Solution-Plasma Synthesis and Characterization of Transition Metals and N-Containing Carbon–Carbon Nanotube Composites
Abstract
1. Introduction
2. Materials and Methods
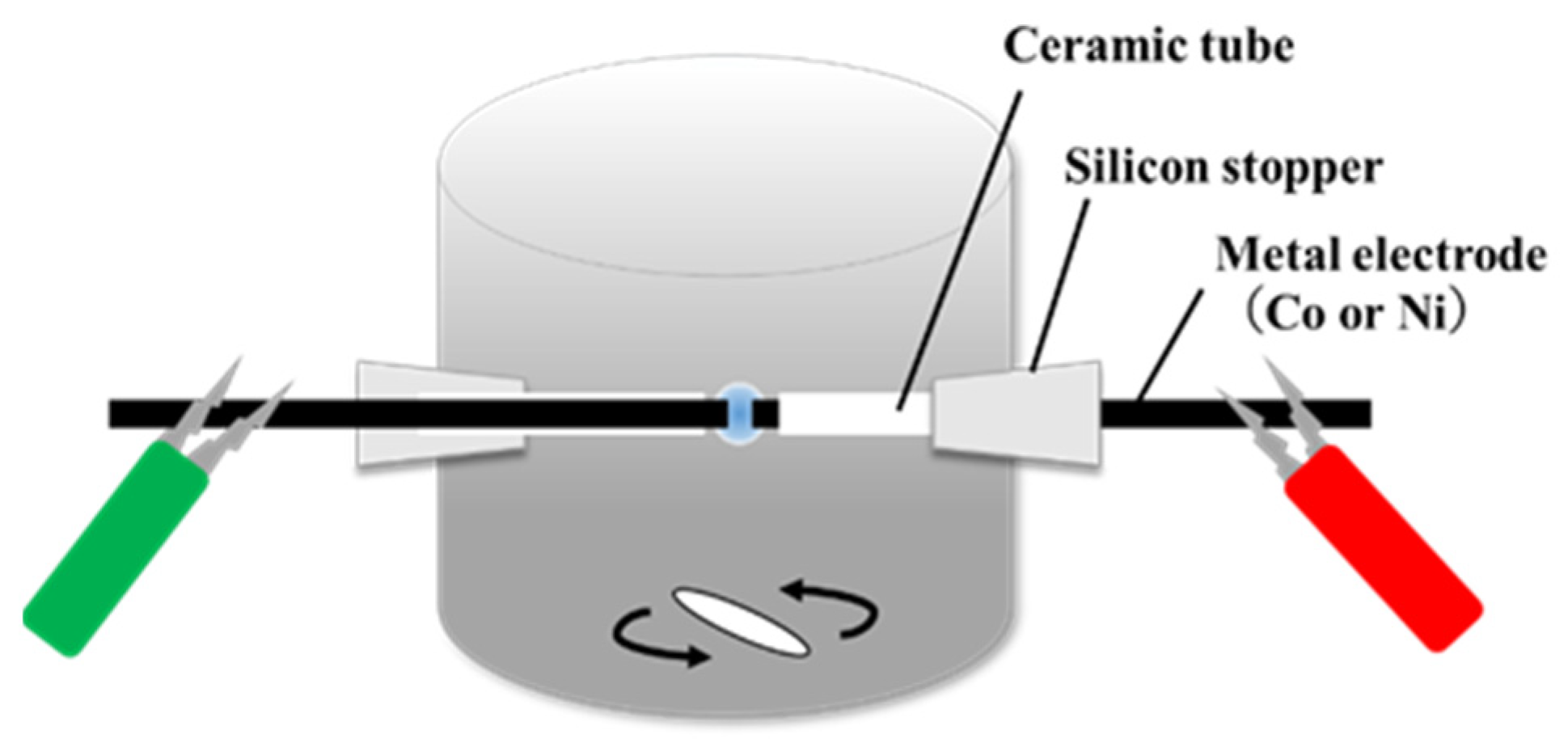
2.1. Synthesis of Carbon Materials
2.2. Evaluation of the Synthesized Carbon Materials
2.3. Electrochemical Measurements
3. Results and Discussion
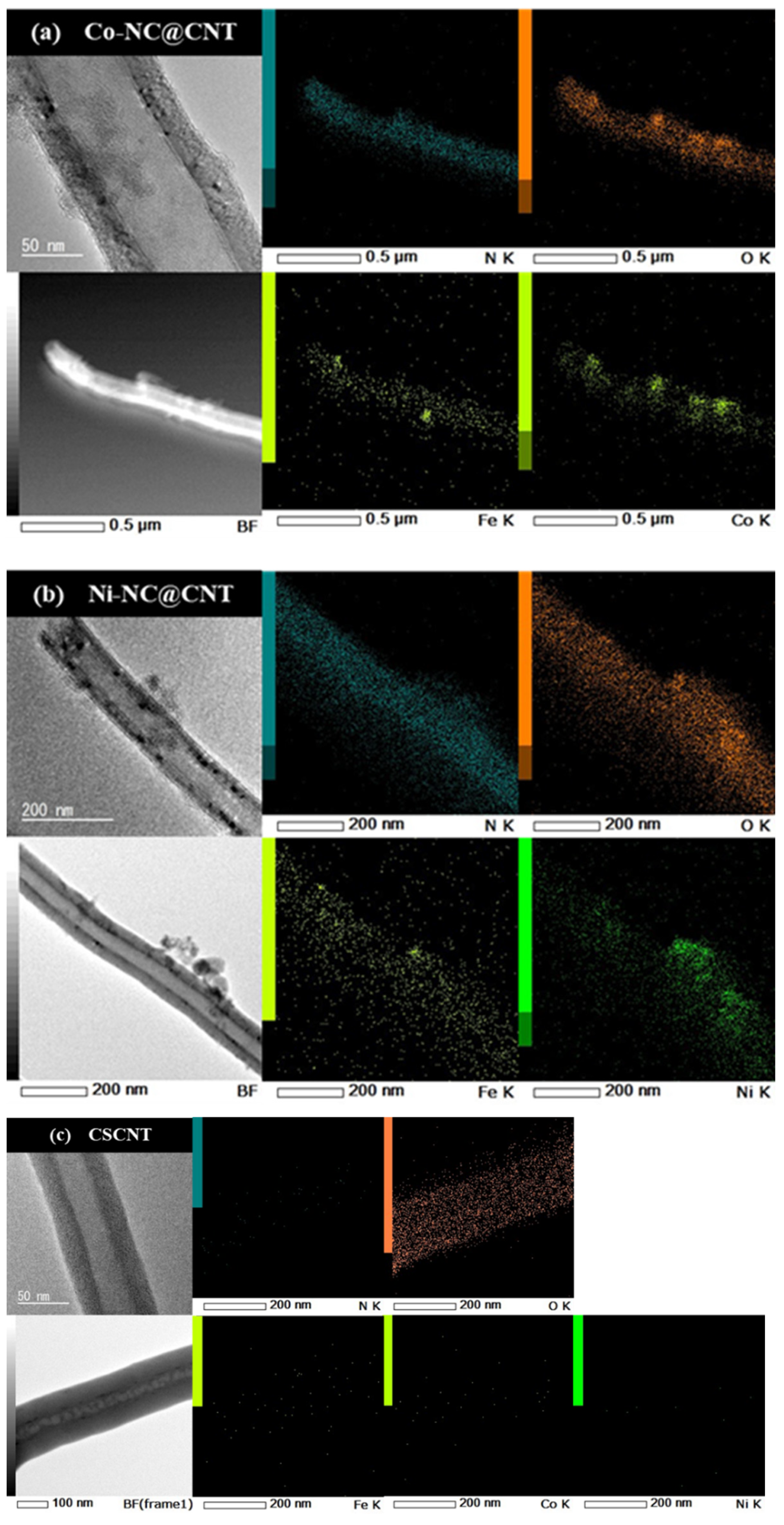
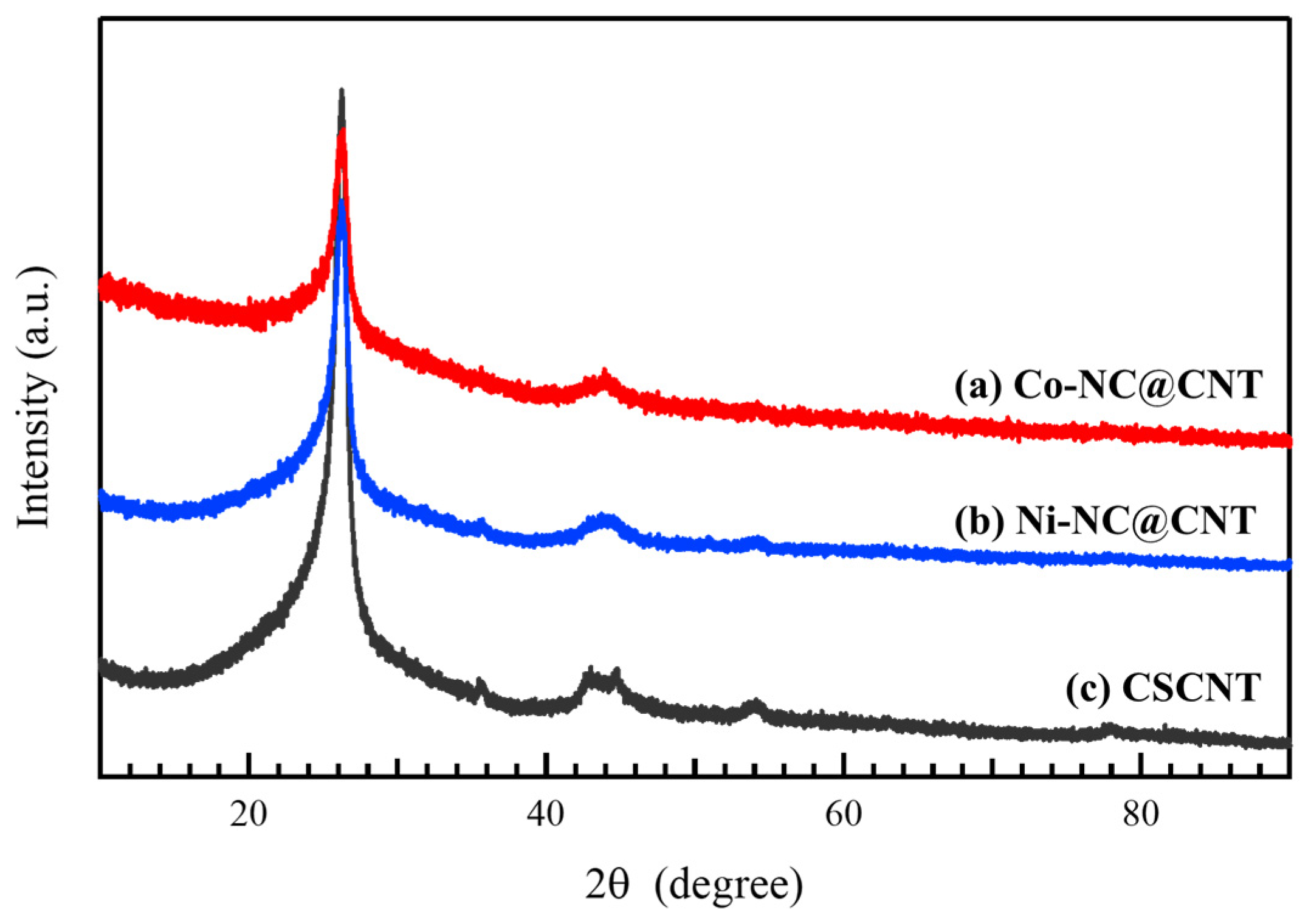
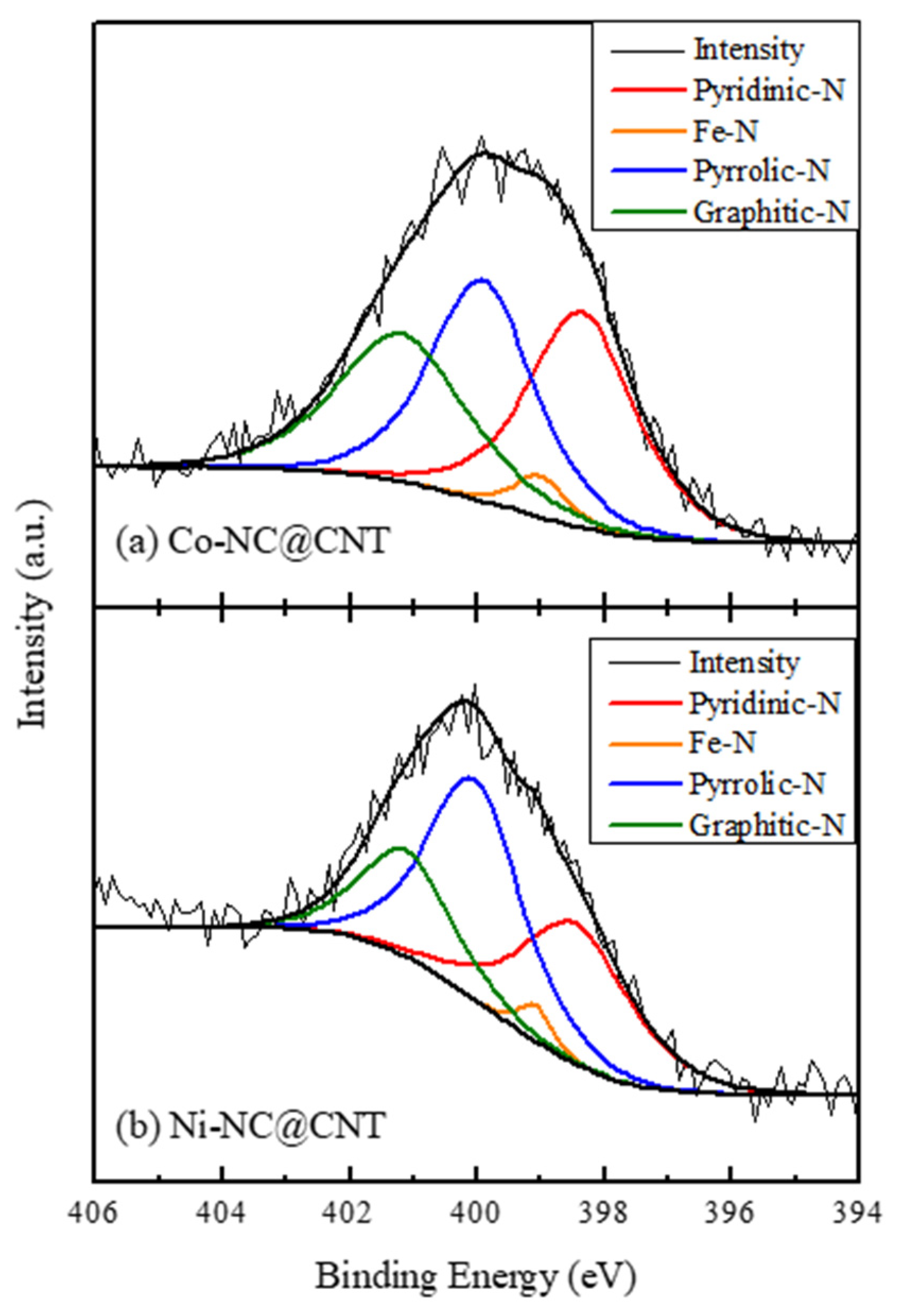
3.1. Characterization of the Synthesized Carbon Materials
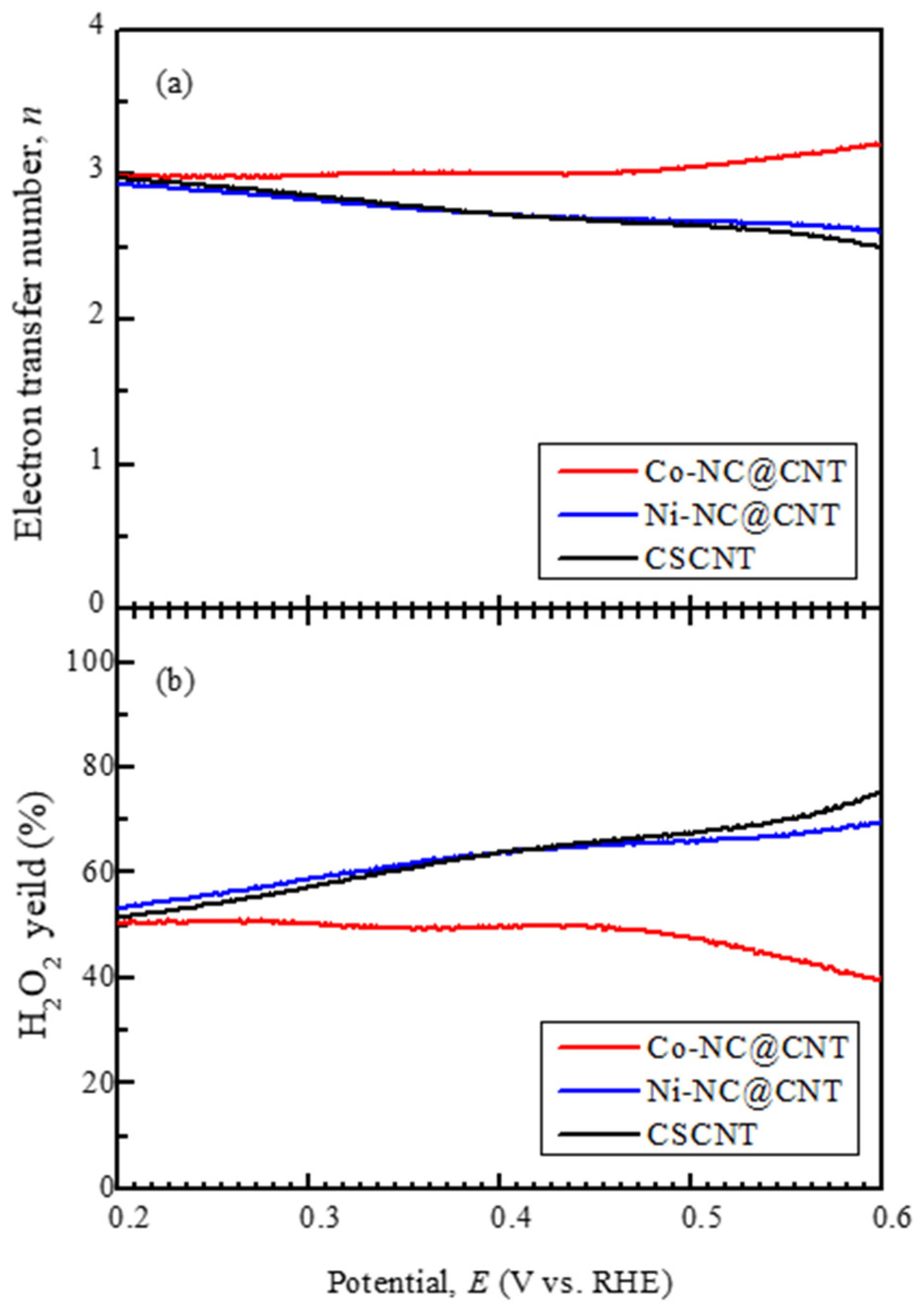
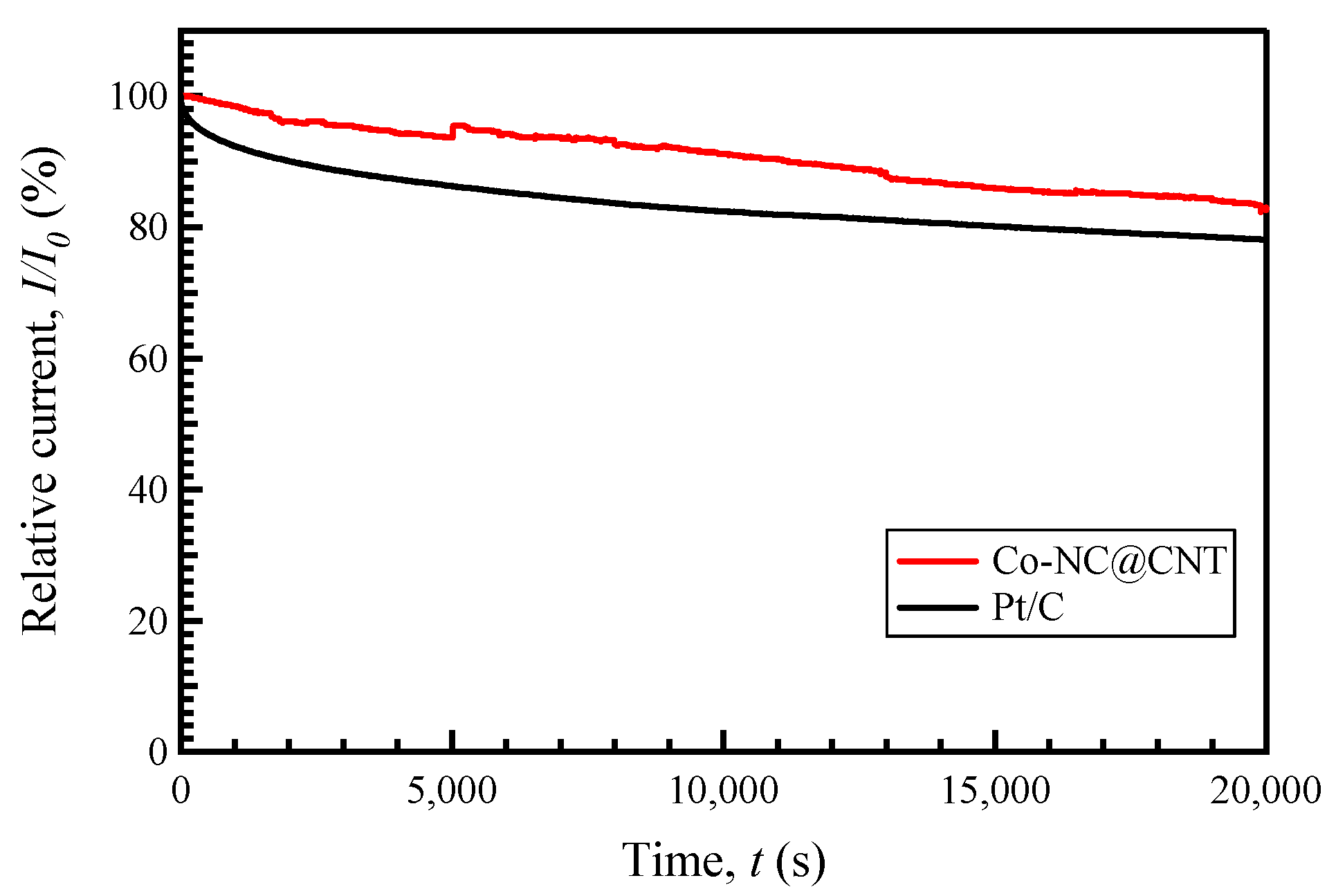
3.2. Electrocatalytic Activity of the Synthesized Carbon Materials
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, J.S.; Kim, S.T.; Cao, R.; Choi, N.S.; Liu, M.; Lee, K.T.; Cho, J. Metal–Air Batteries with High Energy Density: Li–Air versus Zn–Air. Adv. Energy Mater. 2011, 1, 34–50. [Google Scholar] [CrossRef]
- Girishkumar, G.; McCloskey, B.; Luntz, A.; Swanson, S.; Wilcke, W. Lithium−Air Battery: Promise and Challenges. J. Phys. Chem. Lett. 2010, 1, 2193–2203. [Google Scholar] [CrossRef]
- Jung, K.N.; Kim, J.; Yamauchi, Y.; Park, M.S.; Lee, J.-W.; Kim, J. Rechargeable lithium–air batteries: A perspective on the development of oxygen electrodes. J. Mater. Chem. A 2016, 4, 14050–14068. [Google Scholar] [CrossRef]
- Aurbach, C.; McCloskey, B.D.; Nazar, L.F.; Bruce, P.G. Advances in understanding mechanisms underpinning lithium–air batteries. Nat. Energy 2016, 1, 16128. [Google Scholar] [CrossRef]
- Harimech, Z.; Hairch, Y.; Atamanov, M.K.; Toshtay, K.; Azat, S.; Souhair, N.; Amrousse, R. Carbon nanotube iridium–cupric oxide supported catalysts for decomposition of ammonium dinitramide in the liquid phase. Int. J. Energ. Mat. Chem. Prop. 2023, 22, 13–18. [Google Scholar] [CrossRef]
- Cao, R.; Thapa, R.; Kim, H.; Xu, X.; Kim, M.G.; Li, Q.; Park, N.; Liu, M.; Cho, J. Promotion of oxygen reduction by a bio-inspired tethered iron phthalocyanine carbon nanotube-based catalyst. Nat. Commun. 2013, 4, 2076. [Google Scholar] [CrossRef]
- Yasuda, S.; Uchibori, Y.; Wakeshim, M.; Hinatsu, Y.; Ogawa, H.; Yano, M.; Asaoka, H. Enhancement of Fe–N–C carbon catalyst activity for the oxygen reduction reaction: Effective increment of active sites by a short and repeated heating process. RSC Adv. 2018, 8, 37600–37605. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Fe–N-doped carbon-based composite as an efficient and durable electrocatalyst for the oxygen reduction reaction. RSC Adv. 2016, 6, 114553–114559. [Google Scholar] [CrossRef]
- Kim, D.; Li, O.L.; Saito, N. The role of the central Fe atom in the N4-macrocyclic structure for the enhancement of oxygen reduction reaction in a heteroatom nitrogen–carbon nanosphere. Phys. Chem. Chem. Phys. 2014, 16, 14905–14911. [Google Scholar] [CrossRef]
- Kim, H.; Cha, B.; Kim, D. Simultaneous introduction of iodine and Fe-Nx into carbon nanospheres for enhanced catalytic activity towards oxygen reduction using a solution plasma process. Electrochem. Commun. 2023, 156, 107589. [Google Scholar] [CrossRef]
- Kim, S.; Kato, S.; Ishizaki, T.; Li, O.L.; Kang, J. Transition Metal (Fe, Co, Ni) Nanoparticles on Selective Amino-N-Doped Carbon as High-Performance Oxygen Reduction Reaction Electrocatalyst. Nanomaterials 2019, 9, 742. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Jiang, H.; Zhu, Y.; Yang, X.; Li, C. Transition metals (Fe, Co, and Ni) encapsulated in nitrogen-doped carbon nanotubes as bi-functional catalysts for oxygen electrode reactions. J. Mater. Chem. A 2016, 4, 1694–1701. [Google Scholar] [CrossRef]
- Panomsuwan, G.; Saito, N.; Ishizaki, T. Nitrogen-Doped Carbon Nanoparticle–Carbon Nanofiber Composite as an Efficient Metal-Free Cathode Catalyst for Oxygen Reduction Reaction. ACS Appl. Mater. Interfaces 2016, 8, 6962–6971. [Google Scholar] [CrossRef] [PubMed]
- Watthanaphanit, A.; Saito, N. Effect of polymer concentration on the depolymerization of sodium alginate by the solution plasma process. Mater. Lett. 2011, 65, 1037–1040. [Google Scholar] [CrossRef]
- Hyun, K.; Ueno, T.; Saito, N. Synthesis of nitrogen-containing carbon by solution plasma in aniline with high-repetition frequency discharges. Jpn. J. Appl. Phys. 2015, 55, 01AE18. [Google Scholar] [CrossRef]
- Kang, J.; Li, O.L.; Saito, N. Synthesis of structure-controlled carbon nano spheres by solution plasma process. Thin Solid Films 2013, 60, 292–298. [Google Scholar] [CrossRef]
- Hieda, J.; Shirafuji, T.; Noguchi, Y.; Saito, N.; Takai, O. Solution Plasma Surface Modification for Nanocarbon-Composite Materials. J. Jpn. Inst. Metals. 2009, 73, 938–942. [Google Scholar] [CrossRef][Green Version]
- Narahara, M.; Lee, S.Y.; Sasaki, K.; Fukushima, K.; Tanaka, K.; Chae, S.; Hu, X.; Panomsuwan, G.; Ishizaki, T. Solution plasma synthesis of perovskite hydroxide CoSn(OH)6 nanocube electrocatalysts toward the oxygen evolution reaction. Sustain. Energy Fuels 2023, 7, 2582–2593. [Google Scholar] [CrossRef]
- Kang, J.; Li, O.L.; Saito, N. Hierarchical meso–macro structure porous carbon black as electrode materials in Li–air battery. J. Power Sources 2014, 261, 156–161. [Google Scholar] [CrossRef]
- Lee, S.; Saito, N. Enhancement of nitrogen self-doped nanocarbons electrocatalyst via tune-up solution plasma synthesis. RSC Adv. 2018, 8, 35503–35511. [Google Scholar] [CrossRef]
- Hyun, K.; Saito, N. The solution plasma process for heteroatom-carbon nanosheets: The role of precursors. Sci. Rep. 2017, 7, 3825. [Google Scholar] [CrossRef] [PubMed]
- Hyun, K.; Ueno, T.; Li, O.L.; Saito, N. Synthesis of heteroatom-carbon nanosheets by solution plasma processing using N-methyl-2-pyrrolidone as precursor. RSC Adv. 2016, 6, 6990–6996. [Google Scholar] [CrossRef]
- Hyun, K.; Ueno, T.; Panomsuwan, G.; Li, O.L.; Saito, N. Heterocarbon nanosheets incorporating iron phthalocyanine for oxygen reduction reaction in both alkaline and acidic media. Phys. Chem. Chem. Phys. 2016, 18, 10856–10863. [Google Scholar] [CrossRef] [PubMed]
- Chae, S.; Phan, P.Q.; Panomsuwan, G.; Bratescu, M.A.; Hashimoto, T.; Teshima, K.; Saito, N. Single-Walled Carbon Nanotubes Wrapped by Cationic Nitrogen-Doped Carbon for Electrocatalytic Application. ACS Appl. Nano Mater. 2020, 3, 10183–10189. [Google Scholar] [CrossRef]
- Phan, P.Q.; Naraprawatphong, R.; Pornaroontham, P.; Park, J.; Chokradjaroen, C.; Saito, N. N-Doped few-layer graphene encapsulated Pt-based bimetallic nanoparticles via solution plasma as an efficient oxygen catalyst for the oxygen reduction reaction. Mater. Adv. 2021, 2, 322–335. [Google Scholar] [CrossRef]
- Kang, J.; Li, O.L.; Saito, N. A simple synthesis method for nano-metal catalyst supported on mesoporous carbon: The solution plasma process. Nanoscale 2013, 5, 6874–6882. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Kim, K.; Wang, X.; Huda, M.; Sawada, Y.; Matsuo, Y.; Saito, N.; Kawasumi, M. Highly durable graphene-encapsulated platinum-based electrocatalyst for oxygen reduction reactions synthesized by solution plasma process. J. Power Sources 2023, 580, 233419. [Google Scholar] [CrossRef]
- Kim, D.; Li, O.L.; Pootawang, P.; Saito, N. Solution plasma synthesis process of tungsten carbide on N-doped carbon nanocomposite with enhanced catalytic ORR activity and durability. RSC Adv. 2014, 4, 16813–16819. [Google Scholar] [CrossRef]
- Jeong, S.; Kim, S.; Son, H.; Li, O.L. Plasma-engineered cobalt nanoparticle encapsulated N-doped graphene nanoplatelets as high-performance oxygen reduction reaction electrocatalysts for aluminum–air batteries. Catal. Today 2023, 420, 114025. [Google Scholar] [CrossRef]
- Hu, X.; Shi, J.; Zhang, J.; Tang, W.; Zhu, H.; Shen, X.; Saito, N. One-step facile synthesis of carbon-supported PdAu nanoparticles and their electrochemical property and stability. J. Alloys Compds. 2015, 619, 452–457. [Google Scholar] [CrossRef]
- Shi, J.; Hu, X.; Zhang, J.; Tang, W.; Li, H.; Shen, X.; Saito, N. One-step facile synthesis of Pd nanoclusters supported on carbon and their electrochemical property. Prog. Natural Sci. Mater. Int. 2014, 24, 593–598. [Google Scholar] [CrossRef]
- Hu, X.; Shen, X.; Takai, O.; Saito, N. Facile fabrication of PtAu alloy clusters using solution plasma sputtering and their electrocatalytic activity. J. Alloys Compds. 2013, 552, 351–355. [Google Scholar] [CrossRef]
- Yoshida, T.; Yamamoto, N.; Mizutani, T.; Yamamoto, M.; Ogawa, S.; Yagi, S.; Nameki, H.; Yoshida, H. Synthesis of Ag nanoparticles prepared by a solution plasma method and application as a cocatalyst for photocatalytic reduction of carbon dioxide with water. Catal. Today 2018, 303, 320–326. [Google Scholar] [CrossRef]
- Thess, A.; Lee, R.; Nikolaev, P.; Dai, H.; Petit, P.; Robert, J.; Xu, C.; Lee, Y.H.; Kim, S.G.; Rinzler, A.G.; et al. Crystalline Ropes of Metallic Carbon Nanotubes. Science 1996, 273, 483–487. [Google Scholar] [CrossRef] [PubMed]
- Lemes, G.; Sebastian, D.; Pastor, E.; Lazaro, J.M. N-doped graphene catalysts with high nitrogen concentration for the oxygen reduction reaction. J. Power Sources 2019, 438, 227036. [Google Scholar] [CrossRef]
- Zheng, Y.; Liu, J.; Liang, J.; Jaroniecc, M.; Qiao, S.-Z. Graphitic carbon nitride materials: Controllable synthesis and applications in fuel cells and photocatalysis. Energy Environ. Sci. 2012, 5, 6717–6731. [Google Scholar] [CrossRef]
- Guo, C.; Wu, Y.; Li, Z.; Liao, W.; Sun, L.; Wang, C.; Wen, B.; Li, Y.; Chen, C. The Oxygen Reduction Electrocatalytic Activity of Cobalt and Nitrogen Co-doped Carbon Nanocatalyst Synthesized by a Flat Template. Nanoscale Res. Lett. 2017, 12, 144. [Google Scholar] [CrossRef] [PubMed]
- Pariiska, O.; Mazur, D.; Cherchenko, K.; Kurys, Y.; Koshechko, V.; Pokhodenko, V. Efficient Co-N-C electrocatalysts for oxygen reduction derived from deep eutectic solvents. Electrochim. Acta 2022, 413, 140132. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, H.; Diao, P.; Chang, W.; Hong, G.; Li, Y.; Gong, M.; Xie, L.; Zhou, J.; Wang, J.; et al. Oxygen reduction electrocatalyst based on strongly coupled cobalt oxide nanocrystals and carbon nanotubes. J. Am. Chem. Soc. 2012, 134, 15849–15857. [Google Scholar] [CrossRef]
- Liang, Y.; Li, Y.; Wang, H.; Zhou, J.; Wang, J.; Regier, T.; Dai, H. Co3O4 nanocrystals on graphene as a synergistic catalyst for oxygen reduction reaction. Nat. Mater. 2011, 10, 780–786. [Google Scholar] [CrossRef]


| C | N | O | Fe | Co | Ni | |
|---|---|---|---|---|---|---|
| Co-NC@CNT | 91.2 | 0.8 | 7.7 | 0.1 | 0.2 | - |
| Ni-NC@CNT | 89.6 | 0.7 | 9.5 | 0.1 | - | 0.1 |
| Pyridinic-N | Fe-N | Pyrrolic N | Graphitic N | |
|---|---|---|---|---|
| Co-NC@CNT | 34.5 | 3.3 | 33.9 | 28.3 |
| Ni-NC@CNT | 32.8 | 2.6 | 44.1 | 20.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sasaki, K.; Yamamoto, K.; Narahara, M.; Takabe, Y.; Chae, S.; Panomsuwan, G.; Ishizaki, T. Solution-Plasma Synthesis and Characterization of Transition Metals and N-Containing Carbon–Carbon Nanotube Composites. Materials 2024, 17, 320. https://doi.org/10.3390/ma17020320
Sasaki K, Yamamoto K, Narahara M, Takabe Y, Chae S, Panomsuwan G, Ishizaki T. Solution-Plasma Synthesis and Characterization of Transition Metals and N-Containing Carbon–Carbon Nanotube Composites. Materials. 2024; 17(2):320. https://doi.org/10.3390/ma17020320
Chicago/Turabian StyleSasaki, Kodai, Kaiki Yamamoto, Masaki Narahara, Yushi Takabe, Sangwoo Chae, Gasidit Panomsuwan, and Takahiro Ishizaki. 2024. "Solution-Plasma Synthesis and Characterization of Transition Metals and N-Containing Carbon–Carbon Nanotube Composites" Materials 17, no. 2: 320. https://doi.org/10.3390/ma17020320
APA StyleSasaki, K., Yamamoto, K., Narahara, M., Takabe, Y., Chae, S., Panomsuwan, G., & Ishizaki, T. (2024). Solution-Plasma Synthesis and Characterization of Transition Metals and N-Containing Carbon–Carbon Nanotube Composites. Materials, 17(2), 320. https://doi.org/10.3390/ma17020320










