Research on Alkali-Activated Slag Stabilization of Dredged Silt Based on a Response Surface Method
Abstract
1. Introduction
2. Materials and Methods
2.1. Test Materials
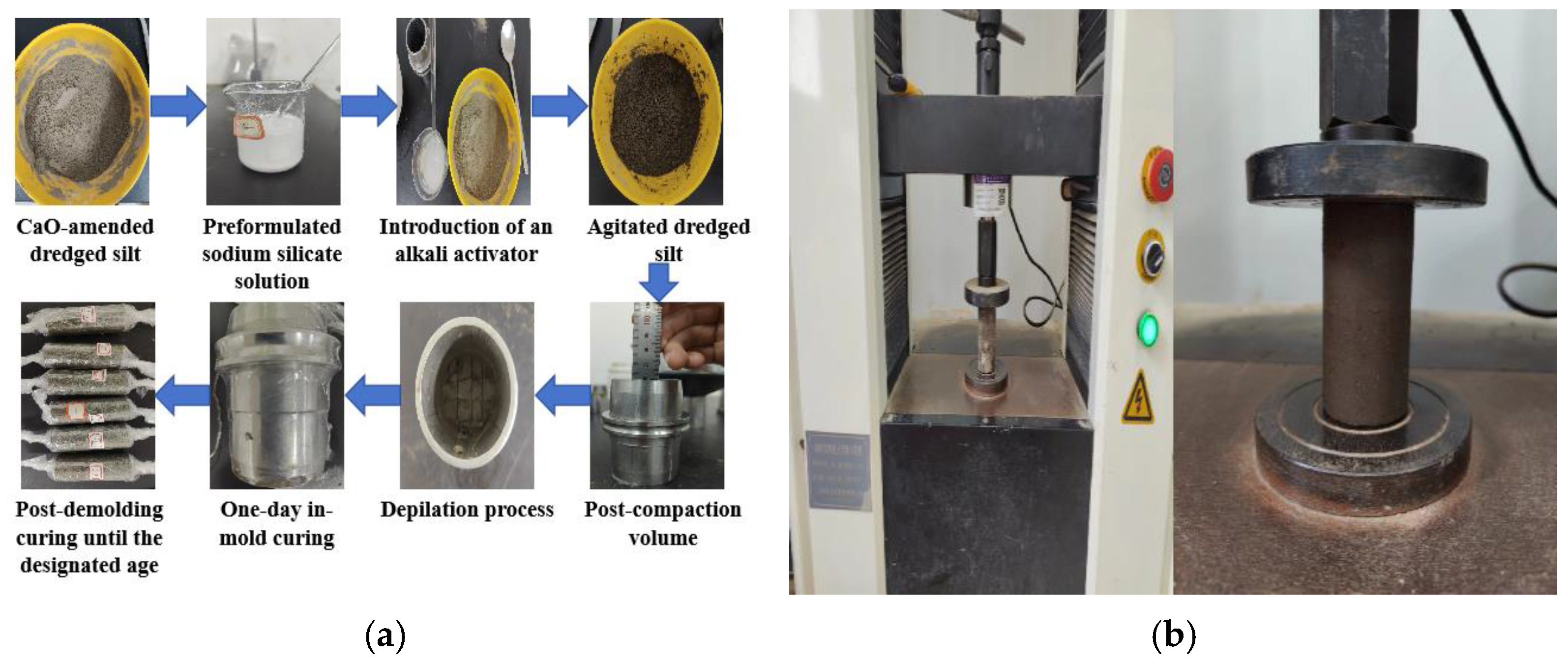
2.2. Sample Preparation
2.3. Single-Addition Experiment
2.4. RSM Experiment
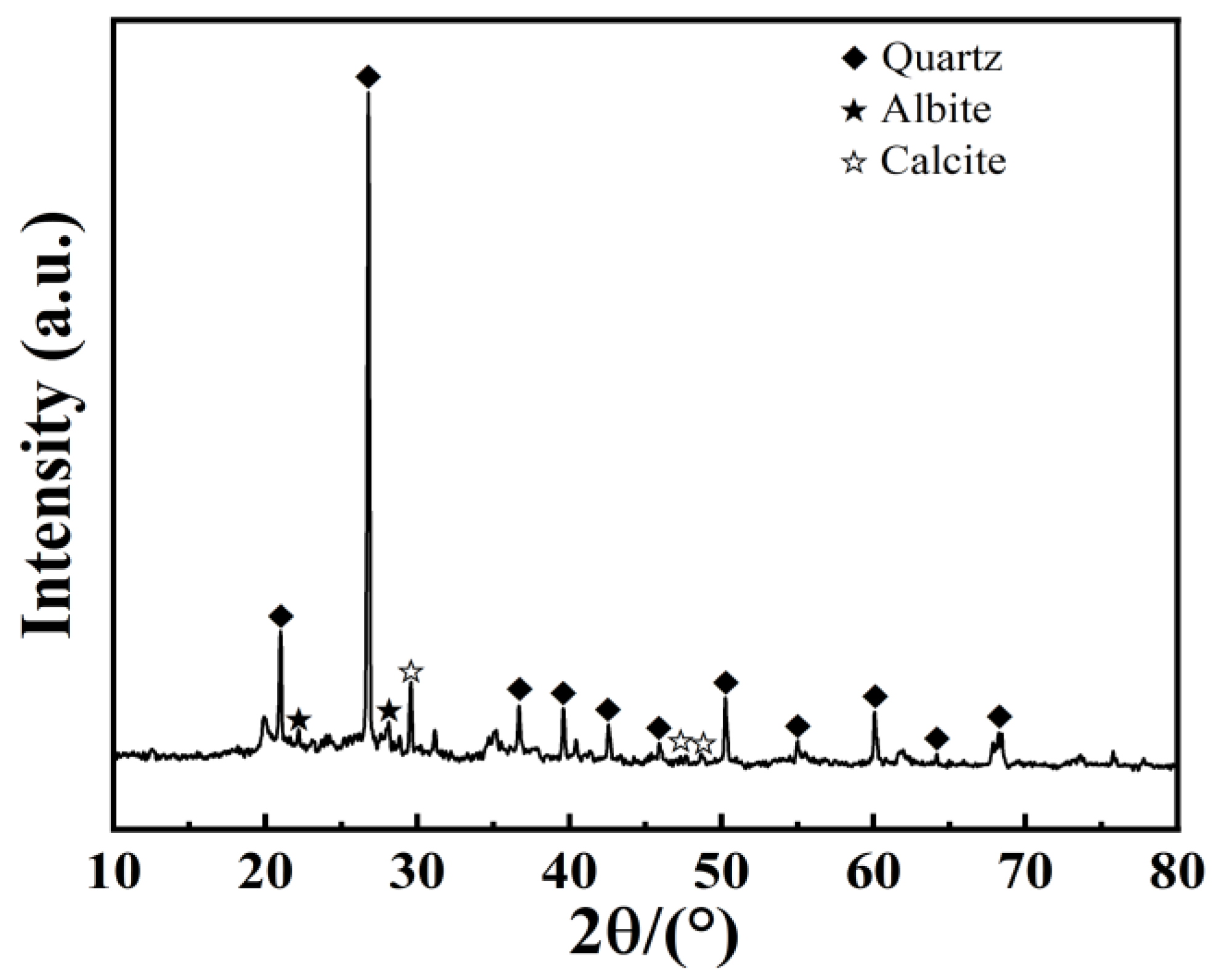
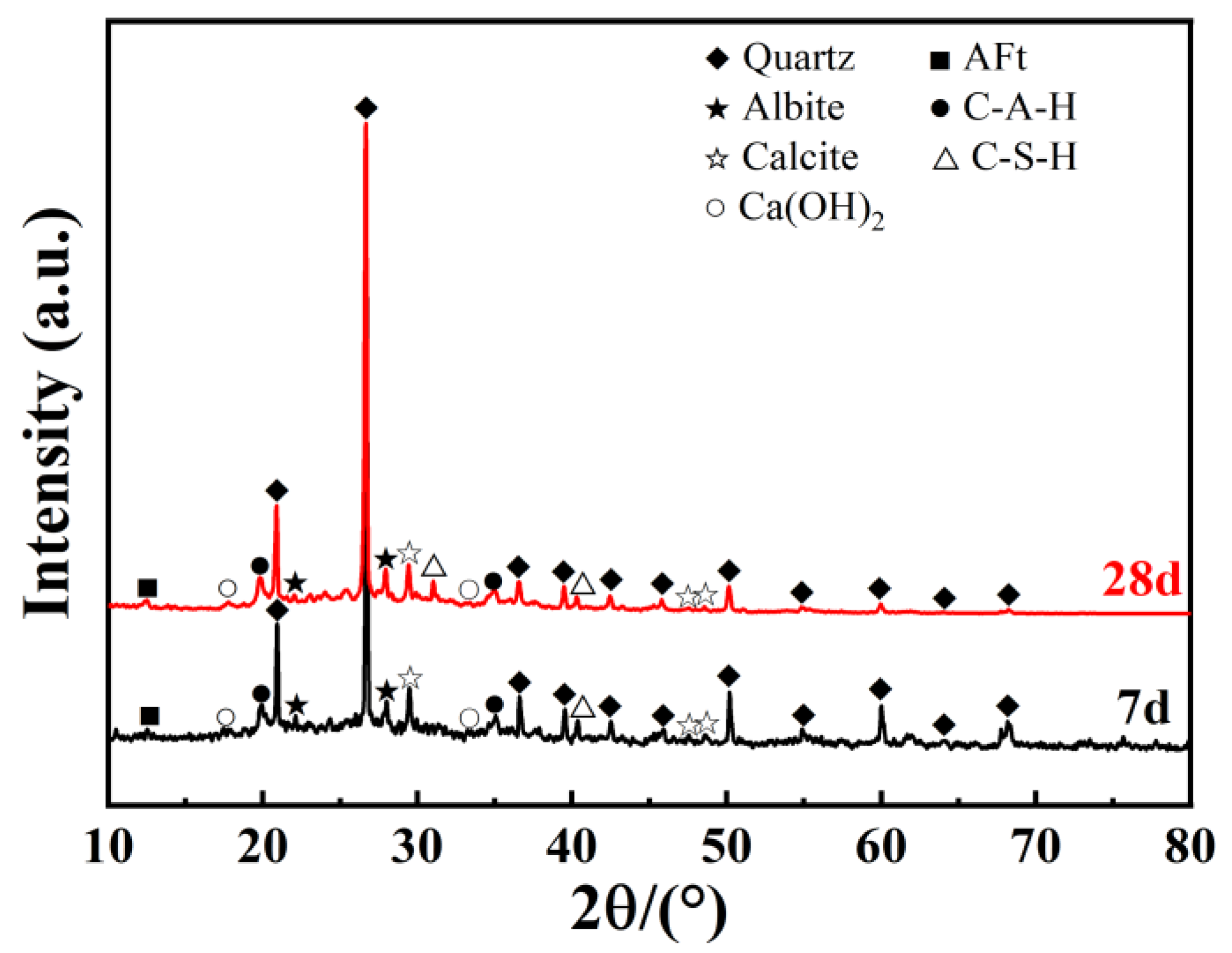
2.5. X-ray Diffraction Test (XRD)
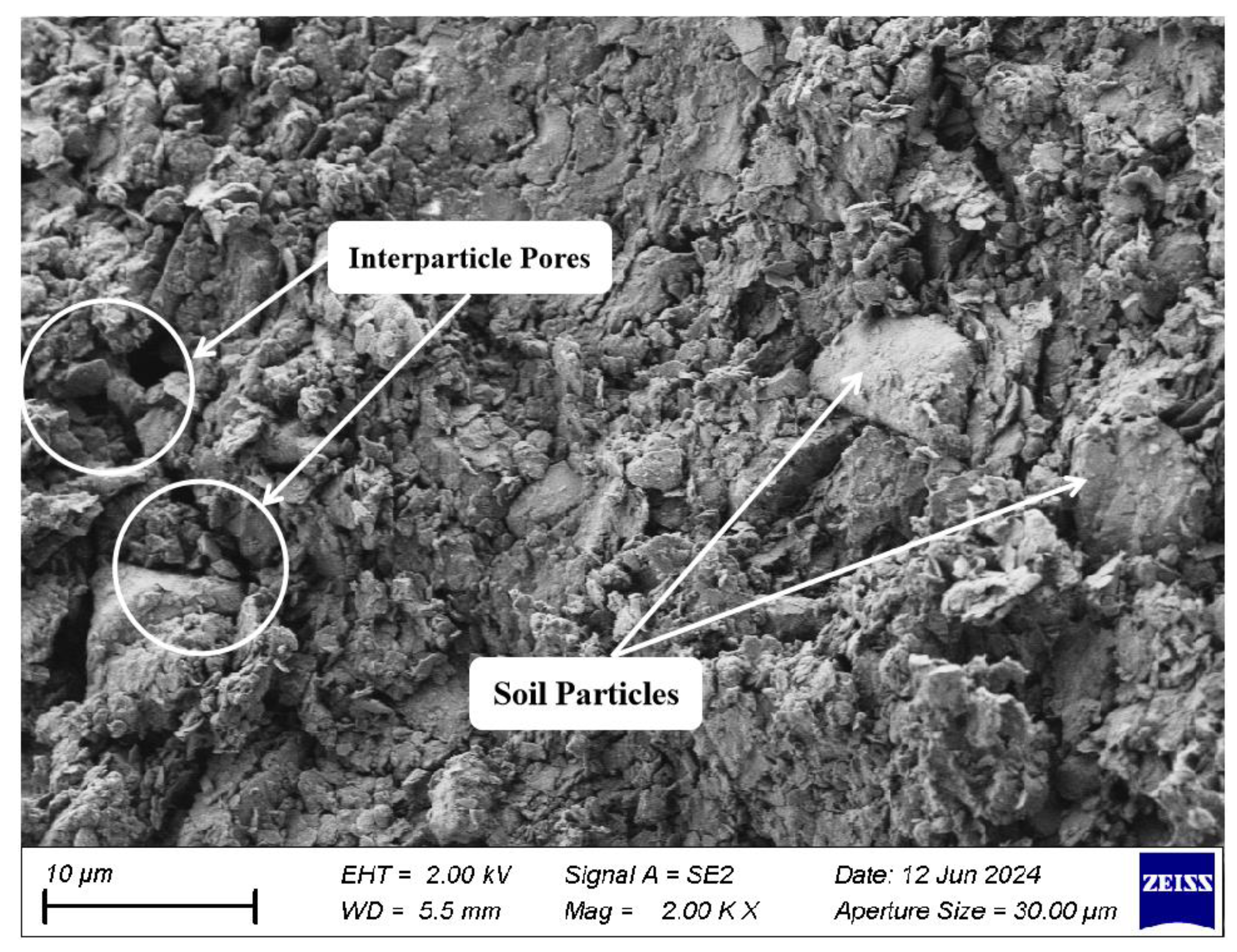
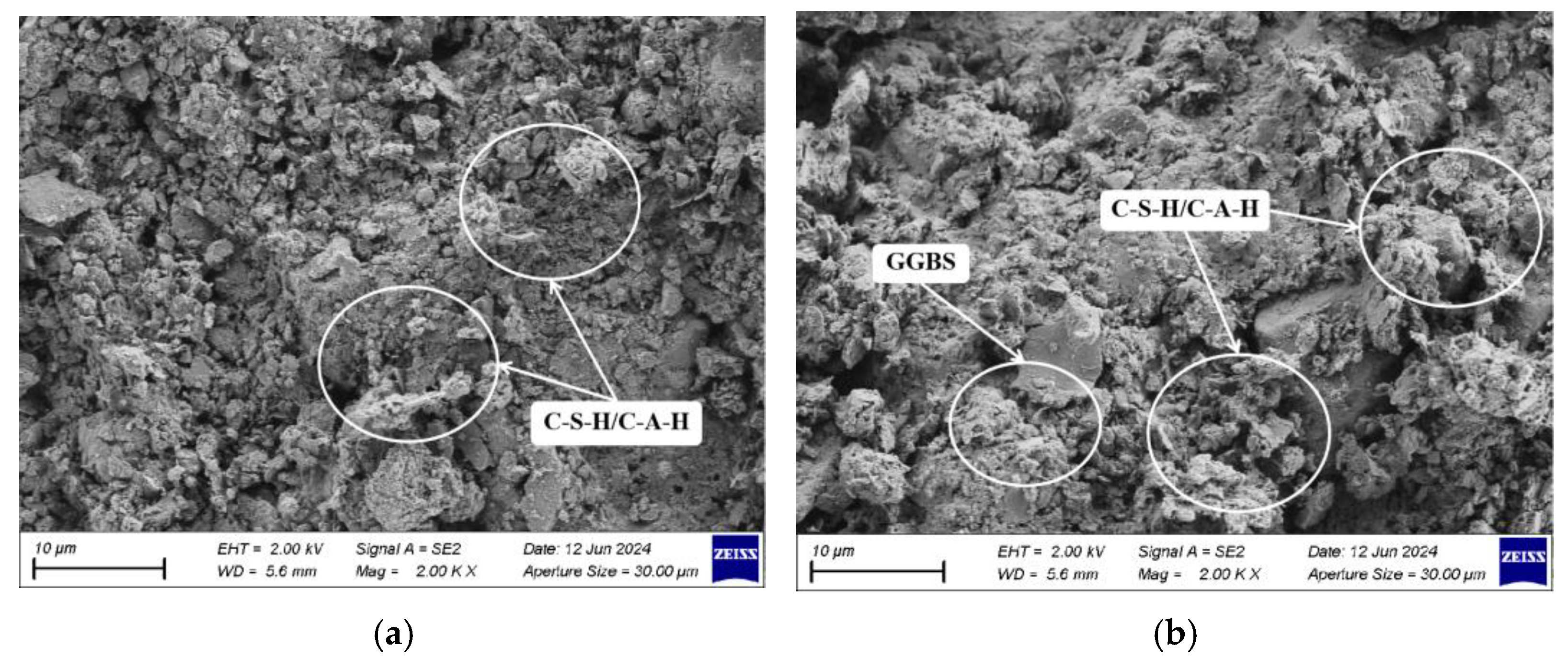
2.6. Scanning Electron Microscopy Test (SEM)
3. Results
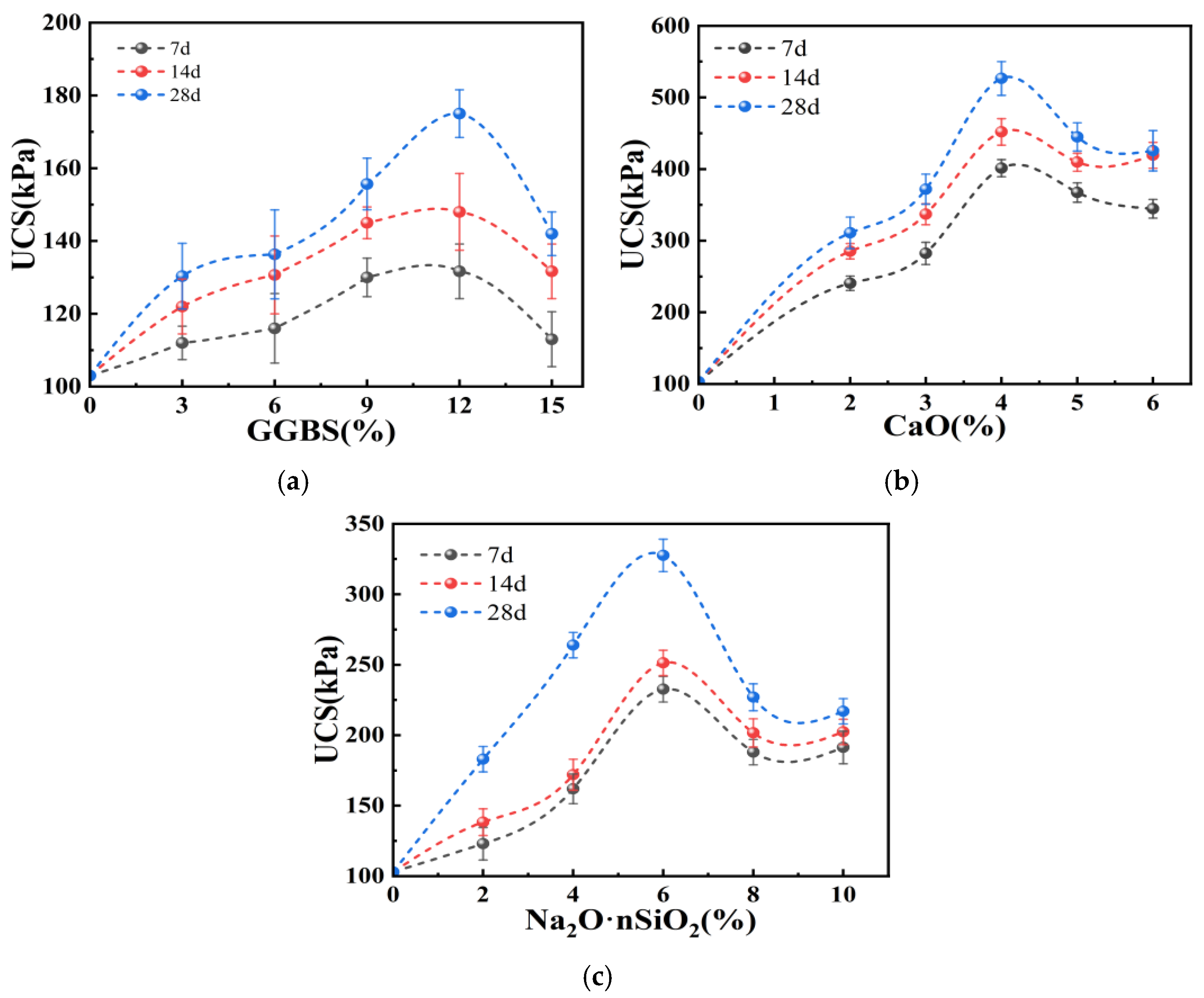
3.1. Results and Analysis of Single-Addition Experiments
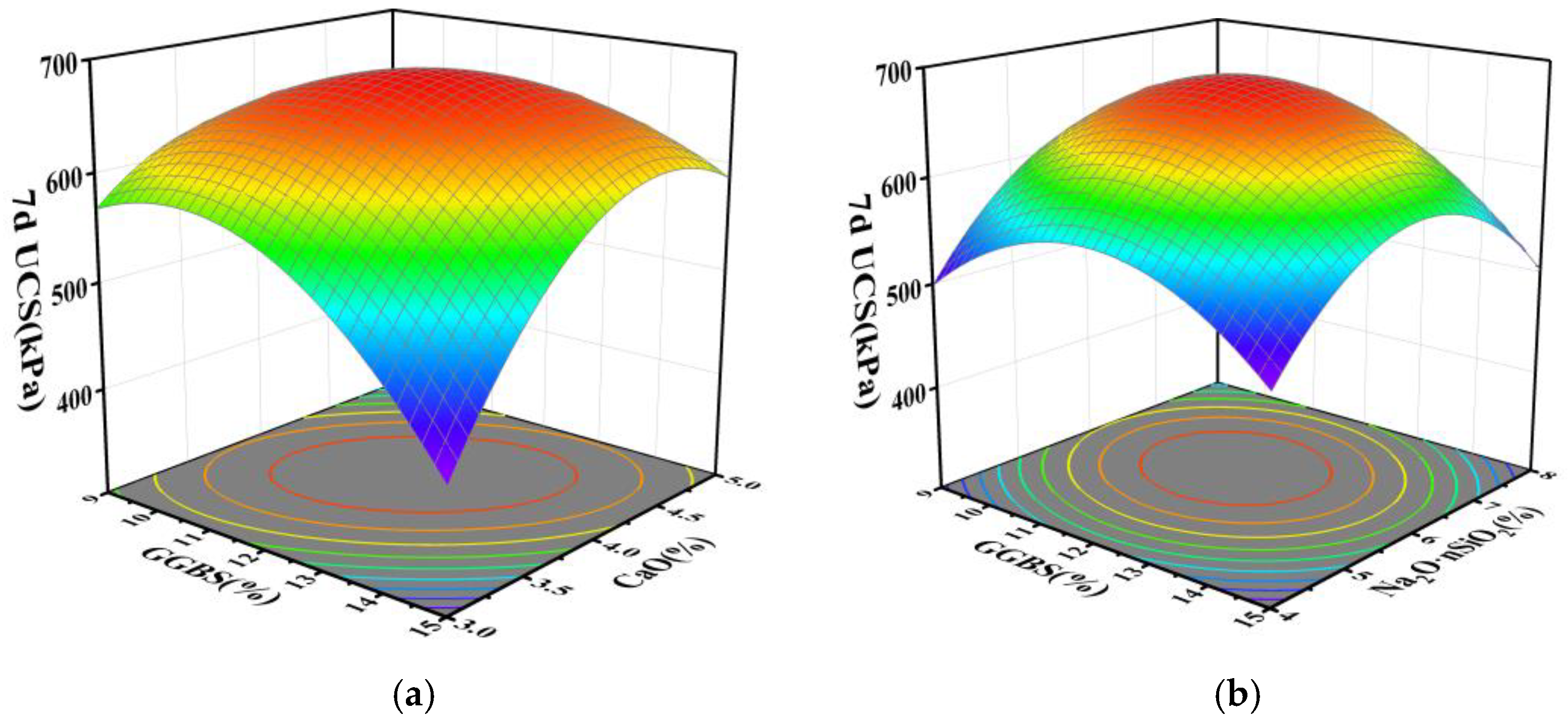
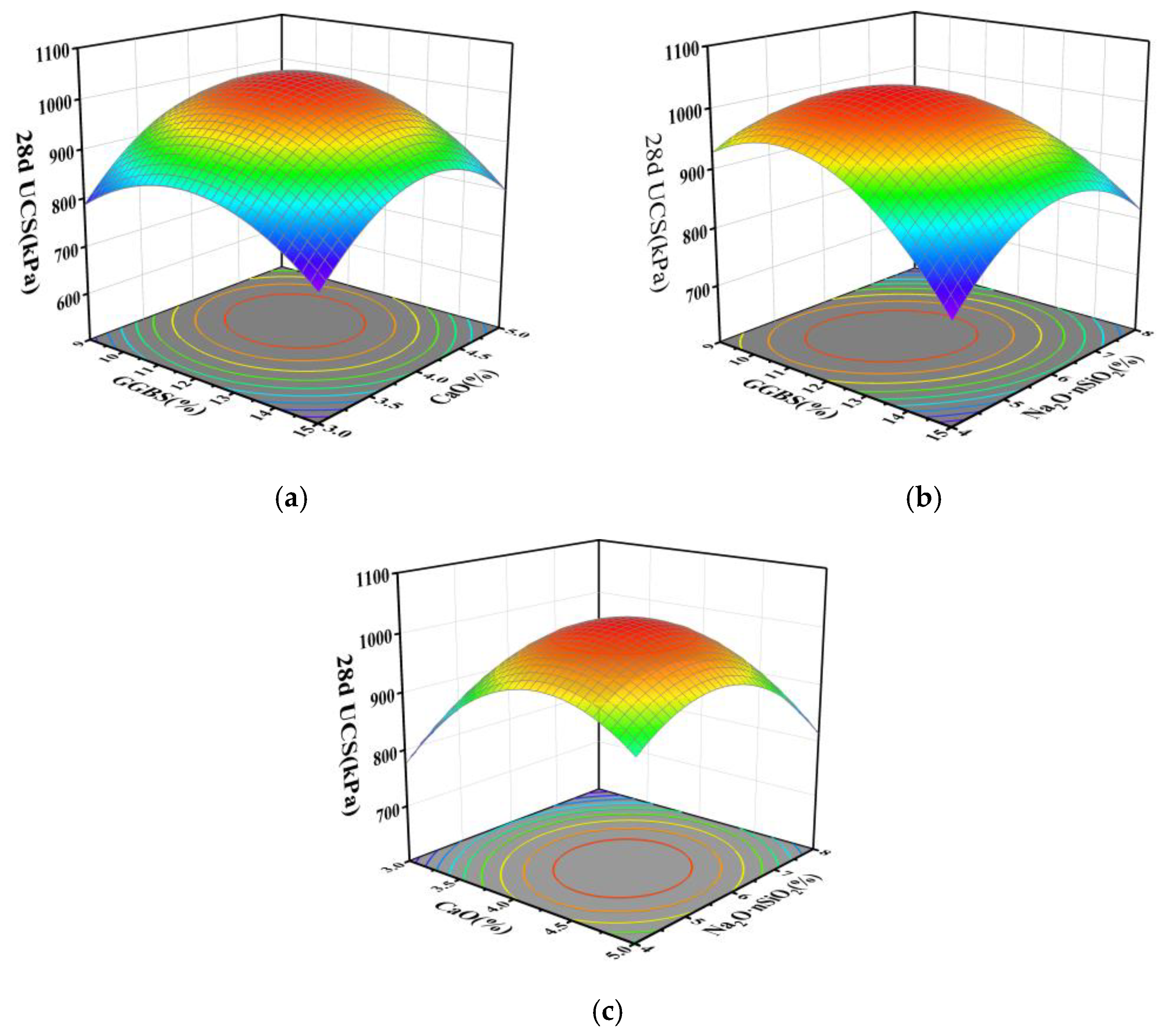
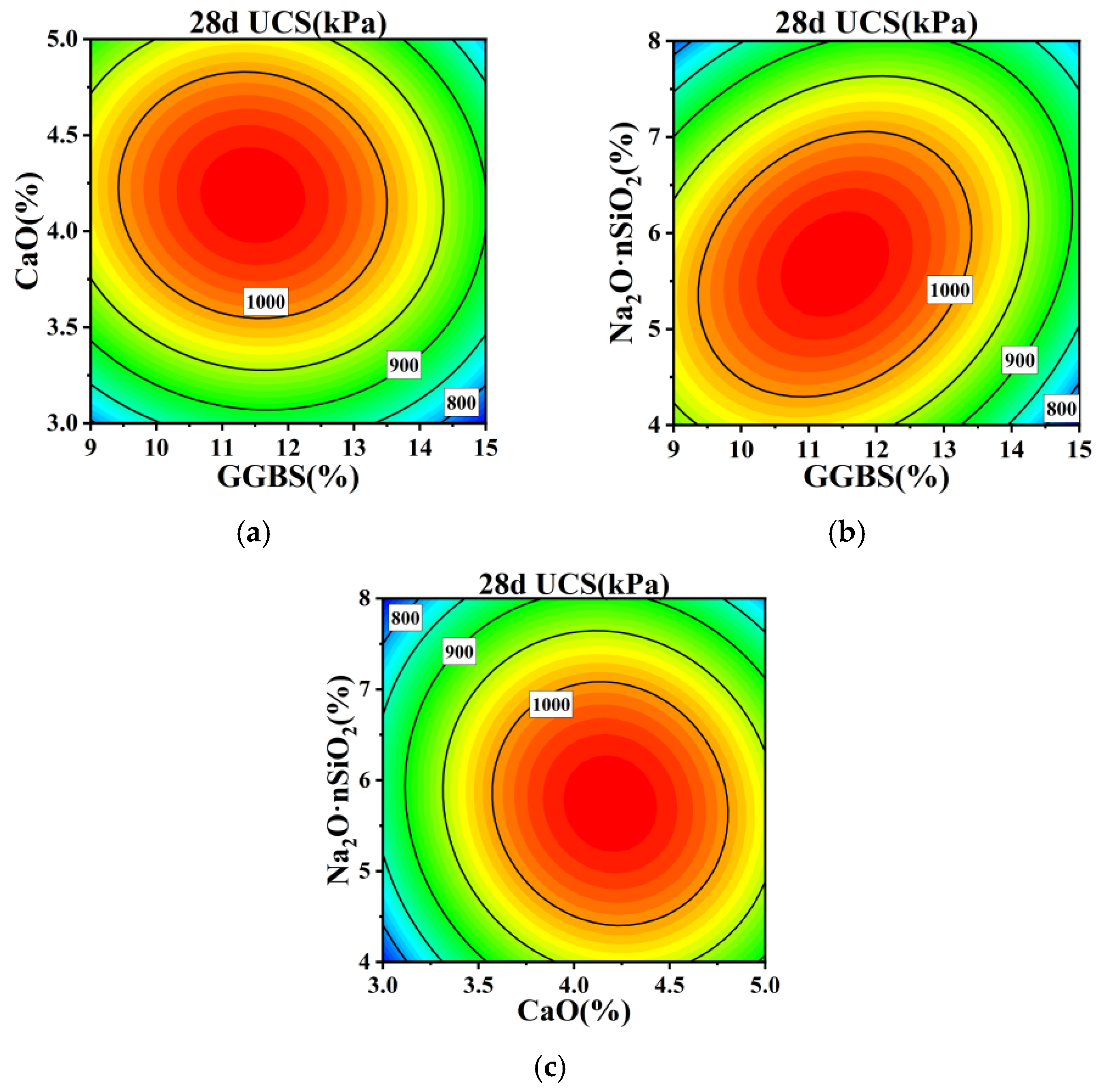
3.2. Results and Analysis of RSM Experiments
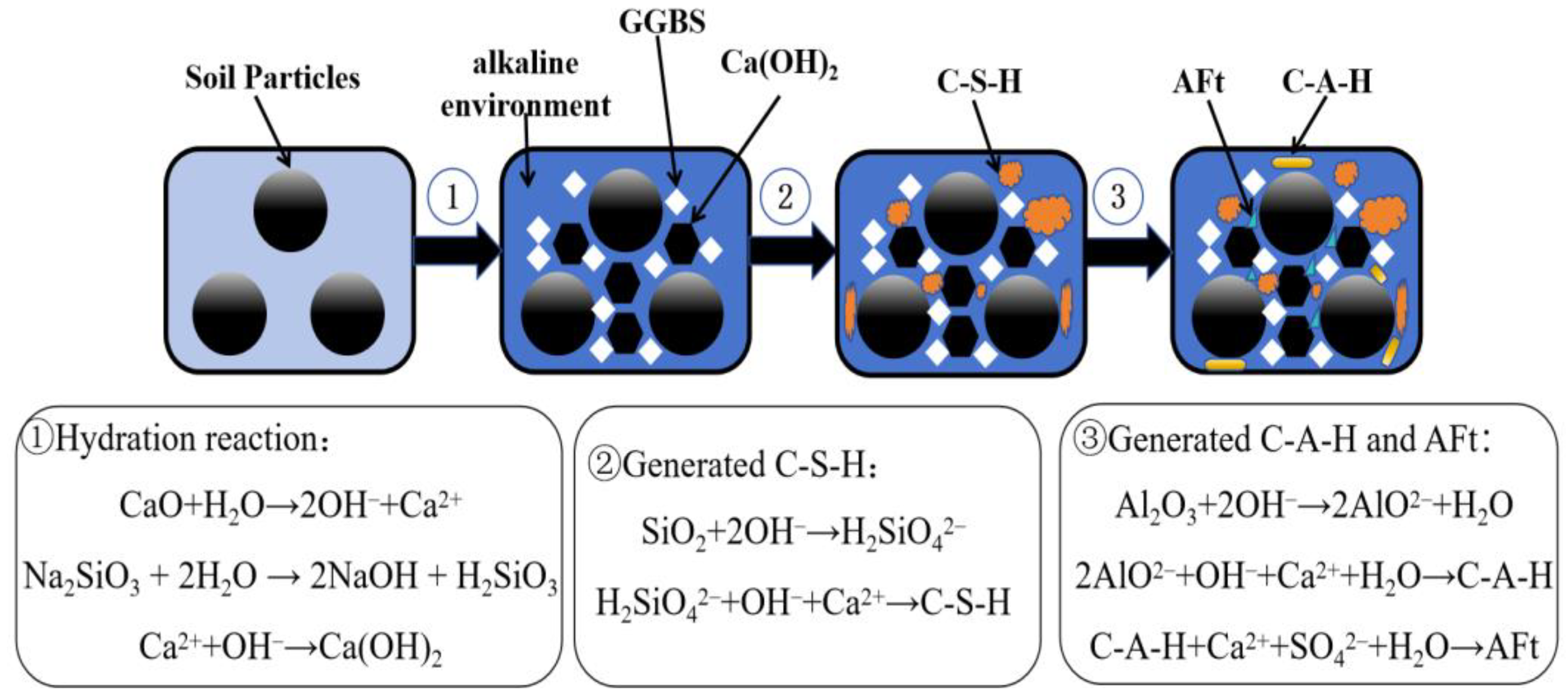
3.3. Analysis of Microstructural Characteristics and Mechanisms
4. Conclusions
- (1)
- In the single-addition experiments, CaO showed the most significant stabilization effect, characterized by rapid hydration reactions and early strength development; the stabilization effect of GGBS was substantially lower than that of CaO, with a gradual increase in strength over the curing period; Na2O·nSiO2 only slightly improved the strength of the stabilized soil at dosages below 6%, with most strength gains occurring in the later stages of curing.
- (2)
- Based on response surface methodology, regression fitting and significance analysis of the experimental results yielded regression equations for the 7 d and 28 d compressive strengths of the stabilized silt, revealing significant interactions between GGBS-CaO and GGBS-Na2O·nSiO2. Optimization of the regression equations determined the optimal mix proportions for the GGBS-CaO-Na2O·nSiO2 interaction affecting the stabilized silt to be 11.5% GGBS, 4.1% CaO, and 5.9% Na2O·nSiO2.
- (3)
- The hydration reactions within the GGBS-CaO-Na2O·nSiO2 composite binder system are mutually enhancing, producing hydration products such as Ca(OH)2, C-S-H, C-A-H, and AFt in an alkaline environment. These products encapsulate, bind, and fill, stabilizing the microstructure of the soil.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, H.; Zhao, M. Review of ground improvement technical and its application in China. J. China Civ. Eng. J. 2016, 49, 96–115. [Google Scholar]
- Zhang, C.; Guan, F.; Li, L.; Huang, Y. The progress in the reutillzation treatment and disposal of dredged sediments in China. J. Environ. Eng. 2014, 32, 95–99. [Google Scholar]
- Xu, R.Q.; Zhu, K.L.; Hang, G.W.; Yan, Z.; Zhang, G.; Yan, J.; Zhang, M.; Zhang, B. Experimental study on solidification and road performance of mucky soil. J. Hunan Univ. (Nat. Sci.) 2022, 49, 167–174. [Google Scholar]
- Gu, C.; Wang, L.W.; Wang, J.; Zhang, T.; Cai, Y.; Lin, G. Model test study on negative friction of single pile in underconsolidated soft clay foundation under different initial moisture content. J. Chin. J. Rock Mech. Eng. 2022, 41, 2554–2566. [Google Scholar]
- Ruan, Q.; Ruan, B.; Zeng, Y.; Wen, K.; Li, X. Experimental research on mechanical properties of cement stabilized muddy clay from Dong-ting Lake area. J. Railw. Sci. Eng. 2014, 11, 106–111. [Google Scholar]
- Chen, M.; Yang, G.; Xu, F. Research progress on solidification treatment of dredged silt. J. South-North Water Transf. Water Sci. Technol. 2018, 16, 128–138. [Google Scholar]
- Sun, H.; Wang, W.; Ling, D. Mechanical properties and microstructure of solidified soil with low cement content. J. Zhejiang Univ. (Eng. Sci.) 2021, 55, 530–538. [Google Scholar]
- Tang, Y.; Liu, H.; Zhu, W. Study on engineering properties of cement-stabilized soil. J. Chin. J. Geotech. Eng. 2000, 22, 549–554. [Google Scholar]
- Huang, Y.; Zhu, W.; Zhang, C.; Wang, W. Mechanical characteristics and strength source of remolded solidified dredged material. J. Rock Soil Mech. 2009, 30, 1352–1356. [Google Scholar]
- Pan, C.; Xie, X.; Gen, J.; Wang, W. Effect of stabilization/solidification on mechanical and phase characteristics of organic river silt by a stabilizer. J. Constr. Build. Mater. 2020, 236, 117538. [Google Scholar] [CrossRef]
- Saini, G.; Vattipalli, U. Assessing properties of alkali activated GGBS based self-compacting geopolymer concrete using nano-silica. J. Case Stud. Constr. Mater. 2020, 12, e00352. [Google Scholar] [CrossRef]
- Li, L.; Han, Q.; Yang, X.; Xiao, H.; Li, W.T.; Huang, S.P. Mechanical properties and micro-mechanisms of RHA-cement solidified sludge. J. China Civ. Eng. J. 2023, 56, 166–176. [Google Scholar]
- Yu, C.; Zhang, L.; Zheng, D.; Yang, F. Research progress of geopolymer materials prepared from solid waste and their applications. Sci. Sin. Technol. 2022, 52, 529–546. [Google Scholar] [CrossRef]
- Chen, R.; Jian, W.; Zhang, X.; Fang, Z.H. Experimental study on performance of sludge stabilized by CSFG-FR synergy. J. Rock Soil Mech. 2022, 43, 1020–1030. [Google Scholar]
- Amar, M.; Benzerzour, M.; Kleib, J.; Abriak, N.E. From dredged sediment to supplementary cementitious material: Characterization, treatment, and reuse. J. Int. J. Sediment Res. 2021, 36, 92–109. [Google Scholar] [CrossRef]
- Lv, O.F.; Wang, Z.S.; Gu, L.Y.; Chen, Y.; Shan, X.K. Effect of sodium sulfate on strength and microstructure of alkali-activated fly ash based geopolymer. J. Cent. South Univ. 2020, 27, 1691–1702. [Google Scholar] [CrossRef]
- Liu, R.; Ding, S.; Yan, P. Influence of Hydration Environment on the Characteristics of Ground Granulated Blast Furnace Slag Hydration Products. J. Bull. Chin. Ceram. Soc. 2015, 52, 1594–1599. [Google Scholar]
- Yi, Y.; Zheng, X.; Liu, S.; Al-Tabbaa, A. Comparison of reactive magnesia- and carbide slag-activated ground granulated blast furnace slag and Portland cement for stabilisation of a natural soil. J. Appl. Clay Sci. 2015, 111, 21–26. [Google Scholar] [CrossRef]
- Liang, S.; Dai, J.; Niu, J.; Wang, M.; Wang, L.; Dong, J. Solidification of additives for zinc-contaminated silt. J. Adv. Mech. Eng. 2018, 10, 1–13. [Google Scholar] [CrossRef]
- He, J.; Wang, X.; Shi, X.; Su, Z. Unconfined compressive strength and microscopic characteristics of soft soil solidified with soda residue and ground granulated blast furnace slag. J. Basic Sci. Eng. 2021, 29, 376–386. [Google Scholar]
- Dong, C.H.; Xie, X.Q.; Wang, X.L.; Zhan, Y.; Yao, Y.J. Application of Box-Behnken design in optimisation for polysaccharides extraction from cultured mycelium of Cordyceps sinensis. J. Food Bioprod. Process. 2009, 87, 139–144. [Google Scholar] [CrossRef]
- Asadzadeh, S.; Khoshbayan, S. Multi-objective optimization of influential factors on production process of foamed concrete using Box-Behnken approach. J. Constr. Build. Mater. 2018, 170, 101–110. [Google Scholar] [CrossRef]
- GB/T 50123-2019; Standard for Geotechnical Testing Methods. National Standard: Beijing, China, 2019.
- Bilim, C.; Karahan, O.; Atis, C.D.; Ilkentapar, S. Influence of admixtures on the properties of alkali-activated slag mortars subjected to different curing conditions. J. Mater. Des. 2013, 44, 540–547. [Google Scholar] [CrossRef]
- JTG/TF20-2015; Pavement Base Construction Technical Instructions. National Standards of the People’s Republic of China: Beijing, China, 2015.
- Cai, G.; Zhou, Y.; Poon, C.S.; Li, J.S. Physical and mechanical performance of quicklime-activated GGBS stabilized marine sediment at high water content. J. Rock Soil Mech. 2022, 43, 327–336. [Google Scholar]
- Li, S.; Zhang, H.; Wang, G.; Deng, R. Experimental study of alkali-activated geopolymer cured silty soil based on response surface method. J. Bull. Chin. Ceram. Soc. 2023, 42, 4438–4448. [Google Scholar]
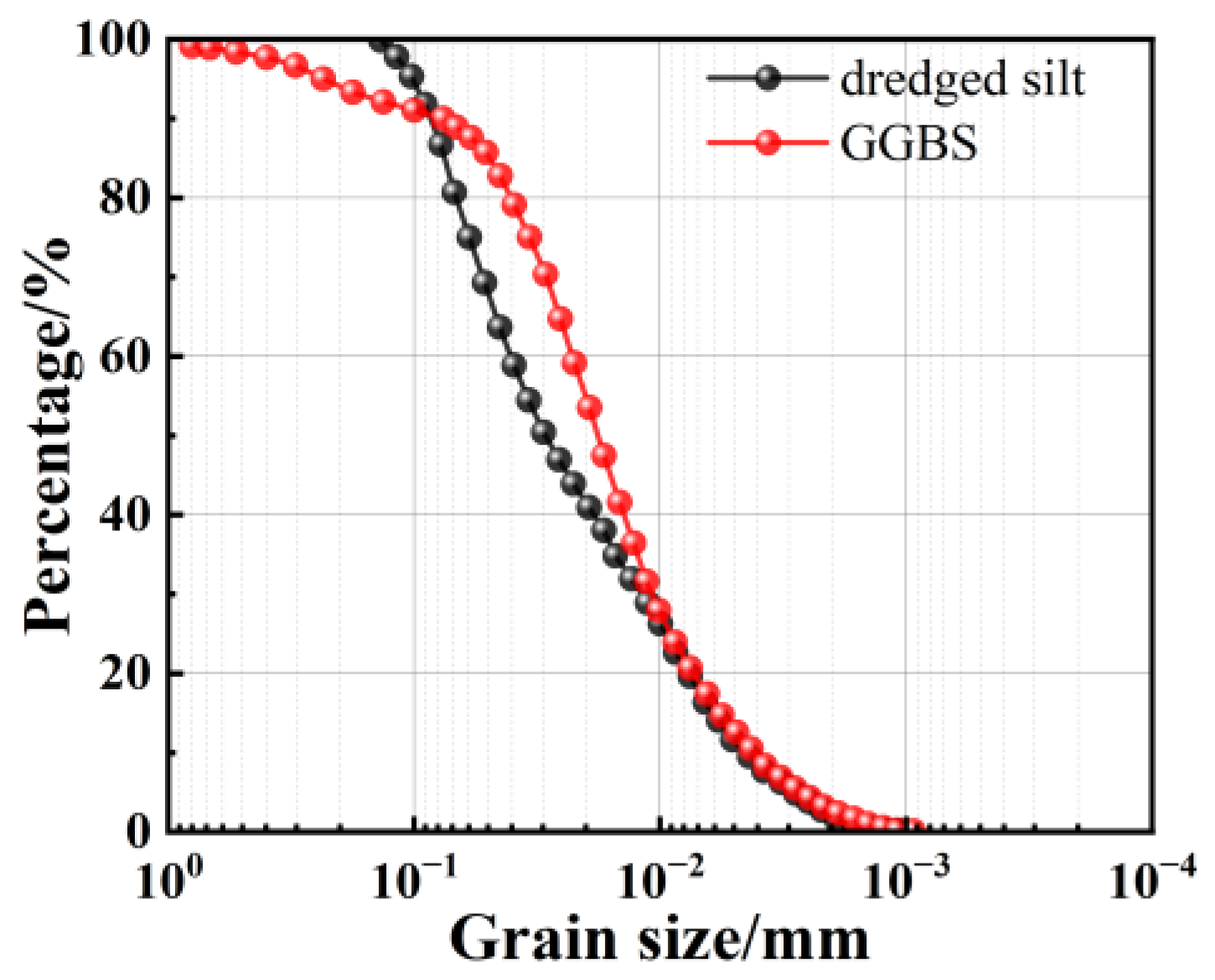





| Moisture Content/% | Relative Density | Liquid Limit/% | Plastic Limit/% | Plasticity Index | Liquidity Index |
|---|---|---|---|---|---|
| 59.2 | 2.61 | 46.1 | 25.9 | 20.2 | 1.64 |
| Materials | Mass Fraction/% | ||||
|---|---|---|---|---|---|
| CaO | SiO2 | Al2O3 | SO3 | Fe2O3 | |
| Dredged silt | 3.2 | 62.6 | 19.5 | 1.3 | 6.5 |
| GGBS | 42.0 | 33.0 | 12.0 | 1.67 | 1.0 |
| Stabilizing Agents | Additive Percentage/% | Curing Period (Days) |
|---|---|---|
| GGBS | 3, 6, 9, 12, 15 | 7, 14, 28 |
| CaO | 2, 3, 4, 5, 6 | |
| Na2O·nSiO2 | 2, 4, 6, 8, 10 |
| Independent Variable | Code Level | ||
|---|---|---|---|
| −1 | 0 | 1 | |
| A (GGBS)/% | 9.00 | 12.00 | 15.00 |
| B (CaO)/% | 3.00 | 4.00 | 5.00 |
| C (Na2O·nSiO2)/% | 4.00 | 6.00 | 8.00 |
| Sample | Independent Variable | Response Value | |||
|---|---|---|---|---|---|
| A/% | B/% | C/% | Y7d/kPa | Y28d/kPa | |
| 1 | −1 | −1 | 0 | 561 | 785 |
| 2 | 1 | −1 | 0 | 395 | 763 |
| 3 | −1 | 1 | 0 | 511 | 875 |
| 4 | 1 | 1 | 0 | 593 | 800 |
| 5 | −1 | 0 | −1 | 495 | 940 |
| 6 | 1 | 0 | −1 | 492 | 757 |
| 7 | −1 | 0 | 1 | 551 | 795 |
| 8 | 1 | 0 | 1 | 505 | 801 |
| 9 | 0 | −1 | −1 | 466 | 762 |
| 10 | 0 | 1 | −1 | 491 | 896 |
| 11 | 0 | −1 | 1 | 500 | 760 |
| 12 | 0 | 1 | 1 | 533 | 825 |
| 13 | 0 | 0 | 0 | 695 | 1014 |
| 14 | 0 | 0 | 0 | 658 | 1013 |
| 15 | 0 | 0 | 0 | 677 | 1024 |
| 16 | 0 | 0 | 0 | 695 | 1013 |
| 17 | 0 | 0 | 0 | 677 | 1048 |
| Source of Variance | Y7d | Y28d | ||||
|---|---|---|---|---|---|---|
| Coefficient | F-Value | p-Value | Coefficient | F-Value | p-Value | |
| Model | 680.4 | 48.31 | <0.0001 | 1022.4 | 61.02 | <0.0001 |
| A | −16.62 | 7.29 | 0.0306 | −34.25 | 26.8 | 0.0013 |
| B | 25.75 | 17.49 | 0.0041 | 40.75 | 37.94 | 0.0005 |
| C | 18.13 | 8.67 | 0.0216 | −21.75 | 10.81 | 0.0133 |
| AB | 62.00 | 50.7 | 0.0002 | −13.25 | 2.01 | 0.1997 |
| AC | −10.75 | 1.52 | 0.2568 | 47.25 | 25.5 | 0.0015 |
| BC | 2.00 | 0.053 | 0.8249 | −17.25 | 3.4 | 0.1078 |
| A2 | −76.08 | 80.35 | <0.0001 | −102.08 | 125.28 | <0.0001 |
| B2 | −89.33 | 110.77 | <0.0001 | −114.58 | 157.85 | <0.0001 |
| C2 | −93.57 | 121.57 | <0.0001 | −97.07 | 113.31 | <0.0001 |
| R2 | 0.9842 | 0.9874 | ||||
| Group | Std.Dev /kPa | Mean/kPa | R2 | Adjusted R2 | Predicted R2 | C.V./% | Adequate Precision |
|---|---|---|---|---|---|---|---|
| 7 d | 17.41 | 558.53 | 0.9842 | 0.9638 | 0.8490 | 3.12 | 20.198 |
| 28 d | 18.71 | 874.76 | 0.9874 | 0.9712 | 0.8657 | 2.14 | 19.398 |
| A/% | B/% | C/% | 28 d | 7 d | ||||
|---|---|---|---|---|---|---|---|---|
| Yp/kPa | Ya/kPa | D/% | Yp/kPa | Ya/kPa | D/% | |||
| 11.5 | 4.1 | 5.9 | 683.4 | 703.4 | 2.92 | 1032.4 | 1066.3 | 3.30 |
| (4) | ||||||||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hu, Q.; Yao, W.; Tao, G. Research on Alkali-Activated Slag Stabilization of Dredged Silt Based on a Response Surface Method. Materials 2024, 17, 4410. https://doi.org/10.3390/ma17174410
Hu Q, Yao W, Tao G. Research on Alkali-Activated Slag Stabilization of Dredged Silt Based on a Response Surface Method. Materials. 2024; 17(17):4410. https://doi.org/10.3390/ma17174410
Chicago/Turabian StyleHu, Qizhi, Wei Yao, and Gaoliang Tao. 2024. "Research on Alkali-Activated Slag Stabilization of Dredged Silt Based on a Response Surface Method" Materials 17, no. 17: 4410. https://doi.org/10.3390/ma17174410
APA StyleHu, Q., Yao, W., & Tao, G. (2024). Research on Alkali-Activated Slag Stabilization of Dredged Silt Based on a Response Surface Method. Materials, 17(17), 4410. https://doi.org/10.3390/ma17174410






