Research on the Corrosion Resistance of Electrodeposited Ni-SiC Composites Constructed for Steel Storage Tank Application
Abstract
1. Introduction
2. Experiment and Method
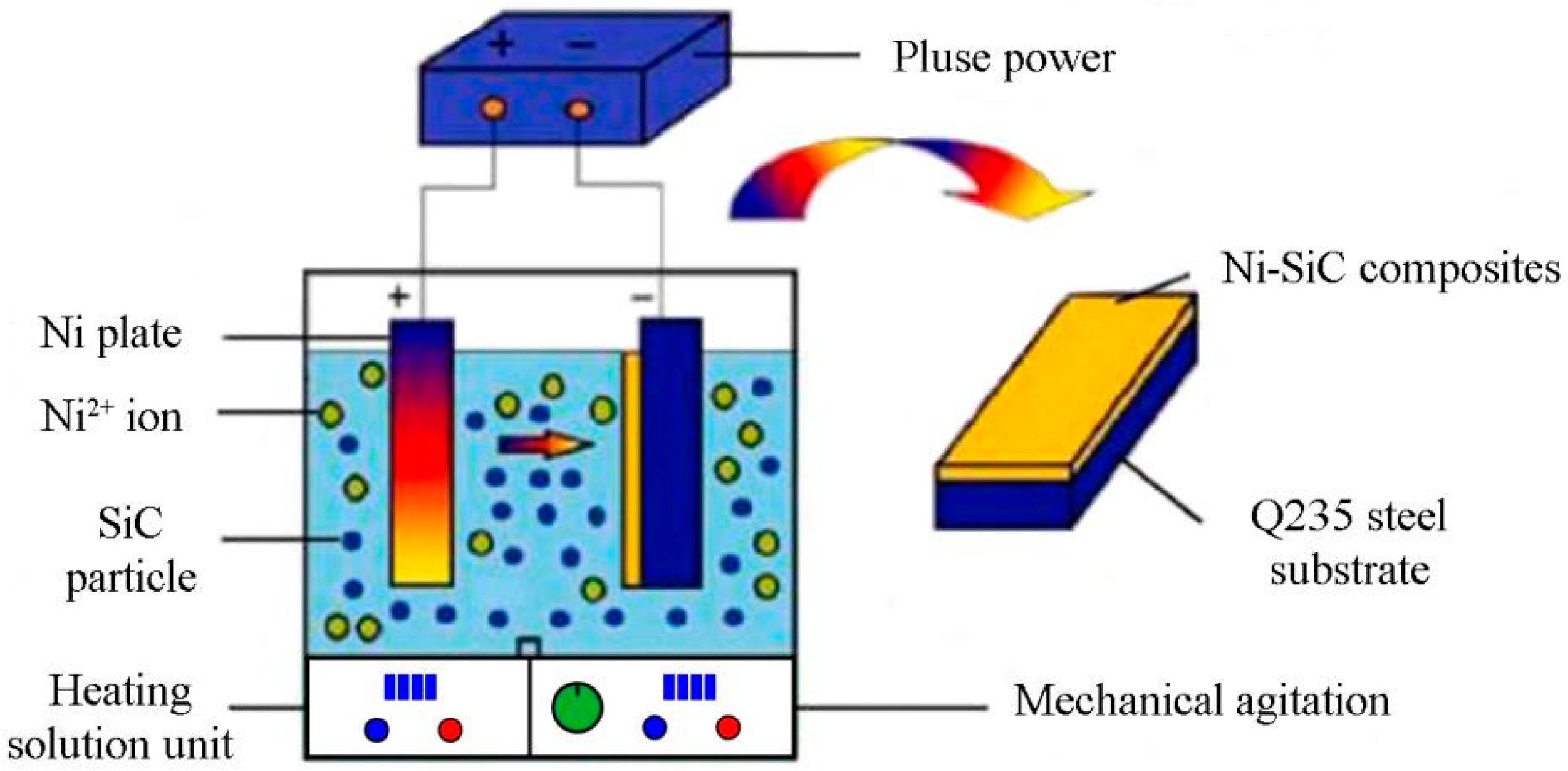
2.1. Preparation of Ni-SiC Composites
2.2. Characterization
3. Results and Discussion
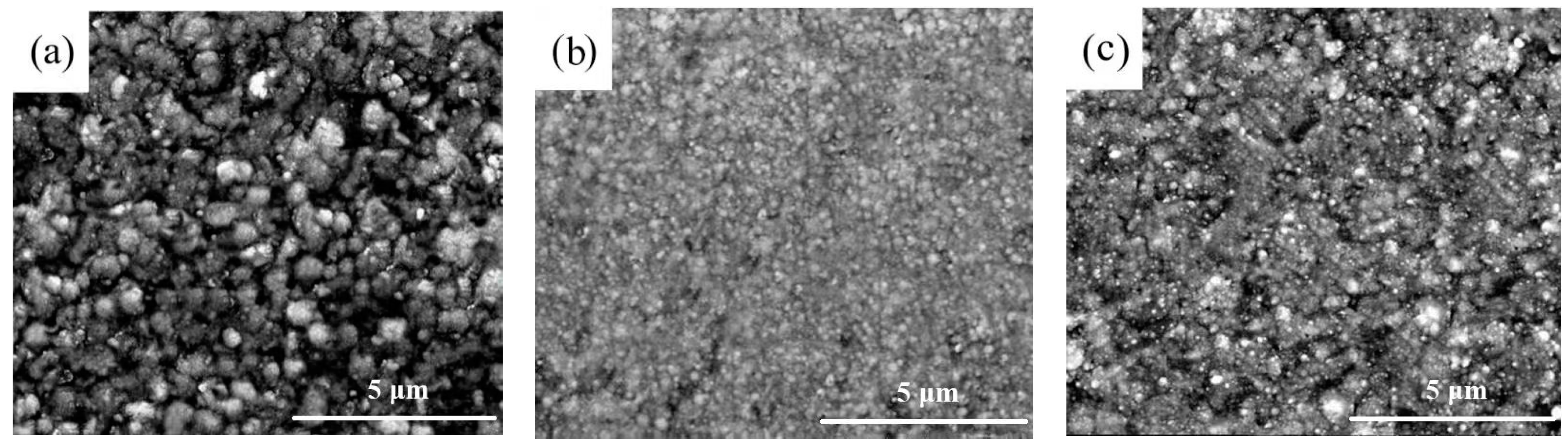
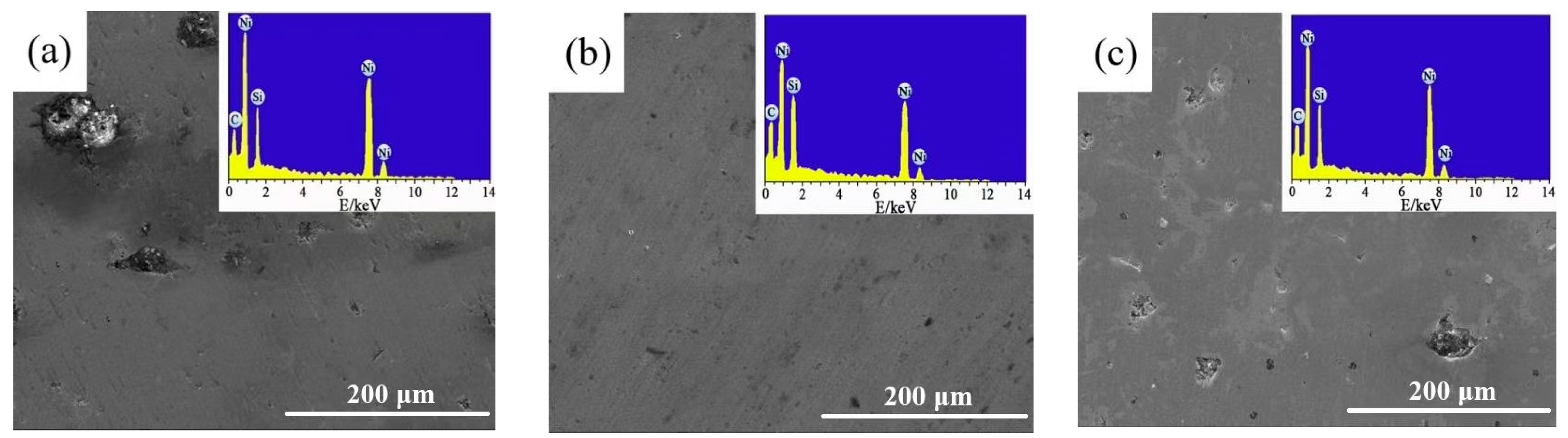
3.1. Surface Morphology Investigation
3.2. SiC Content Detection
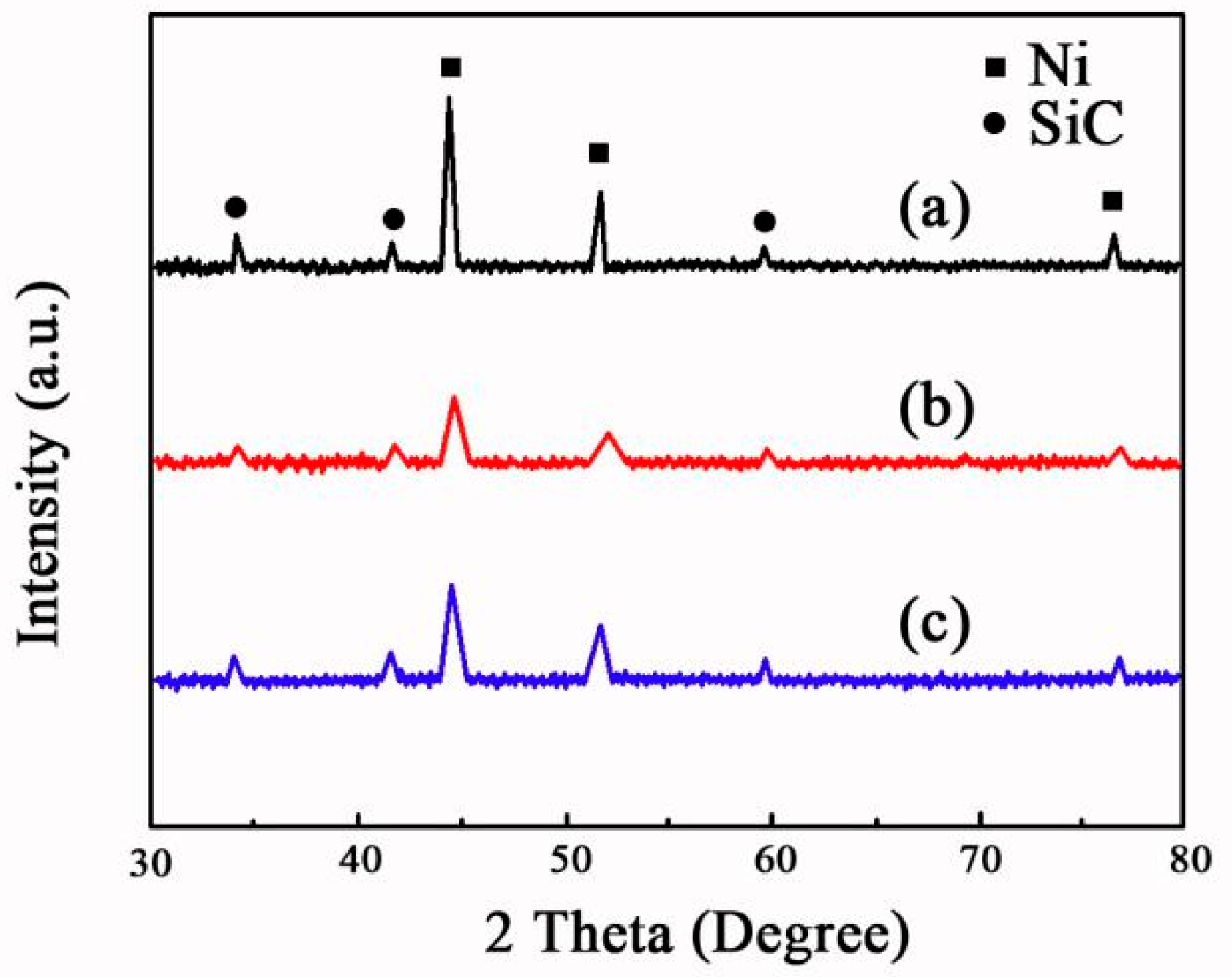
3.3. XRD Patterns Observation
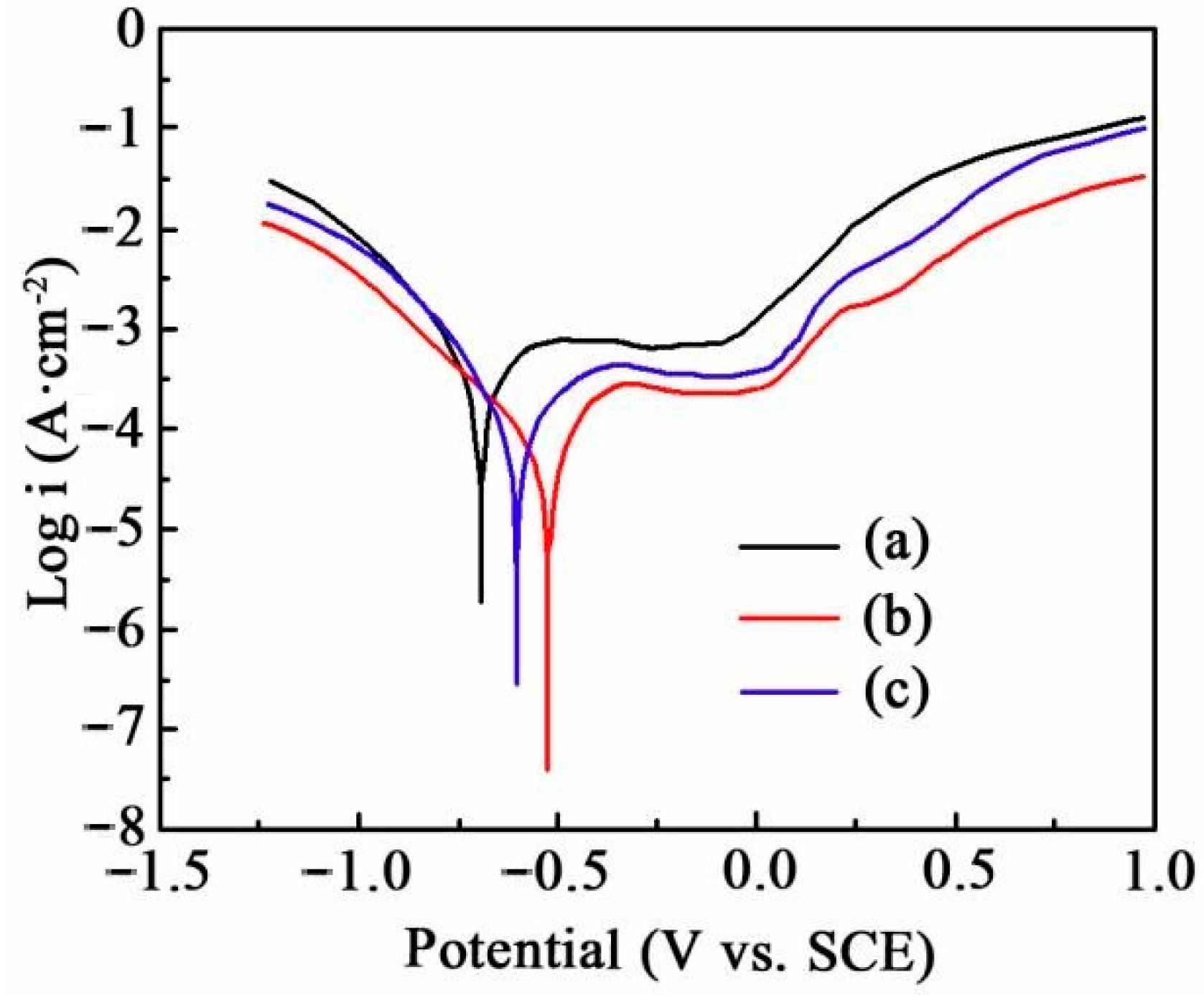
3.4. Corrosion Behaviour Test
3.5. Corrosion Relationship Analysis
4. Conclusions
- The Ni-SiC composites manufactured at 1.8 or 3.2 A/dm2 had uneven and loose microstructures, while the Ni-SiC composites fabricated at 2.5 A/dm2 possessed smooth and compact microstructure. In addition, EDS results shown that the SiC content of Ni-SiC composites prepared at 1.8 or 3.2 A/dm2 were lower than the one obtained at 2.5 A/dm2.
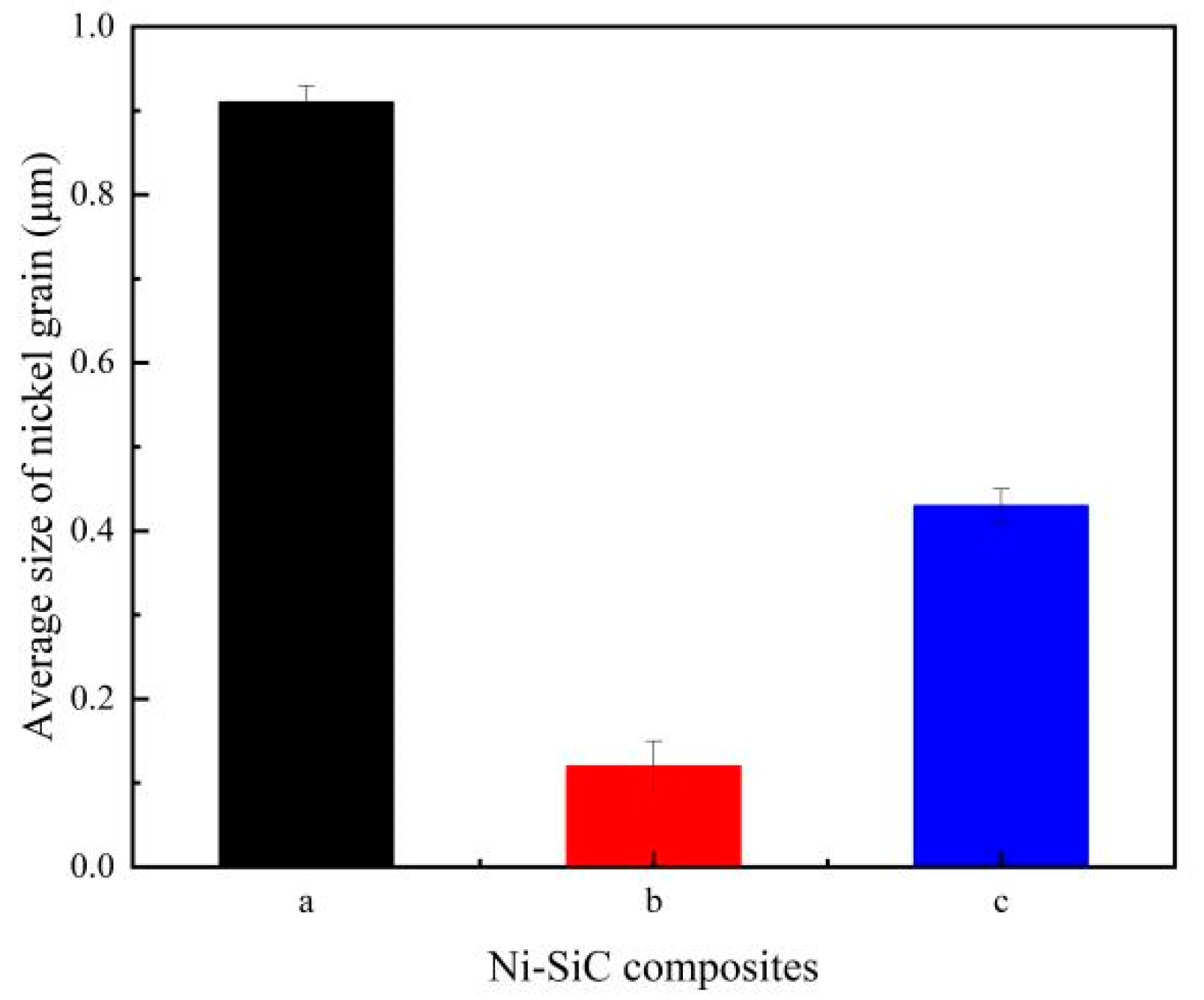
- XRD patterns demonstrated that the SiC phases were successfully incorporated into the Ni matrix during electrodeposition. Furthermore, the average size of nickel grain in the Ni-SiC composites produced at 2.5 A/dm2 was smaller than those deposited at 1.8 or 3.2 A/dm2.
- Electrochemical measurement indicated that the Ni-SiC composites fabricated at 2.5 A/dm2 owned a low corrosion current density, positive corrosion potential, and large impedance value, illustrating the corrosion resistance was excellent. Moreover, the corrosion relationship revealed that the corrosion weight loss of Ni-SiC composites was highly related to SiC content.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Aneziris, O.; Koromila, I.; Nivolianitou, Z. A systematic literature review on LNG safety at ports. Saf. Sci. 2020, 124, 104595. [Google Scholar] [CrossRef]
- Sorensen, P.A.; Kiil, S.; Dam-Johansen, K.; Weinell, C.E. Anticorrosive coatings: A review. J. Coat. Technol. Res. 2009, 6, 135–176. [Google Scholar] [CrossRef]
- Zhang, Z.F.; He, Y.; Bai, Y.; Song, R.X.; He, Y.H.; Liu, B.; Li, H.J.; Shangguan, J.X. Influence of iron element on the structure and corrosion resistance of Ni-P coatings in different corrosive environments. Colloids Surf. A Physicochem. Eng. Asp. 2022, 655, 130100. [Google Scholar] [CrossRef]
- Revathy, S.; Aswathy, S.N.; Sreejakumari, S.S. Recent trends and developments in two-dimensional materials based electrodeposited nickel nanocomposite coatings. FlatChem 2022, 36, 100434. [Google Scholar]
- Fathi, R.; Wei, H.Y.; Saleh, B.; Radhika, N.; Jiang, J.H.; Ma, A.B.; Ahmed, M.H.; Li, Q.; Ostrikov, K.K. Past and present of functionally graded coatings: Advancements and future challenges. Appl. Mater. Today 2022, 26, 101373. [Google Scholar] [CrossRef]
- Mohammad, S.; Seyyed, M.S.H.; Parnia, B.; Nader, S.; Saman, H. Wear and tribological characterization of nickel matrix electrodeposited composites: A review. Wear 2021, 486–487, 204098. [Google Scholar]
- Dong, H.; Guo, P.F.; Han, Y.; Bai, R.X.; Yang, Z.C.; Zhang, S.Q. Enhanced corrosion resistance of high speed laser-cladded Ni/316L alloy coating by heat treatment. J. Mater. Res. Technol. 2023, 24, 952–962. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Shen, X.L. Facile fabrication of robust superhydrophobic coating for enhanced corrosion protection on AZ91 magnesium alloy by electroless Ni-B/Go plating. Surf. Coat. Technol. 2023, 455, 129213. [Google Scholar] [CrossRef]
- Lee, Y.C.; Yen, V.C.; Christian, P.; Jan, J.H. Study of Ni-Cr/CrN bilayer thin films resistor prepared by magnetron sputtering. Vacuum 2023, 213, 112085. [Google Scholar] [CrossRef]
- Li, S.; Li, H.Q.; Zhai, Z.H.; Cao, X.J.; Liu, D.X.; Jiang, J.L. Corrosion resistance and tribological behavior of FeCoCrNi@GO/Ni high entropy alloy-based composite coatings prepared by electrodeposition. Surf. Coat. Technol. 2024, 477, 130379. [Google Scholar] [CrossRef]
- Zhou, H.L.; Yan, S.M.; He, Y.; Fan, Y.; Yan, L.P.; Cheng, X.Y.; Li, Z.Y.; Zhong, J.M.; Song, J.X. Preparation of a superhydrophobic Ni/SH-SiO2 coating by one-step electrodeposition and an analysis of its anti-corrosion performance. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132084. [Google Scholar] [CrossRef]
- Zhao, Y.T.; Jiang, C.H.; Xu, Z.; Cai, F.; Zhang, Z.Q.; Fu, P. Microstructure and corrosion behavior of Ti nanoparticles reinforced Ni-Ti composite coatings by electrodeposition. Mater. Des. 2015, 85, 39–46. [Google Scholar] [CrossRef]
- Frank, C.W.; Wang, S.C.; Zhou, N. The electrodeposition of composite coatings: Diversity, applications and challenges. Curr. Opin. Electrochem. 2020, 20, 8–19. [Google Scholar]
- Ren, A.H.; Kang, M.; Fu, X.Q. Corrosion behaviour of Ni/WC-MoS2 composite coatings prepared by jet electrodeposition with different MoS2 doping concentrations. Appl. Surf. Sci. 2023, 613, 155905. [Google Scholar] [CrossRef]
- Jin, H.; Ji, R.J.; Dong, T.C.; Liu, S.; Liu, Y.H.; Zhang, F.; Ma, C.; Liu, S.G. Fabrication and characterization of WC particles reinforced Ni-Fe composite coating by jet electrodeposition. Surf. Coat. Technol. 2021, 421, 127368. [Google Scholar] [CrossRef]
- Feng, Q.Y.; Li, T.J.; Teng, H.T.; Zhang, X.L.; Zhang, Y.; Liu, C.S.; Jin, J.Z. Investigation on the corrosion and oxidation resistance of Ni-Al2O3 nanocomposite coatings prepared by sediment co-depsoition. Surf. Coat. Technol. 2008, 202, 4137–4144. [Google Scholar] [CrossRef]
- Wu, T.T.; Ma, M.L.; Ding, K.W.; Nan, X.; Wang, Z.; Wei, X.F.; Zhu, X.W. Effect of Y2O3 nanoparticles on the microstructure and corrosion resistance of electrodeposited Ni-Mo-Y2O3 nanocomposite coatings. Int. J. Electrochem. Sci. 2023, 18, 100095. [Google Scholar] [CrossRef]
- Rao, H.; Li, W.P.; Luo, Z.L.; Liu, H.C.; Zhu, L.Q.; Chen, H.N. Nucleation and growth mechanism of Ni/SiC composite coatings electrodeposited with micro- and nano-SiC particles. J. Mater. Res. Technol. 2024, 30, 3079–3091. [Google Scholar] [CrossRef]
- Zhou, Y.; Sun, Z.P.; Yu, Y.; Li, L.; Song, J.L.; Xie, F.Q.; Wu, X.Q. Tribological behavior of Ni-SiC composite coatings produced by circulating-solution electrodeposition technique. Tribol. Int. 2021, 159, 106933. [Google Scholar] [CrossRef]
- Ma, C.Y.; He, H.X.; Xia, F.F.; Xiao, Z.M.; Liu, Y. Performance of Ni-SiC composites deposited using magnetic-field-assisted electrodeposition under different magnetic-field directions. Ceram. Int. 2023, 49, 35907–35916. [Google Scholar] [CrossRef]
- Rita, M.; Prvan, K.K.; Prabhat, C.Y.; Pooja, R. Influence of pulse frequency on microstructure, mechanical properties, and corrosion resistance of electrodeposited Ni-SiC composite coatings. Mater. Today Commun. 2024, 40, 109977. [Google Scholar]
- Ren, A.H.; Fu, X.Q.; Duan, S.L.; Shen, M.Q.; Lin, J.R. Effect of current density on the performance of Ni-Fe-P-CeO2 composite coating prepared by jet electrodepsoition. Int. J. Electrochem. Sci. 2020, 15, 8563–8583. [Google Scholar]
- Li, D.D.; Li, B.S.; Du, S.S.; Zhang, W.W. Synthesis of a novel Ni-B/YSZ metal-ceramic composite coating via single-step electrodeposition at different current density. Ceram. Int. 2019, 45, 24884–24893. [Google Scholar] [CrossRef]
- Morteza, A.; Abbas, C. Electrodeposition of Ni-Mo/Al2O3 nano-composite coatings at various deposition current densities. Appl. Surf. Sci. 2019, 466, 433–440. [Google Scholar]
- Guo, C.; Zuo, Y.; Zhao, X.H.; Zhao, J.M.; Xiong, J.P. The effects of electrodeposition current density on properties of Ni-CNTs composite coatings. Surf. Coat. Technol. 2008, 202, 3246–3250. [Google Scholar] [CrossRef]
- Xia, F.F.; Li, C.Y.; Ma, C.Y.; Li, Q.; Xing, H.Y. Effect of pulse current density on microstructure and wear property of Ni-TiN nanocoatings deposited via pulse electrodeposition. Appl. Surf. Sci. 2021, 538, 148139. [Google Scholar] [CrossRef]
- Bibekananda, N.; Sharmistha, A.; Shahid, A. Mechanical and corrosion resistance behavior of ultrasonication-assisted electrodeposited Ni-SiC nanocomposite coatings. Phys. B Condens. Matter 2024, 675, 415585. [Google Scholar]
- Sun, W.C.; Zhang, P.Z.; Zhao, K.; Tian, M.M.; Wang, Y. Effect of graphite concentration on the friction and wear of Ni-Al2O3/graphite composite coatings by a combination of electrophoresis and electrodeposition. Wear 2015, 342–343, 172–180. [Google Scholar] [CrossRef]
- Zhang, W.W.; Li, B.S. Influence of electrodeposition conditions on the microstructure and hardness of Ni-B/SiC nanocomposite coatings. Int. J. Electrochem. Sci. 2018, 13, 3486–3500. [Google Scholar] [CrossRef]
- Shahzad, K.; Radwan, A.B.; Fayyaz, O.; Shakoor, R.A.; Uzma, M.; Umer, M.A.; Baig, M.N.; Raza, A. Effect of concentration of TiC on the properties of pulse electrodeposited Ni-P-TiC nanocomposite coatings. Ceram. Int. 2021, 47, 19123–19133. [Google Scholar] [CrossRef]
- Zhang, W.W.; Li, B.S.; Mei, T.Y.; Li, M.Y.; Hong, M.; Yuan, Z.W.; Chu, H.W. Effect of graphene oxide and current density on the structure and corrosion properties of nanocrystalline nickel coating fabricated by electrodeposition. Colloids Surf. A Physicochem. Eng. Asp. 2022, 648, 129220. [Google Scholar] [CrossRef]
- Li, C.Y.; Xia, F.F.; Ma, C.Y.; Li, Q. Research on the Corrosion Behavior of Ni-SiC Nanocoating Prepared Using a Jet Electrodeposition Technique. J. Mater. Eng. Perform. 2021, 30, 6336–6344. [Google Scholar] [CrossRef]
- Li, C.Y.; Xia, F.F.; Yao, L.M.; Li, H.X.; Jia, X. Investigation of the mechanical properties and corrosion behaviors of Ni-BN-TiC layers constructed via laser cladding technique. Ceram. Int. 2022, 49, 6671–6677. [Google Scholar] [CrossRef]
- Zhu, X.B.; Cai, C.; Zheng, G.Q.; Zhang, Z.; Li, J.F. Electrodeposition and corrosion behavior of nanostructured Ni-TiN composite films. Trans. Nonferrous Met. Soc. China 2011, 21, 2216–2224. [Google Scholar] [CrossRef]
- Hong, Q.; Wang, D.W.; Yin, S.H. The microstructure, wear and electrochemical properties of electrodeposited Ni-diamond composite coatings: Effect of diamond concentration. Mater. Today Commun. 2023, 34, 105476. [Google Scholar] [CrossRef]
- Nujira, K.; Komsak, H.; Chatpawee, H.O.; Qin, J.Q.; Yuttanant, B.; Napat, T.; Papot, J. Enhanced particle incorporation for co-electrodeposited Ni-P/diamond coatings with a pulse-stirring technique. Appl. Surf. Sci. Adv. 2023, 18, 100499. [Google Scholar]
- Malay, K.D.; Li, R.X.; Qin, J.Q.; Zhang, X.Y.; Kumkumlata, D.; Adiasak, T.; Sarintorn, L.; Yuttanat, B.Y.; Ma, M.Z.; Liu, R.P. Effect of electrodeposition conditions on structure and mechanical properties of Ni-W/diamond composite coatings. Surf. Coat. Technol. 2017, 309, 337–343. [Google Scholar]
- Feng, X.L.; Wang, H.F.; Liu, X.C.; Wang, C.M.; Cui, H.Z.; Song, Q.; Huang, K.; Li, N.; Jiang, X. Effect of Al content on wear and corrosion resistance of Ni-based alloy coatings by laser cladding. Surf. Coat. Technol. 2021, 412, 126976. [Google Scholar] [CrossRef]




| Electrolyte Composition | Concentration (g/L) |
|---|---|
| SiC concentration | 8 |
| NiSO4·6H2O | 230 |
| C6H8O7·H2O | 30 |
| NiCl2·6H2O | 40 |
| H3BO3 | 35 |
| Saccharin | 4 |
| Plating Parameters | Specific |
| Duty cycle (%) | 60 |
| Plating temperature (°C) | 55 |
| Plating time (min) | 45 |
| pH value | 4.1 |
| Rational speed (rad/min) | 600 |
| Frequency (Hz) | 10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cui, L.; Li, X.; Li, C.; Zhu, L.; Zhang, Q.; Li, Z.; Liu, H. Research on the Corrosion Resistance of Electrodeposited Ni-SiC Composites Constructed for Steel Storage Tank Application. Materials 2024, 17, 4616. https://doi.org/10.3390/ma17184616
Cui L, Li X, Li C, Zhu L, Zhang Q, Li Z, Liu H. Research on the Corrosion Resistance of Electrodeposited Ni-SiC Composites Constructed for Steel Storage Tank Application. Materials. 2024; 17(18):4616. https://doi.org/10.3390/ma17184616
Chicago/Turabian StyleCui, Lifu, Xiang Li, Chaoyu Li, Lijie Zhu, Qinggao Zhang, Zheng Li, and Haiyu Liu. 2024. "Research on the Corrosion Resistance of Electrodeposited Ni-SiC Composites Constructed for Steel Storage Tank Application" Materials 17, no. 18: 4616. https://doi.org/10.3390/ma17184616
APA StyleCui, L., Li, X., Li, C., Zhu, L., Zhang, Q., Li, Z., & Liu, H. (2024). Research on the Corrosion Resistance of Electrodeposited Ni-SiC Composites Constructed for Steel Storage Tank Application. Materials, 17(18), 4616. https://doi.org/10.3390/ma17184616





