corundum. Wear 2016, 368–369, 453–460. [Google Scholar] [CrossRef]
- Vadivelan, V.; Kumar, K.V. Equilibrium, kinetics, mechanism, and process design for the sorption of methylene blue onto rice husk. J. Colloid Interface Sci. 2005, 286, 90–100. [Google Scholar] [CrossRef]
- Konstantinou, I.K.; Albanis, T.A. TiO2-assisted photocatalytic degradation of azo dyes in aqueous solution: Kinetic and mechanistic investigations: A review. Appl. Catal. B Environ. 2004, 49, 1–14. [Google Scholar] [CrossRef]
- De Tacconi, N.R.; Boyles, C.A.; Rajeshwar, K. Surface Morphology/Composition and Photoelectrochemical Behavior of Metal−Semiconductor Composite Films. Langmuir 2000, 16, 5665–5672. [Google Scholar] [CrossRef]
- Mallakpour, S.; Nikkhoo, E. Surface Modification of Nano-TiO2 with Trimellitylimido-Amino Acid-Based Diacids for Preventing Aggregation of Nanoparticles. Adv. Powder Technol. 2014, 25, 348–353. [Google Scholar] [CrossRef]
- Cervantes-Avilés, P.; Piñas, N.C.; Ida, J.; Cuevas-Rodríguez, G. Influence of Wastewater Type on the Impact Generated by TiO2 Nanoparticles on the Oxygen Uptake Rate in Activated Sludge Process. J. Environ. Manag. 2017, 190, 35–44. [Google Scholar] [CrossRef]
- Clarke, M.; Elbourne, R.G.P. The Passivation Potential of “65/35” Electrodeposited Sn-Ni Alloy. Corros. Sci. 1968, 8, 39. [Google Scholar] [CrossRef]
- Clarke, M.; Elbourne, R. The peculiarly low passivation potentials of some intermetallic tin compounds. Electrochim. Acta 1971, 16, 1949–1954. [Google Scholar] [CrossRef]
- Tang, Y.; Zuo, Y.; Wang, J.; Zhao, X.; Niu, B.; Lin, B. The metastable pitting potential and its relation to the pitting potential for four materials in chloride solutions. Corros. Sci. 2014, 80, 111–119. [Google Scholar] [CrossRef]
- Tian, W.; Du, N.; Li, S.; Chen, S.; Wu, Q. Metastable pitting corrosion of 304 stainless steel in 3.5% NaCl solution. Corros. Sci. 2014, 85, 372–379. [Google Scholar] [CrossRef]
- Jones, D.A. Principles and Prevention of Corrosion, 2nd ed.; Pearson: London, UK, 1996; p. 185. [Google Scholar]
- Thomas, J.H.; Sharma, S.P. ESCA Study of the Passive Layer on Sn-Ni Alloy. J. Vac. Sci. Technol. 1977, 14, 1168–1172. [Google Scholar] [CrossRef]
- Katamipour, A.; Farzam, M.; Danaee, I. Effects of sonication on anticorrosive and mechanical properties of electrodeposited Ni–Zn–TiO2 nanocomposite coatings. Surf. Coat. Technol. 2014, 254, 358–363. [Google Scholar] [CrossRef]
- Pant, B.; Park, M.; Park, S.-J. Recent Advances in TiO2 Films Prepared by Sol-Gel Methods for Photocatalytic Degradation of Organic Pollutants and Antibacterial Activities. Coatings 2019, 9, 613. [Google Scholar] [CrossRef]










| Electrodeposition Parameters | Experimental Conditions |
|---|---|
| T: | 70 ± 1 (°C) |
| pH: | 4.2–4.4 |
| Agitation: | Magnetic stirring 250 rpm |
| Rotation disc electrode: | 600 rpm |
| Current density (J): | 1 to 5 Adm−2 |
| Duty Cycle (d.c) = Ton/(Ton + Toff): | 50% |
| Frequency of pulses (ν): | 0.1, 1, 10, 100 Hz |
| Pulse on/off time (s): | 5, 0.5, 0.05, 0.005 s |
| Substrate: | Brass disc (diameter 25 mm) |
| Tribological Parameters | Experimental Conditions |
|---|---|
| Load | 2Ν |
| Ball | 100 Cr6 (d = 6 mm) |
| Sliding cycles | 3000 Laps |
| Ball’s linear velocity | 0.1 m/s |
| Ambient conditions | T = 25 °C, Humidity: 42–50% |
| Electrodeposition Conditions | Roughness (μm) |
|---|---|
| DC | 0.16 ± 0.001 |
| PC—0.1 Hz | 0.21 ± 0.05 |
| PC—1 Hz | 0.25 ± 0.04 |
| PC—10 Hz | 0.29 ± 0.03 |
| PC—100 Hz | 0.23± 0.01 |
| Electrodeposition Conditions | Lattice Constant (Å) | Average Grain Size (nm) |
|---|---|---|
| DC | 2.0857 | 36 |
| PC—0.1 Hz | 2.0946 | 32 |
| PC—1 Hz | 2.0994 | 34 |
| PC—10 Hz | 2.1016 | 32 |
| PC—100 Hz | 2.0866 | 32 |
| Sample | OCP (V) | Jpass (A cm−2) | Rp (Ohm/cm2) |
|---|---|---|---|
| Sn–Ni (Pure) | −0.037 | 2.84 × 10−5 | 0.56 × 106 |
| Sn–Ni/TiO2—DC | −0.135 | 3.48 × 10−5 | 0.1 × 106 |
| Sn–Ni/TiO2—PC 0.1 Hz | −0.08 | 3.43 × 10−5 | 0.38 × 105 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rosolymou, E.; Karantonis, A.; Pavlatou, E.A. Effects of Direct and Pulse Plating on the Co-Deposition of Sn–Ni/TiO2 Composite Coatings. Materials 2024, 17, 392. https://doi.org/10.3390/ma17020392
Rosolymou E, Karantonis A, Pavlatou EA. Effects of Direct and Pulse Plating on the Co-Deposition of Sn–Ni/TiO2 Composite Coatings. Materials. 2024; 17(2):392. https://doi.org/10.3390/ma17020392
Chicago/Turabian StyleRosolymou, Eleni, Antonis Karantonis, and Evangelia A. Pavlatou. 2024. "Effects of Direct and Pulse Plating on the Co-Deposition of Sn–Ni/TiO2 Composite Coatings" Materials 17, no. 2: 392. https://doi.org/10.3390/ma17020392
APA StyleRosolymou, E., Karantonis, A., & Pavlatou, E. A. (2024). Effects of Direct and Pulse Plating on the Co-Deposition of Sn–Ni/TiO2 Composite Coatings. Materials, 17(2), 392. https://doi.org/10.3390/ma17020392








