The Effect of Nb Doping on the Properties of Ti-Al Intermetallic Compounds Using First-Principles Calculations
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Crystal Structure and Phase Stability
3.2. Mechanical Properties
3.3. Electronic Structures
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zope, R.R.; Mishin, Y. Interatomic potentials for atomistic simulations of the Ti-Al system. Phys. Rev. B 2003, 68, 024102. [Google Scholar] [CrossRef]
- Ding, J.; Zhang, M.; Ye, T.; Liang, Y.; Ren, Y.; Dong, C.; Lin, J. Microstructure stability and micro-mechanical behavior of as-cast gamma-TiAl alloy during high-temperature low cycle fatigue. Acta Mater. 2018, 145, 504–515. [Google Scholar] [CrossRef]
- Yang, T.; Cao, B.X.; Zhang, T.L.; Zhao, Y.L.; Liu, W.H.; Kong, H.J.; Luan, J.H.; Kai, J.J.; Kuo, W.; Liu, C.T. Chemically complex intermetallic alloys: A new frontier for innovative structural materials. Mater. Today 2022, 52, 161–174. [Google Scholar] [CrossRef]
- Qu, S.J.; Tang, S.Q.; Feng, A.H.; Feng, C.; Shen, J.; Chen, D.L. Microstructural evolution and high-temperature oxidation mechanisms of a titanium aluminide based alloy. Acta Mater. 2018, 148, 300–310. [Google Scholar] [CrossRef]
- Cui, S.; Cui, C.; Xie, J.; Liu, S.; Shi, J. Carbon fibers coated with graphene reinforced TiAl alloy composite with high strength and toughness. Sci. Rep. 2018, 8, 2364. [Google Scholar] [CrossRef]
- Chen, J.; Chen, Q.; Qu, S.J.; Xiang, H.P.; Wang, C.; Gao, J.B.; Feng, A.H.; Chen, D.L. Oxidation mechanisms of an intermetallic alloy at high temperatures. Scr. Mater. 2021, 199, 113852. [Google Scholar] [CrossRef]
- Wu, G.D.; Cui, G.R.; Qu, S.J.; Feng, A.H.; Cao, G.J.; Ge, B.H.; Xiang, H.P.; Shen, J.; Chen, D.L. High-temperature oxidation mechanisms of nano-/submicro-scale lamellar structures in an intermetallic alloy. Scr. Mater. 2019, 171, 102–107. [Google Scholar] [CrossRef]
- Niu, H.Z.; Chen, Y.Y.; Xiao, S.L.; Xu, L.J. Microstructure evolution and mechanical properties of a novel beta γ-TiAl alloy. Intermetallics 2012, 31, 225–231. [Google Scholar] [CrossRef]
- Brotzu, A.; Felli, F.; Pilone, D. Effect of alloying elements on the behaviour of TiAl-based alloys. Intermetallics 2014, 54, 176–180. [Google Scholar] [CrossRef]
- Kim, S.-W.; Hong, J.K.; Na, Y.-S.; Yeom, J.-T.; Kim, S.E. Development of TiAl alloys with excellent mechanical properties and oxidation resistance. Mater. Des. 2014, 54, 814–819. [Google Scholar] [CrossRef]
- Murray, J.L. Calculation of the titanium-aluminum phase diagram. Metall. Trans. A 1988, 19, 243–247. [Google Scholar] [CrossRef]
- Schuster, J.C.; Palm, M. Reassessment of the binary Aluminum-Titanium phase diagram. J. Phase Equilibria Diffus. 2006, 27, 255–277. [Google Scholar] [CrossRef]
- Ghosh, G.; Asta, M. First-principles calculation of structural energetics of Al–TM (TM = Ti, Zr, Hf) intermetallics. Acta Mater. 2005, 53, 3225–3252. [Google Scholar] [CrossRef]
- Jian, Y.; Huang, Z.; Xing, J.; Sun, L.; Liu, Y.; Gao, P. Phase stability, mechanical properties and electronic structures of TiAl binary compounds by first principles calculations. Mater. Chem. Phys. 2019, 221, 311–321. [Google Scholar] [CrossRef]
- Tang, P.Y.; Huang, G.H.; Xie, Q.L.; Guan, N.N.; Ning, F. Elastic Properties, Phonon Focusing and Electronic Structures of Typical Long-Period Superstructures Al5Ti2 and Al11Ti5. Mater. Sci. Forum 2017, 898, 1026–1035. [Google Scholar] [CrossRef]
- Chen, H.; Li, X.; Chen, Z.; Zhang, R.; Ma, X.; Zheng, F.; Ma, Z.; Pan, F.; Lin, X. Investigation on electronic structures and mechanical properties of Nb–doped TiAl2 intermetallic compound. J. Alloys Compd. 2019, 780, 41–48. [Google Scholar] [CrossRef]
- Song, Y.; Yang, R.; Li, D.; Hu, Z.Q.; Guo, Z.X. A first principles study of the influence of alloying elements on TiAl: Site preference. Intermetallics 2000, 8, 563–568. [Google Scholar] [CrossRef]
- Wolf, W.; Podloucky, R.; Rogl, P.; Erschbaumer, H. Atomic modelling of Nb, V, Cr and Mn substitutions in γ-TiAl. 2: Electronic structure and site preference. Intermetallics 1996, 4, 201–209. [Google Scholar] [CrossRef]
- Lee, T.; Kim, S.-W.; Kim, J.Y.; Ko, W.-S.; Ryu, S. First-principles study of the ternary effects on the plasticity of γ-TiAl crystals. Sci. Rep. 2020, 10, 21614. [Google Scholar] [CrossRef]
- Liu, X.; Lin, Q.; Zhang, W.; Horne, C.V.; Cha, L. Microstructure Design and Its Effect on Mechanical Properties in Gamma Titanium Aluminides. Metals 2021, 11, 1644. [Google Scholar] [CrossRef]
- Zhang, Z.; Qu, S.; Cui, G.; Feng, A.; Shen, J.; Chen, D. A New Mechanism of Dynamic Phase Transformations in An Isothermal Forged Beta–Gamma Intermetallic Alloy. Materials 2019, 12, 2787. [Google Scholar] [CrossRef]
- Zhang, Y.; Feng, A.; Qu, S.; Shen, J.; Chen, D. Microstructure and low cycle fatigue of a Ti2AlNb-based lightweight alloy. J. Mater. Sci. Technol. 2020, 44, 140–147. [Google Scholar] [CrossRef]
- Xiang, J.M.; Mi, G.B.; Qu, S.J.; Huang, X.; Chen, Z.; Feng, A.H.; Shen, J.; Chen, D.L. Thermodynamic and microstructural study of Ti2AlNb oxides at 800 °C. Sci. Rep. 2018, 8, 12761. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169–11186. [Google Scholar] [CrossRef] [PubMed]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized Gradient Approximation Made Simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmüller, J. Efficiency of ab-initio total energy calculations for metals and semiconductors using a plane-wave basis set. Comput. Mater. Sci. 1996, 6, 15–50. [Google Scholar] [CrossRef]
- Monkhorst, H.J.; Pack, J.D. Special points for Brillouin-zone integrations. Phys. Rev. B 1976, 13, 5188–5192. [Google Scholar] [CrossRef]
- Methfessel, M.; Paxton, A.T. High-precision sampling for Brillouin-zone integration in metals. Phys. Rev. B 1989, 40, 3616–3621. [Google Scholar] [CrossRef]
- Liu, Y.; Chong, X.; Jiang, Y.; Zhou, R.; Feng, J. Mechanical properties and electronic structures of Fe-Al intermetallic. Phys. B Condens. Matter 2017, 506, 1–11. [Google Scholar] [CrossRef]
- Asta, M.; de Fontaine, D.; van Schilfgaarde, M. First-principles study of phase stability of Ti–Al intermetallic compounds. J. Mater. Res. 1993, 8, 2554–2568. [Google Scholar] [CrossRef]
- Watson, R.E.; Weinert, M. Transition-metal aluminide formation: Ti, V, Fe, and Ni aluminides. Phys. Rev. B 1998, 58, 5981–5988. [Google Scholar] [CrossRef]
- Hayes, F.H. The Al-Ti-V (aluminum-titanium-vanadium) system. J. Phase Equilibria 1995, 16, 163–176. [Google Scholar] [CrossRef]
- Sridharan, S.; Nowotny, H.; Wayne, S.F. Investigations within the quaternary system titanium-nickel-aluminium-carbon. Monatshefte Chem. Chem. Mon. 1983, 114, 127–135. [Google Scholar] [CrossRef]
- Kubaschewski, O.; Dench, W.A. The heats of formation in the systems titanium-aluminium and titanium-iron. Acta Metall. 1955, 3, 339–346. [Google Scholar] [CrossRef]
- Kubaschewski, O.; Heymer, G. Heats of formation of transition-metal aluminides. Trans. Faraday Soc. 1960, 56, 473–478. [Google Scholar] [CrossRef]
- Nassik, M.; Chrifi-Alaoui, F.Z.; Mahdouk, K.; Gachon, J.C. Calorimetric study of the aluminium–titanium system. J. Alloys Compd. 2003, 350, 151–154. [Google Scholar] [CrossRef]
- Braun, J.; Ellner, M. Phase equilibria investigations on the aluminum-rich part of the binary system Ti-Al. Metall. Mater. Trans. A 2001, 32, 1037–1047. [Google Scholar] [CrossRef]
- Braun, J.; Ellner, M. X-ray high-temperature in situ investigation of the aluminide TiAl2 (HfGa2 type). J. Alloys Compd. 2000, 309, 118–122. [Google Scholar] [CrossRef]
- Schuster, J.; Ipser, H. Phases and phase relations in the partial system TiAl3-TiAl. Int. J. Mater. Res. 1990, 81, 389–396. [Google Scholar] [CrossRef]
- Raman, A.; Schubert, K. The constitution of some alloy series related to TiAl3. I. Investigations in some T4-Zn-Al, T4-Zn-Ga, and T4-Ga-Ge systems. Acta Metall. 1965, 56, 99–104. [Google Scholar]
- Menand, A.; Huguet, A.; Nérac-Partaix, A. Interstitial solubility in γ and α2 phases of TiAl-based alloys. Acta Mater. 1996, 44, 4729–4737. [Google Scholar] [CrossRef]
- Amador, C.; Hoyt, J.J.; Chakoumakos, B.C.; de Fontaine, D. Theoretical and Experimental Study of Relaxations in Al3Ti and Al3Zr Ordered Phases. Phys. Rev. Lett. 1995, 74, 4955–4958. [Google Scholar] [CrossRef]
- Colinet, C.; Pasturel, A. Ab initio calculation of the formation energies of L12, D022, D023 and one dimensional long period structures in TiAl3 compound. Intermetallics 2002, 10, 751–764. [Google Scholar] [CrossRef]
- Meschel, S.V.; Kleppa, O.J. The Standard Enthalpies of Formation of Some 3d Transition Metal Aluminides by High-Temperature Direct Synthesis Calorimetry. In Metallic Alloys: Experimental and Theoretical Perspectives; Faulkner, J.S., Jordan, R.G., Eds.; Springer: Dordrecht, The Netherlands, 1994; pp. 103–112. [Google Scholar]
- Patil, S.K.R.; Khare, S.V.; Tuttle, B.R.; Bording, J.K.; Kodambaka, S. Mechanical stability of possible structures of PtN investigated using first-principles calculations. Phys. Rev. B 2006, 73, 104118. [Google Scholar] [CrossRef]
- Nye, J.F.; Lindsay, R.B. Physical Properties of Crystals: Their Representation by Tensors and Matrices. Phys. Today 1957, 10, 26. [Google Scholar] [CrossRef]
- Wu, Z.-j.; Zhao, E.-j.; Xiang, H.-p.; Hao, X.-f.; Liu, X.-j.; Meng, J. Crystal structures and elastic properties of superhard IrN2 and IrN3 from first principles. Phys. Rev. B 2007, 76, 054115. [Google Scholar] [CrossRef]
- Hu, H.; Wu, X.; Wang, R.; Li, W.; Liu, Q. Phase stability, mechanical properties and electronic structure of TiAl alloying with W, Mo, Sc and Yb: First-principles study. J. Alloys Compd. 2016, 658, 689–696. [Google Scholar] [CrossRef]
- Junhua, T.; Kaijin, Z.; Junhui, P. First-Principles Simulation on Structure-Property of Ti-Al Intermetallic Compounds. Chin. J. Comput. Phys. 2017, 34, 365–373. [Google Scholar] [CrossRef]
- He, Y.; Schwarz, R.B.; Migliori, A.; Whang, S.H. Elastic constants of single crystal γ—TiAl. J. Mater. Res. 1995, 10, 1187–1195. [Google Scholar] [CrossRef]
- Tang, P.-Y.; Tang, B.-Y.; Su, X.-P. First-principles studies of typical long-period superstructures Al5Ti3, h-Al2Ti and r-Al2Ti in Al-rich TiAl alloys. Comput. Mater. Sci. 2011, 50, 1467–1476. [Google Scholar] [CrossRef]
- Anderson, O.L. A simplified method for calculating the debye temperature from elastic constants. J. Phys. Chem. Solids 1963, 24, 909–917. [Google Scholar] [CrossRef]
- Li, Y.; Gao, Y.; Xiao, B.; Min, T.; Fan, Z.; Ma, S.; Xu, L. Theoretical study on the stability, elasticity, hardness and electronic structures of W–C binary compounds. J. Alloys Compd. 2010, 502, 28–37. [Google Scholar] [CrossRef]
- Chen, X.-Q.; Niu, H.; Li, D.; Li, Y. Modeling hardness of polycrystalline materials and bulk metallic glasses. Intermetallics 2011, 19, 1275–1281. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B.; Zhou, R.; Pan, W.; Clarke, D.R. Anisotropic elastic and thermal properties of the double perovskite slab–rock salt layer Ln2SrAl2O7 (Ln = La, Nd, Sm, Eu, Gd or Dy) natural superlattice structure. Acta Mater. 2012, 60, 3380–3392. [Google Scholar] [CrossRef]
- Feng, J.; Xiao, B.; Chen, J.; Du, Y.; Yu, J.; Zhou, R. Stability, thermal and mechanical properties of PtxAly compounds. Mater. Des. 2011, 32, 3231–3239. [Google Scholar] [CrossRef]
- Shein, I.R.; Ivanovskii, A.L. Elastic properties of mono- and polycrystalline hexagonal AlB2-like diborides of s, p and d metals from first-principles calculations. J. Phys. Condens. Matter 2008, 20, 415218. [Google Scholar] [CrossRef]
- Young, A.F.; Sanloup, C.; Gregoryanz, E.; Scandolo, S.; Hemley, R.J.; Mao, H.-k. Synthesis of Novel Transition Metal Nitrides IrN2 and OsN2. Phys. Rev. Lett. 2006, 96, 155501. [Google Scholar] [CrossRef]
- Pugh, S.F. XCII. Relations between the elastic moduli and the plastic properties of polycrystalline pure metals. Lond. Edinb. Dublin Philos. Mag. J. Sci. 1954, 45, 823–843. [Google Scholar] [CrossRef]
- Lakes, R.; Wojciechowski, K.W. Negative compressibility, negative Poisson’s ratio, and stability. Phys. Status Solidi 2008, 245, 545–551. [Google Scholar] [CrossRef]
- Kirihara, K.; Nagata, T.; Kimura, K.; Kato, K.; Takata, M.; Nishibori, E.; Sakata, M. Covalent bonds and their crucial effects on pseudogap formation in α-Al(Mn, Re)Si icosahedral quasicrystalline approximant. Phys. Rev. B 2003, 68, 014205. [Google Scholar] [CrossRef]

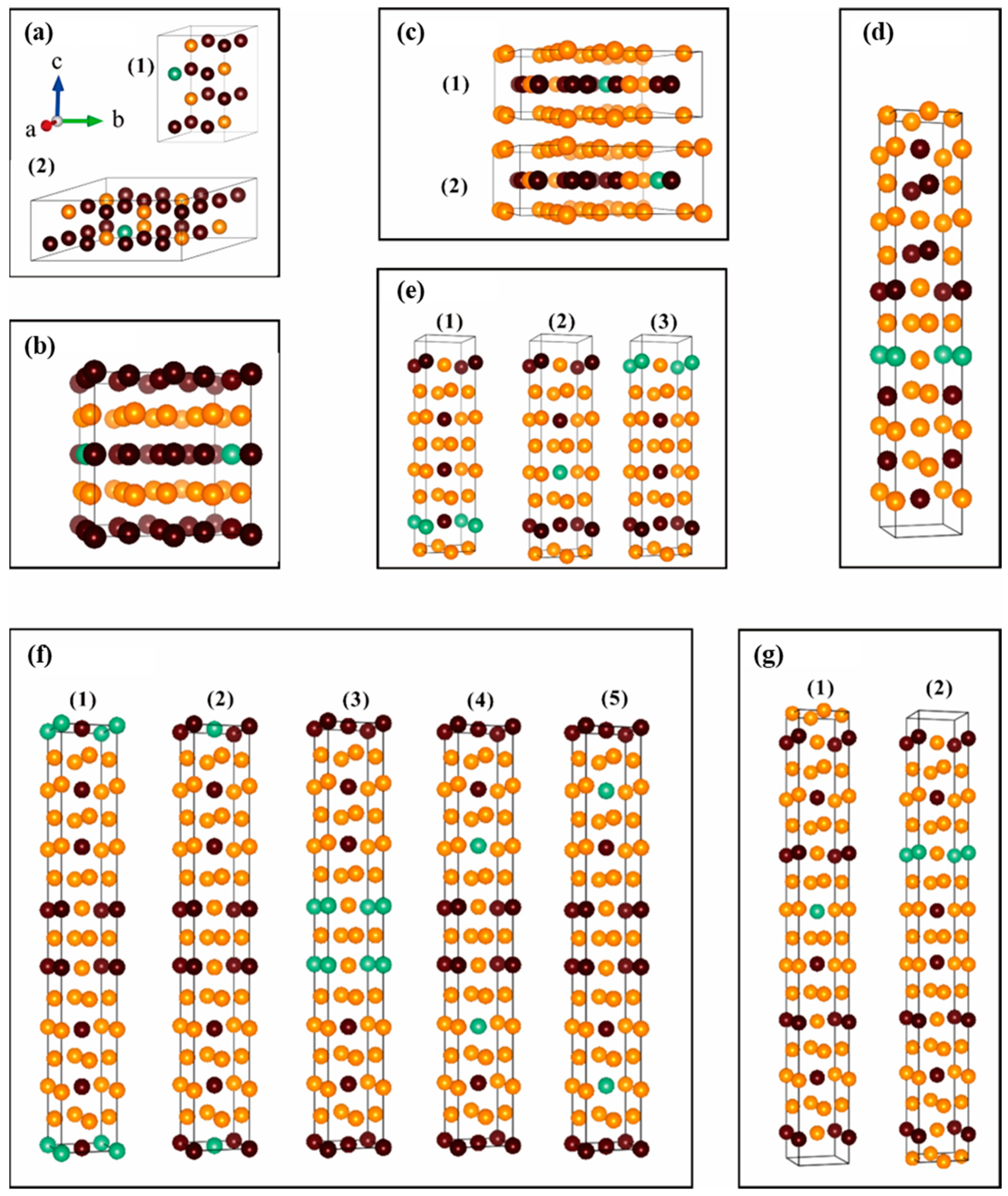
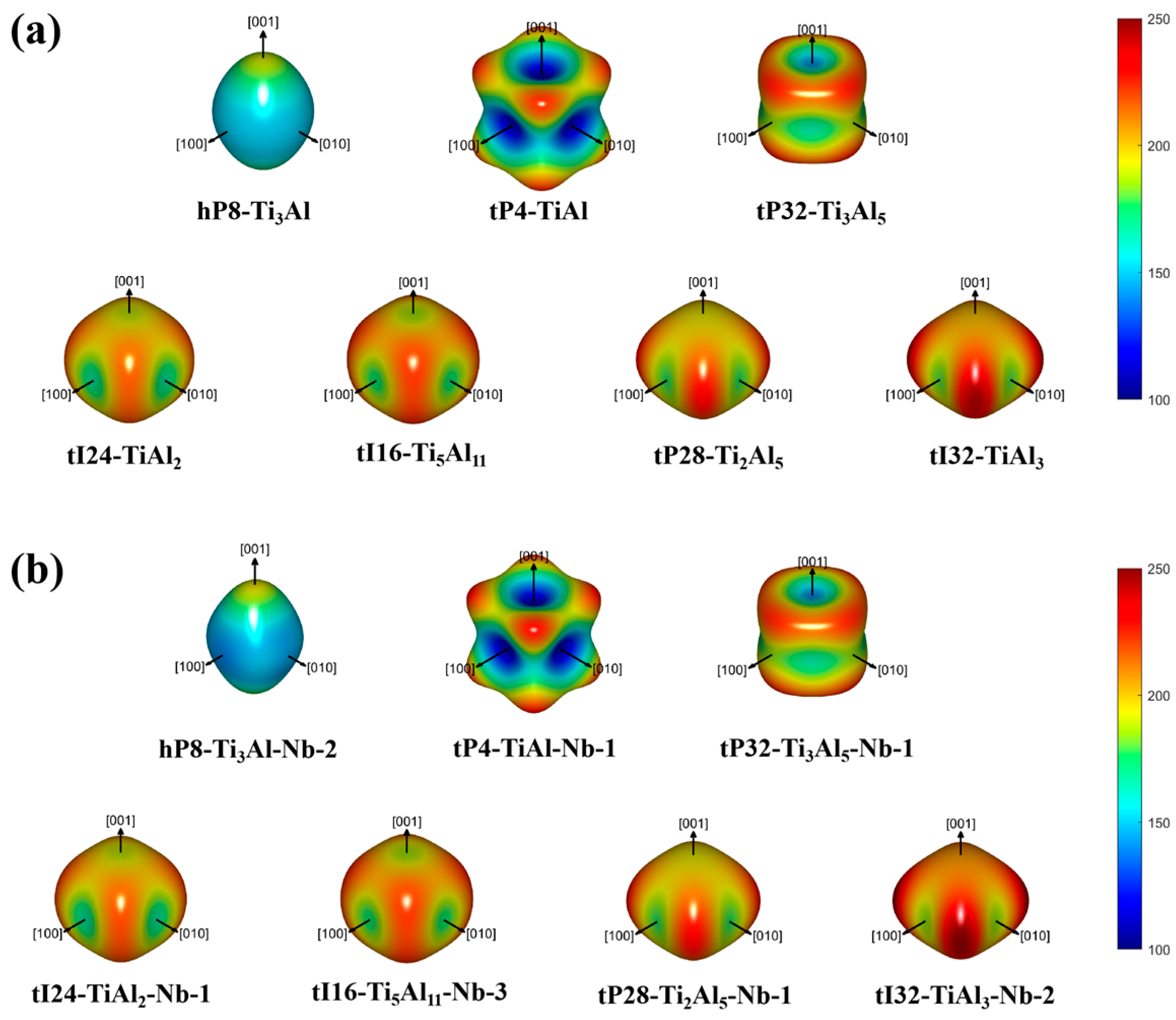
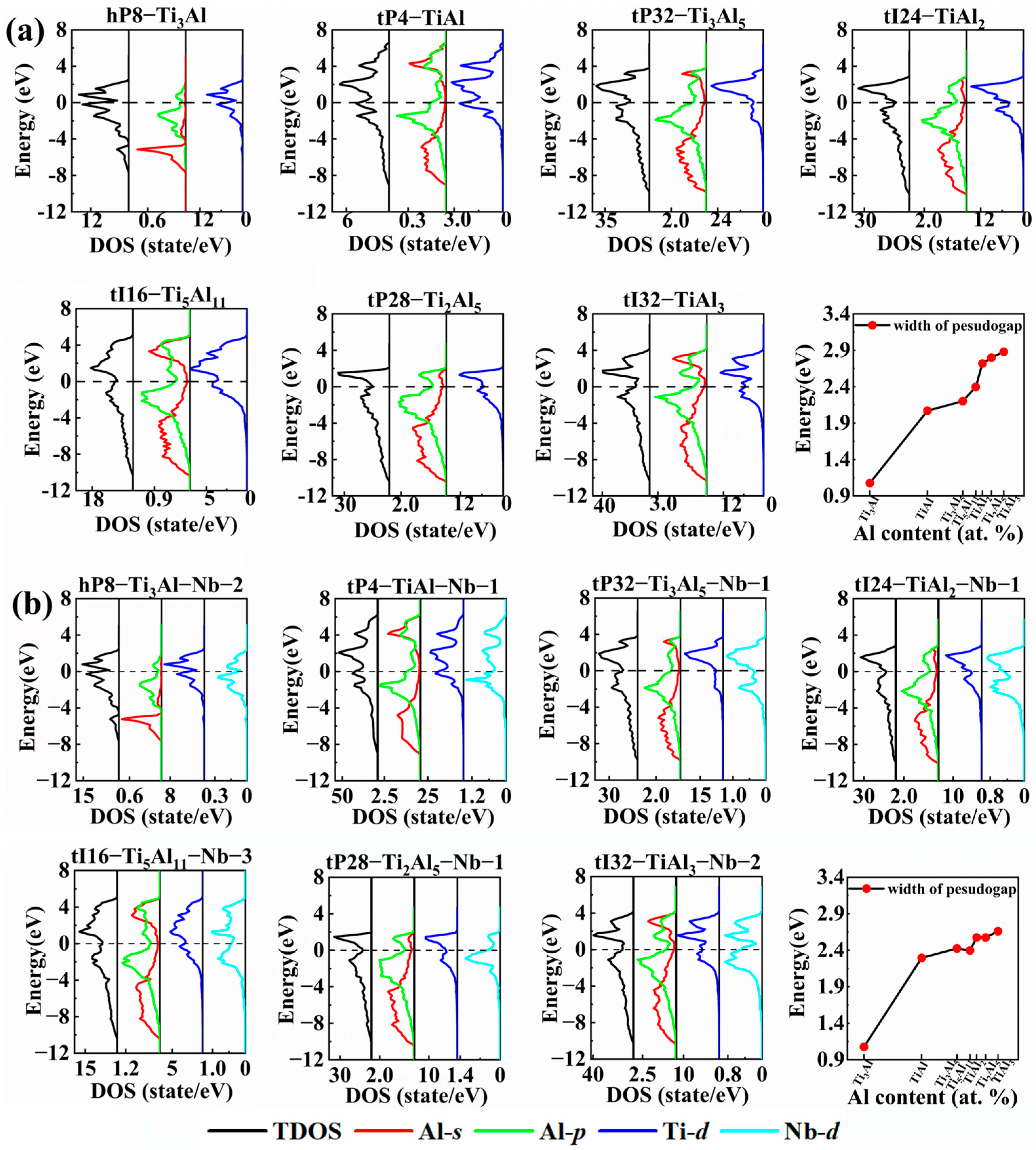
| Pearson Symbol (Space Group) | Lattice Parameters and Formation Enthalpy | Method and Reference | ||||
|---|---|---|---|---|---|---|
| a | b | c | ΔHr | |||
| Ti3Al | hP8 | 5.759 | 4.655 | −27.086 | PAW-GGA | |
| (P63/mmc) | 5.759 | 4.655 | −26.827 | US-PP-GGA [14] | ||
| Ti3Al | hP8 (P63/mmc) | 5.7372 | 4.6825 | −27.395 | US-PP-GGA [13] | |
| 5.6496 | 4.5706 | −28.70 | FP-LMTP-LDA [30] | |||
| 5.6136 | 4.6649 | −26.979 | FLASTO-LDA [31] | |||
| 5.775 | 4.655 | Experiment [32] | ||||
| TiAl | tP4 (P4/mmm) | 3.9893 | 4.074 | −39.23 | PAW-GGA | |
| 3.994 | 4.079 | −38.431 | US-PP-GGA [14] | |||
| 3.9814 | 4.0803 | −39.712 | US-PP-GGA [13] | |||
| 3.9921 | 4.04 | −42.00 | FP-LMTP-LDA [30] | |||
| 3.9716 | 4.051 | −42.00 | FLASTO-LDA [31] | |||
| 4.001 | 4.071 | Experiment [33] | ||||
| −40.1 ± 1 | Experiment [34] | |||||
| −36.4 ± 1 | Experiment [35] | |||||
| −35.1 ± 0.5 | Experiment [36] | |||||
| cP2 (Pm-3m) | 3.1865 | −26.154 | PAW-GGA | |||
| 3.1854 | −25.876 | US-PP-GGA [13] | ||||
| 3.1529 | −25.052 | FLASTO-LDA [31] | ||||
| Ti3Al5 | tP32 (P4/mmm) | 11.283 | 4.0305 | −41.25 | PAW-GGA | |
| 11.286 | 4.0311 | −41.640 | US-PP-GGA [13] | |||
| 11.293 | 4.0381 | Experiment [37] | ||||
| TiAl2 | tI24 (I41/amd) | 3.967 | 24.306 | −41.73 | PAW-GGA | |
| 3.9658 | 24.321 | −42.370 | US-PP-GGA [13] | |||
| 3.9628 | 24.068 | −42.396 | FLASTO-LDA [31] | |||
| 3.9711 | 24.313 | Experiment [38] | ||||
| oC12 (Cmmm) | 12.149 | 3.9305 | 4.0067 | −41.346 | PAW-GGA | |
| 12.164 | 3.936 | 4.011 | −40.896 | US-PP-GGA [14] | ||
| 12.161 | 3.9322 | 4.0018 | −42.013 | US-PP-GGA [13] | ||
| Ti5Al11 | tI16 (I4/mmm) | 3.926 | 16.517 | −39.519 | PAW-GGA | |
| 3.9239 | 16.52 | −40.18 | US-PP-GGA [13] | |||
| 3.923 | 16.519 | PAW-GGA [16] | ||||
| 3.917 | 16.524 | Experiment [39] | ||||
| 3.923 | 16.535 | Experiment [40] | ||||
| Ti2Al5 | tP28 (P4/mmm) | 3.9132 | 29.019 | −39.808 | PAW-GGA | |
| 3.9114 | 29.023 | −39.398 | US-PP-GGA [13] | |||
| 3.912 | 29.004 | PAW-GGA [16] | ||||
| 3.905 | 29.196 | Experiment [40] | ||||
| TiAl3 | tI32 (I4/mmm) | 3.8732 | 33.841 | −38.846 | PAW-GGA | |
| 3.875 | 33.84 | Experiment [41] | ||||
| tI8 (I4/mmm) | 3.9664 | 8.4797 | −38.37 | PAW-GGA | ||
| 3.76 | 8.4976 | −41.44 | FP-LMTO-LDA [42] | |||
| 3.799 | 8.5174 | −39.51 | FLASTO-LDA [31] | |||
| 3.8400–3.8537 | 8.5600–8.6140 | Experiment [43] | ||||
| −36.6 ± 1.3 | Experiment [44] | |||||
| −39.2 ± 1.8 | Experiment [31] | |||||
| cP4 (Pm-3m) | 3.9807 | −35.616 | PAW-GGA | |||
| 3.981 | −36.583 | US-PP-GGA [13] | ||||
| 3.9800–4.0500 | −36.907 | Experiment [43] | ||||
| −36.614 | Experiment [31] | |||||
| x% | a | c | c/a | V | Δa | Δc | Δ(c/a) | ΔV | ΔHr | |
|---|---|---|---|---|---|---|---|---|---|---|
| hP8-Ti3Al-Nb-1 | 6.250% | 5.729 | 9.327 | 1.628 | 267.107 | −0.327% | 0.266% | 1.189% | 0.349% | −26.304 |
| hP8-Ti3Al-Nb-2 | 3.125% | 11.531 | 4.658 | 0.404 | 533.271 | 0.301% | 0.150% | −0.075% | 0.171% | −26.784 |
| tP4-TiAl-Nb-1 | 3.125% | 7.980 | 8.157 | 1.022 | 519.509 | 0.021% | 0.116% | 0.095% | 0.159% | −38.784 |
| tP32-Ti3Al5-Nb-1 | 4.545% | 11.297 | 4.035 | 0.357 | 514.012 | 0.124% | 0.102% | −0.022% | 0.171% | −40.608 |
| tP32-Ti3Al5-Nb-2 | 4.545% | 11.289 | 4.034 | 0.357 | 514.013 | 0.051% | 0.075% | 0.024% | 0.172% | −40.512 |
| tI24-TiAl2-Nb-1 | 4.167% | 3.964 | 24.383 | 6.151 | 383.283 | −0.069% | 0.318% | 0.387% | 0.203% | −40.512 |
| tI16-Ti5Al11-Nb-1 | 6.250% | 3.928 | 16.571 | 4.218 | 255.736 | 0.063% | 0.326% | 0.262% | 0.452% | −38.4 |
| tI16-Ti5Al11-Nb-2 | 6.250% | 3.928 | 16.551 | 4.213 | 255.369 | 0.052% | 0.204% | 0.151% | 0.307% | −37.152 |
| tI16-Ti5Al11-Nb-3 | 6.250% | 3.920 | 16.582 | 4.230 | 254.817 | −0.151% | 0.394% | 0.546% | 0.090% | −38.592 |
| tP28-Ti2Al5-Nb-1 | 3.571% | 3.912 | 29.096 | 7.437 | 445.385 | −0.018% | 0.264% | 0.282% | 0.228% | −38.784 |
| tP28-Ti2Al5-Nb-2 | 3.571% | 3.920 | 28.982 | 7.394 | 445.329 | 0.171% | −0.127% | −0.297% | 0.215% | −37.92 |
| tP28-Ti2Al5-Nb-3 | 3.571% | 3.911 | 29.107 | 7.442 | 445.276 | −0.05% | 0.304% | 0.355% | 0.203% | −36.96 |
| tP28-Ti2Al5-Nb-4 | 3.571% | 3.912 | 29.092 | 7.436 | 445.270 | −0.025% | 0.252% | 0.277% | 0.202% | −36.96 |
| tP28-Ti2Al5-Nb-5 | 3.571% | 3.914 | 29.095 | 7.434 | 445.672 | 0.016% | 0.261% | 0.246% | 0.292% | −36.48 |
| tI32-TiAl3-Nb-1 | 3.125% | 3.874 | 33.871 | 8.772 | 508.253 | 0.013% | 0.414% | 0.401% | 0.112% | −38.208 |
| tI32-TiAl3-Nb-2 | 3.125% | 3.871 | 33.889 | 8.754 | 507.911 | −0.047% | 0.141% | 0.188% | 0.045% | −38.976 |
| Cij (GPa) | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| C11 | C12 | C13 | C22 | C23 | C33 | C44 | C55 | C66 | |
| Ti-Al compounds | |||||||||
| hP8-Ti3Al | 193.9 | 84.1 | 66.5 | 193.9 | 66.5 | 223 | 63.5 | 63.5 | 54.9 a |
| 192.2 | 78.2 | 66.8 | 192.2 | 66.8 | 234.2 | 61.6 | 61.6 | 57.0 b [14] | |
| 202.6 | 67.6 | 78.9 | 202.6 | 78.9 | 202.9 | 61.6 | 61.6 | 67.5 a [49] | |
| tP4-TiAl | 171 | 88.7 | 85.9 | 171 | 85.9 | 165.5 | 114.1 | 114.1 | 69.8 a |
| 168.6 | 88.3 | 80.9 | 168.6 | 80.9 | 174.1 | 111.8 | 111.8 | 73.7 a [14] | |
| 166.4 | 96 | 88.1 | 166.4 | 88.1 | 179.6 | 119.2 | 119.2 | 76.0 a [49] | |
| 173 | 83 | 84 | 168 | 111 | 75 a [48] | ||||
| 186 | 72 | 74 | 176 | 101 | 77 d [50] | ||||
| tP32-Ti3Al5 | 215 | 50 | 71.1 | 215 | 71.1 | 180.1 | 104.8 | 104.8 | 69.7 a |
| 213.7 | 52.7 | 72.1 | 181.8 | 101.4 | 65.8 c [51] | ||||
| tI24-TiAl2 | 199.2 | 69.5 | 58.4 | 199.2 | 58.4 | 214.6 | 88.5 | 88.5 | 98.7 a |
| tI16-Ti5Al11 | 201.6 | 68.8 | 56.6 | 201.6 | 56.6 | 208.9 | 88.5 | 88.5 | 93.9 a |
| 200.6 | 71.8 | 58.8 | 208.5 | 87.6 | 92.6 a [16] | ||||
| tP28-Ti2Al5 | 206.1 | 68.1 | 54 | 206.1 | 54 | 205.5 | 84.5 | 84.5 | 100.2 a |
| 218.5 | 62.9 | 48.8 | 221.1 | 102.3 | 117.0 a [16] | ||||
| tI32-TiAl3 | 208.7 | 71.3 | 47.1 | 208.7 | 47.1 | 215.8 | 89.3 | 89.3 | 116.2 a |
| Nb-doped Ti-Al compounds | |||||||||
| hP8-Ti3Al-Nb-2 | 189.9 | 91.3 | 68.2 | 185 | 66.4 | 226.8 | 60.7 | 62.6 | 53.6 |
| tP4-TiAl-Nb-1 | 171.3 | 93.5 | 87.4 | 171.3 | 87.4 | 167.7 | 114.7 | 114.7 | 73.8 |
| tP32-Ti3Al5-Nb-1 | 216.5 | 51.6 | 73.2 | 218.2 | 72.2 | 181.1 | 104.7 | 104.7 | 67.5 |
| tI24-TiAl2-Nb-1 | 200.8 | 73 | 61 | 201.3 | 61.3 | 216.1 | 88.5 | 88.5 | 100.2 |
| tI16-Ti5Al11-Nb-3 | 208.3 | 74.1 | 61.3 | 208.3 | 61.3 | 212.3 | 92.2 | 92.2 | 99 |
| tP28-Ti2Al5-Nb-1 | 209.2 | 74.4 | 52.4 | 209.2 | 52. 4 | 212.7 | 86.2 | 86.2 | 104.6 |
| tI32-TiAl3-Nb-2 | 214 | 74 | 47.3 | 214 | 47.3 | 221.2 | 90.6 | 90.6 | 117.8 |
| B | G | E | Hv | ν | K | ΘD | |
|---|---|---|---|---|---|---|---|
| Ti-Al compounds | |||||||
| hP8-Ti3Al | 116.1 | 62.2 | 158.3 | 7.7 | 0.273 | 1.87 | 496.5 |
| tP4-TiAl | 114.3 | 69.1 | 172.5 | 10.2 | 0.240 | 1.65 | 554.7 |
| tP32-Ti3Al5 | 110.5 | 81.4 | 196.1 | 15.2 | 0.204 | 1.36 | 620.0 |
| tI24-TiAl2 | 109.5 | 82.7 | 198.2 | 15.9 | 0.198 | 1.32 | 631.7 |
| tI16-Ti5Al11 | 108.5 | 82.2 | 196.9 | 18.2 | 0.197 | 1.32 | 632.8 |
| tP28-Ti2Al5 | 107.7 | 82.7 | 197.4 | 18.4 | 0.194 | 1.30 | 640.2 |
| tI32-TiAl3 | 106.9 | 89.7 | 210.3 | 19.5 | 0.172 | 1.19 | 673.1 |
| Nb-doped Ti-Al compounds | |||||||
| hP8-Ti3Al-Nb-2 | 118.2 | 60.4 | 154.8 | 7.0 | 0.282 | 1.96 | 481.8 |
| tP4-TiAl-Nb | 116.3 | 69.4 | 173.6 | 10.0 | 0.251 | 1.67 | 545.6 |
| tP32-Ti3Al5-Nb-1 | 112.1 | 80.9 | 195.7 | 14.7 | 0.209 | 1.38 | 601.5 |
| tI24-TiAl2-Nb | 112.1 | 82.6 | 199.0 | 15.4 | 0.204 | 1.35 | 614.9 |
| tI16-Ti5Al11-Nb-3 | 113.6 | 84.6 | 203.3 | 15.7 | 0.201 | 1.34 | 617.6 |
| tP28-Ti2Al5-Nb-1 | 109.9 | 84.7 | 202.1 | 16.6 | 0.193 | 1.29 | 628 |
| tI32-TiAl3-Nb-2 | 109.5 | 90.6 | 213.0 | 19.2 | 0.176 | 1.21 | 662.1 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, K.; Xiang, H.; Xu, L.; Feng, A.; Qu, S.; Wang, H.; Chen, D. The Effect of Nb Doping on the Properties of Ti-Al Intermetallic Compounds Using First-Principles Calculations. Materials 2024, 17, 358. https://doi.org/10.3390/ma17020358
Wang K, Xiang H, Xu L, Feng A, Qu S, Wang H, Chen D. The Effect of Nb Doping on the Properties of Ti-Al Intermetallic Compounds Using First-Principles Calculations. Materials. 2024; 17(2):358. https://doi.org/10.3390/ma17020358
Chicago/Turabian StyleWang, Kun, Hongping Xiang, Lin Xu, Aihan Feng, Shoujiang Qu, Hao Wang, and Daolun Chen. 2024. "The Effect of Nb Doping on the Properties of Ti-Al Intermetallic Compounds Using First-Principles Calculations" Materials 17, no. 2: 358. https://doi.org/10.3390/ma17020358
APA StyleWang, K., Xiang, H., Xu, L., Feng, A., Qu, S., Wang, H., & Chen, D. (2024). The Effect of Nb Doping on the Properties of Ti-Al Intermetallic Compounds Using First-Principles Calculations. Materials, 17(2), 358. https://doi.org/10.3390/ma17020358










