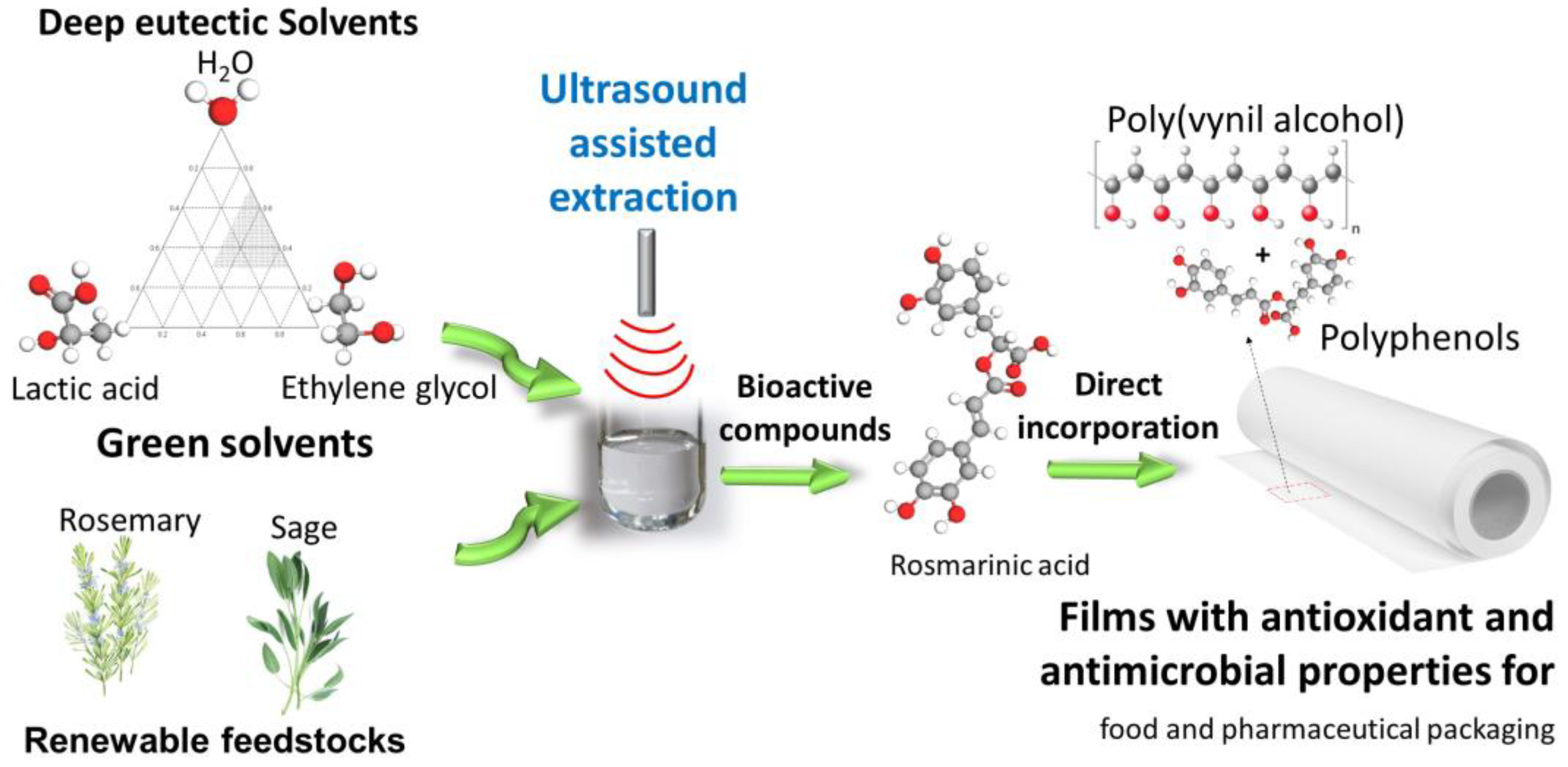
Ecofriendly Preparation of Rosmarinic Acid-poly(vinyl alcohol) Biofilms Using NADES/DES, Ultrasounds and Optimization via a Mixture-Process Design Strategy
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
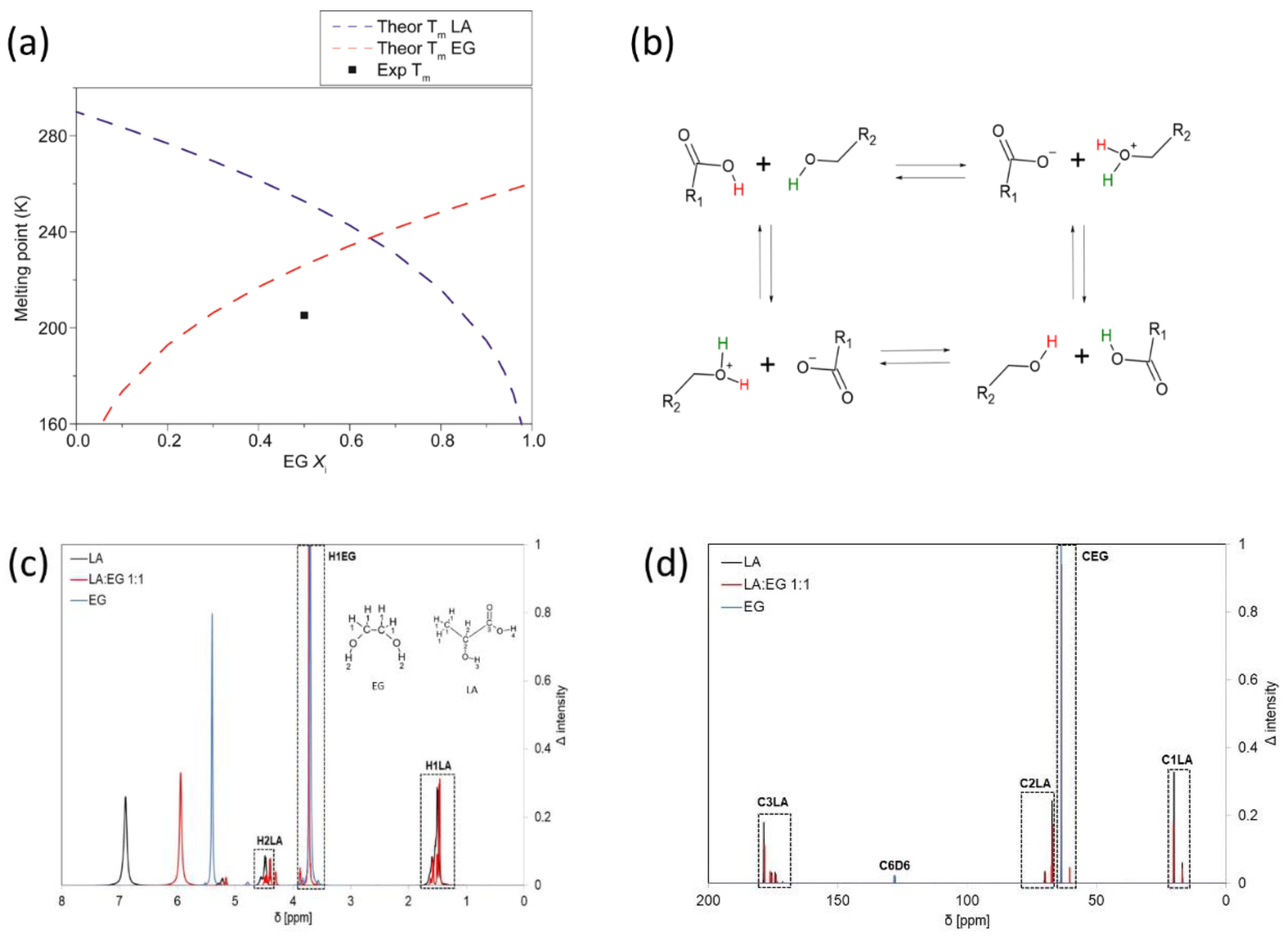
2.2. Solvent Preparation and Characterization
2.3. Extraction Procedure
2.4. Mixture-Process and D-Optimal Design
2.5. HPLC Analysis
2.6. PVA Films Preparation
3. Results and Discussion
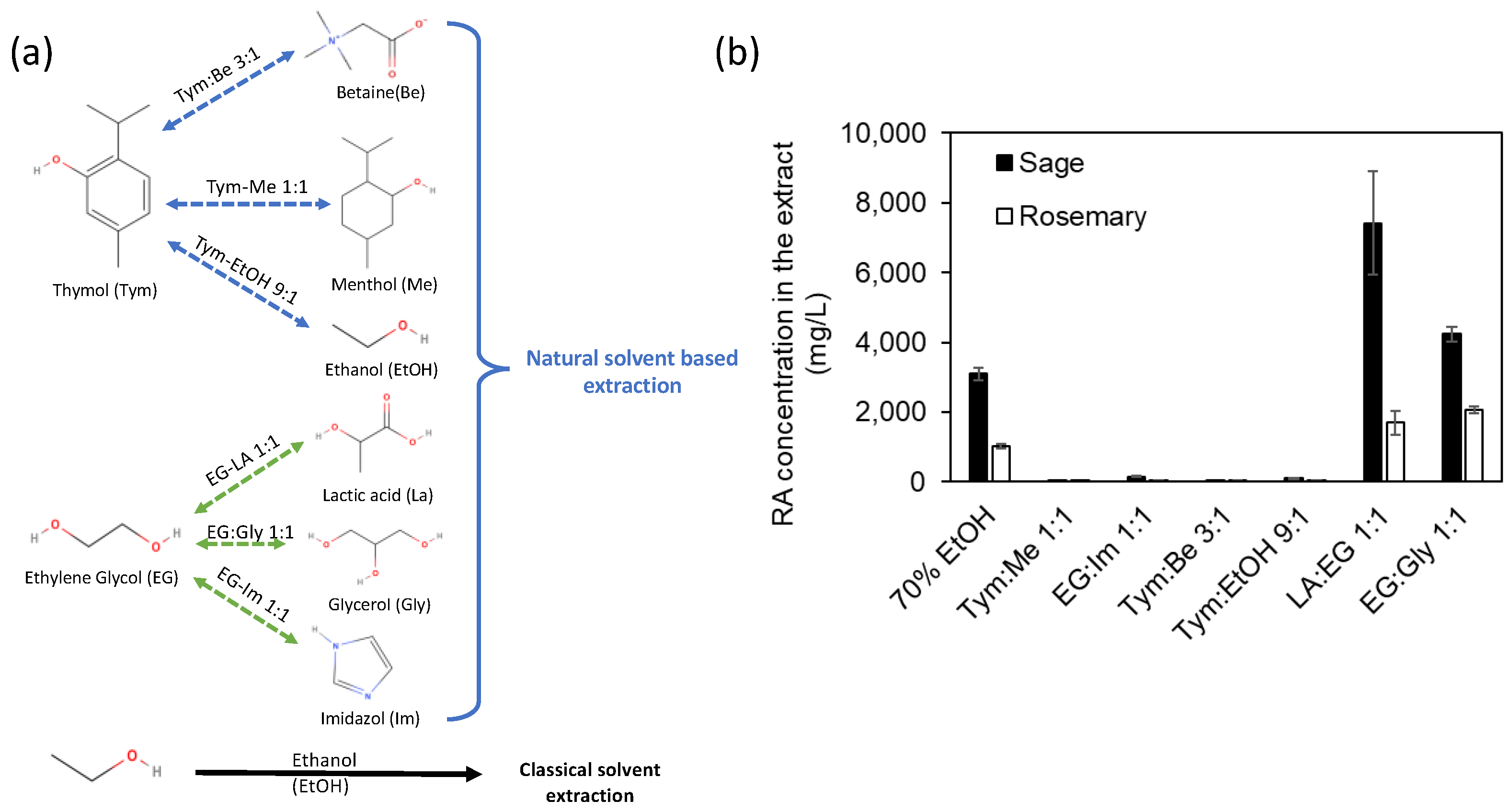
3.1. Screening Step
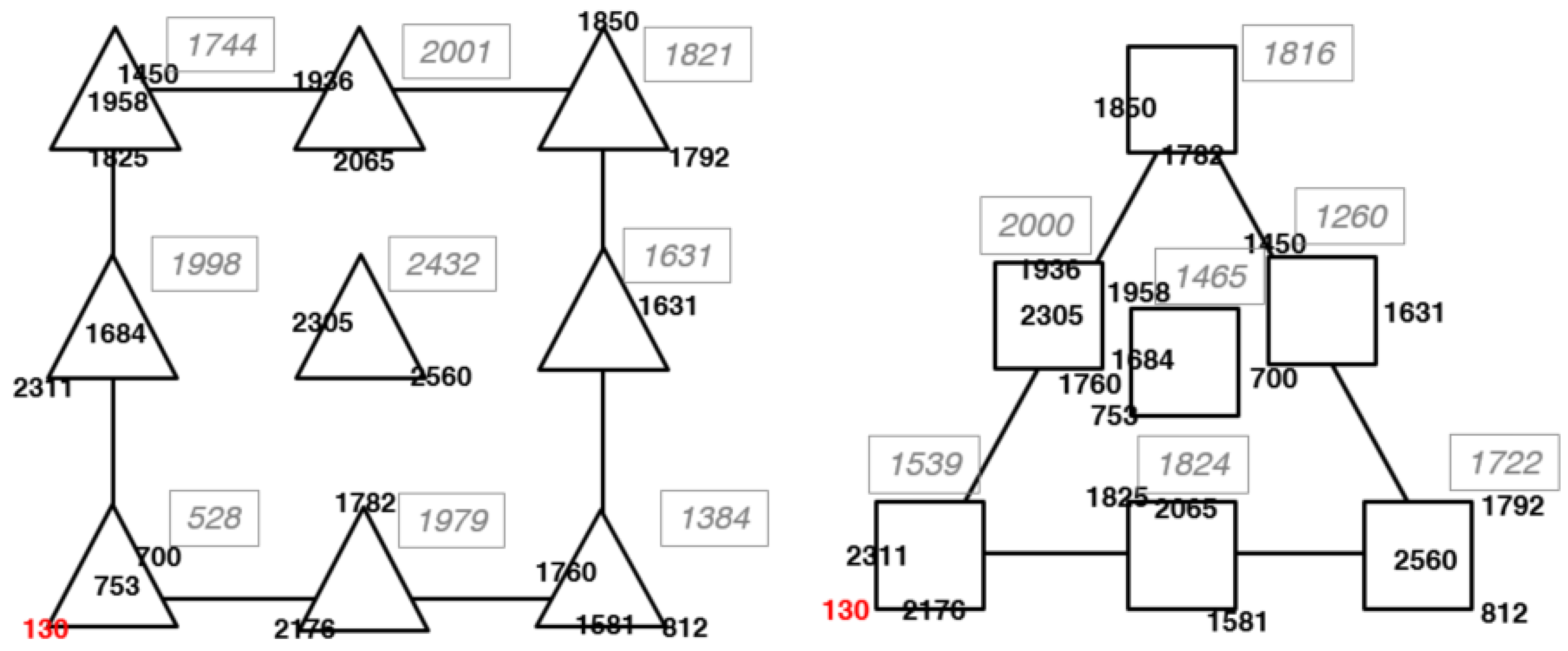
3.2. Optimization: Mixture-Process Design
- -
- PC1 = conc% v/v H2O.
- -
- PC2 = conc% v/v LA.
- -
- PC3 = conc% v/v EG.
- -
- X1 = S/L ratio.
- -
- X2 = time.
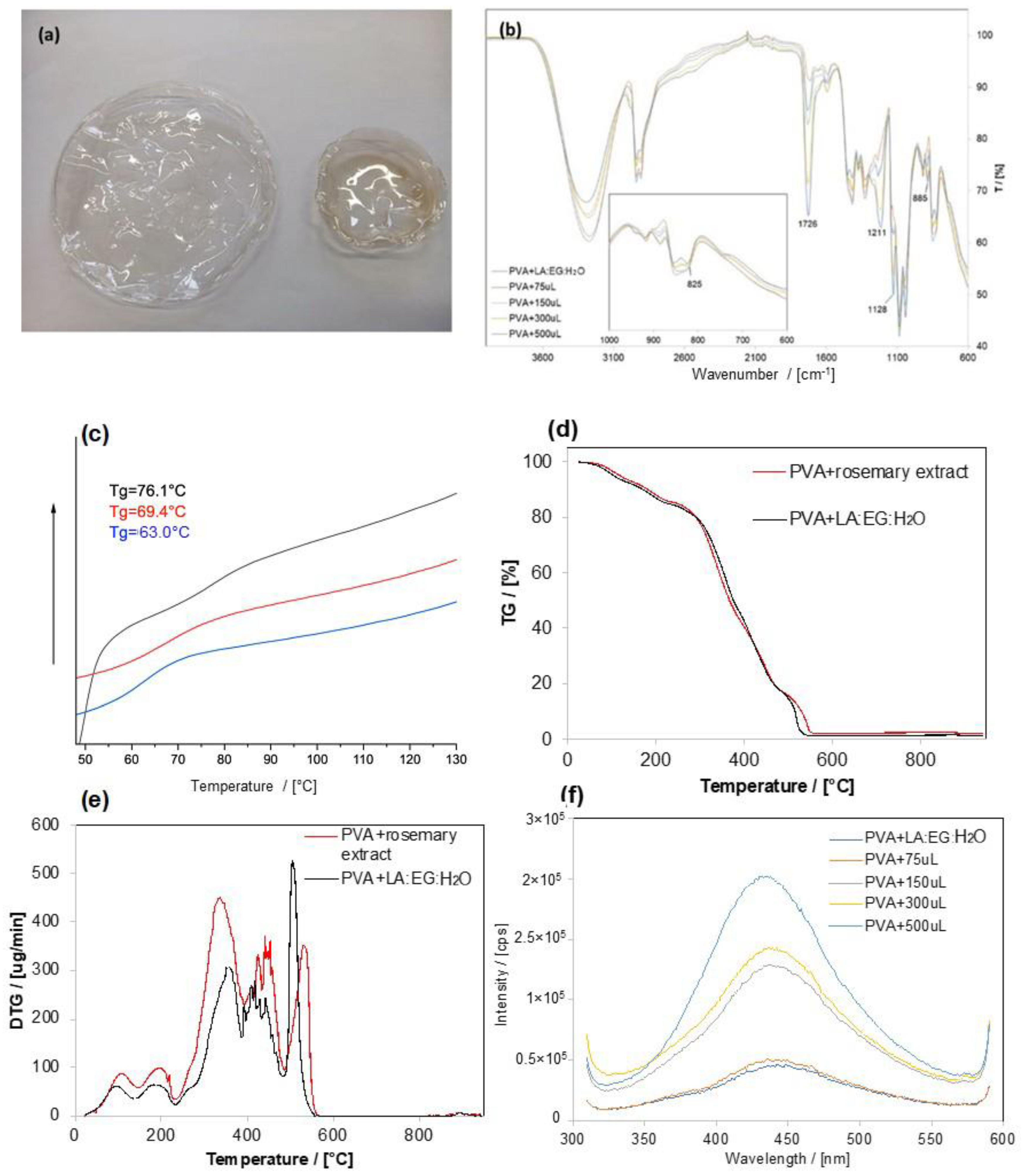
3.3. Inclusion of the Extract into PVA Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Khan, M.K.; Paniwnyk, L.; Hassan, S. Polyphenols as natural antioxidants: Sources, extraction and applications in food, cosmetics and drugs. In Plant Based “Green Chemistry 2.0”; Springer: Berlin/Heidelberg, Germany, 2019; pp. 197–235. [Google Scholar]
- Bernatoniene, J.; Cizauskaite, U.; Ivanauskas, L.; Jakstas, V.; Kalveniene, Z.; Kopustinskiene, D.M. Novel approaches to optimize extraction processes of ursolic, oleanolic and rosmarinic acids from Rosmarinus officinalis leaves. Ind. Crops Prod. 2016, 84, 72–79. [Google Scholar] [CrossRef]
- de Oliveira, A.; da Conceição, E.; Leles, M. Multiresponse optimization of an extraction procedure of carnosol and rosmarinic and carnosic acids from rosemary. Food Chem. 2016, 211, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, S.; Pellegrini, M.; Ricci, A.; Compagnone, D.; Lo Sterzo, C. Chemical composition and antioxidant activity of thyme, hemp and coriander extracts: A comparison study of maceration, Soxhlet, UAE and RSLDE techniques. Foods 2020, 9, 1221. [Google Scholar] [CrossRef] [PubMed]
- Sik, B.; Hanczné, E.L.; Kapcsándi, V.; Ajtony, Z. Conventional and nonconventional extraction techniques for optimal extraction processes of rosmarinic acid from six Lamiaceae plants as determined by HPLC-DAD measurement. J. Pharm. Biomed. Anal. 2020, 184, 113173. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Fan, Y.; Zhang, S.; Li, J.; Zhang, L.; Wu, H. Extraction of rosmarinic acid from Perilla seeds using green protic ionic liquids. Microchem. J. 2021, 170, 106667. [Google Scholar] [CrossRef]
- Dzah, C.S.; Duan, Y.; Zhang, H.; Wen, C.; Zhang, J.; Chen, G.; Ma, H. The effects of ultrasound assisted extraction on yield, antioxidant, anticancer and antimicrobial activity of polyphenol extracts: A review. Food Biosci. 2020, 35, 100547. [Google Scholar] [CrossRef]
- González-Rivera, J.; Husanu, E.; Mero, A.; Ferrari, C.; Duce, C.; Tinè, M.R.; D’Andrea, F.; Pomelli, C.S.; Guazzelli, L. Insights into microwave heating response and thermal decomposition behavior of deep eutectic solvents. J. Mol. Liq. 2020, 300, 112357. [Google Scholar] [CrossRef]
- Alam, M.A.; Muhammad, G.; Khan, M.N.; Mofijur, M.; Lv, Y.; Xiong, W.; Xu, J. Choline chloride-based deep eutectic solvents as green extractants for the isolation of phenolic compounds from biomass. J. Clean. Prod. 2021, 309, 127445. [Google Scholar] [CrossRef]
- Gajardo-Parra, N.F.; Cotroneo-Figueroa, V.P.; Aravena, P.; Vesovic, V.; Canales, R.I. Viscosity of choline chloride-based deep eutectic solvents: Experiments and modeling. J. Chem. Eng. Data 2020, 65, 5581–5592. [Google Scholar] [CrossRef]
- Ma, C.; Laaksonen, A.; Liu, C.; Lu, X.; Ji, X. The peculiar effect of water on ionic liquids and deep eutectic solvents. Chem. Soc. Rev. 2018, 47, 8685–8720. [Google Scholar] [CrossRef]
- Fu, X.; Wang, D.; Belwal, T.; Xu, Y.; Li, L.; Luo, Z. Sonication-synergistic natural deep eutectic solvent as a green and efficient approach for extraction of phenolic compounds from peels of Carya cathayensis Sarg. Food Chem. 2021, 355, 129577. [Google Scholar] [CrossRef]
- de Almeida Pontes, P.V.; Shiwaku, I.A.; Maximo, G.J.; Batista, E.A.C. Choline chloride-based deep eutectic solvents as potential solvent for extraction of phenolic compounds from olive leaves: Extraction optimization and solvent characterization. Food Chem. 2021, 352, 129346. [Google Scholar] [CrossRef] [PubMed]
- Alasalvar, H.; Yildirim, Z. Ultrasound-assisted extraction of antioxidant phenolic compounds from Lavandula angustifolia flowers using natural deep eutectic solvents: An experimental design approach. Sustain. Chem. Pharm. 2021, 22, 100492. [Google Scholar] [CrossRef]
- Oke, E.A.; Ijardar, S.P. Advances in the application of deep eutectic solvents based aqueous biphasic systems: An up-to-date review. Biochem. Eng. J. 2021, 176, 108211. [Google Scholar] [CrossRef]
- Jakubowska, E.; Gierszewska, M.; Szydłowska-Czerniak, A.; Nowaczyk, J.; Olewnik-Kruszkowska, E. Development and characterization of active packaging films based on chitosan, plasticizer, and quercetin for repassed oil storage. Food Chem. 2023, 399, 133934. [Google Scholar] [CrossRef] [PubMed]
- Velásquez, P.; Bustos, D.; Montenegro, G.; Giordano, A. Ultrasound-assisted extraction of anthocyanins using natural deep eutectic solvents and their incorporation in edible films. Molecules 2021, 26, 984. [Google Scholar] [CrossRef]
- Wang, S.; Peng, X.; Zhong, L.; Jing, S.; Cao, X.; Lu, F.; Sun, R. Choline chloride/urea as an effective plasticizer for production of cellulose films. Carbohydr. Polym. 2015, 117, 133–139. [Google Scholar] [CrossRef]
- Almeida, C.M.; Magalhães, J.M.; Souza, H.K.; Gonçalves, M.P. The role of choline chloride-based deep eutectic solvent and curcumin on chitosan films properties. Food Hydrocoll. 2018, 81, 456–466. [Google Scholar] [CrossRef]
- Coiai, S.; Campanella, B.; Paulert, R.; Cicogna, F.; Bramanti, E.; Lazzeri, A.; Pistelli, L.; Coltelli, M.-B. Rosmarinic acid and Ulvan from terrestrial and marine sources in anti-microbial bionanosystems and biomaterials. Appl. Sci. 2021, 11, 9249. [Google Scholar] [CrossRef]
- Martins, R.; Mouro, C.; Pontes, R.; Nunes, J.; Gouveia, I. Natural Deep Eutectic Solvent Extraction of Bioactive Pigments from Spirulina platensis and Electrospinning Ability Assessment. Polymers 2023, 15, 1574. [Google Scholar] [CrossRef]
- Bramanti, E.; Ferrari, C.; Angeli, V.; Onor, M.; Synovec, R.E. Characterization of BSA unfolding and aggregation using a single-capillary viscometer and dynamic surface tension detector. Talanta 2011, 85, 2553–2561. [Google Scholar] [CrossRef] [PubMed]
- Boggia, R.; Leardi, R.; Zunin, P.; Bottino, A.; Capannelli, G. Dehydration of pdo genovese basil leaves (Ocimum basilicum maximum L. cv genovese gigante) by direct osmosis. J. Food Process. Preserv. 2013, 37, 621–629. [Google Scholar] [CrossRef]
- Leardi, R.; Melzi, C.; Polotti, G. CAT (Chemometric Agile Tool). Available online: http://gruppochemiometria.it/index.php/software (accessed on 22 September 2022).
- Jung, D.; Jung, J.B.; Kang, S.; Li, K.; Hwang, I.; Jeong, J.H.; Kim, H.S.; Lee, J. Toxico-metabolomics study of a deep eutectic solvent comprising choline chloride and urea suggests in vivo toxicity involving oxidative stress and ammonia stress. Green Chem. 2021, 23, 1300–1311. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Redovniković, I.R. Enabling technologies for the extraction of grape-pomace anthocyanins using natural deep eutectic solvents in up-to-half-litre batches extraction of grape-pomace anthocyanins using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
- Bosiljkov, T.; Dujmić, F.; Bubalo, M.C.; Hribar, J.; Vidrih, R.; Brnčić, M.; Zlatic, E.; Redovniković, I.R.; Jokić, S. Natural deep eutectic solvents and ultrasound-assisted extraction: Green approaches for extraction of wine lees anthocyanins. Food Bioprod. Process. 2017, 102, 195–203. [Google Scholar] [CrossRef]
- Montgomery, R. Acidic constituents of lactic acid-water systems. J. Am. Chem. Soc. 1952, 74, 1466–1468. [Google Scholar] [CrossRef]
- Brown, L.C.; Hogg, J.M.; Swadźba-Kwaśny, M. Lewis acidic ionic liquids. In Ionic Liquids II; Springer: Cham, Switzerland, 2017; pp. 185–224. [Google Scholar]
- Xu, J.; Xiao, C.; He, X. Controllable synthesis of a novel poly (vinyl alcohol)-based hydrogel containing lactate and PEG moieties. Polym. Eng. Sci. 2014, 54, 1366–1371. [Google Scholar] [CrossRef]
- González-Rivera, J.; Mero, A.; Husanu, E.; Mezzetta, A.; Ferrari, C.; D’Andrea, F.; Bramanti, E.; Pomelli, C.S.; Guazzelli, L. Combining acid-based deep eutectic solvents and microwave irradiation for improved chestnut shell waste valorization. Green Chem. 2021, 23, 10101–10115. [Google Scholar] [CrossRef]
- Agatonovic-Kustrin, S.; Balyklova, K.S.; Gegechkori, V.; Morton, D.W. HPTLC and ATR/FTIR Characterization of Antioxidants in Different Rosemary Extracts. Molecules 2021, 26, 6064. [Google Scholar] [CrossRef]
- Shaulov, A.Y.; Lomakin, S.; Zarkhina, T.; Rakhimkulov, A.; Shilkina, N.; Muravlev, Y.B.; Berlin, A. Carbonization of polyvinyl alcohol in compositions with boron polyoxide. Dokl. Akad. Nauk-Ross. Akad. Nauk 2005, 403, 772–776. [Google Scholar]
- Al Danaf, N.; Abi Melhem, R.; Assaf, K.I.; Nau, W.M.; Patra, D. Photophysical properties of neutral and dissociated forms of rosmarinic acid. J. Lumin. 2016, 175, 50–56. [Google Scholar] [CrossRef]
- Oun, A.A.; Shin, G.H.; Rhim, J.W.; Kim, J.T. Recent advances in polyvinyl alcohol-based composite films and their applications in food packaging. Food Packag. Shelf Life 2022, 34, 100991–101007. [Google Scholar]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campanella, B.; Simoncini, M.; Passaglia, E.; Cicogna, F.; Ciancaleoni, G.; González-Rivera, J.; Bernazzani, L.; Bramanti, E. Ecofriendly Preparation of Rosmarinic Acid-poly(vinyl alcohol) Biofilms Using NADES/DES, Ultrasounds and Optimization via a Mixture-Process Design Strategy. Materials 2024, 17, 377. https://doi.org/10.3390/ma17020377
Campanella B, Simoncini M, Passaglia E, Cicogna F, Ciancaleoni G, González-Rivera J, Bernazzani L, Bramanti E. Ecofriendly Preparation of Rosmarinic Acid-poly(vinyl alcohol) Biofilms Using NADES/DES, Ultrasounds and Optimization via a Mixture-Process Design Strategy. Materials. 2024; 17(2):377. https://doi.org/10.3390/ma17020377
Chicago/Turabian StyleCampanella, Beatrice, Mattia Simoncini, Elisa Passaglia, Francesca Cicogna, Gianluca Ciancaleoni, José González-Rivera, Luca Bernazzani, and Emilia Bramanti. 2024. "Ecofriendly Preparation of Rosmarinic Acid-poly(vinyl alcohol) Biofilms Using NADES/DES, Ultrasounds and Optimization via a Mixture-Process Design Strategy" Materials 17, no. 2: 377. https://doi.org/10.3390/ma17020377
APA StyleCampanella, B., Simoncini, M., Passaglia, E., Cicogna, F., Ciancaleoni, G., González-Rivera, J., Bernazzani, L., & Bramanti, E. (2024). Ecofriendly Preparation of Rosmarinic Acid-poly(vinyl alcohol) Biofilms Using NADES/DES, Ultrasounds and Optimization via a Mixture-Process Design Strategy. Materials, 17(2), 377. https://doi.org/10.3390/ma17020377










