Corrosion Evaluation and Mechanism Research of AISI 8630 Steel in Offshore Oil and Gas Environments
Abstract
1. Introduction
2. Experimental Method
2.1. Corrosion Rate Measurement
- where R—the corrosion rate, mm/year;
- M0—the mass of the sample before the test, g;
- M1—the mass of the sample after the test, g;
- S—the total area of the sample, cm2;
- T—the test time, h;
- D—the density of the material, kg/m3;
- K—a constant equal to 8.76 × 107.
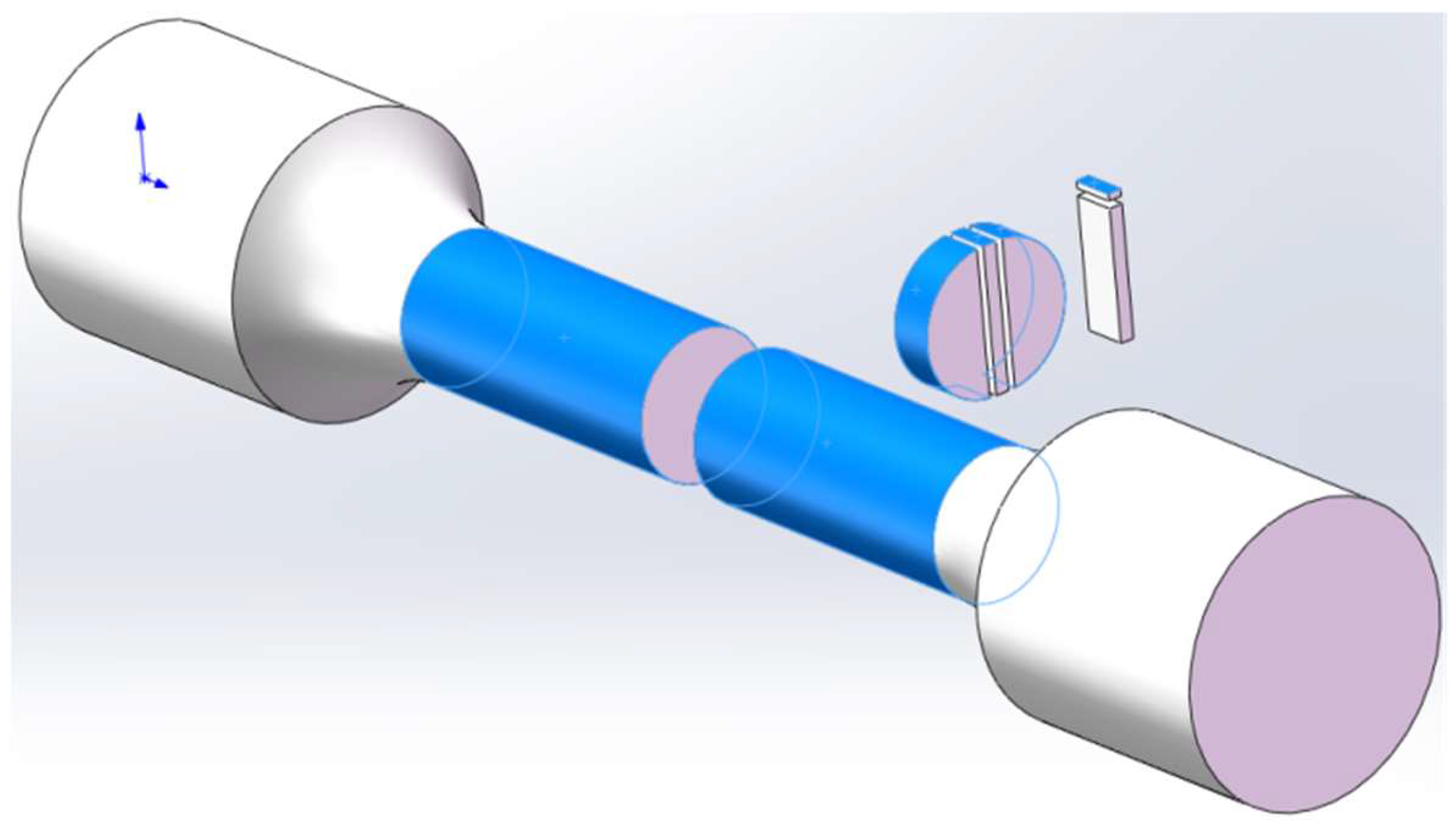
2.2. Hydrogen Sulfide Stress Corrosion Cracking Test
2.3. Macroscopic Observation and X-ray Diffraction
2.4. Sample Preparation and Transmission Electron Microscopy
3. The Results
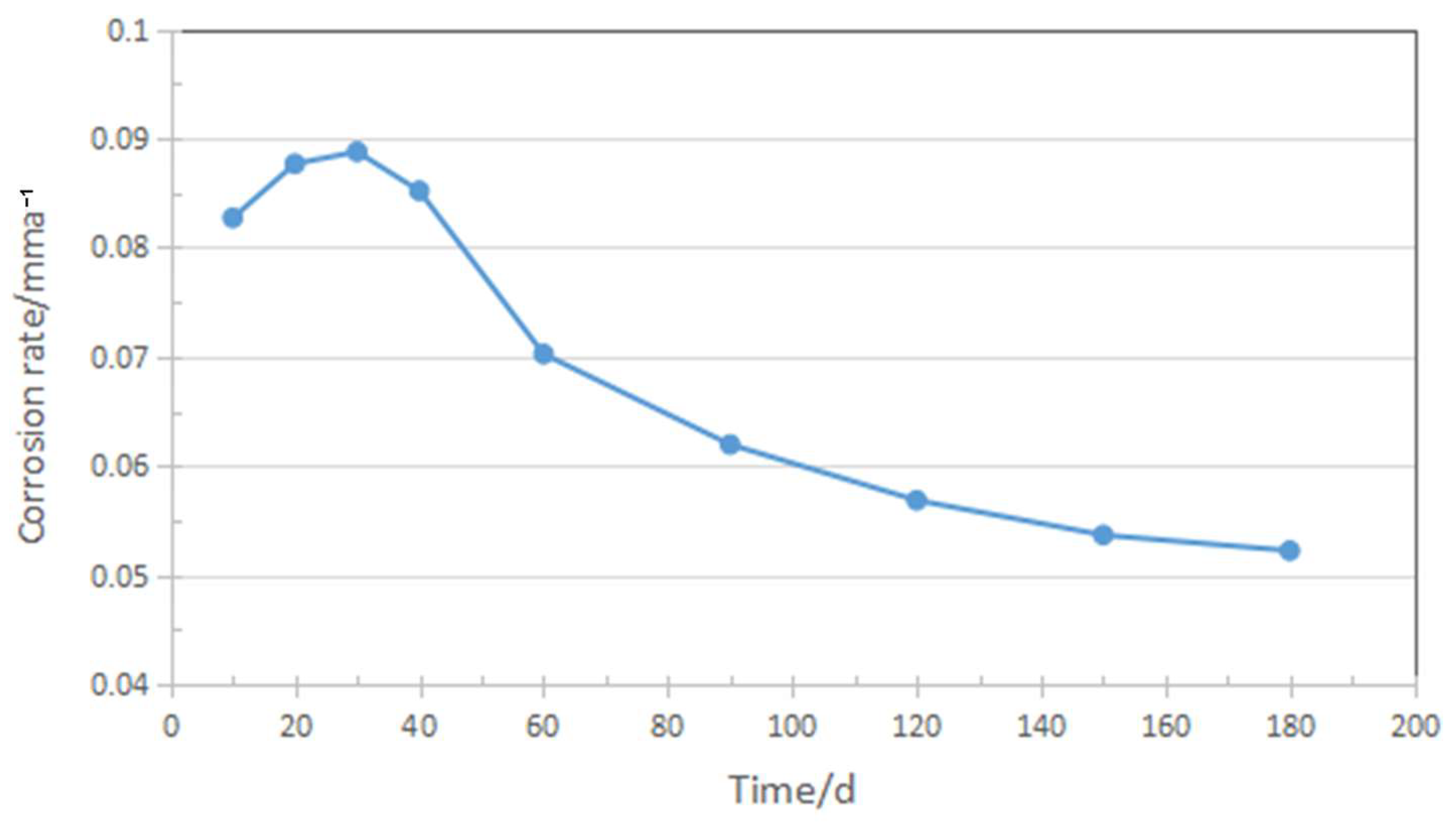
3.1. Corrosion Rate Analysis
3.2. Macroscopic Examination after Corrosion Testing
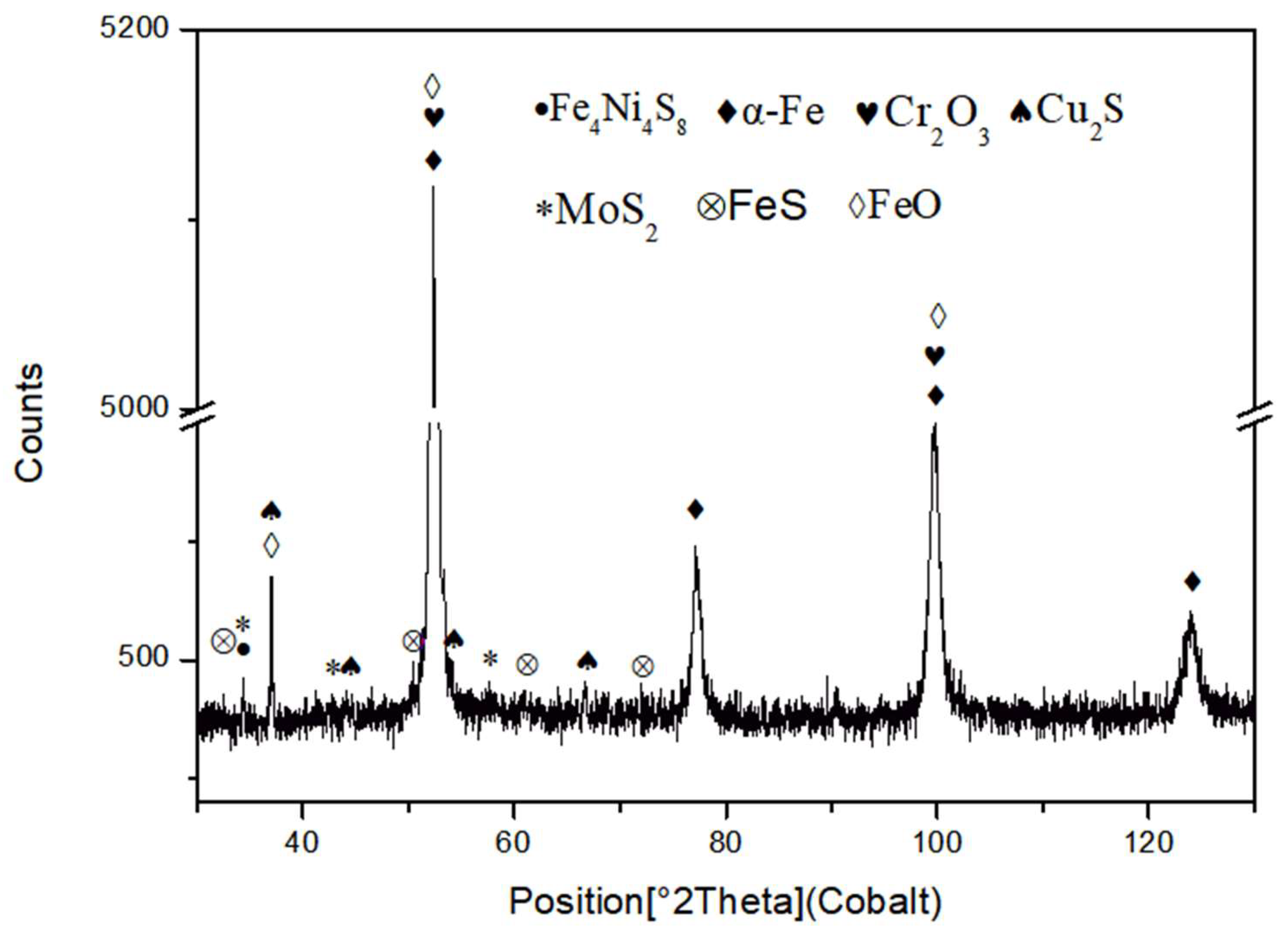
3.3. X-ray Diffraction Analysis of Corrosion Products
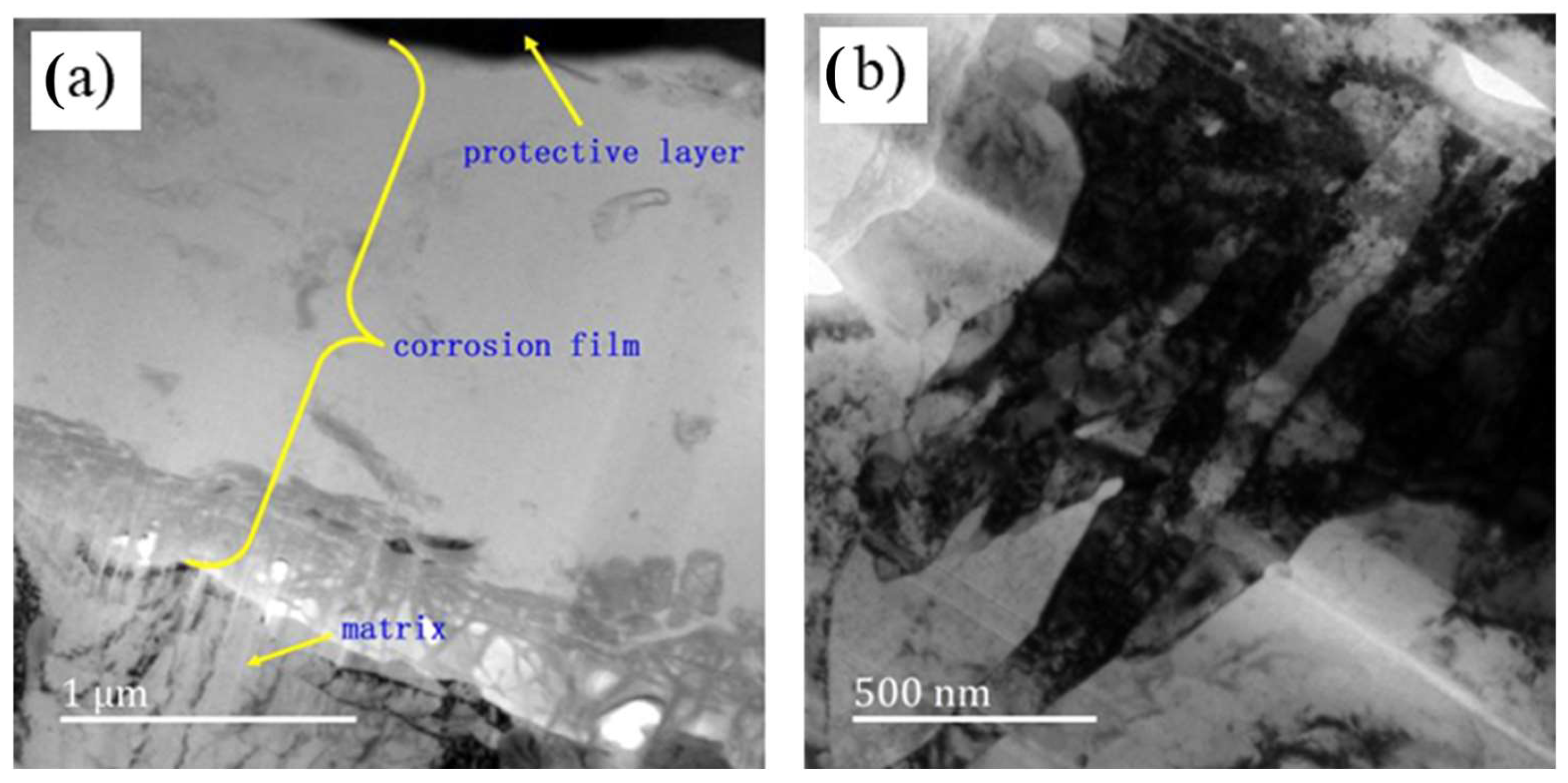
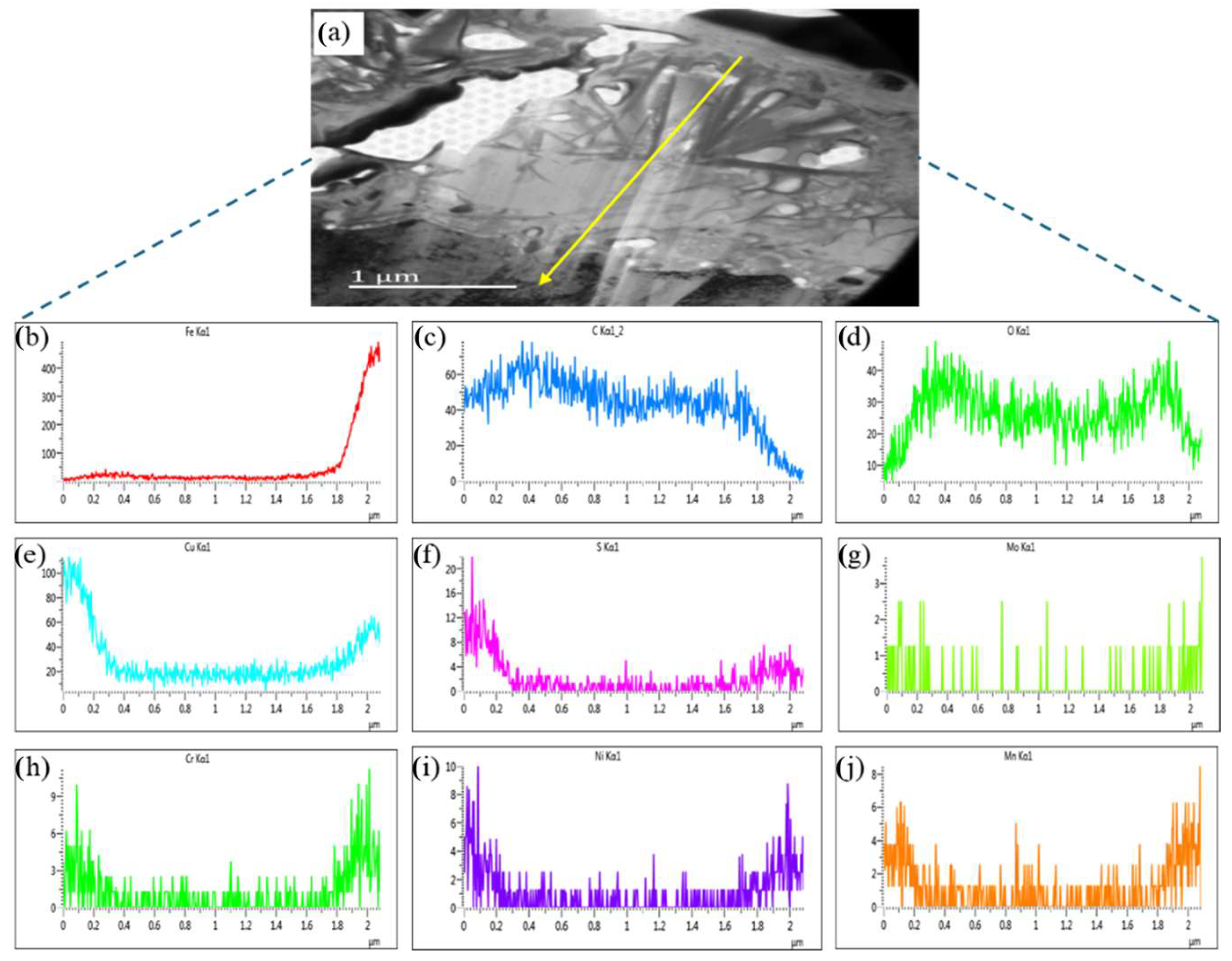
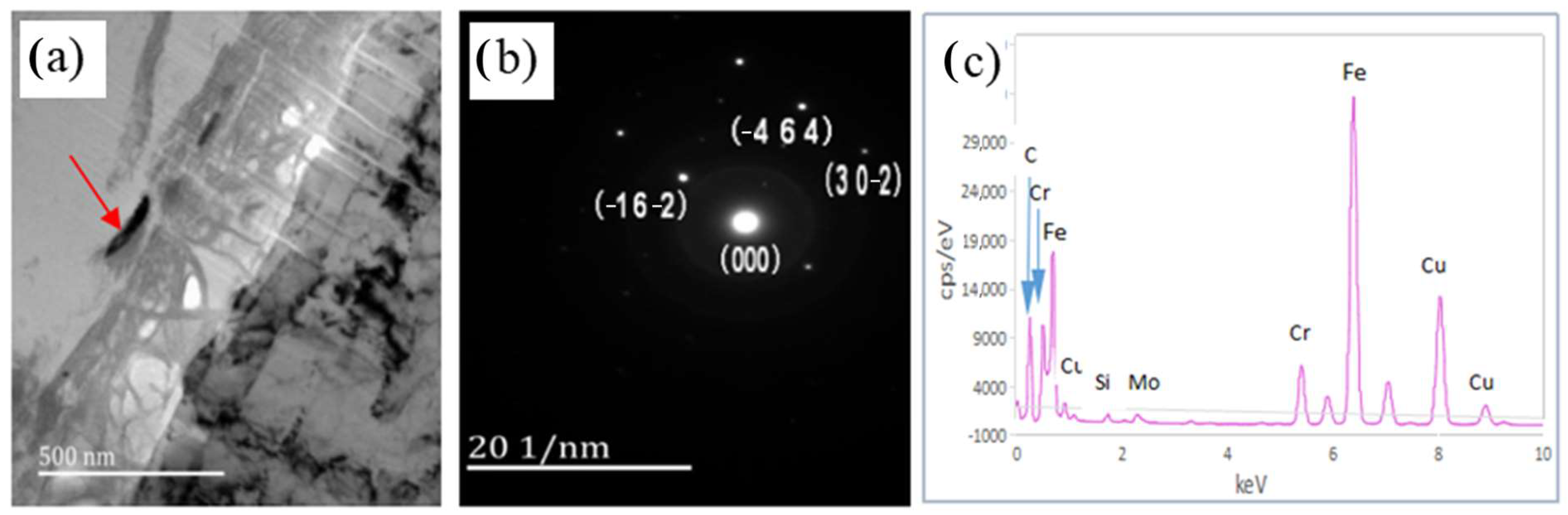
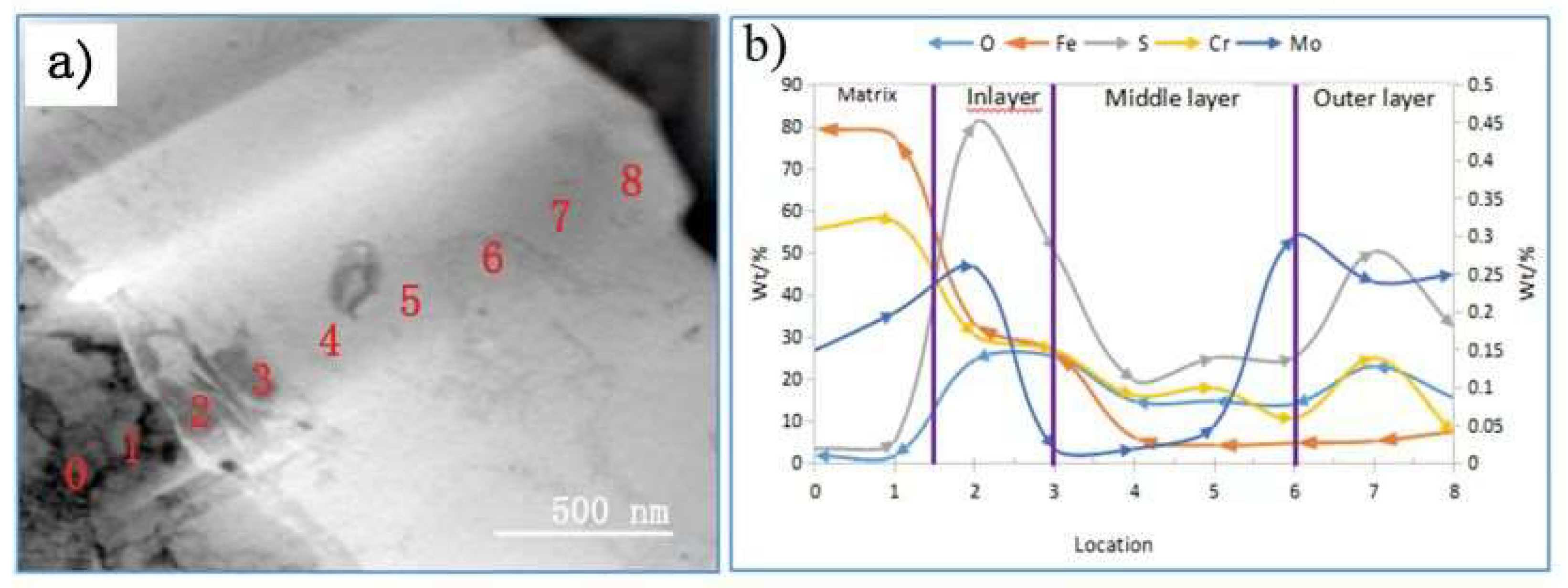
3.4. Transmission Electron Microscopy of the Corrosion Film
4. Analysis and Discussion
4.1. Analysis of the Immersion Corrosion Rate
4.2. Analysis of SSCC Products
4.3. Analysis of the Corrosion Film after SSCC
5. Conclusions
- Corrosion tests were conducted on improved AISI 8630 steel in a 3.5% NaCl solution. A 720-h fixed-load tensile test was performed to evaluate its susceptibility to sulfide stress corrosion cracking (SSCC), following the NACE TM-0177-2016 standard. The results showed a decrease in the corrosion rate from approximately 0.09 mm/year at 30 days to about 0.05 mm/year after 180 days. This reduction is attributed to the formation of a thicker and more adherent corrosion film over time.
- After 720 h of the SSCC test, the corrosion products on the sample surface mainly consisted of FeO, FeS, Cr2O3, MoS2, and Cu2S. The corrosion film comprising iron oxides and sulfides were loose and porous, whereas the layers of Cr2O3 and Cu2S formed a dense, well-adhered rust layer. This dense film significantly contributes to improved corrosion resistance.
- The corrosion film on the SSCC sample after 720 h exhibited a three-layer structure. The innermost layer, with a thickness of 200–300 nm, contained higher concentrations of alloying elements and formed a dense, cohesive rust layer that hindered the diffusion of oxygen and chloride ions, thus enhancing corrosion resistance. The middle layer was thicker and less rich in alloying elements, while the outer layer was loose and porous. This multi-layered structure acts as a barrier, slowing down the diffusion of corrosive species into the steel substrate and reducing crack initiation.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Seyyedattar, M.; Zendehboudi, S.; Butt, S. Technical and Non-technical Challenges of Development of Offshore Petroleum Reservoirs: Characterization and Production. Nat. Resour. Res. 2019, 29, 2147–2189. [Google Scholar] [CrossRef]
- Sharma, A.; Sharma, S. Graphene-based polymer coatings for preventing marine corrosion: A review. J. Coat. Technol. Res. 2023, 20, 413–432. [Google Scholar] [CrossRef]
- Rong, X.; Zhu, H. Research on erosion model based on AISI 8630. J. Phys. Conf. Ser. 2020, 1633, 012018. [Google Scholar] [CrossRef]
- Bala, Y.G.; Sankaranarayanan, S.R.; Pandey, K.S. Assessment of densification and mechanical property of AISI 8630 steel composition on different heat treatments produced through hot upsetting powder preform forging. Met. Mater. Int. 2015, 21, 1044–1053. [Google Scholar] [CrossRef]
- Zhang, M.X.; Liu, H.X.; Karn, S.K.; Duan, J.Z.; Guan, F.; Zhai, X.F.; Zhao, S.J.; Li, K.; Hou, B.R. Corrosion Behavior of Q235 Carbon Steel in Presence of H2S Producing and a Consortium of Microbes Isolated from Inner Rust Layer. Int. J. Electrochem. Sci. 2017, 12, 2315–2328. [Google Scholar] [CrossRef]
- Liu, M.; Cheng, X.Q.; Li, X.G.; Yue, P.; Li, J. Corrosion Behavior and Durability of Low-Alloy Steel Rebars in Marine Environment. J. Mater. Eng. Perform. 2016, 25, 4967–4979. [Google Scholar] [CrossRef]
- Murayama, M.; Nishimura, T.; Tsuzaki, K. Nano-scale chemical analysis of rust on a 2% Si-bearing low alloy steel exposed in a coastal environment. Corros. Sci. 2008, 50, 2159–2165. [Google Scholar] [CrossRef]
- Ghosh, S.; Kain, V. Microstructural changes in AISI 304L stainless steel due to surface machining: Effect on its susceptibility to chloride stress corrosion cracking. J. Nucl. Mater. 2010, 403, 62–67. [Google Scholar] [CrossRef]
- Dai, T.; Thodla, R.; Kovacs Iii, W.; Tummala, K.; Lippold, J. Effect of Postweld Heat Treatment on the Sulfide Stress Cracking of Dissimilar Welds of Nickel Base Alloy 625 on Steels. Corrosion 2019, 75, 641–656. [Google Scholar] [CrossRef]
- ASTM NACE/ASTM G31-2012; Standard Practice for Laboratory Immersion Corrosion Testing of Metals. ASTM International: West Conshohocken, PA, USA, 2012.
- Lv, W.; Pan, C.; Su, W.; Wang, Z.; Liu, S.; Wang, C. Atmospheric corrosion mechanism of 316 stainless steel in simulated marine atmosphere. Corros. Eng. Sci. Technol. 2016, 51, 155–162. [Google Scholar] [CrossRef]
- Li, Z.L.; Xiao, K.; Dong, C.F.; Cheng, X.Q.; Xue, W.; Yu, W. Atmospheric corrosion behavior of low-alloy steels in a tropical marine environment. J. Iron Steel Res. Int. 2019, 26, 1315–1328. [Google Scholar] [CrossRef]
- Zhu, J.; Li, D.; Chang, W.; Wang, Z.; Hu, L.; Zhang, Y.; Wang, M.; Yang, Z.; Song, J.; Chen, S.; et al. In situ marine exposure study on corrosion behaviors of five alloys in coastal waters of western Pacific Ocean. J. Mater. Res. Technol. 2020, 9, 8104–8116. [Google Scholar] [CrossRef]
- Kim, H.; Yarin, A.L.; Lee, M.W. Self-healing corrosion protection film for marine environment. Compos. Part B Eng. 2020, 182, 107598. [Google Scholar] [CrossRef]
- Gkatzogiannis, S.; Weinert, J.; Engelhardt, I.; Knoedel, P.; Ummenhofer, T. Correlation of laboratory and real marine corrosion for the investigation of corrosion fatigue behaviour of steel components. Int. J. Fatigue 2019, 126, 90–102. [Google Scholar] [CrossRef]
- Tewary, N.K.; Kundu, A.; Nandi, R.; Saha, J.K.; Ghosh, S.K. Microstructural characterisation and corrosion performance of old railway girder bridge steel and modern weathering structural steel. Corros. Sci. 2016, 113, 57–63. [Google Scholar] [CrossRef]
- Daniel, E.F.; Wang, C.G.; Li, C.; Dong, J.H.; Udoh, I.I.; Zhang, D.J.; Zhong, W.A.; Zhong, S. Evolution of corrosion degradation in galvanised steel bolts exposed to a tropical marine environment. J. Mater. Res. Technol. 2023, 27, 5177–5190. [Google Scholar] [CrossRef]
- Deng, P.; Li, Z.; Li, X.; Hu, J.; Wang, G. Vertical galvanic corrosion of pipeline steel in simulated marine thermocline. Ocean Eng. 2020, 217, 107584. [Google Scholar] [CrossRef]
- Xu, Y.; Huang, Y.; Cai, F.; Wang, Z.; Lu, D.; Wang, X.; Yang, L. Evaluation of Hydrogen Permeation into High-Strength Steel during Corrosion in Different Marine Corrosion Zones. Appl. Sci. 2022, 12, 2785. [Google Scholar] [CrossRef]
- Tian, H.; Cui, Z.; Ma, H.; Zhao, P.; Yan, M.; Wang, X.; Cui, H. Corrosion evolution and stress corrosion cracking behavior of a low carbon bainite steel in the marine environments: Effect of the marine zones. Corros. Sci. 2022, 206, 110490. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Yu, H.; Liu, J.; Cheng, L.; Hu, S.-E.; Wu, K. Effects of pearlite on corrosion initiation and propagation in weathering steels in marine environments. J. Mater. Sci. 2022, 57, 6039–6055. [Google Scholar] [CrossRef]
- Peng, L.; Stewart, M.G.; Melchers, R.E. Corrosion and capacity prediction of marine steel infrastructure under a changing environment. Struct. Infrastruct. Eng. 2016, 13, 988–1001. [Google Scholar] [CrossRef]
- Hu, J.; Lin, G.; Deng, P.; Li, Z.; Tian, Y. Galvanic Corrosion of E690 Offshore Platform Steel in a Simulated Marine Thermocline. Metals 2024, 14, 287. [Google Scholar] [CrossRef]
- Gartner, N.; Kosec, T.; Legat, A. Monitoring the Corrosion of Steel in Concrete Exposed to a Marine Environment. Materials 2020, 13, 407. [Google Scholar] [CrossRef]
- Liu, H.; Teng, Y.; Guo, J.; Li, N.; Wang, J.; Zhou, Z.; Li, S. Corrosion resistance and corrosion behavior of high-copper-bearing steel in marine environments. Mater. Corros. 2020, 72, 816–828. [Google Scholar] [CrossRef]
- Wu, W.; Dai, Z.; Liu, Z.; Liu, C.; Li, X. Synergy of Cu and Sb to enhance the resistance of 3%Ni weathering steel to marine atmospheric corrosion. Corros. Sci. 2021, 183, 109353. [Google Scholar] [CrossRef]
- Wang, Y.; Lv, P.; Liu, Y.; Zhou, X. Modification and corrosion resistance of halloysite carrier with metal nanoinhibitor in marine corrosion environment. Anti-Corros. Methods Mater. 2022, 69, 371–379. [Google Scholar] [CrossRef]
- Khan, M.S.; Liang, T.; Liu, Y.; Shi, Y.; Zhang, H.; Li, H.; Guo, S.; Pan, H.; Yang, K.; Zhao, Y. Microbiologically Influenced Corrosion Mechanism of Ferrous Alloys in Marine Environment. Metals 2022, 12, 185. [Google Scholar] [CrossRef]
- Takabe, H.; Ueda, M.; Fujimoto, S. Corrosion Behavior under Black Deposit on Low Cr Bearing Steels in NaCl Completion Fluid. ISIJ Int. 2008, 48, 1758–1765. [Google Scholar] [CrossRef]
- Cao, H.; Wang, K.; Song, S.; Zhang, X.; Gao, Q.; Liu, Y. Corrosion behavior research and corrosion prediction of structural steel in marine engineering. Anti-Corros. Methods Mater. 2022, 69, 636–650. [Google Scholar] [CrossRef]
- Xiang, B.; Zhang, J.; Duan, J.Z.; Hou, B.R. Study on corrosion of steels in marine environment by corrosion simulation device. Mater. Corros. 2003, 54, 697–702. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, X.; Cheng, L.; Liu, J.; Wu, K. Role of inclusion and microstructure on corrosion initiation and propagation of weathering steels in marine environment. J. Mater. Res. Technol. 2020, 10, 306–321. [Google Scholar] [CrossRef]
- Xu, X.; Li, J.; Zhang, W.; Liu, Q.; Hu, B.; Wang, X.; Shang, C.; Misra, R.D.K. Structure-Property Relationship in a Micro-laminated Low-Density Steel for Offshore Structure. Steel Res. Int. 2019, 90, 1800515. [Google Scholar] [CrossRef]


| Element | C | Mn | Si | Cr | Mo | Ni | P | S | V | Cu |
|---|---|---|---|---|---|---|---|---|---|---|
| Actual | 0.28 | 0.78 | 0.21 | 0.90 | 0.45 | 0.55 | 0.0048 | 0.0031 | 0.031 | 0.015 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Z.; Wen, L.; Huang, Q.; Guo, L.; Dong, Z.; Zhu, L. Corrosion Evaluation and Mechanism Research of AISI 8630 Steel in Offshore Oil and Gas Environments. Materials 2024, 17, 4907. https://doi.org/10.3390/ma17194907
Zhang Z, Wen L, Huang Q, Guo L, Dong Z, Zhu L. Corrosion Evaluation and Mechanism Research of AISI 8630 Steel in Offshore Oil and Gas Environments. Materials. 2024; 17(19):4907. https://doi.org/10.3390/ma17194907
Chicago/Turabian StyleZhang, Zhao, Liang Wen, Que Huang, Li Guo, Zhizhong Dong, and Lin Zhu. 2024. "Corrosion Evaluation and Mechanism Research of AISI 8630 Steel in Offshore Oil and Gas Environments" Materials 17, no. 19: 4907. https://doi.org/10.3390/ma17194907
APA StyleZhang, Z., Wen, L., Huang, Q., Guo, L., Dong, Z., & Zhu, L. (2024). Corrosion Evaluation and Mechanism Research of AISI 8630 Steel in Offshore Oil and Gas Environments. Materials, 17(19), 4907. https://doi.org/10.3390/ma17194907






